We read the paper reported by Tam et al. in your journal with great interest.1 They updated the guidelines of the Asia Pacific League of Associations for Rheumatology on the management of patients with rheumatic and musculoskeletal diseases during the coronavirus disease 2019 (COVID‐19) pandemic based on the globally accumulated evidence. We would like to emphasize that there is still lack of data about pulmonary sequelae due to COVID‐19 in patients with rheumatic and musculoskeletal diseases. Here we present our case of severe COVID‐19 in dermatomyositis, in which chest computed tomography was examined before COVID‐19 pneumonia, at the time of COVID‐19 pneumonia, and at 4 months after surviving COVID‐19 pneumonia.
A 69‐year‐old Asian man presented with fever and was positive for the reverse transcriptase polymerase chain reaction testing for SARS‐CoV‐2 (nasopharyngeal swab sample). He had a past history of smoking and anti‐transcriptional intermediary factor (TFI)1γ‐positive dermatomyositis complicated with interstitial lung disease (Figure 1A) and was receiving prednisolone (20 mg/d) and azathioprine (50 mg/d) at that time. Dermatomyositis was diagnosed 1 month earlier, based on the Bohan and Peter criteria: symmetrical weakness of limb‐girdle muscles (manual muscle test of 3), positive evidence for typical myositis on muscle biopsy, elevation of serum creatine kinase (515 U/L, normal <248 U/L) and aldolase (7.5 U/L, normal <6.1 U/L), and heliotrope rash (Figure 1D‐G). Anti‐TIF1γ antibody was detected by enzyme‐linked immunosorbent assay (126.0 index, normal <32 index), whereas anti‐melanoma differentiation‐associated gene‐5 (MDA5), anti‐aminoacyl tRNA synthetase (anti‐ARS), and anti‐Mi‐2 antibodies were negative.
FIGURE 1.
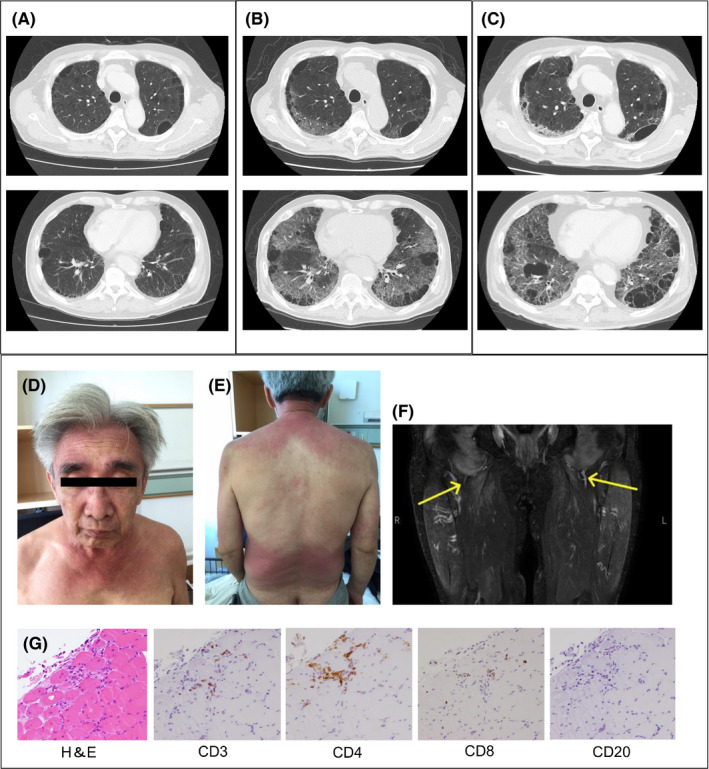
Chest computed tomography (CT) findings of COVID‐19 pneumonia in a patient with dermatomyositis with pre‐existing interstitial lung disease. (A) Chest CT performed before being affected with COVID‐19 pneumonia shows multiple cysts in the upper lobe and honeycomb lung in the lower lobe. (B) Chest CT at the timing of COVID‐19 pneumonia reveals newly emerging ground glass opacities in both lungs. (C) Chest CT at the 4‐month follow‐up after COVID‐19 pneumonia demonstrates residual ground glass opacities and reticular fibrosis changes. (D) Heliotrope rash at diagnosis of dermatomyositis. (E) V‐neck erythema and shawl sign at diagnosis of dermatomyositis. (F) High signal intensity of thighs on T2‐weighted magnetic resonance imaging at diagnosis of dermatomyositis. (G) Muscle biopsy specimens of the right rectus femoris show CD4‐positive T cell‐dominant infiltration into the interstitium of muscle fibers at the margin of the muscle bundle. H&E, hematoxylin and eosin stain
Arterial oxygen saturation of pulse oximetry was 85% at room air, and chest computed tomography performed at admission demonstrated newly emerging bilateral, non‐segmental, diffuse ground glass opacities (Figure 1B). Serum ferritin was elevated (1736 ng/mL, normal <464 ng/mL). He was diagnosed with severe COVID‐19 pneumonia and treated with a high‐flow nasal canula, dexamethasone (6 mg for 10 days), and remdesivir (200 mg on day 1 followed by 100 mg on days 2‐5). Fortunately, he survived and was discharged. Chest computed tomography at 4‐month follow‐up revealed residual ground glass opacities (Figure 1C). Pulmonary function test showed impaired lung diffusing capacity for carbon monoxide (26.8%) and poor 6‐min walking test (320 m). The pulmonary sequelae due to COVID‐19 resulted in significant decrease of his daily activities.
