Abstract
Traditional Indian medical practices (Ayurveda, Siddha, Unani, and homeopathy) are a vast reservoir of knowledge about medicinal plants. The promising pharmacological properties of these plants have paved the way for developing therapy against novel Coronavirus (CoV) infection. The current review will summarize published works of literature on the effects of traditional Indian medicinal plants against acute respiratory infection (COVID‐19, SARS, Influenza, and Respiratory syncytial virus infection) and registered clinical trials of traditional Indian herbal medicines in COVID‐19. The current study aims to comprehensively evaluate the data of traditional Indian medicinal plants to warrant their use in COVID‐19 management. PubMed, Embase, and Cochrane databases were searched along with different clinical trial databases. A total of 22 relevant traditional Indian medicinal plants (35 relevant studies) were included in the current study having potential antiviral properties against virus‐induced respiratory illness along with promising immunomodulatory and thrombolytic properties. Further, 36 randomized and nonrandomized registered clinical trials were also included that were aimed at evaluating the efficacy of herbal plants or their formulations in COVID‐19 management. The antiviral, immunomodulatory, and thrombolytic activities of the traditional Indian medicinal plants laid down a strong rationale for their use in developing therapies against SARS‐CoV‐2 infection. The study identified some important potential traditional Indian medicinal herbs such as Ocimum tenuiflorum, Tinospora cordifolia, Achyranthes bidentata, Cinnamomum cassia, Cydonia oblonga, Embelin ribes, Justicia adhatoda, Momordica charantia, Withania somnifera, Zingiber officinale, Camphor, and Kabusura kudineer, which could be used in therapeutic strategies against SARS‐CoV‐2 infection.
Keywords: Ayurvedic medicine, COVID‐19, homeopathy, medicinal plants, respiratory syncytial virus infections, SARS virus, Unani medicine
1. INTRODUCTION
Infections caused by viruses pose a great challenge due to their wide spectrum of clinical presentations (Zou et al., 2020). Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) that causes coronavirus disease (COVID‐19) reported to affect more than 8 million people worldwide and has shown significantly high morbidity and mortality rates within a very short period (WHO, Situation Report—150, 2020). The critical cases of coronaviruses (CoV) are linked to severe acute respiratory infection (SARI), aberrant immune response (L. Chen et al., 2020; C. Huang et al., 2020; Z. Xu et al., 2020), and thrombosis (Connors & Levy, 2020; Helms et al., 2020). At present, there is no drug therapy available for this disease. The research is currently focused on three strategies, first to test broad‐spectrum antiviral drugs, second in silico screening, and finally repurposing of drugs (Wu et al., 2020).
Apart from these strategies, alternate medicines, for instance, traditional Indian medicines can be explored to find effective drug therapy against the current pandemic. There is a dearth of suitable therapeutic strategies for COVID‐19; therefore, it is imperative to peruse all the available options. Traditional Indian medicinal practices, for instance, Ayurveda, Siddha, Unani, and Homeopathy are some of the oldest practices that emerged in the first two centuries (common era). Thus, Indian traditional medicine is a vast reservoir of medicinal plants. Pharmacopoeia Commission for Indian Medicine & Homoeopathy (PCIM & H) contained the Pharmacopoeias and formularies of traditional Indian medicinal plants (Pharmacopoeia commission for Indian medicine & Homoeopathy, 2020). Practitioners have been using traditional Indian medicinal plants extensively for their antiviral, immunomodulatory, and thrombolytic activities for ages (Arora et al., 2011).
Influenza, CoV, and Respiratory syncytial viruses (RSV) all belong to the category of SARI and imposed a great burden on our healthcare (Boncristiani, Criado, & Arruda, 2009). These viruses affect both upper and lower respiratory tracts. The complications in these diseases range from fever to pneumonia with high morbidity and mortality (V. C. Cheng, Tang, Wu, Chu, & Yuen, 2004; Thompson & Zambon, 2009).
The study aimed to review the efficacy of traditional Indian medicinal plants in these viral‐induced respiratory illnesses (COVID‐19, SARS, RSV, and Influenza). Further, the study also reviewed the registered clinical trials of traditional Indian herbal medicines and their formulations against COVID‐19.
1.1. Similarities between CoVs and other viruses
In novel coronavirus, the positive single‐stranded RNA of length 30 kb is encircled in a nucleocapsid (N protein). An important protein present on the virus called spike protein S is a final determinant of its entry into the cell (de Wit, van Doremalen, Falzarano, & Munster, 2016) leading to dysregulation of multiple pathways (Figure 1).
FIGURE 1.

Pathophysiology of Coronaviruses. (1) SARS‐CoV & SARS‐CoV‐2 interact with ACE‐2 receptor, MERS‐CoV binds to DPP4 receptor; (2) Proteolytic cleavage at SARS‐CoV S protein at S2 position; (3) Membrane fusion followed by endocytosis; (4) Viral genome released; (5) Polyprotein and Structural protein released; (6) Viral replication takes place, Nucleocapsid formed from genomic RNA and nucleocapsid protein and ultimately ERGIC generate viral particle; (7) Double‐layer vesicles contain viral particle formed; (8) Vesicles fuse with plasma membrane and release virus; (9) Viral peptide presentation to antigen‐presenting cells and antigen‐presenting cells present antigen to T‐cells (CD4+ and CD8+); (10) B‐cells activated and expanded, releasing antibodies; (11) Clonal expression of T‐cells, which further releases cytokines and chemokines; (12) Storm of Cytokines and Chemokine shoot up; (13) This results in ARDS which may lead to mortality [Colour figure can be viewed at wileyonlinelibrary.com]
RSV is a single‐stranded, negative‐sense RNA virus whose receptors included toll‐like receptor‐4 (TLR‐4), heparan sulfate proteoglycans (HSPGs), and CX3 chemokine receptor 1 (CX3CR1), which are present at the cell surface. These receptors bind with viral RSV glycoprotein and restrain the virus to the surface of the cell. Nucleolin cell surface proteins help in the virus entry by activating the fusion of host and virus‐cell membranes. Furthermore, the virus hijacks the cellular machinery for its replication. RNA‐dependent replication cycle plays an important role in RSV multiplication (Griffiths, Drews, & Marchant, 2017; Ye & Wang, 2018).
The influenza virus is a single‐stranded RNA virus (Rossman & Lamb, 2011) encoding 11 proteins (Blackburne, Hay, & Goldstein, 2008; Tumpey et al., 2007). The hemagglutinin (HA) protein of the virus complexes binds to the sialic acid residues present on the alveolar epithelium of the target cell to carry out the endocytosis process. The entry of the virus into the lysosome triggers the acidification that activates the matrix protein‐2 viral channel resulting in membrane fusion leading to the detachment of the ribonucleoprotein core of the virus. The ribonucleoprotein is then transported to the nucleus for viral replication (Herold, Becker, Ridge, & Budinger, 2015; Rust, Lakadamyali, Zhang, & Zhuang, 2004).
COVID‐19, influenza, and RSV viruses exhibit a broad range of respiratory illnesses from asymptomatic to severe disease and death. The transmission factors for these viruses are common such as droplets, aerosols, and fomites (Figure 2) (Killingley & Nguyen‐Van‐Tam, 2013; Thompson & Zambon, 2009).
FIGURE 2.
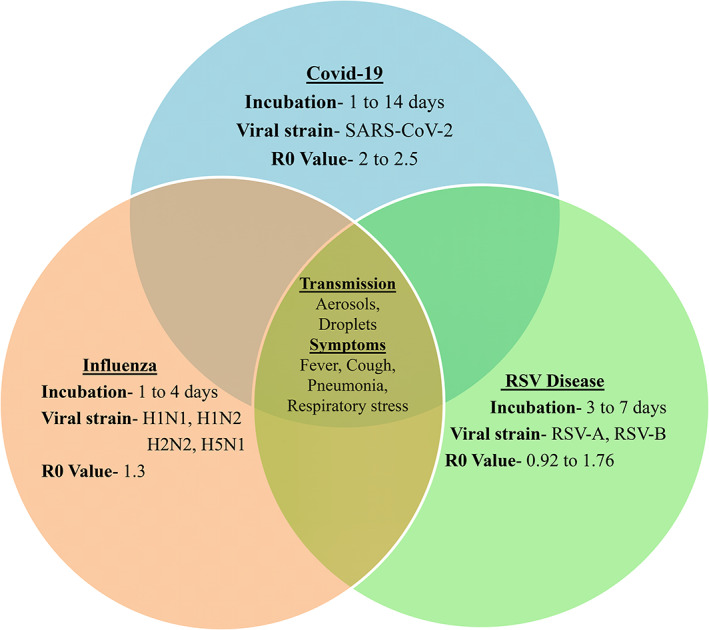
Common features in COVID‐19, RSV disease, and Influenza: Transmission factors and symptoms showed inside the Venn diagram are similar in all these diseases [Colour figure can be viewed at wileyonlinelibrary.com]
2. METHOD
2.1. Search strategy
Our study is a systematic review and follows the PRISMA guidelines (Moher et al., 2009). The PubMed, Embase, and Cochrane databases were searched using the following Mesh terms “medicinal plants” OR “herbal medicine” OR “ayurvedic medicine” OR “Unani medicine” OR “Siddha medicine” OR “homeopathy” OR “antiviral agents” OR “alternate medicine” AND “COVID‐19” OR “coronavirus” OR “Influenza, Human” OR “Respiratory Syncytial Virus Infections.” The present study includes clinical trials (randomized and nonrandomized), in silico, in vitro, and in vivo studies that evaluated the traditional Indian medicinal plants in viral‐induced respiratory illness like COVID‐19, SARS, MERS, influenza, and RSV. Pharmacopeias and formularies of Ayurveda, Siddha, Unani, and Homoeopathy systems of medicine along with traditional Indian medicinal plants databases were also searched. Plant names have been verified and upgraded with the online available databases such as the International plant name index (International Plant Names Index, 2020), plant list (The Plant List, 2013), and Indian medicinal plants database (Indian Medicinal Plants Database, National Medicinal Plants Board, 2020).
The Clinical trial databases were searched for the registered clinical trials of traditional Indian medicinal plants in COVID‐19 included ClinicalTrials.gov, Clinical Trial Registry‐India (CTRI), International Clinical Trials Registry Platform (ICTRP), European Union Clinical Trials Register, UMIN‐Clinical Trials Registry (UMIN‐CTR), and Australian New Zealand Clinical Trials Registry (ANZCTR) using keywords “medicinal plants” OR “herbal medicine” OR “Ayurvedic medicine” OR “Unani medicine” OR “Siddha medicine” OR “homeopathy” AND “COVID‐19” OR “coronavirus” OR“SARS‐CoV‐2” OR “2019‐nCoV” OR “severe acute respiratory syndrome‐coronavirus‐2.”
2.2. Types of studies
There was no restriction for study selection. In silico, in vitro, and in vivo studies were included that evaluated efficacy of traditional Indian medicinal plants in viral‐induced respiratory illnesses. For clinical trial data, randomized and nonrandomized clinical trials were included. Only articles in the English language were included. The databases were searched in the data range between January 1, 2000 and May 26, 2020. Only published articles were included along with all registered Clinical trials of Indian traditional medicinal plants in COVID‐19.
2.3. Types of outcome measures
The studies which evaluated the antiviral activity of traditional Indian medicinal plants in viral‐induced respiratory infections were included in the study. For clinical trials, the primary outcomes' measures were disease severity, mortality rate, clinical symptoms, number of serious patients, and number of days of hospitalization. The secondary outcomes that were evaluated were the number of days of clinical symptoms, comparative assessment of the severity of COVID‐19 in herbal treated, and control groups.
2.4. Study selection
The eligibility of the studies was assessed independently in an unblinded standardized manner. Disagreements between reviewers were resolved by consensus. A total of 1,877 studies were searched using the mentioned criteria out of which 71 studies were scrutinized for the study (Figure 3).
FIGURE 3.

Flow diagram for literature search. (*TIM—traditional Indian medicine; CTRI, Clinical trial registry India; ICTRP, International Clinical Trials Registry Platform; ANZCTR, Australian New Zealand Clinical Trials Registry, University Hospital medical information network clinical trial registry)
3. RESULTS
3.1. Potential traditional Indian medicinal plants for viral‐induced severe acute respiratory infection—A preclinical perspective
3.1.1. Acacia arabica (Lam.) Willd. (Leguminosae)
Acacia arabica is also known as gum arabic tree. It has many medicinal roles in Ayurveda and is used in the management of several health diseases, for instance, asthma, inflammation, cough, flu (Singh, Satapathy, & Prasad, 2020), Peste des petits ruminants (PPR) virus (Balamurugan et al., 2008), and goatpox virus (Bhanuprakash et al., 2008).
Ghoke et al. (2018) evaluated the antiviral activity of the leaves extract of Acacia arabica against the Influenza A virus subtype H9N2 (H9N2) using in ovo model in three different experimental procedures that is, virucidal, prophylactic, and therapeutic. The most suitable nontoxic dose of each extract of this compound was evaluated using viral load along with haemagglutination level and real‐time PCR (polymerase chain reaction). The study reported that its polyphenolic content and crude extract expressed promising antiviral activities against viral infections. Table 1 presents traditional Indian medicinal plants with antiviral properties.
TABLE 1.
Antiviral properties of traditional Indian medicinal plants
| Sr. No. | Indian medicinal plant | Common name | Component and dose of plant used | Assay used | Action | References |
|---|---|---|---|---|---|---|
| 1. | Acacia arabica | Gum arabic tree, Babul | Leaves (polyphenol); 135 mg/0.1 ml | H9N2 strain in embryonated chicken eggs; | Antiviral | Ghoke et al., 2018 |
| 2. | Achyranthes bidentata | Ox knee, putkanda |
Sulfated Achyranthes bidentata polysaccharide (sABPS) roots; 0.244–0.488 μg/ml Root extract |
PRRSV strain in MARC‐15 cells — |
Decrease viral titer Effective in bronchitis, fever, and pneumonia |
C. Liu et al., 2013 Singh et al., 2014 |
| 3. | Aloe vera | Aloe, chhota‐kanvar | Ethanol extract of leaves; 25 or 250 μg/ml | H1N1 in MDCK cells; | Antiviral; | J. G. Choi et al., 2019 |
| Aloin extracted from leaves; 100 μM aloin (in vitro); 2.5 mg/kg body weight (in vivo) | H1N1 in MDCK cells and in mice | Immunomodulator and Antiviral; | C. T. Huang et al., 2019 | |||
| 4. | Andrographis paniculata | Kariyat, Nilavembu kudineer | Andrographolide | SARS‐CoV‐2 in silico study; | High druggability and target accuracy; | Enmozhi, Raja, Sebastine, & Joseph, 2020 |
| Ethanol and water extract of leaves; 8.2 μg/ml (ethanol extract), 380.3 μg/ml (water extract) | H5N1strain in MDCK cells | Antiviral; | Sornpet, Potha, Tragoolpua, & Pringproa, 2017 | |||
| 5. | Bergenia ciliata | fringed elephant's ears, Pasanabheda (Sanskrit) | Methanolic extract of rhizome; 8 to 10 μg/ml | H1N1 strain in MDCK and Varo cell | High IC50 activity | Rajbhandari et al., 2009 |
| 6. | Cinnamomum cassia | Cinnamon, Dalchini | Nanoparticles of bark; 50, 100, and 200 μg/ml | H7N3 strain in varo cells | Antiviral | Fatima, Zaidi, Amraiz, & Afzal, 2016 |
| 7. | Curcuma longa | Turmeric, haldi | AgNPs from rhizomes; 0.12 nM and 0.24 nM | RSV strain in Hep‐2 cells | Immunomodulator and Antiviral; | X. X. Yang, Li, & Huang, 2016 |
| crude extracts of Curcuma longa; 69.3 μg/ml (ethanol extract), 142.3 μg/ml (water extracts) | H5N1 virus infection | Immunomodulator and Antiviral; | Sornpet et al., 2017 | |||
| Curcumin; 30 μM curcumin or MAC | PR8, H1N1 strain in MDCK cell | Antiviral; | Richart et al., 2018 | |||
| Curcumin; 50 mg/kg body weight | Acute Respiratory Distress syndrome; in vivo study | Antiviral; | Avasarala et al., 2015 | |||
| 8. | Cydonia oblonga | Quince | Ethanolic extract of Fruit; 0.5 mg/ml (as phenolics) | A/PR/8/34 strain in chicken erythrocyte Suspension; | Antiviral | Hamauzu, Yasui, Inno, Kume, & Omanyuda, 2005 |
| 9. | Embelia ribes Burm | False black pepper, white‐flowered embelia a | Ethyl acetate extract of Fruit; 0.2 μg/ml | H1N1, H3N2, H5N2 in MDCK cells | Antiviral | Hossan et al., 2018 |
| 10. | Glycyrrhiza glabra | liquorice | Glycyrrhizin component; 4,000 mg/L | FFM‐1 and FFM‐2 in Vero cells; | Block replication, adsorption and penetration; | Cinatl et al., 2003 |
| Water soluble Glycyrrhetinic acid conjugates; 50 μM | H1N1 in MDCK cells; | Decreased viral titer; | Liang et al., 2019 | |||
| Constituents of roots; 1.70 μg/ml (methanol extract), 0.33 μg/ml (aglycone‐enriched fraction) | H1N1 strain in MDCK cells; | NA inhibition | Grienke et al., 2014 | |||
| 11. | Hypericum perforatum | St. John's wort | Methanol and Ethyl acetate; 78.13 μg/ml (H. perforatum ethyl acetate), 39.06 μg/ml (H. perforatum water) 480–120 mg/kg (in vivo) | IBV strain in CEK cells and SPF chickens; | Antiinflammatory, immunomodulator, decreased mRNA expression and viral titer; | H. Chen et al., 2019 |
| Ethanol extract of dry plant; 30 μg/ml (in vitro), 110 mg/kg (in vivo) | H1N1 strain in A549 cells and Balb/c mice; | Antiinflammatory, immunomodulator; | N. Huang et al., 2013 | |||
| Hypericum perforatum extract (HPE) 50–200 mg/kg | influenza A virus (IAV) in Balb/c mice | Immunomodulator and decreased viral titer; | Xiuying et al., 2012 | |||
| 12. | Jatropha curcas | purging nut, jablota | Aqueous and methanolic extract leaf; 15 mg to 1 mg/ml. (methanol extract), 25 and 5 mg/ml (aqueous extract) | H1N1 strain in MDCK cell | Block viral adsorption | Patil et al., 2013 |
| 13. | Justicia adhatoda | Malabar nut | Methanolic extract of leaf; 10 mg/ml | Standard influenza strain in MDCK cells | Reduction in HA, block viral attachment and replication | Chavan & Chowdhary, 2014 |
| 14. | Momordica charantia | Bitter cucumber | Seeds; 1.401 mg/ml | H1N1, H3N2, H5N1 strains in MDCK cells | Decreased viral titer | Pongthanapisith, Ikuta, Puthavathana, & Leelamanit, 2013 |
| 15. | Ocimum tenuiflorum | Holy basil, Thai basil, Tulsi | Leaves (crude extract, terpenoid and polyphenols); 135 mg/0.1 ml | H9N2 strain in embryonated chicken eggs; | Antiviral; | Ghoke et al., 2018; |
| Phytocompounds‐ Apigenin; | H1N1 in silico study; | High binding energy; | Alhazmi, 2015 | |||
| 16. | Psoralea corylifolia | Bawchan seed | Bakuchiol, a naturally occurring phenolic isoprenoid; 12.5–100 μM | H1N1 and H3N2 strains in MDCK cells | Immunomodulator and antioxidant | Shoji et al., 2015 |
| 17. | Syzygium cumini | Jambolan, java plum Indian blackberry | Aqueous crude extract of leaves and bark; 1.28 μg/ml and 8.69 μg/ml* | H5N1 in MDCK cells | Antiviral | Sood et al., 2012 |
| 18. | Tinospora cordifolia | Heart‐leaved moonseed, Gulbel | Berberine, Isocolumbin, Magnoflorine, and Tinocordiside | SARS‐CoV‐2 proteins under in silico studies | Antiviral | Sagar & Kumar, 2020 |
| (1,4)‐alpha‐D‐glucan (alpha‐DG) drug; 10 mg/kg | Lung and Spleen of Endotoxin‐Stimulated Juvenile Rats | Immunomodulator; Antiviral | Velazquez, Kimura, Torbati, Ramachandran, & Totapally, 2009 | |||
| 19. | Viscum album | Mistletoe | Aqueous extract of leaves; 1 μg/ml (aqueous extract) | HPIV2 strain in Varo cells | Reduced viral replication | Karagöz, Onay, Arda, & Kuru, 2003 |
| 20. | Withania somnifera | Ashwagandha | Withaferin A | In silico study | High binding affinity to NA | Cai et al., 2015 |
| 21. | Woodfordia fruticosa | Fire Flame Bush, Red Bell Bush, ban‐mahendi (hindi) | Gallic acid extracted from flowers; 100 μg/ml | Hella, HRV2 and HRV3 cells | Inhibit viral replication and antioxidant | H. J. Choi, Song, Bhatt, & Baek, 2010 |
| 22. | Zingiber officinale | Ginger | Aqueous extract of Fresh ginger rhizomes; 300 μg/ml | HRSV strain in HEp‐2 and A549 cell lines; | Antiviral; | Chang, Wang, Yeh, Shieh, & Chiang, 2013 |
| Aqueous extract of ginger rhizomes; 10% concentration of aqueous extract | H9N2 strain in embryonated chick eggs; | Antiviral; | Rasool et al., 2017 |
Abbreviations: H1N1, H3N2, H5N1, H7N3, H9N2, Influenza A virus subtypes; HPIV‐2, human parainfluenza virus type 2; HRV, Human rhinovirus; IAV, influenza A virus; IBV, Infectious Bronchitis Virus; MDCK, Madin‐Darby canine kidney; PRRSV, porcine reproductive and respiratory syndrome virus; RSV, Respiratory Syncytial Virus Infection; SPF, Specific pathogen free.
