Abstract
Background
The humoral immune response following COVID‐19 vaccination in nursing home residents is poorly known. A longitudinal study compared levels of IgG antibodies against the spike protein (S‐RBD IgG) (S‐RDB protein IgG) after one and two BNT162b2/Pfizer jabs in residents with and without prior COVID‐19.
Methods
In 22 French nursing homes, COVID‐19 was diagnosed with real‐time reverse‐transcriptase polymerase chain reaction (RT‐PCR) for SARS‐CoV‐2. Blood S‐RDB‐protein IgG and nucleocapsid (N) IgG protein (N‐protein IgG) were measured 21–24 days after the first jab (1,004 residents) and 6 weeks after the second (820 residents).
Results
In 735 residents without prior COVID‐19, 41.7% remained seronegative for S‐RDB‐protein IgG after the first jab vs. 2.1% of the 270 RT‐PCR‐positive residents (p < 0.001). After the second jab, 3% of the 586 residents without prior COVID‐19 remained seronegative. However, 26.5% had low S‐RDB‐protein IgG levels (50–1050 UA/ml) vs. 6.4% of the 222 residents with prior COVID‐19. Residents with an older infection (first wave), or with N‐protein IgG at the time of vaccination, had the highest S‐RDB‐protein IgG levels. Residents with a prior COVID‐19 infection had higher S‐RDB‐protein IgG levels after one jab than those without after two jabs.
Interpretation
A single vaccine jab is sufficient to reach a high humoral immune response in residents with prior COVID‐19. Most residents without prior COVID‐19 are seropositive for S‐RDB‐protein IgG after the second jab, but around 30% have low levels. Whether residents with no or low post‐vaccine S‐RDB protein IgG are at higher risk of symptomatic COVID‐19 requires further analysis.
Keywords: antibodies against SARS‐CoV‐2, BNT162b2/Pfizer vaccine, COVID‐19, nursing home, rRT‐PCR
This study compares levels of IgG antibodies against the spike protein (S‐RBD IgG) (S‐RDB protein IgG) after one and two BNT162b2/Pfizer jabs in residents with and without prior COVID‐19. One BNT162b2 jab is sufficient to reach an S‐RBD protein IgG level of 1050 AU/ml in 98% of nursing home residents with prior COVID‐19. After two jabs, levels of S‐RBD IgG are below 1050 AU/ml in 29.5% of residents without prior COVID‐19. Abbreviations: AU/ml, arbitrary units per ml; COVID‐19, coronavirus disease 2019; RT‐PCR, real‐time reverse‐transcriptase polymerase chain reaction; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; S‐RBD IgG, IgG antibodies against the spike protein.

Abbreviations
- NH
Nursing home
- N‐protein IgG
nucleocapsid (N) IgG protein
- RT‐PCR
real‐time reverse‐transcriptase polymerase chain reaction for SARS‐CoV‐2
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus‐2
- S‐RBD‐protein IgG
SARS‐CoV‐2 spike IgG
1. BACKGROUND
Nursing home (NH) residents are at high risk of serious illness and death from COVID‐19 due to severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). 1 In NHs facing a COVID‐19 outbreak, infection prevention and control (IPC) measures based on results of repeated testing by real‐time reverse‐transcriptase polymerase chain reaction for SARS‐CoV‐2 (RT‐PCR) in all residents can limit coronavirus transmission. 2 , 3 Residents with prior COVID‐19 may be protected against reinfection during outbreaks in NHs. 4
Vaccination against SARS‐CoV‐2 is safe and effective in preventing COVID‐19 in adults, 5 and immunocompetent SARS‐CoV‐2‐seropositive adults might only require one jab of the Pfizer‐BioNTech or the Moderna mRNA vaccines to reach immunity. 6 , 7
Age or immune dysregulation may impact immune response to COVID‐19 vaccination. The level of N‐protein IgG produced after SARS‐CoV‐2 infection, associated with a substantially reduced risk of SARS‐CoV‐2 reinfection, 8 declines over time. 9 In the Danish Microbiology Database, protection against reinfection was found to be reduced in participants over 65 years. 10 Age seems also to reduce serological response after vaccination of kidney 11 or liver transplant recipients, 12 and of patients with chronic lymphocytic leukemia, 13 cancer, 14 or hemodialysis. 15
