Abstract
Oropharyngeal dysphagia is one of the complications of endotracheal intubation. As expected, cases of dysphagia following coronavirus disease 2019 (COVID‐19) reported to date have all been intubated. We here report a case of sarcopenic dysphagia following severe COVID‐19 pneumonia in a nonintubated older adult. The patient was an 85‐year‐old male who was readmitted to the hospital with dysphagia and subsequent aspiration pneumonia in the first week after his discharge from the COVID‐19 unit. On physical examination, the patient was sarcopenic and malnourished. Flexible endoscopic evaluation of swallowing (FEES) revealed aspiration into the airway. Enteral feeding was initiated and the infusion rate gradually increased to achieve the desired protein‐energy targets. Control FEES 2 months after discharge showed recovery of swallowing function, with no apparent penetration or aspiration. Clinicians caring for patients with COVID‐19 should be aware that dysphagia, which is associated with increased mortality in older adults, may occur even in the absence of intubation. We recommend that the evaluation of dysphagia be part of the clinical assessment in older COVID‐19 patients with malnutrition or sarcopenia.
Keywords: aspiration pneumonia, COVID‐19, dysphagia, sarcopenia
INTRODUCTION
Coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has become a pandemic of unprecedented damage, resulting in hospitalizations and intensive care unit (ICU) admissions of older adults worldwide. Oropharyngeal dysphagia is one of the complications of endotracheal intubation in ICU patients. So far, three case reports have been published on dysphagia as a complication of COVID‐19 and all three cases have a history of intubation.1, 2, 3 However, to the best of our knowledge, there is no published report on dysphagia in nonintubated COVID‐19 patients. The inflammatory state of COVID‐19, combined with malnutrition and low mobility during hospitalization, may predispose the individual to secondary sarcopenia and sarcopenic dysphagia, presenting a major challenge for clinicians caring for patients with COVID‐19. Herein, we report a case of sarcopenic dysphagia following COVID‐19 infection in a nonintubated older adult. The patient's written informed consent was obtained for this report.
CASE PRESENTATION
An 85‐year‐old male was readmitted to the hospital for post‐COVID‐19 cough and difficulty in swallowing 3 days after his discharge from the COVID‐19 unit, where he had been hospitalized for 6 weeks. He had received remdesivir, favipiravir, dexamethasone, and low‐molecular‐weight heparin for severe COVID‐19 pneumonia, but never required ICU care. During his hospital stay due to COVID‐19 pneumonia, the patient had been confined to a bed, as he had dyspnea on exertion. Physical therapy had not been initiated. Because of his loss of appetite, he had been able to consume approximately 25% of his estimated daily energy and protein requirements. He had not been evaluated for malnutrition or dysphagia. An oral nutrition supplement had not been initiated, and he claimed to have lost >3 kg.
He was a nonsmoker with an unremarkable medical history. He actively worked as a university professor and reminisced about his daily bicycle riding, suggesting that he was in good physical condition up until his initial presentation with COVID‐19. He used to eat an adequate and balanced that which met his daily energy and protein requirements. He had a stable body weight and exercised regularly. Hence, sarcopenia and malnutrition prior to his hospitalization for COVID‐19 were unlikely.
On clinical examination, he was afebrile with a blood pressure of 117/78 mm Hg and a pulse rate of 90 bpm. O2 saturation was 96% on 4 L of oxygen through the nasal cannula. He described difficulty swallowing liquid and solid bolus, which was not present prior to his hospitalization for COVID‐19. There was no sign of olfactory dysfunction. Gag reflex was present bilaterally. His eating assessment tool (EAT‐10) score on admission was 40/40 (EAT‐10 is a screening tool for dysphagia and a score of ≥3 is considered abnormal)4.
The patient had a body mass index of 21.6 kg/m2. His Mini Nutritional Assessment (MNA) score was 6/30, which was consistent with malnutrition. According to the Global Leadership Initiative on Malnutrition (GLIM) criteria, the patient had stage 2 (severe) malnutrition.