3.1.2. Achyranthes bidentata Blume (Amaranthaceae)
Achyranthes bidentata is commonly known as ox knee and is indigenous to the hilly districts of India, China, and Japan. It is widely used as antiarrhythmic, laxative, ecbolic, antihypertensive, antiviral, antispasmodic, anticoagulant, and antitumor (Devi et al., 2007).
Achyranthes bidentata was found to be effective in bronchitis, which is a type of bronchial tube inflammation caused by certain viruses like coronaviruses, influenza, rhinovirus, and adenovirus (Peiris et al., 2003). The root extract was found to be effective in bronchitis, cough, asthma, pneumonia, and fever (Singh et al., 2014).
The sulfated Achyranthes bidentata polysaccharide (ABPS) has a potent antiviral activity. The potency of ABPS was evaluated against porcine reproductive and respiratory syndrome virus (PRRSV) strain that is, (VR2332) in MARC‐145 (African green monkey kidney‐derived cells) cells. The sulfated form showed antiviral activity even at lower concentrations when compared to nonmodified ABPS in MARC‐145 cells (C. Liu et al., 2013).
The immunomodulatory effect of Achyranthes bidentata polysaccharide (ABP) was evaluated in underdeveloped piglets. The study illustrated increased lymphocyte proliferation, serum IgG (Immunoglobulin type G), IgM, IgA, IL‐2 (Interleukin type‐2), and IFN‐γ (Interferon‐gamma) levels. Moreover, it also increased the production of peripheral T‐cells and splenic lymphocytes (Q. Chen, Liu, & He, 2009). Table 2 presents traditional Indian medicinal plants with immunomodulatory properties.
TABLE 2.
Traditional Indian medicinal plants as immunomodulators
| Sr. No. | Plant name | Component | Immunologic RESPONSE | Reference |
|---|---|---|---|---|
| 1. | Achyranthes bidentata | Achyranthes bidentata polypeptide k (ABPPk) from roots | Increased lymphocyte level of IgA, IgG, IgM, IL‐2, and IFN‐γ and also increased splenic lymphocytes and peripheral T‐cells | Q. Cheng et al., 2019 |
| 2. | Andographis paniculata | HN‐02, (andrographolides + 14‐deoxyandrographolide +14‐deoxy‐11,12‐didehydroandrographolide) crude powder |
Improved macrophage phagocyte system‐innate immunity Ameliorate humoral response in Hemagglutination antibody (HA) titer against sheep red blood cells |
Naik & Hule, 2009 |
| 3. | Andographis paniculata | Andrographolide |
In vivo—Decreased production of anti‐HBs antibody and number of IL‐4 inducing splenocytes In vitro—decreased the macrophages activation induced by LPS or IL‐4, cytokine expression of M1 and M2, IL‐12/IL‐10 ratio and mannose receptor (CD206) |
Wang et al., 2010 |
| 4. | Andographis paniculata | Hot water extract of raw herb |
Regulated the population of regulatory T cells, production of cytokines in model of esophageal tumorigenesis Significantly increased the tumors apoptosis by suppressing the growth of cell line‐based xenografts In vitro—proliferation of human peripheral blood mononuclear cells, as well as TNF‐α and IFN‐γ productions |
Yue et al., 2019 |
| 5. | Aloe Vera | Aqueous gel extract from leaves | Increased cell viability of infected macrophages | Farahnejad, Ghazanfari, & Yaraee, 2011 |
| 6. | Aloe Vera | Aloin from leaves | Boosted host immunity with enhance the hemaggluttin‐specific T cell response | C. T. Huang et al., 2019 |
| 7. | Bergenia ciliata | Ethanol leaves extract | CD69 increased on lymphocytes | Tumova & Vokurková, 2018 |
| 8. | Cinnamomum Cassia | 2′‐hydroxycinnamaldehyde (HCA) and 2′‐benzoxycinnamaldehyde (BCA) from stem bark using methanol | Lymphoproliferation suppressed and T‐cell differentiation stimulated | Koh et al., 1998 |
| 9. | Curcuma longa | Aqueous extract of dried rhizome | Stimulative effect on proliferation of PBMC and production of cytokine | Yue, Chan, Hon, Kennelly, et al., 2010; Yue, Chan, Hon, Lee, et al., 2010 |
| 10. | Curcuma longa | Curcuminoids and alpha‐turmerone from dried rhizome using ethanol | Production of cytokines and PBMC proliferation stimulated | Yue, Chan, Hon, Kennelly, et al., 2010; Yue, Chan, Hon, Lee, et al., 2010 |
| 11. | Cydonia oblonga | peel, pulp and seeds hot water extract | IL‐13, TNF‐α, Leukotriene C(4) and prostaglandin, COX‐2 reduced in BMMC | Kawahara & Iizuka, 2011 |
| 12. | Cydonia oblonga | whole crushed | Reduced level of IL‐8 and TNF‐α in human mast cells | Huber et al., 2012 |
| 13. | Embelia ribes | Embelin (present in berries of plant) ‐ in silico study | TNF‐alpha decreased | Dhanjal et al., 2014 |
| 14. | Glycyrhiza glabra | Aqueous root extract | HA titer and antibody‐secreting cells in mouse spleen | Mazumder, Pattnayak, Parvani, Sasmal, & Rathinavelusamy, 2012 |
| 15. | Hypericum perforatum | Hyperforin | Enhances immunity by modulating phagocytosis | Brondz & Brondz, 2012 |
| 16. | Hypericum perforatum | ethanol extract of dry plant | Suppresses IFN‐γ and monocyte chemotactic protein | N. Huang et al., 2013 |
| 17. | Jatropha curcus | Aqueous methanolic extract and its derivatives | Increased level of lymphocytes, macrophages cells, and antibody titer | Abd‐Alla, Moharram, Gaara, & El‐Safty, 2009 |
| 18. | Justicia adhatoda | Chloroform, methanolic and diethyl ether extracts from leaves | Increased percentage of neutrophil adhesion, and it also leads to delayed type hypersensitivity in rats | Vinothapooshan & Sundar, 2011 |
| 19. | Momordica charantia | polysaccharide extract from dried fruit extract using ethanol | Increased the production of serum haemolysin, thymus index, spleen index, and natural killer cell cytotoxicity | Deng et al., 2014 |
| 20. | Ocimum sanctum | Petroleum ether seeds extract | Balanced both cell and humoral‐mediated immune response | Mediratta, Sharma, & Singh, 2002 |
| 21. | Ocimum sanctum | Aqueous leaves extract | Enhanced the lymphocyte and neutrophil counts along with increase in phagocytic index and phagocytic activity | Mukherjee, Dash, & Ram, 2005 |
| 22. | Psoralea corylifolia | Ethanol seeds extract | Stimulated antibody‐dependent cellular cytotoxicity, NK cell activity, antibody complement‐mediated cytotoxicity during development of tumor, and inhibition of growth of tumor | Latha, Evans, Panikkar, & Jayavardhanan, 2000 |
| 23. | Tinospora cordifolia | ethyl acetate, water fractions and hot water stem extract | Increased phagocyctosis percentage | U. Sharma et al., 2012 |
| 24. | Tinospora cordifolia | G1‐4A (polysaccharide) | Upregulation of IL‐β, IL‐12, TNF‐α, IFN‐γ, IL‐10 and nitric oxide | Gupta, Rajan, & Kulkarni, 2017 |
| 25. | Tinospora cordifolia | Guduchi ImP from dry stem powder using water | Significantly increased anti‐guduchi Imp IgG and IgA, anti‐OVA IgG and IgA after administration of guduchi Imp | Aranha & Venkatesh, 2018 |
| 26. | Tinospora cordifolia | Aqueous extract | Increased the production of TNF‐ α, IFN‐ γ and IL‐1 β in cyclophosphamide‐treated mice | Alrumaihi et al., 2019 |
| 27. | Viscum album |
Peptide Iscador (isotonic injectable Preparation and concentrated plant extract) |
Natural killer cell activity enhanced, antibody dependent cellular cytotoxicity stimulated | Kuttan & Kuttan, 1992 |
| 28. | Viscum album | Iscador M (= mali from apple tree mistletoe) and Iscador P (= pini, from pine mistletoe) | Normalization of initial immune indices | Chernyshov et al., 2000 |
| 29. | Withania somnifera | Methanol Root extract |
Increased circulating antibody titer and number of plaque forming cells in spleen, Phagocytic action of peritoneal macrophages increased |
Davis & Kuttan, 2000 |
| 30. | Withania somnifera | Withania somnifera (WS) extract and Withaferin A | Reduction in phagocytosis, up‐regulation of TLR6, restoration of phagocytic activities | J. Kumar, Mitra, Hussain, & Kaul, 2019 |
| 31. | Woodfordia fruticosa | Ethanol Flowers extract | Stimulation of macrophages, bone marrow cells and nonspecific immune responses | Shah & Juvekar, 2010 |
| 32. | Zingiber officinale | Essential oil extract | Recovered the humoral immune activity in immunosuppressed mice | Carrasco et al., 2009 |
| 33. | Zingiber officinale | Ethanol rhizome extract | Regulated expression of IL‐33 and IL‐27 | Jafarzadeh et al., 2014 |
Abbreviations: ABPPk, Achyranthes bidentata polypeptide k; COX‐2‐cyc, looxygenase‐2; HA, Hemagglutination antibody; IFN‐γ, Interferon gamma; IgA, IgG, IgM, Immunoglobulins; IL‐2, IL‐4, IL‐12/IL‐10, IL‐13, IL‐β, IL‐12, IL‐33 and IL‐27, Interleukins; LPS, Lipopolysaccharides; PBMC, peripheral blood mononuclear cell; TLR 6, Toll like receptors 6; TNF‐α, Tumor necrosis factor alpha.
Achyranthes bidentata polypeptide k (ABPPk) has a potential thrombolytic activity. It can prevent oxidative damage of brain endothelial cells caused by ischemia and also leads to NF‐κB (nuclear factor kappa‐light‐chain‐enhancer of activated B cells) and plasminogen activator inhibitor‐1 activation. ABPPk inhibited the neutrophils, basophils, and eosinophils infiltration along with the activation of various MMP‐2/‐9 (matrix metalloproteinases) in the ischemic penumbra and alleviated neurological functions (Q. Cheng et al., 2019). Table 3 presents traditional Indian medicinal plants with thrombolytic properties.
TABLE 3.
Thrombolytic properties of traditional Indian medicinal plants
| Sr. No. | Plant name | Component | Thrombolytic activity | Reference |
|---|---|---|---|---|
| 1. | Achyranthes bidentata | Achyranthes bidentata polypeptide k (ABPPk) from roots | Inhibited oxidative damage induced in endothelial cells of brain, enhanced the tissue factor activation, plasminogen activator inhibitor −1 and MMP‐2/9 activation | Q. Cheng et al., 2019 |
| 2. | Cinnamomum Cassia | Active compounds obtained from methanol extract of twigs | Cinnamic alcohol, methoxycinnamaldehyde, eugenol, coniferaldehyde, amygdalactone, and hydroxycinnamaldehyde effective in inhibition of platelet coagulation | Kim et al., 2010 |
| 3. | Curcuma longa | Aqueous extract | Clot lysis activity (32.94 ± 3.663%) | I. N. Khan et al., 2011 |
| 4. | Cydonia oblonga | Aqueous extract of leaves | Decreased plasma concentration of thromboxane B2 and increased 6‐keto‐prostaglandine F1α | Zhou et al., 2014 |
| 5. | Embelia Ribesm | Ethanol fruit extract |
Reduced adenosine diphosphate and thrombin‐induced platelet aggregation Inhibit malondialdehyde production |
Jagtap, Sancheti, & Phadke, 2012 |
| 6. | Glycyrrhiza glabra | Glycyrrhetinic acid | Inhibit factor Xa and platelet aggregation | Jiang, Wang, Shen, Xiao, & Li, 2014 |
| 7. | Justicia adhatoda | Methanolic leaves extract | Lysis of almost 80% clot | Shahriar 2013 |
| 8. | Momordica charantia | Seed extract | Role of seed extract in intrinsic pathway of blood coagulation cascade enhanced the clotting time of blood | Manjappa et al., 2015 |
| 9. | Tinospora cordifolia | Caps HT2, a herbal formulation | Inhibited platelet aggregation, increased the synthesis of lipoprotein lipase enzyme, and delaying calcification of plasma | Mary, Babu, & Padikkala, 2003 |
| 10. | Withania somnifera | Withaferin A (WFA) | Increased bleeding time in an in vivo and ex vivo, prevented PAI‐1 production induced by TNF‐α, decreased PAI‐1/t‐PA ratio significantly | Ku & Bae, 2014 |
| 11. | Zingiber officinale | Aqueous roots extract | Lowered serum PGE(2), TXB(2), decreased in serum cholesterol | Thomson et al., 2002 |
Abbreviations: ABPPk, Achyranthes bidentata polypeptide k; MMP‐2/9, Matrix metalloproteinase‐2/9; TNF‐α, Tumor necrosis factor alpha; PGE(2), Prostaglandin E2; TXB(2), Thromboxane B2.
3.1.3. Aloe vera (L.) Burm.f. (Xanthorrhoeaceae)
Traditionally, Aloe vera acts as an antioxidant, antibacterial (Nejatzadeh‐Barandozi, 2013), and antiviral. The activity of ethanol extract of Aloe vera was evaluated against influenza A virus in Madin‐Darby canine kidney (MDCK) cells. The extract significantly inhibited autophagy induced by the viral strain (J. G. Choi et al., 2019). C. T. Huang et al. (2019) assessed the antiviral efficacy of Aloin (Aloe vera derivative) against Influenza A virus subtype H1N1 (H1N1) in MDCK cells. Aloin significantly decreased viral infections. Also, when H1N1 induced mice were treated with Aloin, the viral load and mortality significantly decreased. These results supported the application of the derivative in clinical use.
Neuraminidase (NA) is an enzyme that hydrolyses sialic acid residue of viruses and receptors of the host. It also helps in virus movement and plays an important role in escaping viruses from the host cell after replication. Aloin inhibited the NA, which further leads to immune suppression, and inhibition of virus replication. Aloin increased the immunity along with an increased hemagglutinin‐specific T‐cell response to the infection (C. T. Huang et al., 2019). Aloe vera aqueous gel extract from leaves showed a significant immunomodulatory effect by increasing the cell viability of infected macrophages (Farahnejad et al., 2011).
3.1.4. Andrographis paniculata (Burm.f.) Nees (Acanthaceae)
Generally called Kariyat, Andrographis paniculata is a herb that is harvested annually and has a common occurrence throughout India (Rajagopal, Kumar, Deevi, Satyanarayana, & Rajagopalan, 2003).
Sornpet et al. (2017) studied the crude extract of Andrographis paniculata in Influenza A virus subtype H5N1 (H5N1) strain using MDCK cells. The study observed a significant decrease in the virus titer in the treatment group. Enmozhi et al. (2020) observed andrographolide (an Andrographis paniculata extract) fitted with the inhibitor site of one of the proteases that play a central role in the SARS‐CoV‐2 virus that is, main protease (Mpro). In silico studies highlighted Andrographolide scored high in the docking study. The docking affinity of the compound was found to be significantly higher than the synthetic compounds like disulfiram, tideglusib, and a combination of lopinavir, oseltamivir, and ritonavir. The in silico study of andrographolide showed significantly high druggability, permeability, solubility, and target accuracy.
Andrographis paniculata has also been observed to inhibit increased levels of NOD‐like receptor protein‐3 (NLRP3), caspase‐1, and interleukin‐1β molecules involved in SARS‐CoV mechanism (Y. T. Liu, Chen, et al., 2020; Z. Liu, Xiao, et al., 2020).
The hot water extract of Andrographis paniculata illustrated significant immunomodulatory activity through its multitarget efficacy in esophageal cancer management (Yue et al., 2019). HN‐02 (a mixture of different Andrographis paniculata extract) ameliorated cyclophosphamide‐induced immune suppression and stimulated the humoral and cell‐mediated immunity (Naik & Hule, 2009). Andrographolide regulated the macrophage activation via mitogen‐activated protein kinase (MAPK) and phosphoinositide 3‐kinase (PI3K) signaling along with targeted antibody secretion (Wang et al., 2010).
3.1.5. Bergenia ciliata (Haw.) Sternb. (Saxifragaceae)
Bergenia Ciliata is commonly known as fringed elephant's ears, is native to China, and the Himalayas, and is commonly used in the management of fever, diarrhea, and chest infection (Saha & Verma, 2011).
Rajbhandari et al. (2009) assessed the antiviral potency of methanol extract of Bergenia ciliata rhizome against human influenza virus A/WSN/33 (H1N1) in MDCK and Vero cells. The extract significantly inhibited the virus strain with the concentration ranging from 30 to 50 μg ml−1 that significantly lowers the cell viability by 50%and half‐maximal inhibitory concentration (IC50) values from 8 to 10 μg ml−1.