Few data exist on post‐vaccine immunity in NH residents, and the size of the study is usually low. After one jab, binding antibodies against S‐RDB‐protein were approximately fourfold lower in residents compared with healthcare professionals. 16 , 17 Although the increment of antibody levels after the second immunisation may be higher in older participants, the absolute mean titer of this group seems to remain lower in NH residents aged over 80 years than in those under 60. 18
A pilot study showed that NH residents having had COVID‐19 in the past 9–12 months had much higher levels of antibodies against the SARS‐CoV‐2 S‐RDB‐protein (S‐RDB‐protein IgG) after a single BNT162b2 jab than residents not having had COVID‐19. 16
It remains therefore necessary (a) to confirm in a large sample of residents whether the S‐protein IgG response after a single BNT162b2 jab may be influenced by prior COVID‐19 infection, (b) to determine in former COVID‐19 residents whether the S‐protein IgG response after a single BNT162b2 jab may be influenced by the time elapsed since the COVID‐19 infection and by the persistence of a natural immunity against N‐protein, and (c) to assess the S‐protein IgG response after the second vaccination in residents with vs. without prior COVID‐19.
We first compared S‐ and N‐protein IgG levels 3 weeks after a single BNT162b2 jab in a large group of residents with vs. without prior COVID‐19 confirmed by RT‐PCR (RT‐PCR‐positive residents). Two periods were considered—the past 9 to 12 months (older infections) and the past 3 to 7 months (newer infections)—corresponding to the first and second waves of the epidemic in our region (March‐June 2020 and September‐December 2020). Secondly, we assessed the S‐protein IgG response in these residents 6 weeks after the second vaccination.
2. METHODS
2.1. Design
A longitudinal study was carried out on NHs having faced a COVID‐19 outbreak in 2020. First, we compared N‐ and S‐protein IgG levels three weeks after a single BNT162b2 jab in residents without prior COVID‐19 (with repeated negative RT‐PCR and negative N‐protein IgG measured 3 weeks after the jab) vs. residents with prior COVID‐19 (confirmed either by a positive RT‐PCR or by detectable N‐protein IgG). Two periods were considered—the past 9 to 12 months (older infections) and the past 3 to 7 months (newer infections)—corresponding to the first and second waves of the epidemic in our region.
Second, S‐protein levels were compared six weeks after a second BNT162b2 jab in residents with vs. without prior COVID‐19.
2.2. Settings
We included 22 NHs of the Montpellier area (France), each with at least five residents with a previous diagnosis of COVID‐19 confirmed by RT‐PCR on a nasopharyngeal swab test between March and December 2020 (PCR‐positive residents). This study follows previous studies conducted in the same NHs with a COVID‐19 outbreak between March and June 2020. 16 , 19 , 20
2.3. Participants
As previously reported, 16 , 19 , 20 as soon as a resident developed COVID‐19 in a NH, all residents and staff members were repeatedly tested using RT‐PCR on nasopharyngeal swab until no new cases were diagnosed. This was the procedure recommended by the Health Agency of our region, in accordance with the European Geriatric Medicine Society guidance. 3 Comorbidities were assessed according to a previous paper. 2 The same population was studied during the first COVID‐19 wave.
All residents who had not had COVID‐19 in the last 3 months, and who signed an informed consent, were offered the first jab of the BNT162b2 vaccine between January and March 2021. According to the national recommendations in NHs, the second jab, irrespective of prior COVID‐19 history, was offered three weeks later. Residents and their family, relatives, or legal representative were informed of the possibility to measure the antibody response after the first and second jabs and of the fact that the residents’ anonymised clinical and biological data would be used for research purposes. The study was approved by the Montpellier University Hospital institutional review board (IRB‐MTP_2020_06_202000534 and IRB‐MTP _2021_04_202000534).