The European Working Group on Sarcopenia in Older People (EWGSOP)5 uses low muscle strength as the main parameter of sarcopenia. Low muscle quantity and quality confirm the diagnosis and low physical performance indicates severity. EWGSOP also provides cut‐off points for the above‐ mentioned parameters; a handgrip strength <27 kg for men indicates low skeletal muscle strength. Our patient had a handgrip strength of 21.4 kg and a calf circumference of 29 cm, which were abnormal (cut‐off point for calf circumference is 31 cm for both men and women). His appendicular skeletal muscle mass adjusted for height squared was 5.7 kg/m2 (for men, <7 kg/m2 is considered low) and his gait speed was <0.8 m/s. The patient was diagnosed with severe sarcopenia, as he had decreased muscle strength and muscle mass combined with low physical performance. Acute stroke was ruled out with diffusion‐weighted magnetic resonance imaging. Computed tomography of the lungs was consistent with aspiration pneumonia in the right lower lobe, for which he was started on meropenem. He was consulted with the Ear Nose Throat clinic and a clinical bedside assessment of swallowing was performed. Cough response was observed when swallowing food with liquid and thickened liquid consistencies. Flexible endoscopic evaluation of swallowing (FEES) revealed the presence of residue in valleculae and over the epiglottis with apparent aspiration into the airway (Figure 1A and B). The penetration‐aspiration scale (PAS) score was 6 with 10 ml of yogurt and 7 with 10 ml of water (PAS is an 8‐point scale that is used to define the depth of airway invasion; a score of 8 being the worst). The patient was diagnosed with oropharyngeal sarcopenic dysphagia and subsequent aspiration pneumonia after recovery from COVID‐19, induced by prolonged immobilization and malnutrition due to hospitalization. Initially, enteral nutrition was started through a nasogastric feeding tube. A high‐protein formula was initiated, and the infusion rate was gradually increased to reach 30 kcal and 1.5–1.8 g protein per kg body weight daily to achieve the desired protein‐energy targets.6, 7 β‐Hydroxy‐β‐methylbutyrate (HMB) supplementation 1.5 g twice daily (3 g/day), and vitamin D supplementation were also started. A dysphagia rehabilitation program including tongue‐hold swallow, tongue base, and shaker exercises was provided until discharge. Percutaneous endoscopic gastrostomy (PEG) tube placement was performed 1 week after his admission when he was no longer on supplemental oxygen. On the next day, he was started on enteral feeds through the PEG tube and discharged home with a reassessment scheduled for 2 months later.
FIGURE 1.
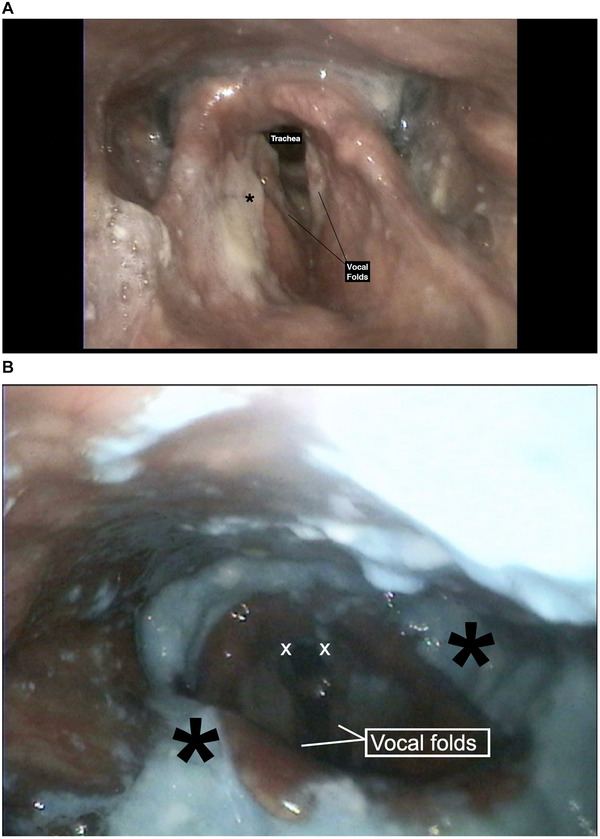
(A) Laryngoscopic evaluation showing laryngeal mucus that does not trigger cough. Black asterisk: Thick mucus over ventricular folds. (B) The presence of residue in valleculae and over the epiglottis with apparent aspiration into the airway. White X: Blue‐dyed food located in the posterior glottis. Black asterisk: Residue collected in the valleculae and pyriform sinuses
At the follow‐up visit 2 months after discharge, he was observed to have gained 4 kg and had a handgrip strength of 28 kg. Control FEES revealed postswallow clearance of blue‐dyed water (Figure 2A). No penetration or aspiration was apparent with blue‐dyed yogurt. The residue of material was visible on the laryngeal side of the epiglottis and valleculae after the swallow (Figure 2B). PAS score was 2 with water and 3 with yogurt. Oral feeding was introduced along with enteral feeding via the PEG tube. The PEG tube was removed when the patient resumed adequate oral intake.