One of the studies evaluated the immunostimulatory activity of lyophilized ethanol extract of Bergenia ciliata leaves. The findings illustrated the increased production of CD69, a human transmembrane glycoprotein that is involved in lymphocyte growth and function (Tumova & Vokurková, 2018).
3.1.6. Cinnamomum cassia Nees ex Blume (Lauraceae)
Traditionally, Cinnamon is used as a healthy herbal spice. It is extracted from the inner bark of a tree that belongs to Cinnamomum (Bae, Nam, & Park, 2002).
Fatima et al. (2016) determined the antiviral properties and characteristics of Cinnamon silver (Ag) nanoparticles against influenza virus infection in Vero cells. The nanoparticles illustrated significant antiviral properties in treated groups along with a significant safety profile. This possible strategy of synthesized nanoparticles may be an option for the effective treatment of virus infections.
Koh et al. (1998) determined two cinnamaldehyde derivatives that is, 2′‐hydroxycinnamaldehyde (HCA) and 2′‐benzoxy‐cinnamaldehyde (BCA) from Cinnamomum cassia and evaluated their immunomodulatory effects. The study concluded that these derivatives have the potency to block the proliferation of lymphocytes and T‐cells differentiation by inhibiting the signaling pathway of cell growth.
Thirteen compounds were extracted from Cinnamomum cassia out of which hydroxycinnamaldehyde, methoxycinnamaldehyde, amygdalactone, eugenol, coniferaldehyde, and cinnamic alcohol were active compounds that significantly inhibited arachidonic acid‐induced platelets aggregation and a thromboxane A2 induced aggregation in the preliminary testing (Kim et al., 2010).
3.1.7. Curcuma longa L. (Zingiberaceae)
Curcumin belongs to the ginger family and is prepared from Curcuma longa rhizomes. It has various noted pharmacological properties like antiinflammatory, antioxidant, antibacterial, and antiviral (Araújo & Leon, 2001).
Sornpet et al. (2017) used the crude extracts of Curcuma longa against H5N1 virus infection to evaluate its antiviral activity in MDCK cells. The study found that the treatment of Curcumin exhibits a significant rise in the level of TNF‐α (tumor necrosis factor) and IFN‐β at mRNA level in cells, which indicates its role in inhibiting virus replication.
X. X. Yang et al. (2016) used Curcumin‐modified silver nanoparticles (cAgNPs) to evaluate its effectiveness against RSV infection in human epithelial type 2 (HEp‐2) cells and found decreased viral loads in the cell line.
Avasarala et al. (2015) observed the antiviral efficacy of curcumin in a rodent model of acute pneumonia (Acute Respiratory Distress Syndrome). Curcumin significantly alleviated the fibrosis and inflammation in the lungs.
Richart et al. (2018) evaluated the synergic virucidal activity of curcumin and monoacetylcurcumin (a structural analog of curcumin) against influenza virus infection in MDCK cells. The combination significantly inhibited viral NA activity and PI3K/Akt (phosphatidylinositol 3‐kinase (PI3K)/protein kinase B) pathway (important for viral replication) thereby decreasing viral replication.
Alpha‐turmerone (Curcuma longa extract) showed a significant immunomodulatory effect in human peripheral blood mononuclear cells (PBMC). Alpha‐turmerone triggered peripheral blood mononuclear cells (PBMCs) proliferation and the release of various cytokines, for instance, TNF‐α, IFN‐γ, and IL‐2 (Yue, Chan, Hon, Kennelly, et al., 2010; Yue, Chan, Hon, Lee, et al., 2010). Polar fractions of Curcuma longa hot water extracts modulated the cytokine production and showed stimulatory effects on PBMCs that further advocated its use as an adjuvant in cancer therapy (Yue, Chan, Hon, Kennelly, et al., 2010; Yue, Chan, Hon, Lee, et al., 2010).
I. N. Khan et al. (2011) determined the thrombolytic activity of Curcuma longa in an in vitro thrombolytic model. The study found that Curcuma longa exhibited moderate clot lysis activity that was 32.94 ± 3.663% as compared to streptokinase (standard) that showed 86.2 ± 10.7% clot lysis effect.
3.1.8. Cydonia oblonga Mill. (Rosaceae)
Cydonia oblonga, commonly known as Quince, mainly found in sub‐Himalayan regions is effective against diabetes, inflammation, cancer, and ailments related to the heart and brain (R. Sharma, Joshi, & Rana, 2011).
Phenolic extract of Quince fruit significantly decreased the influenza viral titer (hemagglutinin =27) when compared to the control group (HA = 210). One of the components of phenolic extract that is, procyanidins was found to have strong antiviral activity and prevent infection in the throat (Hamauzu et al., 2005).
Cydonia oblonga increased thrombolysis and shortened euglobulin lysis time. It also reduced the arterial and venous thrombus weights in a dose‐dependent manner. Furthermore, treatment of Cydonia decreased the thromboxane B2 (TXB2) level and increased the level of 6‐keto‐prostaglandin F1α (6‐keto‐PGF1α) in plasma (Zhou et al., 2014).
3.1.9. Embelia ribes Burm.f. (Primulaceae)
Embelia Ribes commonly called white‐flowered embelia or false black pepper was found in northern western ghats of Maharashtra state of India. The National Medicinal Plant Board put it on a priority species list for cultivation. It has great importance in traditional medicine as analgesic, antiinflammatory, antibacterial, and antioxidant (Mhaskar et al., 2011).
Hossan et al. (2018) assessed ethyl acetate extract of Embelia ribes fruits using different strains of influenza (H1N1, H3N2, and H5N2) in MDCK cells. Selectivity index (SI) and IC50 values of the fruit extract of Embelia ribes and Embelin (Embelia ribes extract) have shown potential antiviral properties of these extracts. H5N2 strain was the most vulnerable to embelin (SI = 31), while the H3N2 virus strain was more resistant (SI = 5).
During inflammation, TNF‐α converting enzyme cleaves the transmembrane protein that is, TNF‐α to release it in the extracellular space. Embelia Ribes was found to reduce the secretion of TNF‐α thereby act as an antiinflammatory and immunomodulatory agent (Dhanjal et al., 2014).
Adenosine diphosphate and thrombin‐induced platelet aggregation were significantly reduced by ethanol extract of Embelin ribes and moreover, it also inhibited malondialdehyde production in platelets. Arteriovenous shunt and stasis‐induced thrombosis were significantly reduced by embelin in rats (Jagtap et al., 2012).
3.1.10. Glycyrrhiza glabra L. (Leguminosae)
Glycyrrhiza glabra, locally known as “liquorice” and “mulaithi,” is found mainly in the Punjab and Sub‐Himalayan regions of India (Shibata, 2000). This herb is employed abundantly in traditional Indian medicine as antiinflammatory and antiviral agents (Damle, 2014).
Glycyrrhizin extracted from Glycyrrhiza glabra showed potency in inhibiting adsorption, penetration, and replication of SARS‐CoV in Vero cell lines (SI = 67). The extract exhibited the virucidal property by inhibiting the cellular signaling pathways of the virus. Moreover, glycyrrhizin and glycyrrhetinic acid, an aglycone metabolite, increased nitrous oxide expression (Cinatl et al., 2003).
Grienke et al. (2014) determined the antiviral efficacy of Glycyrrhiza glabra roots extract. The study used NA inhibition in chemiluminescence (CL)‐based assay along with additional tests. Out of different compounds, three compounds showed promising results (Tanimoto Combo value>0.70).
Liang et al. (2019) evaluated 18 water‐soluble β‐cyclodextrin (CD)‐G (Glycyrrhiza glabra conjugates) for antiviral activities in A/WSN/33 (H1N1) virus. A total of six conjugates showed potential antiviral activity when evaluated in a cytopathic effect assay.
The aqueous root extract of Glycyrrhiza glabra is a potent immunomodulator that significantly increased HA titer and antibody‐secreting cells along with Zinc in a mouse spleen (Mazumder et al., 2012).
The glycyrrhetinic acid showed antithrombotic activity by inhibiting the platelet aggregation and the factor involved in the clotting pathway that is, factor Xa in the rat venous stasis model (Jiang et al., 2014).
3.1.11. Hypericum perforatum L. (Hypericaceae)
Generally known as St John's Wort, Hypericum perforatum is a herb, which grows mainly in Asia, Africa, and Australia and has antiinflammatory, antidepressant, anxiolytic, and nootropic activities (V. Kumar, Singh, & Bhattacharya, 2001).
H. Chen et al. (2019) assessed Hypericum perforatum ethyl acetate (HPE) extract in specific pathogen‐free (SPF) chicken embryos along with kidney cells using infectious bronchitis strain (IBS‐M41). Under in vitro conditions, HPE significantly reduced viral titer and mRNA expression. Under in vivo conditions, HPE ameliorated IBS‐M41‐induced inflammation in the trachea and in the kidney that could be attributed to its immunomodulatory effect and antiviral effect. Hypericum perforatum's components, for instance, hyperoside, quercitrin, quercetin, pseudohypericin, and hypericin, were found to be potential anti‐IBV (Infectious Bronchitis Virus) agents.
N. Huang et al. (2013) studied ethanol extract of Hypericum perforatum against H1N1 strain in A549 human bronchial alveolar epithelial cells and mice. In cell lines, Hypericum perforatum suppressed IFN‐γ and monocyte chemotactic protein. In mice, the treatment group showed a minor reduction in viral titer.
Xiuying et al. (2012) observed significant antiviral potency of Hypericum perforatum extracts in Influenza A Virus (IAV)‐induced mice. The expression of IL‐10 and IFN‐γ increased, whereas expression of TNF‐α and IL‐6 decreased significantly. It resulted in decreased lung index and viral load.
Hyperforin (Hypericum perforatum extract) significantly increases immunity by modulating phagocytosis. It has an important antibiotic along with immunomodulatory properties administer to individuals exposed to Gram‐positive Coccaceae, including methicillin‐resistant Staphylococcus aureus (Brondz & Brondz, 2012).
3.1.12. Jatropha curcas L. (Euphorbiaceae)
Commonly known as purging nut, it is mainly cultivated in drier sites of central and western parts of India. It has a medicinal value in tumors and certain infections (Dahake, Roy, Patil, Rajopadhye, & Chowdhary, 2013).
Patil et al. (2013) assessed the aqueous and methanolic extract of Jatropha curcas leaves against the Influenza A (H1N1) virus in MDCK cells. The aqueous and methanolic extract of Jatropha curcas showed more potent antiviral activity in the simultaneously treated group and postpenetration exposure group than the prepenetration exposure group. The study concluded that the extracts of Jatropha curcas at the time of simultaneous exposure may exert their effect by inhibiting the absorption of viruses in the cells, mediated by sialic acid.
The aqueous methanolic extracts and derivatives of Jatropha curcas illustrated a significant increase in antibody production, lymphocyte, and macrophage cells, in SPF chicks of 1 day old. These chicks were observed to be protected against the Newcastle disease virus (Abd‐Alla et al., 2009).
3.1.13. Justicia adhatoda L. (Acanthaceae)
Justicia adhatoda generally known as Malabar nut is a herb that grows in the open tree canopy in tropical to subtropical areas mainly in the Indo‐China region. It acts as an antimicrobial, antiinflammatory, and beneficial for cardiovascular diseases (Dhankhar et al., 2011). Chavan and Chowdhary (2014) assessed the activity of methanolic leaf extract of Justicia adhatoda against standard influenza strain in MDCK cells and found a reduction in HA and virus replication.
Administration of Justicia adhatoda extracts in Wistar rats enhanced the adhesion of neutrophils to nylon fibers. Moreover, the test samples also induced delayed‐type hypersensitivity reactions in erythrocytes of sheep that showed its immunomodulatory activity in the host (Vinothapooshan & Sundar, 2011).
The methanolic fraction of Justicia adhatoda exhibited a significant thrombolytic activity (53.23%) using streptokinase as the standard (Shahriar, 2013).
3.1.14. Momordica charantia L. (Cucurbitaceae)
Momordica charantia is found mainly in some parts of Asia and Africa and used for its edible fruit. It acts as an antidiabetic, anticancer, and anti‐infectious agent (Grover & Yadav, 2004).
Pongthanapisith et al. (2013) assessed the antiviral efficacy of Momordica charantia against H1N1 and H3N2 viral infections. The study concluded that the purified protein from the seeds of Momordica charantia significantly inhibited H1N1 and H3N2 and also its subtypes under in vitro conditions without exhibiting any cytotoxicity. The plant has a broad range of antiviral activity and can be used as a treatment option for various emerging viral infections.
Administration of polysaccharides of Momordica charantia in cyclophosphamide‐induced immunosuppressed mice significantly enhanced the secretion of serum hemolysin. Moreover, it normalizes the thymus index, spleen index, and natural killer (NK)‐cell cytotoxicity (Deng et al., 2014).
The seed extract of Momordica charantia prolonged the clotting time of activated partial thromboplastin time through blood coagulation cascade (Manjappa et al., 2015).
3.1.15. Ocimum tenuiflorum L. (Lamiaceae)
Ocimum tenuiflorum is widely distributed in India and its medicinal roles have been mentioned in Indian Ayurveda (Wealth of India, 1991). There are different parts of Ocimum tenuiflorum used for the treatment of several ailments like asthma, bronchitis, cough, cold, and digestive disorders (Ghosh, 1995).
An in silico study concluded the potential role of Ocimum tenuiflorum extracts in inhibition of ACE‐2 (angiotensin‐converting enzyme −2) receptor of SARS‐CoV‐2 (Varshney, Varshney, & Nath, 2020).
Out of different extracts, crude extract and terpenoid extract of Ocimum tenuiflorum exhibited a significant reduction in copy numbers of viruses genome at the lowest dose tested. The crude and terpenoid extract prepared from the Ocimum tenuiflorum leaves had strong antiviral potency against the H9N2 virus (Ghoke et al., 2018).
Alhazmi (2015) evaluated the antiviral properties of all phytocompounds isolated from Ocimum tenuiflorum plant using a protein of H1N1 strain under in silico study. The study reported that Apigenin has good binding properties in comparison to Oseltamivir and Zanamivir. Therefore, this compound needs further investigation under in vitro as well as in vivo conditions.
Intraperitoneal administration of seed oil extract from Ocimum tenuiflorum at the dose of 3 ml/kg in sensitized rats exhibited significant augmentation of antibody titer and reduction in the release of histamines from peritoneal mast cells and also inhibited the migration of leukocyte (Mediratta et al., 2002). Aqueous extract of Ocimum tenuiflorum showed significant immunomodulatory potential by increasing the lymphocytes and neutrophils population. It also augmented the phagocytic index as well as phagocytic activity (Mukherjee et al., 2005).
3.1.16. Psoralea corylifolia L. (Leguminosae)
Psoralea corylifolia is a plant used in Indian traditional medicine. (+)‐(S)‐Bakuchiol is a phenolic isoprenoid that is isolated from Psoralea corylifolia seeds and helpful in traditional Indian medicine to cure numerous diseases (Z. Chen et al., 2010; H. L. Chen, Feng, & Li, 2010; Poláková, Fauger, Sayag, & Jourdan, 2015).
Shoji et al. (2015) examined the antiviral activity of Bakuchiol in MDCK cells. The study found that (+)‐(S)‐Bakuchiol and its enantiomer (−)‐(R)‐Bakuchiol significantly prevented the activity of influenza A viral infection by inhibiting the mRNA expression and protein expression of the virus. (+)‐(S)‐Bakuchiol showed more potency as compared to (−)‐(R)‐Bakuchiol. These results showed that it may be used as a good antiviral candidate against viral infectious diseases.
Administration of Psoralea corylifolia extract inhibited the cancer cell growth and induced the antibody‐dependent cellular cytotoxicity, NK‐cell activity, antibody complement‐mediated cytotoxicity, and activation of B‐cells for the production of antibodies (Latha et al., 2000).
3.1.17. Syzygium cumini (L.) Skeels (Myrtaceae)
Generally called jambolan or java plum, Syzygium cumini has a potential activity in respiratory illness, diabetes, and inflammation (Ayyanar & Subash‐Babu, 2012).
Sood et al. (2012) determined the antiviral efficacy of crude extracts of leaves and bark of Syzygium cumini against the avian influenza virus (H5N1) in MDCK cells. Cytopathic effect (CPE) reduction assay, yield reduction assay, and egg‐based in ovo assay were used to examine the virucidal, pre‐ and postexposure antiviral activities of the extracts. The complete inhibition of the virus was shown by bark and leaves extracts. The efficacy was further supported by egg‐based in ovo assay and virus yield reduction assay.
3.1.18. Tinospora cordifolia (Willd.) Miers (Menispermaceae)
Tinospora cordifolia, generally called heart‐leaved moonseed, is a shrub that mainly grows in higher altitudes of India. It is found to be efficacious in ameliorating diabetes, inflammation, oxidation, cancer, and immune imbalance (Ayurvedic Pharmacopoeia Committee, 2001; Parthipan, Aravindhan, & Rajendran, 2011; Rana, Thakur, Sood, Sharma, & Sharma, 2012; Upadhyay, Kumar, Kumar, & Mishra, 2010).
An in silico study concluded four natural compounds (Berberine, Isocolumbin, Magnoflorine, and Tinocordiside) of Tinospora cordifolia showed high binding affinity toward four important targets of SARS‐CoV‐2 that are responsible for attachment (6VSb, 6M0J) and replication (6M71, 6Y84). The reported efficacy of the natural compounds is either equivalent or superior to some of the important antiviral drugs such as Favipiravir, Lopinavir, and Remdesivir (Sagar & Kumar, 2020).
(1,4)‐alpha‐D‐glucan (alpha‐DG) drug was extracted from Tinospora cordifolia and its antiviral efficacy was evaluated in the lungs of E. coli endotoxin‐stimulated juvenile rats. Quantification of protein using enzyme‐linked immunosorbent assay (ELISA) showed a decline in pro‐inflammatory and antiinflammatory cytokines in a drug‐treated group when compared to endotoxemia (Velazquez et al., 2009).
G1‐4A a polysaccharide of Tinospora cordifolia effectively activated macrophages via TLR4‐MyD88 dependent pathway and plays an important role in innate immunity (Gupta et al., 2017).
Tinospora cordifolia aqueous extract efficiently ameliorated cyclophosphamide‐induced immunosuppression in a rodent model (Alrumaihi et al., 2019).
Further, as a herbal formulation, Tinospora cordifolia prevented platelet aggregation, increased the production of lipoprotein lipase enzyme, and delayed plasma calcification. This process resulted in inhibition of atherogenicity (Mary et al., 2003).
3.1.19. Viscum album L. (Santalaceae)
Viscum album belongs to the family Viscaceae and it has various therapeutic roles as a traditional Indian medicinal plant in many diseases like asthma, vertigo, lumbago, and hypertension (Duke, 1987; Zarkovic, Zarkovic, Grainca, Kissel, & Jurin, 1997).
Different extracts of Viscum album, for instance, distilled water, chloroform, ethanol, acetone, and petroleum ether were examined for antiviral potential against human parainfluenza virus type 2 (HPIV‐2) growths in Vero cells. The study observed that out of five extracts, the aqueous extract was the most potent selective inhibitor followed by chloroform extract (Karagöz et al., 2003).
The peptide of the Viscum album at 2 micrograms/ml enhanced the NK‐cell activity in animals. It also induced antibody cellular cytotoxicity and increased the antibody titer in peptide‐treated animals (Kuttan & Kuttan, 1992). Children who were exposed to radioactive fallout when treated with Viscum album resulted in the regulation of immune indices in the controlled range (Chernyshov et al., 2000).