We used a control group of younger healthcare workers to assess the differences with NH residents who had never had a positive RT‐PCR and who had undetectable N‐protein IgG levels after vaccination.
2.4. Outcomes
S‐RDB‐protein IgG against the SARS‐CoV‐2 receptor‐binding domain (RBD) of the S1 subunit was detected using the SARS‐CoV‐2 IgG II Quant assay (Abbott Diagnostics). Results were expressed as arbitrary units per ml (AU/ml; positive threshold: 50 AU/ml; upper limit: 40,000 AU/ml; a level ≥1,050 AU/ml was considered as a significant response 21 and a level ≥4160 AU/ml indicated a high neutralising effect according to the manufacturer). N‐protein IgG was detected using the SARS‐CoV‐2 IgG assay (Abbott Diagnostics). Results were expressed as a signal to cutoff ratio (S/CO; Abbott Alinity; positive threshold: 0.8 S/CO). 22
The humoral immune response was first assessed 3 weeks after the first jab, i.e. just before the second one. The boost induces a second wave, generating longer‐lived plasma cells that provide long‐lived immunity, but a waning of antibodies during the first. This is why it is necessary to study the S‐RBD protein level 6 weeks after the second jab. 23
2.5. Sample size
The sample size was not calculated since we aimed to study all possible NH residents.
2.6. Statistical analysis
Qualitative variables were described with frequency and proportions for each category. The description of quantitative variables was performed using mean and standard deviation and/or median, minimum, and maximum values. N‐protein and S‐RBD‐protein IgG levels were compared in residents (a) with and without prior COVID‐19, (b) with prior COVID‐19 during the first or second wave of the epidemic, and (c) who remained or did not remain seropositive for N‐protein IgG. Wilcoxon‐Mann‐Whitney two‐sided tests were used, qualitative variables were compared using a chi‐square test, and the statistical significance threshold was set at 5%. Analyses were performed using the SAS Enterprise Guide, v7.3 (SAS Institute Inc.).
3. vc RESULTS
3.1. Demographic characteristics of the residents
Among the 1,243 eligible residents, 1,004 were analysed to assess the antibody response after the first jab. Seven hundred and thirty‐five residents had always tested negative for SARS‐CoV‐2 RNA by RT‐PCR, 94 had tested positive 9 to 12 months before the vaccine, and 176 had tested positive 3 to 7 months before. Among those 1,004 residents, 808 accepted the second jab and agreed to participate in the study (Figure 1). Most residents were females.
FIGURE 1.
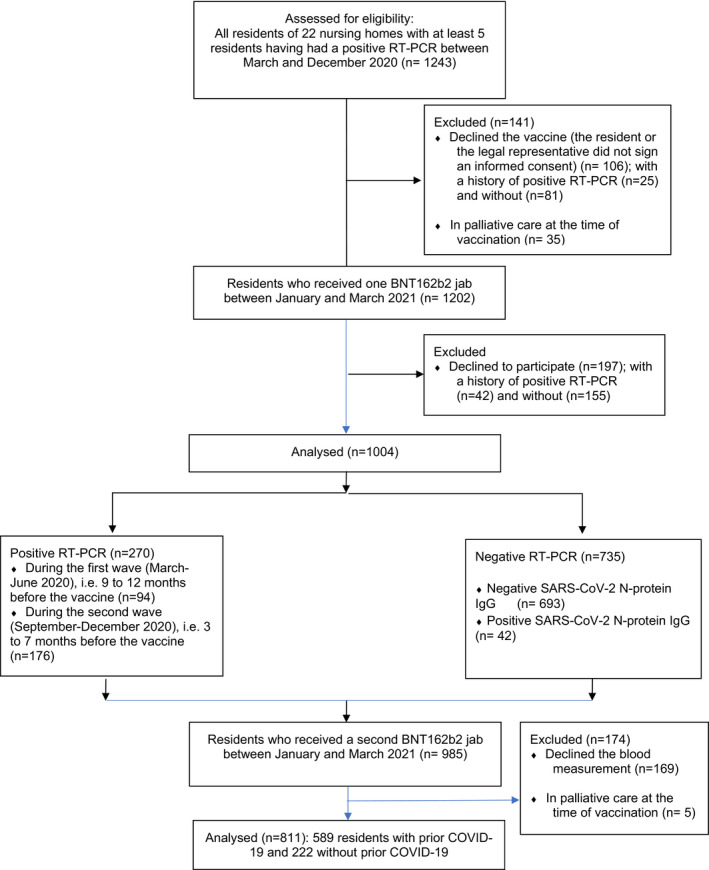
Flow Diagram of the sample of residents from 22 nursing homes having faced a COVID‐19 outbreak between March and December 2020
3.2. N‐Protein IgG levels 6 weeks after the first BNT162b2 jab
N‐protein IgG were detectable (≥ 0.8 signal to cutoff ratio) in 42 (5.7%) of the 735 negative RT‐PCR residents. These residents were then considered as having a prior infection by SARS‐CoV‐2. Residents with an older infection were less frequently seropositive for N‐protein IgG than residents with a newer infection (61.1% vs. 83.7%, p < .001) (Table 1). The N‐protein IgG median level was lower in residents with an older infection than in those with a newer infection (p < 0.001).