FIGURE 2.
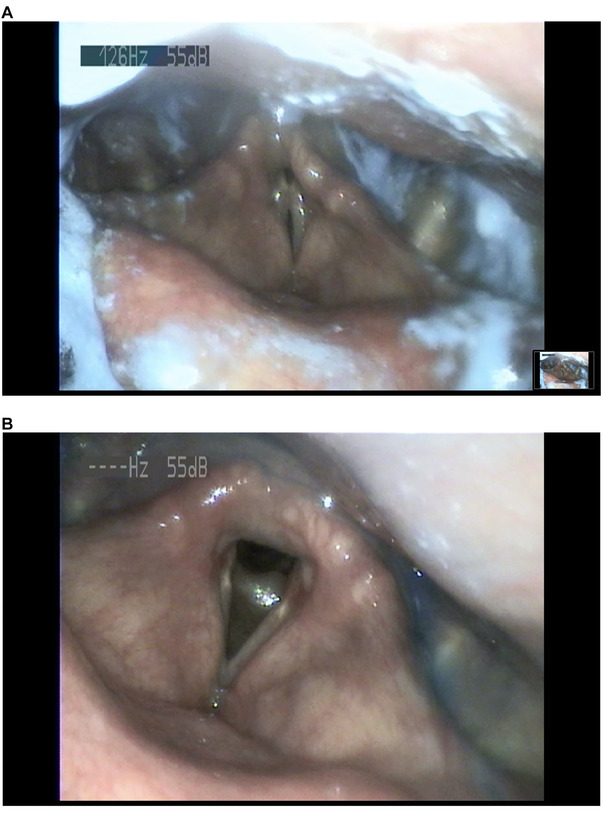
Control flexible endoscopic evaluation of swallowing 2 months after discharge. (A) Trace amounts of blue‐dyed water in the airway that are cleared completely at the end of the swallow (Penetration‐aspiration scale score: 2). (B) No apparent penetration or aspiration even after the fifth spoon of blue‐dyed yogurt. The residue of material is visible on the laryngeal side of the epiglottis and valleculae after swallow (Penetration‐aspiration scale score: 3)
DISCUSSION
Sarcopenia is either primary (age‐related) or secondary, as in the setting of an inflammatory systemic disease.5 Reports indicating an increased risk for sarcopenia among survivors of COVID‐19 came as no surprise because proinflammatory cytokines play a crucial role in sarcopenia pathogenesis.5, 8 Physical inactivity and malnutrition due to hospitalization are also associated with secondary sarcopenia, with more pronounced effects on older adults.5
Sarcopenia may lead to dysphagia through the weakening of the muscles responsible for the coordination of swallowing, namely sarcopenic dysphagia. Maeda et al8 have shown that sarcopenia and physical function are associated with dysphagia in the absence of stroke, neurodegenerative disease, or cancer.
Swallowing is a complex motor event thay requires the harmonious contraction of the tongue muscles, suprahyoids, thyrohyoids, pharyngeal elevators, and intrinsic laryngeal muscles9. Geniohyoid muscle atrophy and decreased tongue pressure have specifically been associated with dysphagia and aspiration in older adults.10, 11 Sarcopenic dysphagia may result in recurrent aspiration pneumonia, which is associated with increased mortality.12
Sarcopenia and malnutrition share common pathophysiological mechanisms including inflammation and oxidative stress.5 In a study from Wuhan, China,13 the prevalence of malnutrition in older patients with COVID‐19 was found to be as high as 52.7%. The long hospitalization period, during which the nutrition needs of the patient were not met, may have contributed to the development of malnutrition and subsequent sarcopenic dysphagia. As dysphagia eventually leads to malnutrition because of inadequate dietary intake, reverse causality is also possible.
SARS‐CoV‐2 is a neurotropic virus that has been shown to cause peripheral nerve disease.14 Glossopharyngeal and vagal neuropathy, which are among the neurological manifestations of COVID‐19, may induce dysphagia.14 The cytokine storm of severe disease may also aggravate neurological damage. Although no major pathologies were apparent in the central nervous system, we could not rule out peripheral neuropathy in our patient.