3.1.20. Withania somnifera (L.) Dunal (Solanaceae)
Withania somnifera (Ashwagandha) has several medicinal roles like antiinflammatory, antioxidant, and immunomodulatory (Malik et al., 2007; Mishra, Singh, & Dagenais, 2000).
It has an active component Withaferin A (WA) that acts as an antiviral agent. Withaferin A decreased activity of NA in H1N1 influenza. The docking and molecular dynamics simulation studies found that Withaferin A has a high binding affinity toward NA and showed several important molecular relationships with the residues that are important in the course of simulations (Cai et al., 2015).
Withania somnifera extract and Withaferin A significantly decreased the toxicity mediated by zinc oxide nanoparticles under in vivo conditions. The extracts also restored TLR6 and phagocytic activities (J. Kumar et al., 2019). Administration of Withania somnifera extract in mice increased the number of plaque‐forming cells, circulation of antibody titer in mice spleen, and phagocytosis activity of macrophages (Davis & Kuttan, 2000).
As a thrombolytic agent, Withaferin A increased bleeding time and inhibited aggregation of platelets, the formation of thrombus, and fibrin polymerization (Ku & Bae, 2014).
3.1.21. Woodfordia fruticosa (L.) Kurz (Lythraceae)
Woodfordia fruticosa is found throughout the region of India, East Asia, and some parts of Africa. The flower of the plant has a medicinal role in diseases like menorrhagia, stomach troubles, and liver diseases (Das et al., 2007).
Gallic acid was prepared from the flower of Woodfordia fruticosa and evaluated for its antiviral potential against human rhinoviruses (HRVs). It was administered at different time points that is, during and after infection of HRV in human epithelioid carcinoma cervix cells. Finally, it inhibited the infection of two types of human rhinovirus without showing any cytotoxicity (H. J. Choi et al., 2010).
Ethanol extract of Woodfordia exhibited significant immunomodulatory activity by exhilarating the nonspecific immune responses. The extract significantly increased bone marrow cell proliferation and ameliorated cyclophosphamide‐induced myelosuppression (Shah & Juvekar, 2010).
3.1.22. Zingiber officinale Roscoe (Zingiberaceae)
Generally known as Ginger, Zingiber officinale acts as an antiemetic, antiviral, and antibacterial agent (Ali, Blunden, Tanira, & Nemmar, 2008). Chang et al. (2013) evaluated the antiviral efficacy of fresh ginger against HRSV (human respiratory syncytial virus)‐induced infection in two important cancer cell lines that is, A549 and HEp‐2 cell lines in a dose‐dependent manner. The extract is effective against the HRSV‐induced infection by blocking the attachment of virus and internalization. Another study used an aqueous extract against the antiavian influenza virus (H9N2) in chick embryos. The study observed that the ginger aqueous extract significantly exhibited antiviral activity against H9N2 (Rasool et al., 2017).
Administration of ginger essential oil orally for a week induced humoral immunity (with sheep red blood cells) in immunosuppressed mice (Carrasco et al., 2009). Jafarzadeh et al. (2014) showed that the administration of extract of ginger in the experimental mice model of autoimmune encephalomyelitis decreased the IL‐27 and IL‐33 expression and also modulated the disease severity.
Zingiber officinale has thrombolytic activity. In one of the studies, the use of 500 mg/kg of the ginger extract significantly decreased the serum level of prostaglandin‐E(2) and platelet thromboxane‐B(2) levels. The higher doses of the ginger extract significantly decreased the serum cholesterol level (Thomson et al., 2002).
3.2. Potential traditional Indian herbs and formula used in clinical trials
3.2.1. Kashaya (decoction)
One of the studies depicted that silver nano‐particles of Piper longum fruit extract has potential antibacterial activity and cytotoxicity activity against MCF‐7 (breast cancer cell lines) cell lines (Reddy, Vali, Rani, & Rani, 2014) while Tinospora cordifolia has promising antiviral, immunomodulatory, and thrombolytic properties (Alrumaihi et al., 2019; Gupta et al., 2017; Mary et al., 2003; Velazquez et al., 2009).
3.2.2. ZingiVir H (a poly herbomineral drug)
ZingiVir H tablets showed a significant role against respiratory infections, acute bronchitis, RSV, and Influenza virus (Clinical Trial of Ayurvedic Medicine Zingivir‐H Tablet Starts on COVID‐19 Patients, 2020). A total of seven studies have been registered for Ayurvedic drugs, which include kashaya (decoction) of Tinospora cordifolia, ZingiVir H, MyVir tablets, Dabur Chyawanprash, Shanshamani Vati or Sudarshana Ghanavati or Ashwagandha, Yashtimadhu tablet, Guduchi tablet. These Ayurvedic formulations consist of polyherbal drugs that act as an immune booster and fight against infectious diseases. Supportive data are scarce for the use of Ayurvedic drugs against COVID‐19. However, the Ministry of AYUSH published guidelines for safety precaution of its use against COVID‐19 (Biswkarma & Wadhawan, 2020).
3.2.3. Dabur Chyawanprash with milk
Dabur Chyawanprash is a formulation of herbals that are broadly used as a dietary supplement in India. The popularity of herbal remedies is now extended after the occurrence of COVID‐19. It has been used for health benefits from ancient times for boosting the immunity of the body. Dabur Chyawanprash is a composition of more than 40 herbs that are clinically tested and found to enhance two times immunity from common routine infections (Phelamei, 2020).
Various existing works of the literature showed the useful effects of IgG and colostrums found in milk against respiratory tract infections. Previous studies reported that dietary bovine colostrums decreased the human RSV infection and increased the CD8+ T‐cell response during infection (den Hartog et al., 2014; M. L. Xu, Kim, Wi, & Kim, 2015).
3.2.4. Aayudh Advance
Aayudh Advance acts as a sniper's shot toward pathogens. Due to pathogens, normal cells of the body can die and can make more harm to the body, which leads to deterioration of health. So Aayudh Advance helps in fighting against pathogens (Aayudh advance, 2020). It was found in a human clinical trial that Aayudh Advance decreased the viral load in the body by 300% and remarkably increased the recovery of patients without showing any side effects or adverse effects. Therefore Aayudh Advance can be used for the treatment of viral diseases (CTRI, CTRI/2020/05/025161).
3.2.5. Samshamani Vati
Samshamani Vati is recommended for the treatment of fever and anemia at a dose of 500 mg twice a day (Maurya, Kumar, Bhatt, & Saxena, 2020).
3.2.6. Anu taila nasal drops
According to the traditional Ayurveda, Anu taila is recommended as a nasal drop to regulate the mucus discharge and other symptoms related to COVID‐19. Maharishi Charak has shown the importance of Anu taila in the treatment of congestion of nasal route (Shukla & Tripathi, 2017).
3.2.7. Sudarshana Ghana Vati
Sudarshana Ghana Vati is broadly used for the treatment of fever in different dosage forms. Previous studies showed its significant activity against Staphylococcus aureus and also equally beneficial against other diseases like Salmonella Typhi, Escherichia coli, and Pseudomonas aeruginosa (Kiran, Vivek, Deepak, & Khemchand, 2017).
3.2.8. AYUSH‐64
Administration of AYUSH‐64 for 1 week showed significant improvement in symptoms of Influenza. The study did not report any adverse drug reaction (Gundeti et al., 2020).
3.2.9. Kabasura kudineer
In silico study of 74 compounds depicted that Kaba Sura Kudineer Chooranam (KSKC) has the potency to inhibit the main protease of COVID‐19 (Lakshmanan, Moulishankar, & Suresh, 2020).
3.2.10. Vitamin C
Vitamin C can be beneficial in the management of viral diseases due to its proven antiviral and immunomodulatory activities (Righi et al., 2020).
3.2.11. Zinc supplementation
Zinc acts as an antiviral against various viral diseases like papillomavirus, herpes simplex virus, RSV, human immunodeficiency virus (HIV), and hepatitis C virus. Zinc showed its effect by inhibiting the fusion with the membrane of the host, decreasing the function of viral polymerase, and blocking the release of viral particles (Ishida, 2019; Read, Obeid, Ahlenstiel, & Ahlenstiel, 2019; Skalny et al., 2020; Suara & Crowe, 2004).
3.2.12. Camphor
In silico study depicted that camphor has a good affinity for the protease of COVID‐19 and ACE‐2 receptor (Omar, Bouziane, Bouslama, & Djemel, 2020).
3.2.13. Ayush kwath
Ayush kwath is an Ayurvedic formulation consisting of four medicinal plants (Ocimum sanctum Linn., Cinnamomum zeylanicum Breyn., Zingiber officinale Rosc., and Piper nigrum Linn.). The formulation has important antiviral, thrombolytic, antiinflammatory, and immunomodulatory activities. Therefore, it has been recommended by the Government of India as an immune booster against COVID‐19 (Gautam, Gautam, Chhetri, & Bhattarai, 2020).
3.2.14. Arsenic Alba‐30
Arsenic Alba‐30 is an important Unani medicine, known for its biological and antioxidant activities. It acts at the subcellular and cellular levels by regulating certain genes that are involved in the enzymatic activity to prevent oxidative damage and genotoxic effects induced by arsenic trioxide (Kundu, Mitra, & Bukhsh, 2000).
3.2.15. Joshanda
“Joshanda” (decoction) means boiling and “Andah” means prepared by; therefore, it means prepared by boiling. The preparations were used predominantly for influenza, common cold, cough, fever, and respiratory ailments (I. Ahmad, Shamsi, & Shadab, 2020). One of the important preparations called Tiryaq‐e‐Arba consists of Gentiana lutea L., Aristolochia longa L, Commiphora myrrha (Nees) Engl, and Laurus nobilis L. This preparation is mainly used as an immune booster to fight epidemics (A. A. Khan, Bashir, & Akhtar, 2020).
Khameera Marwareed, an Unani formulation, contains three constituents of animal origin, 11 constituents of plant origin, and one constituent of mineral origin (S. Ahmad et al., 2010). These are recommended for the clinical manifestations of infectious diseases (Ansari, Ahmed, Ahmed, & Khan, 2020).
3.2.16. Bryonia alba L
Bryonia alba L contains several pharmacologically important constituents such as bryonicine, saponarin, vitexin, isovitexin, lutonarin, isoorientin, glycosides 22‐deoxocucurbitosides A and B, and arvenin IV and has significant antiviral activity against human coronavirus and H1N1 (Manvi & Prasad Garg, 2011).
3.2.17. Gelsemium sempervirens
Gelsemium sempervirens is used in homeopathic medicines and might be promising against COVID‐19 (Dey, Dey & Sihag 2020). The known pharmacological action of antimony potassium tartrate is well documented and it is used especially as an antihelminthic in animals (Antimony Potassium Tartrate ‐ an overview|ScienceDirect Topics, 2020). The documented toxicological studies of antimony potassium tartrate give a detailed picture of its toxicological and pathophysiological effects as well as its harmful dosage. Homeopathic dilutions are much safer and well below their toxic proportions (Antimony Potassium Tartrate [UK PID], 2020). There is a substantial basis to consider Homeopathic administration of antimonium tartrate for prophylaxis as well as an add‐on treatment of COVID‐19 infection (Deodhar & Deodhar, 2020). Crotalus horridus from rattlesnake venom may be able to inhibit the replication of HIV (P., 2015).
3.2.18. Neolamarckia cadamba
Neolamarckia cadamba is used predominantly in Indian traditional medicine for cough, fever, gastrointestinal ailments, and skin diseases (Umachigi et al., 2007).
3.2.19. AOIM‐Z tablet
AOIM‐Z tablet is manufactured by Solumiks Herbaceuticals and has antioxidant and immunomodulatory properties. It enhances the host defense and is used against respiratory tract infections (Aoim‐Z tablets, 2020).
3.2.20. Ayurvedic kadha
Ayurvedic kadha consists of basil, cinnamon, black pepper, dry ginger, and raisins are effective as an immune booster (Ministry of AYUSH, 2020).
3.3. Clinical trials on traditional Indian medicinal plants and their formulations
Numerous clinical trials are under investigation to elucidate the efficacy of traditional Indian medicinal plants against COVID‐19. In the majority of the clinical trials, traditional Indian medicines against COVID‐19 are studied as add‐on therapy in addition to standard of care medicines or as a prophylactic/preventive measure in a high‐risk population. Traditional Indian medicines are studied as stand‐alone in asymptomatic patients or patients with mild to moderate disease (Refer to Table 4).
TABLE 4.
Clinical trials of traditional Indian medicinal plants in COVID‐19
| Trial registration | Study design/n/Site | Intervention/comparator agent | Therapy type | |
|---|---|---|---|---|
| 1 | CTRI/2020/04/024882 |
Nonrandomized, active controlled trial N = 60 Haryana |
Kashaya (decoction) of Tinospora cordifolia stem added with finely powdered dried Piper longum fruit Standard of care |
Add‐on to standard of care |
| 2 | CTRI/2020/04/024883 |
Other N = 112 Karnataka |
ZingiVir H (a poly herbomineral drug) | Add‐on therapy |
| 3 | CTRI/2020/05/024967 |
Single arm trial N = 30 Karnataka |
MyVir tablets Standard treatment as per hospital protocol for COVID 19 Cases |
Standalone |
| 4 | CTRI/2020/05/024981 |
Randomized, parallel group trial N = 600 Gujarat, Maharashtra, Rajasthan, |
Dabur Chyawanprash twice daily followed by milk Milk (twice daily) |
Preventive remedy |
| 5 | CTRI/2020/05/025161 |
Randomized, parallel group, active controlled trial N = 120 Gujarat |
Herbal formulation—Aayudh Advance As standard treatment suggested by WHO |
Supplemental treatment |
| 6 | CTRI/2020/05/025171 |
Randomized, parallel group trial N = 50,000 New Delhi |
Samshamani Vati, Anutaila, rock salt and turmeric, Ayush preventive guidelines Conventional preventive medicine guidelines |
Preventive remedy |
| 7 | CTRI/2020/05/025069 |
Single arm trial N = 1,324 New Delhi |
Shanshamani Vati or Sudarshana Ghanavati or Ashwagandha | Preventive remedy |
| 8 | CTRI/2020/05/025093 |
Other N = 1,200 Andhra Pradesh |
Yashtimadhu tablet | Preventive remedy |
| 9 | CTRI/2020/05/025166 |
Randomized, parallel group trial N = 1,200 Andhra Pradesh |
Ashwagandha tablet | Preventive remedy |
| 10 | CTRI/2020/05/025088 |
Randomized, parallel group trial N = 1,200 Andhra Pradesh |
Guduchi tablet | Preventive remedy |
| 11 | CTRI/2020/05/025156 |
Randomized, parallel group, active controlled trial N = 60 Maharashtra |
AYUSH‐64 Standard treatment for COVID‐19 infection |
Add‐on to standard treatment |
| 12 | CTRI/2020/05/025213 |
Single arm trial N = 1,500 Himachal Pradesh |
Guduchi ghanvati | Preventive remedy |
| 13 | CTRI/2020/05/025214 |
Randomized, parallel group, active controlled trial N = 80 Chandigarh |
AYUSH 64 Conventional standard therapy as per ICMR/WHO parameters |
Add‐on to standard of care |
| 14 | CTRI/2020/05/025298 |
Nonrandomized, active controlled trial N = 21,500 Tamil Nadu |
Kabasura kudineer Nilavembu kudineer Personal Sanitation and Environmental Sanitation |
Prophylaxis |
| 15 | CTRI/2020/05/025215 |
Randomized, parallel group trial N = 50 Tamil Nadu |
Kabasura Kudineer Vitamin C, zinc supplementation |
Add‐on |
| 16 | CTRI/2020/05/025222 |
Single arm trial N = 275 Maharashtra |
AOIM‐Z Tablets | Preventive remedy |
| 17 | CTRI/2020/05/025276 |
Single arm trial N = 50 New Delhi |
SanshamaniVati (Tinosporacordifolia) with Nagaradikwath (Decoction of Zingiberofficinale, Terminaliachebula, Tinosporacordifolia), AmalakiChurna (Powder of Phyllanthusemblica), Golden Milk (milk with Curcuma longa) | Standalone |
| 18 | CTRI/2020/05/025273 |
Randomized, parallel group, placebo‐controlled trial N = 120 Rajasthan |
Pure Ashwagandha, Pure Giloy Extract, Pure Tulsi Extract, AnuTaila drops, Nasal Drop Powder SwasariRas Placebo therapy placebo of same dosage form by Oral/nasal route |
Standalone |
| 19 | CTRI/2020/05/025275 |
Randomized, parallel group trial N = 200 New Delhi |
Ayurveda Rasayana along with conventional guidelines for health care workers. Conventional guidelines for health care workers as per the WHO |
Preventive remedy |
| 20 | CTRI/2020/04/024857 |
Cluster‐randomized trial N = 100 Maharashtra |
ArsAlb, Camphora, Bryonia alba, Helleborusniger, Justicia Adhatoda | Standalone |
| 21 | CTRI/2020/04/024905 |
Randomized, parallel group, placebo‐controlled trial N = 100 Uttar Pradesh |
Arsenic Album, Bryonia Alba, Gelsemium, AntimoniumTartaricum, Crotalus Horridus Placebo |
Add‐on |
| 22 | CTRI/2020/04/024947 |
Randomized, parallel group, active‐controlled trial N = 100 Maharashtra |
Cadamba drug therapy | Standalone |
| 23 | CTRI/2020/04/024925 |
Randomized, parallel group, placebo‐controlled trial N = 100 Maharashtra |
Homoeopathic medicines will be given as adjuvant to the standard care Placebo |
Add‐on to standard treatment |
| 24 | CTRI/2020/04/024926 |
Single‐arm trial N = 100 Uttar Pradesh |
Arsenic Album, Bryonia Alba, Gelsemium, Antimonium Tartaricum, Crotalus horridus | Standalone |
| 25 | CTRI/2020/05/024986 |
Single‐arm trial N = 10,000 New Delhi |
Arsenic album 30c | Preventive remedy |
| 26 | CTRI/2020/05/025254 |
Nonrandomized, multiple arm trial N = 40,000 Uttar Pradesh, Karnataka, Telangana, Jammu & Kashmir, Maharashtra, New Delhi |
Unani Joshanda (Decoction) and Tiryaq‐e‐Arba Unani Joshanda (Decoction) and Khameera Marwareed |
Prophylaxis |
| 27 | CTRI/2020/05/025205 |
Cluster randomized trial N = 33,000 Chennai, Delhi, Andhra Pradesh, Hyderabad, Jaipur, Kolkata, Kerala, Uttar Pradesh, Mumbai, Noida, Gujarat |
Arsenicum album 30C The control group clusters will receive no treatment. However, this will be under observation similar to medicine group. |
Preventive remedy |
| 28 | CTRI/2020/05/025272 |
Cluster randomized trial N = 800 Kerala |
Arsenicum album 30 Placebo |
Prophylaxis |
| 29 | CTRI/2020/05/025341 |
Randomized, parallel group trial N = 30 Uttar Pradesh |
Kiratiktadi Kwath, Ashwagandha churna, Yoga exercises, Immuno‐booster Ayush Kwath as ministry of ayush guidelines Modern treatment as per UP Govt. norms to asymptomatic and mild cases |
Standalone |
| 30 | CTRI/2020/05/025338 |
Single‐arm trial N = 40 New Delhi |
Ayush 64 | Standalone |
| 31 | CTRI/2020/05/025332 |
Randomized, parallel group, active‐controlled trial N = 400 Mumbai |
Ashwagandha (Withania somnifera) Hydroxychloroquine |
Prophylaxis |
| 32 | CTRI/2020/05/024969 |
Randomized, parallel group, placebo‐controlled trial N = 100 Uttar Pradesh |
Homoeopathic medicine Placebo |
Add‐on to standard treatment |
| 33 | NCT04387643 |
Observational model: Other Time perspective: Prospective N = 52 Jaipur, India |
Dietary supplement: Ayurvedic Kadha | Preventive |
| 34 | NCT04395976 |
Randomized, open label, parallel assignment trial N = 120 London, UK |
Ayurveda (each participants will be treated with individualized care) | Prophylaxis |
| 35 | NCT04345549 |
Open‐label single‐group assignment N = 18 United Kingdom |
Individualized Ayurveda | Supportive care |
| 36 | NCT04351542 |
Randomized trial N = 32 United Kingdom |
Dietary supplement: Ayurveda Usual care |
Supportive care |
A total of 32 clinical trials of traditional Indian medicines are registered with Clinical Trial Registry‐India (CTRI). Ayurveda interventions include: decoction of Tinospora cordifolia stem and Piper longum fruit as add‐on to standard of care (CTRI, CTRI/2020/04/024882), a poly herbo‐mineral drug ZingiVir H as an adjuvant therapy (CTRI, CTRI/2020/04/024883), MyVir tablets to ameliorate immunity in quarantine patients (CTRI, CTRI/2020/05/024967), Chyawanprash as a preventive remedy (CTRI, CTRI/2020/05/024981; CTRI, CTRI/2020/05/025275), supplemental treatment of Aayudh Advance (herbal formulation consisting of essential oils) (CTRI, CTRI/2020/05/025161), effect of Shanshamani Vati or Sudarshana Ghanavati or Ashwagandha in prevention of infection in containment areas (CTRI, CTRI/2020/05/025069), Guduchi ghanvati in prevention of infection in containment areas (CTRI, CTRI/2020/05/025213), AOIM‐Z Tablets for prevention of COVID‐19 infection in high risk healthy police personnel (CTRI, CTRI/2020/05/025222), and efficacy of Kiratikta and Ashwagandha Churna with Yoga modalities in management of COVID‐19 patients (CTRI, CTRI/2020/05/025341). Two randomized controlled trials to assess the potency and safety of Ayush‐64 as an add‐on to standard therapy are registered (CTRI, CTRI/2020/05/025156; CTRI, CTRI/2020/05/025214). A randomized exploratory clinical study evaluates the immune‐stimulatory potential of Ayurveda management protocol (Tablet Samshamani Vati, application of Anutaila to each nostril, gargle with warm water mixed with rock salt and turmeric, Ayush preventive guidelines for COVID‐19 with Yoga and Pranayama) (CTRI, CTRI/2020/05/025171). A pilot study is evaluating the efficacy of Ayurveda protocol: Tinospora cordifolia with a decoction of Zingiber officinale, Terminalia chebula, Tinospora cordifolia; powder of Phyllanthus emblica with water; and golden milk (milk with Curcuma longa) (CTRI, CTRI/2020/05/025276). Another randomized trial evaluates the effect of the Ayurvedic treatment regime (pure ashwagandha, pure giloy extract, pure tulsi extract, anu taila drops, and nasal drop powder Swasari Ras) in COVID‐19 (CTRI, CTRI/2020/05/025273). Three observational studies that evaluate the intake of Yashtimadhu, Ashwagandha, and Guduchi tablets as a preventive measure are registered (CTRI, CTRI/2020/05/025093; CTRI, CTRI/2020/05/025166; CTRI, CTRI/2020/05/025088).