TABLE 1.
Demographic characteristics with N‐Protein IgG and S‐Protein levels (First jab)
| Repeated negative RT‐PCR in the last 12 months (n = 735) | Positive RT‐PCR (n = 270) | p value | |||
|---|---|---|---|---|---|
|
Negative N‐Protein IgG a (n = 693) |
Positive N‐Protein IgG b (n = 42) |
9 to 12 months ago c (n = 94) |
3 to 7 months ago d (n = 176) |
||
| Sex | |||||
| Female (%) | 484 (69.8) | 35 (83.3) | 66 (70.2) | 139 (78.0) | 0.03 |
| Male (%) | 209 (30.2) | 7 (16.7) | 28 (29.8) | 37 (21.0) | |
| Age, mean (SD), range, year |
86.2 (0.0) 51–106 |
85.3 (7.0) 72–100 |
86.6 (9.2) 54–100 |
87.8 (8.2) 54–100 |
0.05 |
| SARS‐CoV−2 N‐Protein IgG level ≥0.8 signal to cutoff ratio, No. (%) | 0 | 42 (100) | 55 (61.1) | 144 (83.7) | <0.001 between c and d |
| SARS‐CoV−2 N‐Protein IgG level, median (IQR) [range], AU/ml | 0 |
3.4 (0.8;7.3), [0.8–7.3] |
1.0 (0.4;2.6) [0.02–6.1] |
3.91 (1.9;5.3) [0.01–8] | <0.001 between c and b , d |
| SARS‐CoV−2 S‐protein IgG level | |||||
| 0–50 AU/ml | 288 (41.7) | 5 (11.9) | 8 (4.6) | 2 (2.1) | |
| 50–1050 AU/ml | 343 (49.6) | 5 (11.9) | 15 (8.6) | 6 (6.4) | |
| 1050–4160 AU/ml | 47 (6.8) | 1 (2.4) | 5 (2.8) | 3 (3.2) | |
| ≥4160 AU/ml | 13 (1.9) | 31 (73.8) | 147 (84.0) | 83 (88.3) | |
| SARS‐CoV−2 S‐protein level median (Q1‐Q3) [range], AU/ml |
82 (16–296) [0–40,000] |
21,736 (16,229) [0–40,000] |
40,000 (9,146–40,000) [6–14142] | 20,796 (4157;40000) [1–40,000] | |
Corresponds to residents with negative RT‐PCR in the last 12 months and with negative N‐Protein IgG.
Corresponds to residents with negative RT‐PCR in the last 12 months and with positive N‐Protein IgG.
Corresponds to residents with posittive RT‐PCR in the last 9‐12 months.
Corresponds to residents with positive RT‐PCR in the 3 to 7 months ago.