Our present case suggests that COVID‐19 pneumonia can cause irreversible lung damage and critically affect the quality of life of patients with dermatomyositis with pre‐existing interstitial lung disease. Recent studies have reported that half of the survivors of COVID‐19 showed residual lung damage on chest computed tomography at 3‐month follow‐up.2 Not only SARS‐CoV but also MERS‐CoV were reported to cause irreversible lung damage in survivors.3 This nature of coronavirus is obviously distinct from other viruses such as influenza virus which usually show complete radiologic resolution of pneumonia after treatment.2 How is COVID‐19 pneumonia pathologically different from other virus‐related pneumonias? What are the background factors that make COVID‐19 pneumonia prone to irreversible interstitial lung disease? Are there any differences in the following background factors as a susceptibility for irreversible interstitial lung disease after COVID‐19 pneumonia? (ⅰ) Presence or absence of pre‐existing interstitial lung disease? (ⅱ) Presence or absence of rheumatic and musculoskeletal diseases? (ⅲ) Presence or absence of pre‐existing interstitial lung disease with rheumatic and musculoskeletal diseases? There have been no reports to answer these questions.
Considering the similarities of lung lesions in anti‐MDA5 antibody‐positive dermatomyositis to COVID‐19 pneumonia,4 we suggest that viral infection may be one of the environmental factors that cause irreversible interstitial lung disease of dermatomyositis in susceptible individuals. Interestingly, MDA5 is involved in the recognition of viral RNAs including coronavirus and picornavirus, and plays a role for the production of interferons in response to those viruses.5 Of note, SARS‐CoV‐2 induces MDA5‐dependent interferon responses in lung cells.6 Furthermore, a recent study has shown that anti‐MDA5 antibody was positive in half of the patients with COVID‐19 and the presence of anti‐MDA5 antibody was associated with uncontrolled hyperinflammation and rapidly progressive interstitial lung disease.7 Thus, viral infection may be the pathogenic cause of interstitial lung disease associated with dermatomyositis.
In any case, for the management of patients with rheumatic and musculoskeletal diseases during the COVID‐19 pandemic, early dissemination of SARS‐CoV‐2 vaccines is desired to reduce the risk of severe COVID‐19 pneumonia and to prevent irreversible lung damage in patients with rheumatic and musculoskeletal diseases.
PATIENT CONSENT FOR PUBLICATION
The authors obtained written informed consent from the patient.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
MA wrote the manuscript. SH and MA made the figure. SH, MA, TS, MHK, HT, KI, HO, and YO were responsible for the clinical care of the patient. MA and YO made critical revisions to the paper to enhance intellectual content. All authors read and approved the final manuscript.
REFERENCES
- 1.Tam LS, Tanaka Y, Handa R, et al. Updated APLAR consensus statements on care for patients with rheumatic diseases during the COVID‐19 pandemic. Int J Rheum Dis. 2021;24(6):733‐745. 10.1111/1756-185X.14124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.So M, Kabata H, Fukunaga K, Takagi H, Kuno T. Radiological and functional lung sequelae of COVID‐19: a systematic review and meta‐analysis. BMC Pulm Med. 2021;21:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonio GE, Wong KT, Hui DS, et al. Thin‐section CT in patients with severe acute respiratory syndrome following hospital discharge: preliminary experience. Radiology. 2003;228:810‐815. [DOI] [PubMed] [Google Scholar]
- 4.Kondo Y, Kaneko Y, Takei H, et al. COVID‐19 shares clinical features with anti‐melanoma differentiation‐associated protein 5 positive dermatomyositis and adult Still's disease. Clin Exp Rheumatol. 2021;39:631‐638. [PubMed] [Google Scholar]
- 5.Xu L, Wang L, Lv C, Tan W. Anti‐MDA‐5‐positive dermatomyositis associated rapidly progressive interstitial lung disease, a virus‐triggered autoimmune‐like symptom? Rheumatology. 2021:keab224. 10.1093/rheumatology/keab224 [DOI] [PubMed] [Google Scholar]
- 6.Rebendenne A, Valadão ALC, Tauziet M, et al. ARS‐CoV‐2 triggers an MDA‐5‐dependent interferon response which is unable to control replication in lung epithelial cells. J Virol. 2021;95:e02415‐e2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C, Wang Q, Wang Y, et al. Analysis of the correlation between Anti‐Mda5 antibody and the severity of Covid‐19: a retrospective study. medRxiv. 2020. https://www.medrxiv.org/content/10.1101/2020.07.29.20164780v2 [Google Scholar]