A nonrandomized population‐based prospective study will evaluate the effectiveness of Unani medicine as a prophylactic in the population at a high risk of COVID‐19. The interventions are (1) Unani Joshanda (decoction) and Tiryaq‐e‐Arba, (2) Unani Joshanda (decoction) and Khameera Marwareed (CTRI, CTRI/2020/05/025254).
There are two trials registered, which aimed to evaluate the effect of Siddha intervention in COVID‐19. The first trial evaluates the effect of Kabasura kudineer Nilavembu kudineer as a prophylactic measure in a high‐risk population (CTRI, CTRI/2020/05/025298) and the other assesses the effect of Kabasura kudineer supplementation to manage asymptomatic COVID‐19 patients (CTRI, CTRI/2020/05/025215).
Homeopathic interventions include Arsenic alba, Camphor, Bryonia alba, Helleborus niger, Justicia Adhatoda, Arsenic Album, Gelsemium, Antimonium Tartaricum, Crotalus horridus; Cadamba drug therapy; Arsenic Album 30c (CTRI, CTRI/2020/04/024857; CTRI, CTRI/2020/04/024905; CTRI, CTRI/2020/04/024947; CTRI, CTRI/2020/04/024925; CTRI, CTRI/2020/04/024926; CTRI, CTRI/2020/05/024986; CTRI, CTRI/2020/05/025205; CTRI, CTRI/2020/05/025272; CTRI, CTRI/2020/05/024969).
A total of four clinical trials of traditional Indian medicines are registered with ClinicalTrials.gov. The first trial is an observational study to evaluate the effect of dietary supplement Ayurvedic Kadha in protecting health care workers against COVID‐19 (ClinicalTrials.gov, NCT04387643). The second trial is to study Ayurveda as prophylaxis for suspected COVID‐19 patients (ClinicalTrials.gov, NCT04395976). Two trials are registered to study the effect of Ayurveda as supportive care in the management of flu‐like symptoms (ClinicalTrials.gov, NCT04345549; ClinicalTrials.gov, NCT04351542).
4. DISCUSSION
Despite a large number of drugs under evaluation, currently, there is no standard therapy for COVID‐19 (Sanders, Monogue, Jodlowski, & Cutrell, 2020). An important strategy could be the use of herbal medicines that shows potential pharmacological activity against the acute viral infections that mainly persist in the lower respiratory tract. Studies are supporting the use of traditional Chinese medicines (TCM) in ameliorating clinical symptoms in COVID‐19. The National Health Commission of China reported that traditional Chinese medicine was found effective in more than 60,000 confirmed cases of COVID‐19 (National Health Commission of the People's Republic of China, 2020). The Chinese medicinal plants have been studied extensively in COVID‐19, for instance, Sangju yin, Yinqiao san, Maxinshigan Tang, and Baihegujin Tang (Z. Xu et al., 2020).
Correspondingly, the Indian subcontinent is a huge repository of medicinal herbs that have been in use to counteract numerous virus‐related infections of the respiratory tract for ages. There is an unmet need for antiviral agents against COVID‐19 that must be effective, safe, and cost‐effective (Mitjà & Clotet, 2020).
The current review puts light on some of the important traditional Indian medicinal herbs that can be a potential antiviral agent against SARS‐CoV‐2. For instance, in silico studies revealed the potential role of extracts of Andrographis paniculata, Camphor, Ocimum tenuiflorum, and Kabusura kudineer in inhibition of COVID‐19. Andrographolide (Andrographis paniculata) fitted well with Mpro of SARS‐CoV‐2 with high druggability and permeability (Enmozhi et al., 2020) that has a vital role in posttranslational modification of replicase protein in SARS‐CoV‐2 (Peele et al., 2020). Another important drug target is the ACE‐2 receptor that is the entry gate for SARS‐CoV‐2 and SARS‐CoV. The inhibition of this receptor can prevent the orchestrated multiple organ damage induced by these coronaviruses (Li, Tang, Li, & Liu, 2020). In silico studies concluded that Camphor (Omar et al., 2020), Ocimum tenuiflorum extract (Varshney et al., 2020), and Kabusura kudineer (Lakshmanan et al., 2020) bind with the ACE‐2 receptor of the coronaviruses that supports their promising role in drug development for COVID‐19. The four natural compounds of Tinospora cordifolia are found to bind with the important proteins of SARS‐CoV‐2 (Sagar & Kumar, 2020).
Glycyrrhiza glabra inhibited the cellular pathways of SARS‐CoV and prevented its adsorption, penetration, and replication. The findings strongly suggest the use of this herb for the treatment of SARS‐CoV‐2 infection (Cinatl et al., 2003).
Apart from these medicinal herbs, which were evaluated against SARS‐CoV‐2 and SARS‐CoV in different in silico and in vitro studies, numerous other traditional Indian medicinal herbal plants proved efficacious against other types of acute respiratory infections. For instance, Acacia arabica decreased the viral titer of Influenza A virus in ovo model (Ghoke et al., 2018) while Aloe vera extracts inhibited the viral‐induced autophagy and H1N1 infection, thereby reducing mortality under in vitro condition (C. T. Huang et al., 2019). Bergenia ciliata decreased H1N1 proliferation (Rajbhandari et al., 2009) and Cinnamon silver (Ag) nanoparticles showed antiviral activity against influenza with a significant safety profile (Fatima et al., 2016). In RSV infection, silver nanoparticles of Curcuma longa decreased the viral load (X. X. Yang et al., 2016) and also the viral titer in influenza by inhibiting PI3K signaling pathway (Richart et al., 2018). Cydonia oblonga and Embelia ribes significantly lowered the viral load of different types of Influenza virus under in vitro conditions (Hamauzu et al., 2005; Hossan et al., 2018). Hypericum perforatum inhibited IFN‐γ and chemotactic protein in influenza (N. Huang et al., 2013) while Jatropha curcas inhibited the absorption of H1N1 (Patil et al., 2013). Justicia adhatoda, Momordica charantia, Psoralea corylifolia, Syzygium cumini, Withania somnifera, and Zingiber officinale inhibited the virus titer of different types of Influenza viruses (Cai et al., 2015; Chavan & Chowdhary, 2014; Pongthanapisith et al., 2013; Rasool et al., 2017; Shoji et al., 2015; Sood et al., 2012). Viscum album significantly reduced the replication of HPIV2 virus under in vitro conditions (Karagöz et al., 2003). Though these traditional Indian medicinal plants have not been evaluated against SARS‐CoV‐2, they provide compelling evidence to assess their efficacy against the virus.
The severity of the novel coronavirus infection is associated with high expression of IFN‐γ‐induced protein‐10, macrophage inflammatory protein 1α, monocyte chemo‐attractant protein‐1, granulocyte‐colony stimulating factor, TNF‐α, IL‐2R, IL‐6, and interstitial mononuclear inflammatory infiltrates (L. Chen et al., 2020; C. Huang et al., 2020; Z. Xu et al., 2020). The traditional Indian medicinal plants have a promising immunomodulatory activity, for instance, Achyranthes bidentata increased the lymphocyte proliferation and release of certain cytokines (Q. Chen et al., 2009) while Andographis paniculata and Aloe Vera can activate the humoral and innate immunity on exposure to viral infections (C. T. Huang et al., 2019; Naik & Hule, 2009). Bergenia ciliata and Cinnamomum Cassia affect the lymphocyte growth and functions (Koh et al., 1998; Tumova & Vokurková, 2018), whereas Curcuma longa increased the PBMC proliferation (Sornpet et al., 2017). Hypericum perforatum alters the production of cytokines (N. Huang et al., 2013) while Jatropha curcus was found to activate antibody secretion and lymphocyte activation against the Newcastle disease virus (Abd‐Alla et al., 2009). Justicia adhatoda can activate delayed‐type hypersensitivity (Vinothapooshan & Sundar, 2011) while Momordica charantia ameliorated the cyclophosphamide‐induced immunosuppression in rats (Deng et al., 2014). Ocimum tenuiflorum can affect the neutrophil and lymphocyte population (Mukherjee et al., 2005), whereas Psoralea corylifolia induced the NK‐cell activity and antibodies‐dependent cytotoxicity in cancer cells (Latha et al., 2000). Tinospora cordifolia activated the innate immune response via TLR4‐MY88 axis (Gupta et al., 2017) and ameliorated cyclophosphamide‐induced immunosuppression in rats (Alrumaihi et al., 2019). Viscum album regulated immune indices in radioactive exposed children (Chernyshov et al., 2000) while Withania somnifera has a beneficial role in humoral immunity (Davis & Kuttan, 2000). Woodfordia fruticosa is useful in immunocompromised conditions (Shah & Juvekar, 2010), and Zingiber officinale induced humoral immunity in immunosuppressed mice (Carrasco et al., 2009). These medicinal plants exhibit significant immunomodulatory activity that could be beneficial in ameliorating the cytokine storm induced by the SARS‐CoV‐2 virus.
Also, the severity of the disease is associated with a high prevalence of clinically relevant thrombosis in COVID‐19 patients. Numerous COVID‐19 patients develop ARDS that results in life‐threatening thrombotic complications (Connors & Levy, 2020; Helms et al., 2020). The traditional medicinal plants showed significant thrombolytic activity, which is attributed to the decreased thromboxane B2 (TXB2), serum level of prostaglandin‐E(2), factor Xa, and increased level of 6‐keto‐prostaglandin F1α (6‐keto‐PGF1α) that improved blood circulation and reduced weight of thrombus. For instance, Achyranthes bidentata can activate plasminogen activator inhibitor‐1 in brain ischemia (Q. Cheng et al., 2019) while Cinnamomum cassia inhibited arachidonic acid and thromboxane A2‐induced platelet aggregation (Kim et al., 2010). Cydonia oblonga dose‐dependently reduced venous and arterial thrombus along with decreased thromboxane B2 (TXB2) and increased level of 6‐keto‐prostaglandin F1α (6‐keto‐PGF1α) (Zhou et al., 2014). Embelin ribes inhibited malondialdehyde production in platelets and also reduced arteriovenous shunt and stasis induced thrombosis in rats (Jagtap et al., 2012). Justicia adhatoda, Momordica charantia, Tinospora cordifolia, and Withania somnifera exhibit significant thrombolytic activity (Ku & Bae, 2014; Manjappa et al., 2015; Mary et al., 2003; Shahriar, 2013). Zingiber officinale significantly decreased the serum level of prostaglandin‐E(2) and platelet thromboxane‐B(2) levels (Thomson et al., 2002).
Apart from preclinical studies, numerous traditional Indian herbal medicines are considered to be evaluated under clinical trials. These studies attempt to establish the safety and efficacy of traditional Indian herbal medicines. The potential use of these traditional Indian medicinal plants cannot be proposed for the management of mild or moderate or in critical conditions but these plant products can be used as an add‐on therapy. More mechanism‐based studies are required to identify the effective components of these plants that could be effective against SARS‐CoV‐2 (Xu & Zhang 2020).
5. CONCLUSION
Our study found 22 potential traditional Indian medicinal plants and 36 clinical trials of traditional Indian medicinal plants currently registered in different clinical trial databases for the development of drug therapies against COVID‐19.
The most promising traditional Indian medicinal plants for the management of COVID‐19 are Ocimum tenuiflorum and Tinospora cordifolia as these herbal plants exhibited significant binding affinity, immunomodulatory, and thrombolytic activities.
Further, Achyranthes bidentata, Cinnamomum cassia, Cydonia oblonga, Embelin ribes, Justicia adhatoda, Momordica charantia, Withania somnifera, and Zingiber officinale exhibit significant antiviral properties against different viruses known to cause acute respiratory infection. These herbs also exhibit significant immunomodulatory and thrombolytic activities.
In silico studies showed that traditional Indian herbal formulas such as Camphor and Kabusura kudineer found to bind with the important protease of the SARS‐CoV‐2 thereby could be promising for the treatment of the viral disease.
The potential use of these traditional Indian medicinal herbs cannot be proposed for the management of mild, moderate, or critical COVID‐19. These types of recommendations need more supportive studies. But these plant traditional Indian medicinal products can be used as an add‐on therapy.
Further, the clinical trials have undertaken some of the potential Indian herbs and formulas against COVID‐19. There is an urgent need for prospective cohort studies to further determine the potential of these traditional Indian medicines. Since there is the availability of the population sample of COVID‐19, a sequence of prospective studies with well‐designed studies should start producing authentic proof for this herbal prevention of COVID‐19 or related respiratory infectious diseases shortly.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR'S CONTRIBUTION
Rahul Soloman Singh, Ashutosh Singh—Conception or design of the work, Data collection, Data analysis and interpretation; Harpinder Kaur, Gitika Batra—Data collection, Data analysis and interpretation; Phulen Sarma—Drafting the article, Critical revision of the article, Data analysis; Hardeep Kaur, Anusuya Bhattacharyya, Amit Raj Sharma, Subodh Kumar, Sujata Upadhyay, Vinod Tiwari—Data collection, Data analysis; Pramod Avti, Ajay Prakash—Drafting the article, Critical revision of the article; Bikash Medhi—Conception or design of the work, Drafting the article, Critical revision of the article, Final approval of the version to be published.
Singh, R. S., Singh, A., Kaur, H., Batra, G., Sarma, P., Kaur, H., Bhattacharyya, A., Sharma, A. R., Kumar, S., Upadhyay, S., Tiwari, V., Avti, P., Prakash, A., & Medhi, B. (2021). Promising traditional Indian medicinal plants for the management of novel Coronavirus disease: A systematic review. Phytotherapy Research, 35(8), 4456–4484. 10.1002/ptr.7150
Rahul Soloman Singh and Ashutosh Singh contributed equally to this study.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Aayudh advance . (2020). Aayudh. Retrieved from https://www.aayudhhealth.com/aayudh-advance
- Abd‐Alla, H. I., Moharram, F. A., Gaara, A. H., & El‐Safty, M. M. (2009). Phytoconstituents of Jatropha curcas L. leaves and their immunomodulatory activity on humoral and cell‐mediated immune response in chicks. Zeitschrift fur Naturforschung C: Journal of Biosciences, 64(7–8), 495–501. 10.1515/znc-2009-7-805 [DOI] [PubMed] [Google Scholar]
- Ahmad, I., Shamsi, S., & Shadab, M. (2020). Development of Standard Manufacturing Process of Joshanda. International Journal of Pharmacy & Biomedical Research, 7(4), 1–5. 10.18782/2394-3726.1097 [DOI] [Google Scholar]
- Ahmad, S., Rehman, S., Ahmad, A. M., Siddiqui, K. M., Shaukat, S., Khan, M. S., … Jahangir, T. (2010). Khamiras, a natural cardiac tonic: An overview. Journal of Pharmacy & Bioallied Sciences, 2(2), 93–99. 10.4103/0975-7406.67009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhazmi, M. I. (2015). Molecular docking of selected phytocompounds with H1N1 Proteins. Bioinformation, 11(4), 196–202. 10.6026/97320630011196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, B. H., Blunden, G., Tanira, M. O., & Nemmar, A. (2008). Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food and Chemical Toxicology, 46(2), 409–420. 10.1016/j.fct.2007.09.085 [DOI] [PubMed] [Google Scholar]
- Alrumaihi, F., Allemailem, K. S., Almatroudi, A., Alsahli, M. A., Khan, A., & Khan, M. A. (2019). Tinospora cordifolia aqueous extract alleviates cyclophosphamide‐ induced immune suppression, toxicity and systemic candidiasis in immunosuppressed mice: In vivo study in comparison to antifungal drug fluconazole. Current Pharmaceutical Biotechnology, 20(12), 1055–1063. 10.2174/1389201019666190722151126 [DOI] [PubMed] [Google Scholar]
- Ansari, A. P., Ahmed, N. Z., Ahmed, K. K., & Khan, A. A. (2020). An Insight on Wabāi Amrād (Epidemic Diseases) and COVID‐19 Like Conditions–Unani Perspective. International Journal of Current Research, 12(17), 109–119. [Google Scholar]
- Antimony Potassium Tartrate ‐ an overview | ScienceDirect Topics . (2020). Science Direct‐ Journals & Books. Retrieved from https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/antimony-potassium‐tartrate#:%7E:text=Antimony%20potassium%20tartrate%20was%20once,expectorant%20in%20certain%20cough%20syrups.&text=The%20dose%20of%20tartar%20emetic%20varies%20greatly%20according%20to%20these%20different%20uses
- Antimony Potassium Tartrate (UK PID) . (2020). WHO‐ Internationally Peer Reviewed Chemical Safety Information. Retrieved from http://www.inchem.org/documents/ukpids/ukpids/ukpid37.htm
- Aoim‐Z tablets . (2020). Ayurmedinfo. Retrieved from https://www.ayurmedinfo.com/2016/09/15/aoim-z-tablets-uses-ingredients-dose-side-effects/#Benefits
- Aranha, I., & Venkatesh, Y. P. (2018). Humoral immune and adjuvant responses of mucosally‐administered Tinospora cordifolia immunomodulatory protein in BALB/c mice. Journal of Ayurveda and Integrative Medicine, 11(2), 140–146. 10.1016/j.jaim.2017.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo, C. C., & Leon, L. L. (2001). Biological activities of Curcuma longa L. Memórias do Instituto Oswaldo Cruz, 96(5), 723–728. 10.1590/s0074-02762001000500026 [DOI] [PubMed] [Google Scholar]
- Arora, R., Chawla, R., Marwah, R., Arora, P., Sharma, R. K., Kaushik, V., … Bhardwaj, J. R. (2011). Potential of Complementary and Alternative Medicine in Preventive Management of Novel H1N1 Flu (Swine Flu) Pandemic: Thwarting Potential Disasters in the Bud. Evidence‐based complementary and alternative medicine. eCAM, 586506. 10.1155/2011/586506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avasarala, S., Zhang, F., Liu, G., Wang, R., London, S. D., & London, L. (2015). Correction: Curcumin modulates the inflammatory response and inhibits subsequent fibrosis in a mouse model of viral‐induced acute respiratory distress syndrome. PLoS One, 10(8), e0134982. 10.1371/journal.pone.0134982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayurvedic Pharmacopoeia Committee . (2001). Ayurvedic Pharmacopoeia of India. Government of India, Ministry of Health and Family Welfare. New Delhi, India: Department of AYUSH.