3.3. S‐RBD‐protein IgG levels 6 weeks after the first BNT162b2 jab
S‐RBD‐protein IgG was undetectable (< 50 AU/ml) in 41.7% of the RT‐PCR‐negative residents who tested negative for N‐protein IgG, in 11.9% of the residents with a negative RT‐PCR but who were positive for N‐protein IgG, in 2.1% of residents with an older infection, and in 4.6% with a newer infection (Table 1). The coefficient of variation of S‐RBD‐protein IgG in the whole sample is 176.2% 3 weeks after the first jab and 125.9% 6 weeks after the second jab.
Median values of S‐RBD‐protein IgG were slightly higher in female vs. male residents without prior COVID‐19 (2552 UA/ml vs. 2305 UA/ml) and in those with prior COVID‐19 (30,531 UA/ml vs. 28,599 UA/ml).
We compared 42 healthcare workers aged 23 to 67 years who did not report any COVID‐19 symptoms and who had negative N‐protein IgG. Their median S‐RBD‐protein IgG level 6 weeks after the vaccination was 10,444 AU/ml (Q1‐Q3: 5419–16,117). None of them had a level under 1050 AU/ml (vs. 29.4% of the residents), six (14.3%) had a level ranging from 1050 to 4160 AU/ml, and 36 (85.7% vs. 28.9% of the residents) had a level over 4160 AU/ml.
A low S‐RBD‐protein IgG level (≤ 1,050 UA/ml) was more often observed in residents without prior COVID‐19 (negative for both RT‐PCR and N‐protein IgG tests) than in residents with a previous positive RT‐PCR (91.3% vs. 11.5%, p < 0.001). RT‐PCR‐positive residents with an older infection more often had a high S‐RBD‐protein IgG level than those with a newer infection (88.3% vs. 84.0%, p < 0.001), and RT‐PCR‐negative residents tested positive for N‐protein IgG (88.3% vs. 73.8%, p < 0.001).
The predictive value of a high S‐RBD‐protein IgG level (≥4,160 UA/ml) by a prior RT‐PCR in the last 3 to 12 months was high (positive predictive value of 85.5% [83.3%–87.7%] and negative predictive value of 94.0% [92.5%–95.5%]).
Comorbidities were studied in 433 residents during the first wave (94.9% were tested). We found no significant link between comorbidities and S‐RBD‐protein IgG levels after the first or second jab, in the residents with or without prior COVID‐19 (Tables [Link], [Link], [Link], [Link]).
In the 181 positive PCR residents of the first wave, 40% were asymptomatic. Asymptomatic residents had non‐significantly different S‐protein IgG levels 6 weeks after the second jab when compared to symptomatic residents (Fisher's test = 0.27, Table S5).
3.4. Link between S‐ and N‐Protein IgG levels after the first BNT162b2 jab
Among the RT‐PCR‐positive residents, the median levels of S‐RBD‐protein IgG after vaccine were ranked in the following order: (a) residents with a newer infection and no detectable N‐protein IgG (median value of 230 AU/ml), (b) residents with a newer infection and detectable N‐protein IgG (median value of 20,685 AU/ml), (c) residents with an older infection and no detectable N‐protein IgG (median value of 27,313 AU/ml), and (d) residents with an older infection and detectable N‐protein IgG (median value of 40,000 AU/ml)(Table 2, Figure 2).
TABLE 2.
S‐Protein IgG levels in RT‐PCR‐positive residents 3 weeks after the first jab
| Negative N‐Protein IgG in the last 9 to 12 months a | Positive N‐Protein IgG in the last 9 to 12 months b | Negative N‐Protein IgG in the last 3 to 7 months c | Positive N‐Protein IgG in the last 3 to 7 months d | p value | |
|---|---|---|---|---|---|
| 39 | 55 | 32 | 144 | ||
| IgG level ≥50 AU/ml No. (%) | 34 (97.1) | 55 (100.0) | 21 (75.0) | 144 (100.0) | p < 0.001 between c and a , b , d |
| median level [range], AU/ml |
20,685 [13,−40,000] |
40,000 [67– 40,000] |
230 [1– 40,000] |
27313 [210–40,000] |
p < 0.001 between c and a , b , d |
AU, arbitrary units; IQR, interquartile range.