During his hospital admission for COVID‐19, the patient received a high dose of dexamethasone that was gradually tapered over 2 weeks. Treatment with dexamethasone may also have contributed to his muscle loss.
CONCLUSION
During this unprecedented crisis, during which hospitals are overloaded and health systems on the verge of collapse, clinicians are being forced to prioritize patient care. Assessment of malnutrition and sarcopenia may be overlooked while struggling to keep the patient alive, as was the case with our patient.
Clinicians caring for patients with COVID‐19 should be aware that dysphagia, which is associated with increased mortality in older adults, may occur even in the absence of intubation. We recommend that the assessment of swallowing function be part of a clinical routine in older COVID‐19 patients with malnutrition or sarcopenia.
FUNDING INFORMATION
None declared.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Büşra Can, Narkiza Ismagulova, Aslı Tufan, and İsmail Cinel contributed to the conception and design of the research; Necati Enver contributed to the acquisition and analysis of the data; Büşra Can and Aslı Tufan drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Can B, İsmagulova N, Enver N, Tufan A, Cinel İ. Sarcopenic dysphagia following COVID‐19 infection: A new danger. Nutr Clin Pract. 2021;36:828–832. 10.1002/ncp.10731
REFERENCES
- 1.Cavalagli A, Peiti G, Conti C, Penati R, Vavassori F, Taveggia G. Cranial nerves impairment in post‐acute oropharyngeal dysphagia after COVID‐19: a case report. Eur J Phys Rehabil Med. 2020;56(6):853‐857. [DOI] [PubMed] [Google Scholar]
- 2.Ishkanian A, Mehl A. Clinical Conundrum: Dysphagia in a Patient with COVID‐19 and Progressive Muscle Weakness [published online ahead of print, 2020 Oct 31]. Dysphagia. 2020;1–2. 10.1007/s00455-020-10205-zect [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoyagi Y, Ohashi M, Funahashi R, Otaka Y, Saitoh E. Oropharyngeal Dysphagia and Aspiration Pneumonia Following Coronavirus Disease 2019: A Case Report. Dysphagia. 2020;35(4):545–548. 10.1007/s00455-020-10140-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belafsky PC, Mouadeb DA, Rees CJ, et al. Validity and reliability of the eating assessment tool (EAT‐10) Ann Otol Rhinol Laryngol. 2008;117(12):919‐924. [DOI] [PubMed] [Google Scholar]
- 5.Cruz‐Jentoft AJ, Bahat G, Bauer J, et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis [published erratum appears in Age Ageing. 2019;48(4):601]. Age Ageing. 2019;48(1):16‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mechanick JI, Carbone S, Dickerson RN, et al. ASPEN COVID‐19 task force on nutrition research. clinical nutrition research and the COVID‐19 pandemic: a scoping review of the ASPEN COVID‐19 task force on nutrition research. JPEN J Parenter Enteral Nutr. 2021;45(1):13‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morley JE, Kalantar‐Zadeh K, Anker SD. COVID‐19: a major cause of cachexia and sarcopenia? J Cachexia Sarcopenia Muscle. 2020;11(4):863‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maeda K, Akagi J. Sarcopenia is an independent risk factor of dysphagia in hospitalized older people. Geriatr Gerontol Int. 2016;16(4):515‐521. [DOI] [PubMed] [Google Scholar]
- 9.Dodds WJ. Physiology of swallowing. Dysphagia. 1989;3(4):171‐178. [DOI] [PubMed] [Google Scholar]
- 10.Feng X, Todd T, Lintzenich CR, et al. Aging‐related geniohyoid muscle atrophy is related to aspiration status in healthy older adults. J Gerontol A Biol Sci Med Sci. 2013;68(7):853‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshida M, Kikutani T, Tsuga K, Utanohara Y, Hayashi R, Akagawa Y. Decreased tongue pressure reflects symptom of dysphagia. Dysphagia. 2006;21(1):61‐65. [DOI] [PubMed] [Google Scholar]
- 12.DeLegge MH. Aspiration pneumonia: incidence, mortality, and at‐risk populations. JPEN Parenter Enteral Nutr. 2002;26(6_suppl):S19‐24. discussion S24‐5. [DOI] [PubMed] [Google Scholar]
- 13.Li T, Zhang Y, Gong C, et al. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID‐19 in Wuhan, China. Eur J Clin Nutr. 2020:74(6):871‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]