- Ayyanar, M., & Subash‐Babu, P. (2012). Syzygium cumini (L.) Skeels: A review of its phytochemical constituents and traditional uses. Asian Pacific Journal of Tropical Biomedicine, 2(3), 240–246. 10.1016/S2221-1691(12)60050-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae, C. H., Nam, S. H., & Park, S. M. (2002). Formation of silver nanoparticles by laser ablation of a silver target in NaCl solution. Applied Surface Science, 197, 628–634. [Google Scholar]
- Balamurugan, V., Sen, A., Saravanan, P., Bhanuprakash, V., Patra, R. C., Swamp, D., … Singh, R. K. (2008). Potential effect of Acacia arabica. on Peste des Petits Ruminants. Virus Replication. Pharmaceutical Biology, 46(3), 171–179. [Google Scholar]
- Bhanuprakash, V., Hosamani, M., Balamurugan, V., Gandhale, P., Naresh, R., Swarup, D., & Singh, R. K. (2008). In vitro antiviral activity of plant extracts on goatpox virus replication. Indian Journal of Experimental Biology, 46(2), 120–127. [PubMed] [Google Scholar]
- Biswkarma, V. K., & Wadhawan, S. (2020). Study of clinical trials for the management of COVID‐19 outbreak registered in the clinical trial registry‐India. Indian Journal of Pharmacy and Pharmacology, 7(2), 100–112. [Google Scholar]
- Blackburne, B. P., Hay, A. J., & Goldstein, R. A. (2008). Changing selective pressure during antigenic changes in human influenza H3. PLoS Pathogens, 4(5), e1000058. 10.1371/journal.ppat.1000058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boncristiani, H. F., Criado, M. F., & Arruda, E. (2009). Respiratory viruses. Encyclopedia of Microbiology, 500–518. 10.1016/B978-012373944-5.00314-X [DOI] [Google Scholar]
- Brondz, I., & Brondz, A. (2012). Enhancement of the Immunity in AIDS and Other Immunocompromised Patients by Hyperforin an Antibiotic from Hypericum perforatum L. Paper presented at: 2nd ARIP European Conference on Antimicrobial Resistance & Infection Prevention, Vilnius (pp. 4–5).
- Cai, Z., Zhang, G., Tang, B., Liu, Y., Fu, X., & Zhang, X. (2015). Promising anti‐influenza properties of active constituent of withania somnifera ayurvedic herb in targeting neuraminidase of H1N1 influenza: Computational study. Cell Biochemistry and Biophysics, 72(3), 727–739. 10.1007/s12013-015-0524-9 [DOI] [PubMed] [Google Scholar]
- Carrasco, F. R., Schmidt, G., Romero, A. L., Sartoretto, J. L., Caparroz‐Assef, S. M., Bersani‐Amado, C. A., & Cuman, R. K. (2009). Immunomodulatory activity of Zingiber officinale Roscoe, Salvia officinalis L. and Syzygium aromaticum L. essential oils: Evidence for humor‐ and cell‐mediated responses. The Journal of Pharmacy and Pharmacology, 61(7), 961–967. 10.1211/jpp/61.07.0017 [DOI] [PubMed] [Google Scholar]
- Chang, J. S., Wang, K. C., Yeh, C. F., Shieh, D. E., & Chiang, L. C. (2013). Fresh ginger (Zingiber officinale) has anti‐viral activity against human respiratory syncytial virus in human respiratory tract cell lines. Journal of Ethnopharmacology, 145(1), 146–151. 10.1016/j.jep.2012.10.043 [DOI] [PubMed] [Google Scholar]
- Chavan, R., & Chowdhary, A. (2014). In vitro inhibitory activity of Justicia adhatoda extracts against influenza virus infection and hemagglutination. International Journal of Pharmaceutical Sciences Review and Research, 25(2), 231–236. [Google Scholar]
- Chen, H., Muhammad, I., Zhang, Y., Ren, Y., Zhang, R., Huang, X., … Li, G. (2019). Antiviral activity against infectious bronchitis virus and bioactive components of Hypericum perforatum L. Frontiers in Pharmacology, 10, 1272. 10.3389/fphar.2019.01272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. L., Feng, H. J., & Li, Y. C. (2010). Vitro antitumor activity and synthesis of the key intermediate of bakuchiol. Yao xue xue bao = Acta Pharmaceutica Sinica, 45(4), 467–470. [PubMed] [Google Scholar]
- Chen, L., Liu, H. G., Liu, W., Liu, J., Liu, K., Shang, J., … Wei, S. (2020). Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua jie he he hu xi za zhi = Zhonghua jiehe he huxi zazhi = Chinese Journal of tuberculosis and respiratory diseases, 43(3), 203–208. 10.3760/cma.j.issn.1001-0939.2020.03.013 [DOI] [PubMed] [Google Scholar]
- Chen, Q., Liu, Z., & He, J. H. (2009). Achyranthes bidentata polysaccharide enhances immune response in weaned piglets. Immunopharmacology and Immunotoxicology, 31(2), 253–260. 10.1080/08923970802439795 [DOI] [PubMed] [Google Scholar]
- Chen, Z., Jin, K., Gao, L., Lou, G., Jin, Y., Yu, Y., & Lou, Y. (2010). Anti‐tumor effects of bakuchiol, an analogue of resveratrol, on human lung adenocarcinoma A549 cell line. European Journal of Pharmacology, 643(2–3), 170–179. 10.1016/j.ejphar.2010.06.025 [DOI] [PubMed] [Google Scholar]
- Cheng, Q., Tong, F., Shen, Y., He, C., Wang, C., & Ding, F. (2019). Achyranthes bidentata polypeptide k improves long‐term neurological outcomes through reducing downstream microvascular thrombosis in experimental ischemic stroke. Brain Research, 1706, 166–176. 10.1016/j.brainres.2018.11.010 [DOI] [PubMed] [Google Scholar]
- Cheng, V. C., Tang, B. S., Wu, A. K., Chu, C. M., & Yuen, K. Y. (2004). Medical treatment of viral pneumonia including SARS in immunocompetent adult. The Journal of Infection, 49(4), 262–273. 10.1016/j.jinf.2004.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyshov, V. P., Heusser, P., Omelchenko, L. I., Chernyshova, L. I., Vodyanik, M. A., Vykhovanets, E. V., … Schaefermeyer, G. (2000). Immunomodulatory and clinical effects of Viscum album (Iscador M and Iscador P) in children with recurrent respiratory infections as a result of the chernobyl nuclear accident. American Journal of Therapeutics, 7(3), 195–203. 10.1097/00045391-200007030-00007 [DOI] [PubMed] [Google Scholar]
- Choi, H. J., Song, J. H., Bhatt, L. R., & Baek, S. H. (2010). Anti‐human rhinovirus activity of gallic acid possessing antioxidant capacity. Phytotherapy Research, 24(9), 1292–1296. 10.1002/ptr.3101 [DOI] [PubMed] [Google Scholar]
- Choi, J. G., Lee, H., Kim, Y. S., Hwang, Y. H., Oh, Y. C., Lee, B., … Ma, J. Y. (2019). Aloe vera and its components inhibit influenza A virus‐induced autophagy and replication. The American Journal of Chinese Medicine, 47(6), 1307–1324. 10.1142/S0192415X19500678 [DOI] [PubMed] [Google Scholar]
- Cinatl, J., Morgenstern, B., Bauer, G., Chandra, P., Rabenau, H., & Doerr, H. W. (2003). Glycyrrhizin, an active component of liquorice roots, and replication of SARS‐associated coronavirus. Lancet (London, England), 361(9374), 2045–2046. 10.1016/s0140-6736(03)13615-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical trial of Ayurvedic Medicine Zingivir‐H tablet starts on COVID‐19 patients . (2020). Medical Dialogues. Retrieved from https://medicaldialogues.in/ayush/ayurveda/news/clinical-trial-of-ayurvedic-medicine-zingivir-h-tablet-starts-on-covid-19-patients-65439
- Clinical Trials Registry‐India . (n.d.‐a). A Pilot study to estimate the effectiveness of Ayurvedic intervention in COVID‐19 positive cases. Identifier CTRI/2020/05/025276. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐b). A prospective non‐randomised open label controlled interventional study on the effect of Siddha intervention as a prophylactic measure among high risk population (Health Care Workers/ Containment Zone population) exposed to COVID 19. Identifier CTRI/2020/05/025298. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐c). A Prospective, Open label observational clinical study to evaluate the efficacy and safety of MyVir tablets to improve immunity in quarantine patients of COVID‐19. Identifier CTRI/2020/05/024967. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐d). A prospective, single centre, randomized open labelled comparative clinical study to evaluate the effectiveness of Siddha medicine, Kabasura kudineer and vitamin c‐zinc supplementation in the management of asymptomatic COVID 19 patients. Identifier CTRI/2020/05/025215. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php.
- Clinical Trials Registry‐India . (n.d.‐e). A Randomized and Comparative Study to assess Safety and Efficacy of Supplemental Treatment of a herbal formulation ‐ Aayudh Advance comprising essential oils in patients with Corona Virus 2019 (Covid‐19). Identifier CTRI/2020/05/025161. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php. [DOI] [PMC free article] [PubMed]
- Clinical Trials Registry‐India . (n.d.‐f). A Randomized, Open Label, Parallel Efficacy, Active Control, Exploratory Clinical Trial to Evaluate Efficacy and Safety of an Ayurvedic Formulation (AYUSH 64) as Adjunct Treatment to Standard of Care for the management of Mild to Moderate COVID‐19 Patients. Identifier CTRI/2020/05/025214. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐g). A Single Blind, Single Arm Clinical Trial to ascertain the effect of Homoeopathic Medicines in arresting the pathogenesis of disease in asymptomatic Corona virus and suspected Corona virus Patients. Identifier CTRI/2020/04/024926. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐h). Ashwagandha for the Prophylaxis against SARS‐CoV‐2 Infection: A Randomized Hydroxychloroquine Controlled Clinical Trial in Health Care Providers. Identifier CTRI/2020/05/025332. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐i). Clinical evaluation of Dabur Chyawanprash (DCP) as a preventive remedy in pandemic of COVID‐19 – An Open label, Multi centric, Randomized, Comparative, Prospective, Interventional Community based Clinical Study on Healthy individuals. Identifier CTRI/2020/05/024981. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐j). Drug Proving & checking its effectiveness in treatment of COVID 19. Identifier CTRI/2020/04/024947. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐k). Effect of adjuvant homoeopathy with standard treatment protocol in management of covid‐19: A randomized, open label, placebo controlled, parallel group study. Identifier CTRI/2020/05/024969. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐l). Effect of an Ayurvedic Formulation as add‐on to standard of care in COVID‐19 positive patients in a tertiary hospital. Identifier CTRI/2020/04/024882. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐m). Effectiveness of Arsenicum Album 30c in Prevention of Covid‐19 in Individuals Residing in Hot Spots of Red Zones– A Multicentric, Randomised, Cluster Level, Controlled Trial. Identifier CTRI/2020/05/025205. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐n). Effectiveness of Arsenicum Album 30c In Prevention Of Covid‐19 In Individuals Residing In Hot spots Of Red Zones In Delhi– A Prospective Cohort Study. Identifier CTRI/2020/05/024986. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐o). Effectiveness of Homoeopathy as an ancillary mode of treatment and management in combating Corona Virus infection ‐ A Randomized, placebo‐controlled, Single Blind Trial. Identifier CTRI/2020/04/024905. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐p). Efficacy Of Homoeopathic Prophylactic Intervention On Covid‐19 Pandemic‐A Double Blind Randomised Controlled Trial. Identifier CTRI/2020/05/025272. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐q). Efficacy of Kiratiktadi Kwath & Ashwagandha Churna with Yoga modalities in management of COVID ‐19 patients. Identifier CTRI/2020/05/025341. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐r). Evaluation of Clinical Efficacy of AOIM – Z Tablets for Prevention of COVID – 19 Pandemic in High Risk Healthy Police Personnel – Single Arm, Open Labelled, Prospective Exploratory Interventional Clinical Study. Identifier CTRI/2020/05/025222. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐s). Evaluation of Efficacy and Safety of Ayurveda Intervention (Ayush‐64) add‐on therapy for patients with COVID‐19 infection (Stage I)‐A Randomized controlled clinical trial. Identifier CTRI/2020/05/025156. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐t). Evaluation of Efficacy and Safety of Ayurveda Intervention (Ayush −64) in the management of COVID‐19 infection (Asymptomatic &Mild to Moderate symptoms)‐ An open label single arm prospective clinical trial. Identifier CTRI/2020/05/025338. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐u). Evaluation of protective potential of an Ayurvedic Rasayan (Chyawanprash) in the prevention of COVID‐19 among Health Care Personnel – An open label, prospective Randomized controlled parallel group study. Identifier CTRI/2020/05/025275. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐v). Evaluation of the Immuno‐stimulatory (Shareera Bala) potential of Ayurveda management protocol in Cohort of Delhi Police ‐ An Exploratory clinical Study. Identifier CTRI/2020/05/025171. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐w). Homoeopathy As An Adjuvant To Standard Treatment Protocol In Management Of Corona Virus Infection‐ A Randomised, Placebo Controlled, Open Label Study. Identifier CTRI/2020/04/024925. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐x). Impact of Ayurvedic intervention (Guduchighanvati) in prevention of COVID‐19 infection in containment areas of Himachal Pradesh‐A community based study. Identifier CTRI/2020/05/025213. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐y). Impact of Ayurvedic Interventions in prevention of COVID‐19 infection in containment areas of Delhi‐ A community based study. Identifier CTRI/2020/05/025069. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐z). Impact of Indian traditional Ayurvedic treatment regime for nCoV‐2 (COVID‐19). Identifier CTRI/2020/05/025273. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐aa). Observational Study of Ashwagandha tablet intake as a preventive measure in pandemic of COVID‐19 – An open label, Randomized, Controlled, Prospective, Interventional, Community‐based Clinical study on healthy subjects. Identifier CTRI/2020/05/025166. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐ab). Observational Study of Guduchi tablet intake as a preventive measure in pandemic of COVID‐19 – An open label, Randomized, Controlled, Prospective, Interventional, Community‐based Clinical study on healthy subjects. Identifier CTRI/2020/05/025088. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐ac). Observational Study of Yashtimadhu tablet intake as a preventive measure in pandemic of COVID‐19 – An open label, Randomized, Controlled, Prospective, interventional, Community‐based Cclinicalstudy on healthy subjects. Identifier CTRI/2020/05/025093. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐ad). Population based Prospective Study on effectiveness and outcomes of Unani Medicine prophylactic interventions on population at risk of COVID‐19. Identifier CTRI/2020/05/025254. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐ae). Proving the efficacy of Homeopathic treatment in prevention and cure of COVID‐19. Identifier CTRI/2020/04/024857. Retrieved from http://ctri.nic.in/Clinicaltrials/advsearch.php
- Clinical Trials Registry‐India . (n.d.‐af). Randomized controlled Single blinded prospective multi‐centre clinical trial to investigate the safety and efficacy of ZingiVir‐H as an adjuvant therapy in hospitalized adults diagnosed with coronavirus disease 2019 (COVID‐19). Identifier CTRI/2020/04/024883. Retrieved fromhttp://ctri.nic.in/Clinicaltrials/advsearch.php
- ClinicalTrials.gov . (n.d.‐a). National Library of Medicine (U.S.). Ayurveda as Prophylaxis for Suspected COVID‐19 Patients. Identifier NCT04395976. Retrieved from https://clinicaltrials.gov/ct2/show/NCT04395976
- ClinicalTrials.gov . (n.d.‐b). National Library of Medicine (U.S.). Ayurveda Self‐Management for Flu Like Symptoms During the Covid‐19 Outbreak. Identifier NCT04345549. Retrieved from https://clinicaltrials.gov/ct2/show/NCT04345549
- ClinicalTrials.gov . (n.d.‐c). National Library of Medicine (U.S.). Ayurveda for Flu Like Illness During Covid‐19 Outbreak. Identifier NCT04351542. Retrieved from https://clinicaltrials.gov/ct2/show/NCT04351542
- ClinicalTrials.gov . (n.d.‐d). National Library of Medicine (U.S.).Protecting Health Care Workers During the COVID‐19 Outbreak. Identifier NCT04387643. Retrieved from https://clinicaltrials.gov/ct2/show/NCT04387643
- Connors, J. M., & Levy, J. H. (2020). COVID‐19 and its implications for thrombosis and anticoagulation. Blood, 135(23), 2033–2040. 10.1182/blood.2020006000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus disease (COVID‐19), “Situation Report – 150” World Health Organization . (2020). Retrieved from https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/situation‐reports
- Dahake, R., Roy, S., Patil, D., Rajopadhye, S., & Chowdhary, A. (2013). Potential anti‐HIV activity of Jatropha curcas Linn. Leaf extracts. Journal of Antivirals & Antiretrovirals, 5(7), 160–165. [Google Scholar]
- Damle, M. (2014). Glycyrrhiza glabra (Liquorice)‐a potent medicinal herb. International Journal of Herbal Medicine, 2(2), 132–136. [Google Scholar]
- Das, P. K., Goswami, S., Chinniah, A., Panda, N., Banerjee, S., Sahu, N. P., & Achari, B. (2007). Woodfordia fruticosa: Traditional uses and recent findings. Journal of Ethnopharmacology, 110(2), 189–199. 10.1016/j.jep.2006.12.029 [DOI] [PubMed] [Google Scholar]
- Davis, L., & Kuttan, G. (2000). Immunomodulatory activity of Withania somnifera. Journal of Ethnopharmacology, 71(1–2), 193–200. 10.1016/s0378-8741(99)00206-8 [DOI] [PubMed] [Google Scholar]
- de Wit, E., van Doremalen, N., Falzarano, D., & Munster, V. J. (2016). SARS and MERS: Recent insights into emerging coronaviruses. Nature Reviews. Microbiology, 14(8), 523–534. 10.1038/nrmicro.2016.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hartog, G., Jacobino, S., Bont, L., Cox, L., Ulfman, L. H., Leusen, J. H., & van Neerven, R. J. (2014). Specificity and effector functions of human RSV‐Specific IgG from bovine milk. PLoS One, 9(11), e112047. 10.1371/journal.pone.0112047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, Y. Y., Yi, Y., Zhang, L. F., Zhang, R. F., Zhang, Y., Wei, Z. C., … Zhang, M. W. (2014). Immunomodulatory activity and partial characterisation of polysaccharides from Momordica charantia. Molecules (Basel, Switzerland), 19(9), 13432–13447. 10.3390/molecules190913432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deodhar, K, & Deodhar, N. (2020). Utility of homeopathic medication ‘Antimonium Tartaricum’ in the treatment of COVID‐19 syndrome.