Corresponds to residents with negative N‐Protein IgG in the last 9 to 12 months.
Corresponds to residents with positive N‐Protein IgG in the last 9 to 12 month.
Corresponds to residents with negative N‐Protein IgG in the last 3 to 7 months.
Corresponds to residents positive N‐Protein IgG in the last 3 to 7 months.
FIGURE 2.
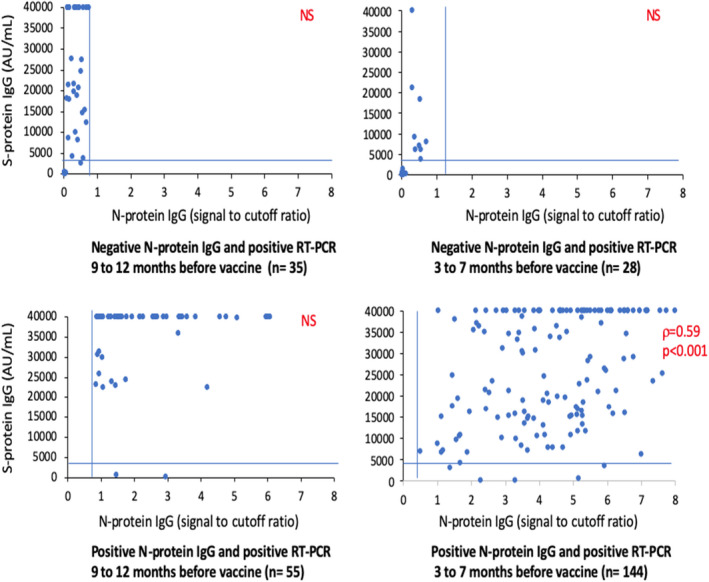
Correlation between N‐ and S‐protein IgG depending on the time of the COVID‐19 infection, 3 weeks after the first jab. Legend: N‐protein IgG levels: positive value when ≥0.8 signal to cutoff ratio (vertical bars); S‐protein IgG levels: high values when ≥4160 arbitrary unit (AU)/ml (horizontal bar)
The predictive value of a high S‐RBD‐protein IgG level (≥4,160 UA/ml) by N‐protein IgG (≥ 0.8 signal to cutoff ratio) was high (positive predictive value of 92.5% [90.8%–94.2%] and negative predictive value of 93.0% [91.4%–94.6%]).
3.5. S‐RBD‐protein IgG levels 6 weeks after the second BNT162b2 jab
Six weeks after the second BNT162b2 jab, only around 3% of the 554 residents without prior COVID‐19 had undetectable S‐RBD‐protein IgG levels (<50 AU/ml) vs. none of the residents with a prior positive PCR (Table 3). The prevalence of residents with low S‐RBD‐protein IgG levels (≤1,050 AU/ml) was significantly lower in residents without prior COVID‐19 than in those with a positive PCR in the last 3 to 12 months (28.3% vs. 2.7%, p < 0.001). The median value of S‐RBD‐protein IgG in residents without prior COVID‐19 after two jabs was 10‐fold lower than that in residents with prior COVID‐19 after one jab (2,384 AU/ml vs. 23,259 AU/ml).
TABLE 3.