- Devi, P. U., Murugan, S., Suja, S., Selvi, S., Chinnaswamy, P., & Vijayanand, E. (2007). Antibacterial, in vitro lipid per oxidation and phytochemical observation on Achyranthes bidentata Blume. Pakistan Journal of Nutrition, 6(5), 447–451. [Google Scholar]
- Dey, J. K., Dey, S. K., & Sihag, H. (2020). Current Insights into the novel Coronavirus disease 2019 (COVID‐19) and its homoeopathic management. Homœopathic Links, 33(3), 171–179. [Google Scholar]
- Dhanjal, J. K., Nigam, N., Sharma, S., Chaudhary, A., Kaul, S. C., Grover, A., & Wadhwa, R. (2014). Embelin inhibits TNF‐α converting enzyme and cancer cell metastasis: Molecular dynamics and experimental evidence. BMC Cancer, 14, 775. 10.1186/1471-2407-14-775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhankhar, S., Kaur, R., Ruhil, S., Balhara, M., Dhankhar, S., & Chhillar, A. K. (2011). A review on Justicia adhatoda: A potential source of natural medicine. African Journal of Plant Science, 5(11), 620–627. [Google Scholar]
- Duke, J. A. (1987). CRC Handbook of Medicinal Herbs (5th ed.). Boca Raton, FL: CRC Press. [Google Scholar]
- Enmozhi, S. K., Raja, K., Sebastine, I., & Joseph, J. (2020). Andrographolide as a potential inhibitor of SARS‐CoV‐2 main protease: An in silico approach. Journal of Biomolecular Structure & Dynamics, 1–7. 10.1080/07391102.2020.1760136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahnejad, Z., Ghazanfari, T., & Yaraee, R. (2011). Immunomodulatory effects of Aloe vera and its fractions on response of macrophages against Candida albicans . Immunopharmacology and Immunotoxicology, 33(4), 676–681. 10.3109/08923973.2011.560158 [DOI] [PubMed] [Google Scholar]
- Fatima, M., Zaidi, N. U., Amraiz, D., & Afzal, F. (2016). In vitro antiviral activity of Cinnamomum cassia and its nanoparticles against H7N3 Influenza A virus. Journal of Microbiology and Biotechnology, 26(1), 151–159. 10.4014/jmb.1508.08024 [DOI] [PubMed] [Google Scholar]
- Gautam, S., Gautam, A., Chhetri, S., & Bhattarai, U. (2020). Immunity Against COVID‐19: Potential Role of Ayush Kwath. Journal of Ayurveda and Integrative Medicine. 10.1016/j.jaim.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoke, S. S., Sood, R., Kumar, N., Pateriya, A. K., Bhatia, S., Mishra, A., … Singh, V. P. (2018). Evaluation of antiviral activity of Ocimum sanctum and Acacia arabica leaves extracts against H9N2 virus using embryonated chicken egg model. BMC Complementary and Alternative Medicine, 18(1), 174. 10.1186/s12906-018-2238-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, G. R. (1995). Tulasi (NO Labiatae, Genus‐Ocimum). New Approaches to Medicine and Health, 3(1), 23–29. [Google Scholar]
- Grienke, U., Braun, H., Seidel, N., Kirchmair, J., Richter, M., Krumbholz, A., … Rollinger, J. M. (2014). Computer‐guided approach to access the anti‐influenza activity of licorice constituents. Journal of Natural Products, 77(3), 563–570. 10.1021/np400817j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths, C., Drews, S. J., & Marchant, D. J. (2017). Respiratory Syncytial virus: Infection, detection, and new options for prevention and treatment. Clinical Microbiology Reviews, 30(1), 277–319. 10.1128/CMR.00010-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover, J. K., & Yadav, S. P. (2004). Pharmacological actions and potential uses of Momordica charantia: A review. Journal of Ethnopharmacology, 93(1), 123–132. 10.1016/j.jep.2004.03.035 [DOI] [PubMed] [Google Scholar]
- Gundeti, M. S., Bhurke, L. W., Mundada, P. S., Murudkar, S., Surve, A., Sharma, R., … Dhiman, K. S. (2020). AYUSH 64, a polyherbal ayurvedic formulation in Influenza like Illness: Results of a pilot study. Journal of Ayurveda and Integrative Medicine. (S0975‐9476(20)), 30025–5. 10.1016/j.jaim.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, P. K., Rajan, M., & Kulkarni, S. (2017). Activation of murine macrophages by G1‐4A, a polysaccharide from Tinospora cordifolia, in TLR4/MyD88 dependent manner. International Immunopharmacology, 50, 168–177. 10.1016/j.intimp.2017.06.025 [DOI] [PubMed] [Google Scholar]
- Hamauzu, Y., Yasui, H., Inno, T., Kume, C., & Omanyuda, M. (2005). Phenolic profile, antioxidant property, and anti‐influenza viral activity of Chinese quince (Pseudocydonia sinensis Schneid.), quince (Cydonia oblonga Mill.), and apple (Malus domestica Mill.) fruits. Journal of Agricultural and Food Chemistry, 53(4), 928–934. [DOI] [PubMed] [Google Scholar]
- Helms, J., Tacquard, C., Severac, F., Leonard‐Lorant, I., Ohana, M., Delabranche, X., … CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) . (2020). High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: A multicenter prospective cohort study. Intensive Care Medicine, 46(6), 1089–1098. 10.1007/s00134-020-06062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold, S., Becker, C., Ridge, K. M., & Budinger, G. R. (2015). Influenza virus‐induced lung injury: Pathogenesis and implications for treatment. The European Respiratory Journal, 45(5), 1463–1478. 10.1183/09031936.00186214 [DOI] [PubMed] [Google Scholar]
- Hossan, M. S., Fatima, A., Rahmatullah, M., Khoo, T. J., Nissapatorn, V., Galochkina, A. V., … Wiart, C. (2018). Antiviral activity of Embelia ribes Burm. f. against influenza virus in vitro. Archives of Virology, 163(8), 2121–2131. 10.1007/s00705-018-3842-6 [DOI] [PubMed] [Google Scholar]
- Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England), 395(10223), 497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. T., Hung, C. Y., Hseih, Y. C., Chang, C. S., Velu, A. B., He, Y. C., … Dutta, A. (2019). Effect of aloin on viral neuraminidase and hemagglutinin‐specific T cell immunity in acute influenza. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology, 64, 152904. 10.1016/j.phymed.2019.152904 [DOI] [PubMed] [Google Scholar]
- Huang, N., Singh, N., Yoon, K., Loiacono, C. M., Kohut, M. L., & Birt, D. F. (2013). The immuno‐regulatory impact of orally‐administered Hypericum perforatum extract on Balb/C mice inoculated with H1n1 influenza A virus. PLoS One, 8(9), e76491. 10.1371/journal.pone.0076491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, R., Stintzing, F. C., Briemle, D., Beckmann, C., Meyer, U., & Gründemann, C. (2012). In vitro antiallergic effects of aqueous fermented preparations from Citrus and Cydonia fruits. Planta Medica, 78(4), 334–340. 10.1055/s-0031-1280455 [DOI] [PubMed] [Google Scholar]
- Indian Medicinal Plants Database, National Medicinal Plants Board . (2020). Indian Medicinal Plants Database. Retrieved from http://www.medicinalplants.in/
- International Plant Names Index . (2020). The Royal Botanic Gardens, Kew, Harvard University Herbaria & Libraries and Australian National Botanic Gardens. Retrieved from https://www.ipni.org/
- Ishida, T. (2019). Review on the role of Zn2+ ions in viral pathogenesis and the effect of Zn2+ ions for host cell‐virus growth inhibition. American Journal of Biomedical Science & Research, 2, 28–37. 10.34297/AJBSR.2019.02.000566 [DOI] [Google Scholar]
- Jafarzadeh, A., Mohammadi‐Kordkhayli, M., Ahangar‐Parvin, R., Azizi, V., Khoramdel‐Azad, H., Shamsizadeh, A., … Khaksari, M. (2014). Ginger extracts influence the expression of IL‐27 and IL‐33 in the central nervous system in experimental autoimmune encephalomyelitis and ameliorates the clinical symptoms of disease. Journal of Neuroimmunology, 276(1–2), 80–88. 10.1016/j.jneuroim.2014.08.614 [DOI] [PubMed] [Google Scholar]
- Jagtap, A. G., Sancheti, J. S., & Phadke, A. S. (2012). Antiplatelet and antithrombotic activity of ethanol extract of Embelia ribes. International Journal of Research in Phytochemistry and Pharmacology, 2(3), 150–156. [Google Scholar]
- Jiang, L., Wang, Q., Shen, S., Xiao, T., & Li, Y. (2014). Discovery of glycyrrhetinic acid as an orally active, direct inhibitor of blood coagulation factor xa. Thrombosis Research, 133(3), 501–506. 10.1016/j.thromres.2013.12.025 [DOI] [PubMed] [Google Scholar]
- Karagöz, A., Onay, E., Arda, N., & Kuru, A. (2003). Antiviral potency of mistletoe (Viscum album ssp. album) extracts against human parainfluenza virus type 2 in Vero cells. Phytotherapy Research, 17(5), 560–562. 10.1002/ptr.1163 [DOI] [PubMed] [Google Scholar]
- Kawahara, T., & Iizuka, T. (2011). Inhibitory effect of hot‐water extract of quince (Cydonia oblonga) on immunoglobulin E‐dependent late‐phase immune reactions of mast cells. Cytotechnology, 63(2), 143–152. 10.1007/s10616-010-9323-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, A. A., Bashir, F., & Akhtar, J. (2020). TIRYAQ E ARBA: A classical Unani formulation to boost immunity. Journal of Drug Delivery and Therapeutics, 10(4‐s), 259–263. [Google Scholar]
- Khan, I. N., Habib, M. R., Rahman, M. M., Mannan, A., Sarker, M. M., & Hawlader, S. (2011). Thrombolytic potential of Ocimum sanctum L., Curcuma longa L., Azadirachta indica L. and Anacardium occidentale L. Journal of Basic and Clinical Pharmacy, 2(3), 125–127. [PMC free article] [PubMed] [Google Scholar]
- Killingley, B., & Nguyen‐Van‐Tam, J. (2013). Routes of influenza transmission. Influenza and Other Respiratory Viruses, 2(Suppl 2), 42–51. 10.1111/irv.12080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. Y., Koo, Y. K., Koo, J. Y., Ngoc, T. M., Kang, S. S., Bae, K., … Yun‐Choi, H. S. (2010). Platelet anti‐aggregation activities of compounds from Cinnamomum cassia. Journal of Medicinal Food, 13(5), 1069–1074. 10.1089/jmf.2009.1365 [DOI] [PubMed] [Google Scholar]
- Kiran, V., Vivek, T., Deepak, K., & Khemchand, S. (2017). Comparative antimicrobial potential of Tribhuvana‐Mishrana and its ingredients against clinical bacteria. International Journal of Ayurvedic Medicine, 8(1), 8–11. [Google Scholar]
- Koh, W. S., Yoon, S. Y., Kwon, B. M., Jeong, T. C., Nam, K. S., & Han, M. Y. (1998). Cinnamaldehyde inhibits lymphocyte proliferation and modulates T‐cell differentiation. International Journal of Immunopharmacology, 20(11), 643–660. 10.1016/s0192-0561(98)00064-2 [DOI] [PubMed] [Google Scholar]
- Ku, S. K., & Bae, J. S. (2014). Antiplatelet, anticoagulant, and profibrinolytic activities of withaferin A. Vascular Pharmacology, 60(3), 120–126. 10.1016/j.vph.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Kumar, J., Mitra, M. D., Hussain, A., & Kaul, G. (2019). Exploration of immunomodulatory and protective effect of Withania somnifera on trace metal oxide (zinc oxide nanoparticles) induced toxicity in Balb/c mice. Molecular Biology Reports, 46(2), 2447–2459. 10.1007/s11033-019-04705-x [DOI] [PubMed] [Google Scholar]
- Kumar, V., Singh, P. N., & Bhattacharya, S. K. (2001). Anti‐inflammatory and analgesic activity of Indian Hypericum perforatum L. Indian Journal of Experimental Biology, 39(4), 339–343. [PubMed] [Google Scholar]
- Kundu, S. N., Mitra, K., & Bukhsh, A. R. (2000). Efficacy of a potentized homoeopathic drug (Arsenicum‐album‐30) in reducing cytotoxic effects produced by arsenic trioxide in mice: III. Enzymatic changes and recovery of tissue damage in liver. Complementary Therapies in Medicine, 8(2), 76–81. 10.1054/ctim.2000.0367 [DOI] [PubMed] [Google Scholar]
- Kuttan, G., & Kuttan, R. (1992). Immunomodulatory activity of a peptide isolated from Viscum album extract (NSC 635 089). Immunological Investigations, 21(4), 285–296. 10.3109/08820139209069368 [DOI] [PubMed] [Google Scholar]
- Lakshmanan, K., Moulishankar, A., & Suresh, J. (2020). Screening of Kabasura Kudineer Chooranam against COVID‐19 through targeting of main protease and RNA‐dependent RNA polymerase of SARS‐Cov‐2 by molecular docking studies.
- Latha, P. G., Evans, D. A., Panikkar, K. R., & Jayavardhanan, K. K. (2000). Immunomodulatory and antitumour properties of Psoralea corylifolia seeds. Fitoterapia, 71(3), 223–231. 10.1016/s0367-326x(99)00151-3 [DOI] [PubMed] [Google Scholar]
- Li, S. R., Tang, Z. J., Li, Z. H., & Liu, X. (2020). Searching therapeutic strategy of new coronavirus pneumonia from angiotensin‐converting enzyme 2: The target of COVID‐19 and SARS‐CoV. European Journal of Clinical Microbiology & Infectious Diseases, 39(6), 1021–1026. 10.1007/s10096-020-03883-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, S., Li, M., Yu, X., Jin, H., Zhang, Y., Zhang, L., … Xiao, S. (2019). Synthesis and structure‐activity relationship studies of water‐soluble β‐cyclodextrin‐glycyrrhetinic acid conjugates as potential anti‐influenza virus agents. European Journal of Medicinal Chemistry, 166, 328–338. 10.1016/j.ejmech.2019.01.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C., Chen, H., Chen, K., Gao, Y., Gao, S., Liu, X., & Li, J. (2013). Sulfated modification can enhance antiviral activities of Achyranthes bidentata polysaccharide against porcine reproductive and respiratory syndrome virus (PRRSV) in vitro. International Journal of Biological Macromolecules, 52, 21–24. 10.1016/j.ijbiomac.2012.09.020 [DOI] [PubMed] [Google Scholar]
- Liu, Y. T., Chen, H. W., Lii, C. K., Jhuang, J. H., Huang, C. S., Li, M. L., & Yao, H. T. (2020). A diterpenoid, 14‐Deoxy‐11, 12‐didehydroandrographolide, in Andrographis paniculata reduces steatohepatitis and liver injury in mice fed a high‐fat and high‐cholesterol diet. Nutrients, 12(2), 523. 10.3390/nu12020523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z., Xiao, X., Wei, X., Li, J., Yang, J., Tan, H., … Liu, L. (2020). Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS‐CoV‐2. Journal of Medical Virology, 92(6), 595–601. 10.1002/jmv.25726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik, F., Singh, J., Khajuria, A., Suri, K. A., Satti, N. K., Singh, S., … Qazi, G. N. (2007). A standardized root extract of Withania somnifera and its major constituent withanolide‐A elicit humoral and cell‐mediated immune responses by up regulation of Th1‐dominant polarization in BALB/c mice. Life Sciences, 80(16), 1525–1538. 10.1016/j.lfs.2007.01.029 [DOI] [PubMed] [Google Scholar]
- Manjappa, B., Gangaraju, S., Girish, K. S., Kemparaju, K., Gonchigar, S. J., Shankar, R. L., … Sannaningaiah, D. (2015). Momordica charantia seed extract exhibits strong anticoagulant effect by specifically interfering in intrinsic pathway of blood coagulation and dissolves fibrin clot. Blood Coagulation & Fibrinolysis: An International Journal in Haemostasis and Thrombosis, 26(2), 191–199. 10.1097/MBC.0000000000000191 [DOI] [PubMed] [Google Scholar]
- Manvi, M, & Prasad Garg, G. (2011). Evaluation of pharmacognostical parameters and Hepatoprotective activity in Bryonia alba Linn. Journal of Chemical and Pharmaceutical Research, 3(6), 99–109. [Google Scholar]
- Mary, N. K., Babu, B. H., & Padikkala, J. (2003). Antiatherogenic effect of Caps HT2, a herbal Ayurvedic medicine formulation. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology, 10(6–7), 474–482. 10.1078/094471103322331412 [DOI] [PubMed] [Google Scholar]
- Maurya, V. K., Kumar, S., Bhatt, M. L., & Saxena, S. K. (2020). Therapeutic Development and Drugs for the Treatment of COVID‐19. In Coronavirus Disease 2019 (COVID‐19) (pp. 109–126). Singapore: Springer. [Google Scholar]
- Mazumder, P. M., Pattnayak, S., Parvani, H., Sasmal, D., & Rathinavelusamy, P. (2012). Evaluation of immunomodulatory activity of Glycyrhiza glabra L roots in combination with zing. Asian Pacific Journal of Tropical Biomedicine, 2(1), S15–S20. [Google Scholar]
- Mediratta, P. K., Sharma, K. K., & Singh, S. (2002). Evaluation of immunomodulatory potential of Ocimum sanctum seed oil and its possible mechanism of action. Journal of Ethnopharmacology, 80(1), 15–20. 10.1016/s0378-8741(01)00373-7 [DOI] [PubMed] [Google Scholar]
- Mhaskar, M., Joshi, S., Chavan, B., Joglekar, A., Barve, N., & Patwardhan, A. (2011). Status of Embelia ribes Burm f.(Vidanga), an important medicinal species of commerce from northern Western Ghats of India. Current Science, 547–552. [Google Scholar]
- Ministry of AYUSH . (2020). Ayurveda's immunity boosting measures for self care during COVID 19 crisis. Retrieved from https://www.ayush.gov.in/docs/123.pdf
- Mishra, L. C., Singh, B. B., & Dagenais, S. (2000). Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): A review. Alternative Medicine Review: A Journal of Clinical Therapeutic, 5(4), 334–346. [PubMed] [Google Scholar]
- Mitjà, O., & Clotet, B. (2020). Use of antiviral drugs to reduce COVID‐19 transmission. The Lancet. Global Health, 8(5), e639–e640. 10.1016/S2214-109X(20)30114-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & PRISMA Group . (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. BMJ, 339, b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee, R., Dash, P. K., & Ram, G. C. (2005). Immunotherapeutic potential of Ocimum sanctum (L) in bovine subclinical mastitis. Research in Veterinary Science, 79(1), 37–43. 10.1016/j.rvsc.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Naik, S. R., & Hule, A. (2009). Evaluation of immunomodulatory activity of an extract of andrographolides from Andographis paniculata. Planta Medica, 75(8), 785–791. 10.1055/s-0029-1185398 [DOI] [PubMed] [Google Scholar]
- National Health Commission of the People's Republic of China . (2020). Transcript of press conference in 17, February. Retrieved from http://www.nhc.gov.cn/xcs/s3574/202002/f12a62d10c2a48c6895cedf2faea6e1f.shtml.2020
- Nejatzadeh‐Barandozi, F. (2013). Antibacterial activities and antioxidant capacity of Aloe vera. Organic and Medicinal Chemistry Letters, 3(1), 5. 10.1186/2191-2858-3-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar, S., Bouziane, I., Bouslama, Z., & Djemel, A. (2020). In‐silico identification of potent inhibitors of COVID‐19 main protease (Mpro) and angiotensin converting enzyme 2 (ACE2) from natural products: Quercetin, hispidulin, and cirsimaritin exhibited better potential inhibition than hydroxy‐chloroquine against COVID‐19 main protease active site and ACE2.
- P . (2015, April 11). Homeopathic medicine from snake venom can arrest spread of HIV, claim researchers. DNA India. Retrieved from https://www.dnaindia.com/node/2076711%20accessed%20on%2013.10.19
- Parthipan, M., Aravindhan, V., & Rajendran, A. (2011). Medico‐botanical study of Yercaud hills in the eastern Ghats of Tamil Nadu, India. Ancient Science of Life, 30(4), 104–109. [PMC free article] [PubMed] [Google Scholar]
- Patil, D., Roy, S., Dahake, R., Rajopadhye, S., Kothari, S., Deshmukh, R., & Chowdhary, A. (2013). Evaluation of Jatropha curcas Linn. leaf extracts for its cytotoxicity and potential to inhibit hemagglutinin protein of influenza virus. Indian Journal of Virology: An Official Organ of Indian Virological Society, 24(2), 220–226. 10.1007/s13337-013-0154-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peele, K. A., Potla Durthi, C., Srihansa, T., Krupanidhi, S., Ayyagari, V. S., Babu, D. J., … Venkateswarulu, T. C. (2020). Molecular docking and dynamic simulations for antiviral compounds against SARS‐CoV‐2: A computational study. Informatics in Medicine Unlocked, 19, 100345. 10.1016/j.imu.2020.100345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris, J. S., Lai, S. T., Poon, L. L., Guan, Y., Yam, L. Y., Lim, W., … SARS study group . (2003). Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet (London, England), 361(9366), 1319–1325. 10.1016/s0140-6736(03)13077-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHARMACOPOEIA COMMISSION FOR INDIAN MEDICINE & HOMOEOPATHY Ministry of AYUSH. Government of India, https://pcimh.gov.in/show_content.php?lang=1&level=1&ls_id=5&lid=5. [Google Scholar]
- Phelamei, S. (2020, September 15). Can Chyawanprash help prevent or cure coronavirus infection? Benefits and uses of Ayurvedic medicine. Retrieved from Timesnownews.com.
- Poláková, K., Fauger, A., Sayag, M., & Jourdan, E. (2015). A dermocosmetic containing bakuchiol, Ginkgo biloba extract and mannitol improves the efficacy of adapalene in patients with acne vulgaris: Result from a controlled randomized trial. Clinical, Cosmetic and Investigational Dermatology, 8, 187–191. 10.2147/CCID.S81691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongthanapisith, V., Ikuta, K., Puthavathana, P., & Leelamanit, W. (2013). Antiviral protein of momordica charantia L. inhibits different subtypes of Influenza A. Evidence‐based Complementary and Alternative Medicine: Ecam, 2013, 729081. 10.1155/2013/729081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal, S., Kumar, R. A., Deevi, D. S., Satyanarayana, C., & Rajagopalan, R. (2003). Andrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculata. Journal of Experimental Therapeutics & Oncology, 3(3), 147–158. 10.1046/j.1359-4117.2003.01090.x [DOI] [PubMed] [Google Scholar]
- Rajbhandari, M., Mentel, R., Jha, P. K., Chaudhary, R. P., Bhattarai, S., Gewali, M. B., … Lindequist, U. (2009). Antiviral activity of some plants used in Nepalese traditional medicine. Evidence‐based Complementary and Alternative Medicine: Ecam, 6(4), 517–522. 10.1093/ecam/nem156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana, V., Thakur, K., Sood, R., Sharma, V., & Sharma, T. R. (2012). Genetic diversity analysis of Tinospora cordifolia germplasm collected from northwestern Himalayan region of India. Journal of Genetics, 91(1), 99–103. 10.1007/s12041-012-0137-7 [DOI] [PubMed] [Google Scholar]
- Rasool, A., Khan, M. U., Ali, M. A., Anjum, A. A., Ahmed, I., Aslam, A., … Nawaz, M. (2017). Anti‐avian influenza virus H9N2 activity of aqueous extracts of Zingiber officinalis (Ginger) and Allium sativum (Garlic) in chick embryos. Pakistan Journal of Pharmaceutical Sciences, 30(4), 1341–1344. [PubMed] [Google Scholar]
- Read, S. A., Obeid, S., Ahlenstiel, C., & Ahlenstiel, G. (2019). The role of zinc in antiviral immunity. Advances in Nutrition, 10(4), 696–710. 10.1093/advances/nmz013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, N. J., Vali, D. N., Rani, M., & Rani, S. S. (2014). Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit. Materials Science and Engineering: C, 34, 115–122. [DOI] [PubMed] [Google Scholar]
- Richart, S. M., Li, Y. L., Mizushina, Y., Chang, Y. Y., Chung, T. Y., Chen, G. H., … Hsu, W. L. (2018). Synergic effect of curcumin and its structural analogue (Monoacetylcurcumin) on anti‐influenza virus infection. Journal of Food and Drug Analysis, 26(3), 1015–1023. 10.1016/j.jfda.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righi, N. C., Schuch, F. B., De Nardi, A. T., Pippi, C. M., de Almeida Righi, G., Puntel, G. O., … Signori, L. U. (2020). Effects of vitamin C on oxidative stress, inflammation, muscle soreness, and strength following acute exercise: Meta‐analyses of randomized clinical trials. European Journal of Nutrition, 59(7), 2827–2839. 10.1007/s00394-020-02215-2 [DOI] [PubMed] [Google Scholar]
- Rossman, J. S., & Lamb, R. A. (2011). Influenza virus assembly and budding. Virology, 411(2), 229–236. 10.1016/j.virol.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust, M. J., Lakadamyali, M., Zhang, F., & Zhuang, X. (2004). Assembly of endocytic machinery around individual influenza viruses during viral entry. Nature Structural & Molecular Biology, 11(6), 567–573. 10.1038/nsmb769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar, V., & Kumar, A. H. (2020). Efficacy of natural compounds from Tinospora cordifolia against SARS‐CoV‐2 protease, surface glycoprotein and RNA polymerase. Virology, 1–10. [Google Scholar]
- Saha, S., & Verma, R. J. (2011). Bergenia ciliata extract prevents ethylene glycol induced histopathological changes in the kidney. Acta Poloniae Pharmaceutica, 68(5), 711–715. [PubMed] [Google Scholar]
- Sanders, J. M., Monogue, M. L., Jodlowski, T. Z., & Cutrell, J. B. (2020). Pharmacologic treatments for Coronavirus disease 2019 (COVID‐19): A review. JAMA, 323(18), 1824–1836. 10.1001/jama.2020.6019 [DOI] [PubMed] [Google Scholar]
- Shah, A. S., & Juvekar, A. R. (2010). In vitro and in vivo immunostimulatory activity of Woodfordia fruticosa flowers on non‐specific immunity. Pharmaceutical Biology, 48(9), 1066–1072. 10.3109/13880200903490497 [DOI] [PubMed] [Google Scholar]
- Shahriar, M. (2013). Phytochemical screenings and thrombolytic activity of the leaf extracts of Adhatoda vasica. International Journal of Sciences and Technology. The Experiment, 7(4), 438–441. [Google Scholar]
- Sharma, R., Joshi, V. K., & Rana, J. C. (2011). Nutritional composition and processed products of quince (Cydonia oblonga Mill.). Indian Journal of Natural Products and Resources, 2, 354–357. [Google Scholar]
- Sharma, U., Bala, M., Kumar, N., Singh, B., Munshi, R. K., & Bhalerao, S. (2012). Immunomodulatory active compounds from Tinospora cordifolia. Journal of Ethnopharmacology, 141(3), 918–926. 10.1016/j.jep.2012.03.027 [DOI] [PubMed] [Google Scholar]
- Shibata, S. (2000). A drug over the millennia: Pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi: Journal of the Pharmaceutical Society of Japan, 120(10), 849–862. 10.1248/yakushi1947.120.10_849 [DOI] [PubMed] [Google Scholar]
- Shoji, M., Arakaki, Y., Esumi, T., Kohnomi, S., Yamamoto, C., Suzuki, Y., … Kuzuhara, T. (2015). Bakuchiol is a phenolic isoprenoid with novel enantiomer‐selective anti‐influenza A virus activity involving Nrf2 activation. The Journal of Biological Chemistry, 290(46), 28001–28017. 10.1074/jbc.M115.669465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla, V., & Tripathi, R. D. (2017). Charaka Saṁhitā (Vol. 1, 2nd ed.). Delhi, India: Chaukhambha Sanskrit Pratishthan. [Google Scholar]
- Singh, P, & Attri, B. L (2014). Survey on traditional uses of medicinal plants of Bageshwar Valley (Kumaun Himalaya) of Uttarakhand, India. International Journal of Conservation Science, 5(2), [Google Scholar]
- Singh, R., Satapathy, T., & Prasad, J. (2020). Development, characterization and evaluation of microparticles containing Acacia arabica. Materials Today: Proceedings, 33, 5397–5402. [Google Scholar]
- Skalny, A. V., Rink, L., Ajsuvakova, O. P., Aschner, M., Gritsenko, V. A., Alekseenko, S. I., … Tinkov, A. A. (2020). Zinc and respiratory tract infections: Perspectives for COVID‐19 (Review). International Journal of Molecular Medicine, 46(1), 17–26. 10.3892/ijmm.2020.4575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood, R., Swarup, D., Bhatia, S., Kulkarni, D. D., Dey, S., Saini, M., & Dubey, S. C. (2012). Antiviral activity of crude extracts of Eugenia jambolana Lam. against highly pathogenic avian influenza (H5N1) virus. Indian Journal of Experimental Biology, 50(3), 179–186. [PubMed] [Google Scholar]
- Sornpet, B., Potha, T., Tragoolpua, Y., & Pringproa, K. (2017). Antiviral activity of five Asian medicinal pant crude extracts against highly pathogenic H5N1 avian influenza virus. Asian Pacific Journal of Tropical Medicine, 10(9), 871–876. 10.1016/j.apjtm.2017.08.010 [DOI] [PubMed] [Google Scholar]
- Suara, R. O., & Crowe, J. E. (2004). Effect of zinc salts on respiratory syncytial virus replication. Antimicrobial Agents and Chemotherapy, 48, 783–790. 10.1128/AAC.48.3.783-790.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Plant List . (2013). Version 1.1. Retrieved from http://www.theplantlist.org/
- Thompson, C., & Zambon, M. (2009). Influenza, respiratory syncytial virus and SARS. Medicine, 37(12), 679–685. 10.1016/j.mpmed.2009.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson, M., Al‐Qattan, K. K., Al‐Sawan, S. M., Alnaqeeb, M. A., Khan, I., & Ali, M. (2002). The use of ginger (Zingiber officinale Rosc.) as a potential anti‐inflammatory and antithrombotic agent. Prostaglandins, Leukotrienes, and Essential Fatty Acids, 67(6), 475–478. 10.1054/plef.2002.0441 [DOI] [PubMed] [Google Scholar]
- Tumova, L., & Vokurková, D. (2018). Immunostimulant activity of Bergenia Extracts. Pharmacognosy Magazine, 14(56), 328. [Google Scholar]
- Tumpey, T. M., Maines, T. R., Van Hoeven, N., Glaser, L., Solórzano, A., Pappas, C., … García‐Sastre, A. (2007). A two‐amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science (New York, N.Y.), 315(5812), 655–659. 10.1126/science.1136212 [DOI] [PubMed] [Google Scholar]
- Umachigi, S. P., Kumar, G. S., Jayaveera, K. N., Kishore, D. V. K., Ashok, K. C. K., & Dhanpal, R. (2007). Antimicrobial, wound healing and antioxidant activities of Anthocephalus cadamba . African Journal of Traditional, Complementary, and Alternative Medicines, 4, 481–487. [PMC free article] [PubMed] [Google Scholar]
- Upadhyay, A. K., Kumar, K., Kumar, A., & Mishra, H. S. (2010). Tinospora cordifolia (Willd.) Hook. f. and Thoms. (Guduchi) ‐ validation of the Ayurvedic pharmacology through experimental and clinical studies. International Journal of Ayurveda Research, 1(2), 112–121. 10.4103/0974-7788.64405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney, K. K., Varshney M., & Nath, B. (2020). Molecular Modeling of Isolated Phytochemicals from Ocimum sanctum Towards Exploring Potential Inhibitors of SARS Coronavirus Main Protease and Papain‐Like Protease to Treat COVID‐19. Retrieved from SSRN: https://ssrn.com/abstract=3554371
- Velazquez, E. A., Kimura, D., Torbati, D., Ramachandran, C., & Totapally, B. R. (2009). Immunological response to (1,4)‐alpha‐D‐glucan in the lung and spleen of endotoxin‐stimulated juvenile rats. Basic & Clinical Pharmacology & Toxicology, 105(5), 301–306. 10.1111/j.1742-7843.2009.00447.x [DOI] [PubMed] [Google Scholar]
- Vinothapooshan, G., & Sundar, K. (2011). Anti‐ulcer activity of Adhatoda vasica leaves against gastric ulcer in rats. Journal of Global Pharma Technology, 3, 7–13. [Google Scholar]
- Wang, W., Wang, J., Dong, S. F., Liu, C. H., Italiani, P., Sun, S. H., … Qu, D. (2010). Immunomodulatory activity of andrographolide on macrophage activation and specific antibody response. Acta Pharmacologica Sinica, 31(2), 191–201. 10.1038/aps.2009.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wealth of India . (1991). New Delhi, CSIR. Publication and information directorate, 7, 79–89.
- Wu, C., Liu, Y., Yang, Y., Zhang, P., Zhong, W., Wang, Y., … Li, H. (2020). Analysis of therapeutic targets for SARS‐CoV‐2 and discovery of potential drugs by computational methods. Acta Pharmaceutica Sinica B, 10(5), 766–788. 10.1016/j.apsb.2020.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiuying, P., Jianping, L., Ruofeng, S., Liye, Z., Xuehong, W., & Yan, L. (2012). Therapeutic efficacy of Hypericum perforatum L. extract for mice infected with an influenza A virus. Canadian Journal of Physiology and Pharmacology, 90(2), 123–130. 10.1139/y11-111 [DOI] [PubMed] [Google Scholar]
- Xu, J., & Zhang, Y. (2020). Traditional Chinese Medicine treatment of COVID‐19. Complementary Therapies in Clinical Practice, 39, 101165. 10.1016/j.ctcp.2020.101165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, M. L., Kim, H. J., Wi, G. R., & Kim, H. J. (2015). The effect of dietary bovine colostrum on respiratory syncytial virus infection and immune responses following the infection in the mouse. Journal of Microbiology (Seoul, Korea), 53(9), 661–666. 10.1007/s12275-015-5353-4 [DOI] [PubMed] [Google Scholar]
- Xu, Z., Shi, L., Wang, Y., Zhang, J., Huang, L., Zhang, C., … Wang, F. S. (2020). Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. The Lancet. Respiratory Medicine, 8(4), 420–422. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. X., Li, C. M., & Huang, C. Z. (2016). Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale, 8(5), 3040–3048. 10.1039/c5nr07918g [DOI] [PubMed] [Google Scholar]
- Ye, S., & Wang, T. (2018). Laboratory epidemiology of respiratory viruses in a large children's hospital: A STROBE‐compliant article. Medicine, 97(30), e11385. 10.1097/MD.0000000000011385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue, G. G., Chan, B. C., Hon, P. M., Kennelly, E. J., Yeung, S. K., Cassileth, B. R., … Lau, C. B. (2010). Immunostimulatory activities of polysaccharide extract isolated from Curcuma longa. International Journal of Biological Macromolecules, 47(3), 342–347. 10.1016/j.ijbiomac.2010.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue, G. G., Chan, B. C., Hon, P. M., Lee, M. Y., Fung, K. P., Leung, P. C., & Lau, C. B. (2010). Evaluation of in vitro anti‐proliferative and immunomodulatory activities of compounds isolated from Curcuma longa. Food and Chemical Toxicology, 48(8–9), 2011–2020. 10.1016/j.fct.2010.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue, G. G., Li, L., Lee, J. K., Kwok, H. F., Wong, E. C., Li, M., … Lau, C. B. (2019). Multiple modulatory activities of Andrographis paniculata on immune responses and xenograft growth in esophageal cancer preclinical models. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology, 60, 152886. 10.1016/j.phymed.2019.152886 [DOI] [PubMed] [Google Scholar]
- Zarkovic, N., Zarkovic, K., Grainca, S., Kissel, D., & Jurin, M. (1997). The Viscum album preparation Isorel inhibits the growth of melanoma B16F10 by influencing the tumour‐host relationship. Anti‐Cancer Drugs, 8(Suppl 1), S17–S22. 10.1097/00001813-199704001-00005 [DOI] [PubMed] [Google Scholar]
- Zhou, W., Abdurahman, A., Umar, A., Iskander, G., Abdusalam, E., Berké, B., … Moore, N. (2014). Effects of Cydonia oblonga Miller extracts on blood hemostasis, coagulation and fibrinolysis in mice, and experimental thrombosis in rats. Journal of Ethnopharmacology, 154(1), 163–169. 10.1016/j.jep.2014.03.056 [DOI] [PubMed] [Google Scholar]
- Zou, L., Ruan, F., Huang, M., Liang, L., Huang, H., Hong, Z., … Wu, J. (2020). SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. The New England Journal of Medicine, 382(12), 1177–1179. 10.1056/NEJMc2001737 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