Demographic characteristics with N‐Protein IgG and S‐Protein levels (second jab)
| Repeated negative RT‐PCR in the last 12 months (n = 589) | Positive RT‐PCR (n = 222) | p value | |||
|---|---|---|---|---|---|
|
Negative N‐Protein IgG a (n = 554) |
Positive N‐Protein IgG b (n = 35) |
9 to 12 months ago c (n = 72) |
3 to 7 months ago d (n = 150) |
||
| Sex | |||||
| Female (%) | 387 (69.9) | 32 (91.4) | 52 (72.2) | 121 (80.7) | <0.01 |
| Male (%) | 167 (30.1) | 3 (8.6) | 20 (27.8) | 29 (19.3) | |
| Age, mean (SD), year | 86.2 (9.0) | 85.6 (7.2) | 87.4 (8.6) | 88.0 (8.5) | NS |
| SARS‐CoV−2 S‐protein IgG level | |||||
| 0–50 AU/ml | 17 (3.1) | 1 (2.9) | 0 (0) | 1 (0.7) | |
| 50–1050 AU/ml | 146 (26.3) | 2 (5.7) | 0 (0) | 5 (3.3) | |
| 1050–4160 AU/ml | 231 (41.7) | 5 (14.3) | 6 (8.3) | 12 (8.0) | |
| ≥4160 AU/ml | 160 (28.9) | 27 (77.1) | 66 (91.7) | 132 (88.0) | |
| SARS‐CoV−2 S‐protein level median (Q1‐Q3) [range], AU/ml | 2384 (827; 4902)[0–40,000] | 18239 (5876; 38418) [0; 40000][0–40,000] | 39525 (19601; 4000) [6–14142] | 28863 (12083; 40000)[1–40,000] | |
Corresponds to residents with negative N‐Protein IgG.
Corresponds to residents with positive N‐Protein IgG.
Corresponds to residents with positive RT‐PCR 9 to 12 months ago.
Corresponds to residents with positive RT‐PCR 3 to 7 months ago.
4. DISCUSSION
Recent studies conducted in small samples of immunocompetent adults showed higher levels of S‐RBD‐protein IgG after a single jab in individuals with prior COVID‐19 than in those without prior COVID‐19. 5 , 24 Our pilot study with 136 residents is the only one to have included NH residents. 16 The current study is innovative because it includes a large sample of NH residents and evaluates the effect on antibody response of (a) the time elapsed since COVID‐19 infection and vaccination, and (b) the persistence of a natural immunity against SARS‐CoV‐2 N‐protein at the time of the first vaccination. The large sample allows the assessment of the percentage of residents with undetectable S‐RBD‐protein IgG (< 50 AU/ml) or with low levels of S‐RBD‐protein (≤1050 AU/ml) after two jabs among those with vs. without prior COVID‐19. It also enables the comparison of antibody response after one jab in residents without COVID‐19 and after two jabs in residents with prior COVID‐19.
The present study shows that 3 weeks after the first jab: (a) 41.7% of residents without prior COVID‐19 have undetectable S‐RBD‐protein IgG and 91.3% have low levels, (b) among residents with prior COVID‐19, those seropositive for N‐protein at the time of the vaccine and those with an older infection (in the last 9 to 12 months) have the highest S‐RBD‐protein IgG levels, (c) the second jab significantly boosts the antibody response of residents without prior COVID‐19 (6 weeks after the second jab, only 3.1% remain seronegative for S‐RBD‐protein IgG). However, 29.4% of residents have low S‐RBD‐protein IgG levels, and (d) in residents with prior COVID‐19, the second jab leads to very few residents with low S‐RBD‐protein IgG levels. The median value of S‐RBD‐protein IgG levels after one jab in residents with prior COVID‐19 exceeds that of two jabs in those without prior COVID‐19.
4.1. Findings of the present study in light of current publications on residents with prior COVID‐19
Three weeks after a single jab of BNT162b2 vaccine, residents with an older infection were less often seropositive for N‐protein IgG than those with a newer infection (61.1% vs. 85.7%). This result accords with the time‐related decline of natural SARS‐CoV‐2 antibodies observed not only in the general population with prior COVID‐19 25 but also in NHs: one study showed that 91% of residents were still seropositive for N‐protein 6 months after COVID‐19, but with a decreasing antibody titer over time. 26 Serum IgG levels are a measurement of the response. The rapid decay of N‐protein IgG in the 90 days after mild COVID‐19 infection has suggested a short‐lasting humoral immunity against SARS‐CoV‐2. 27 The present study is quite reassuring as it indicates a natural immunity in 61% of NH residents 9 to 12 months after infection, which is in line with the results shown after other acute viral infections. 28 , 29 The S‐RBD‐protein IgG levels of NH residents without prior COVID‐19 are significantly lower than those of healthcare workers without prior COVID‐19.
This study shows a link between N‐protein IgG and S‐RBD‐protein IgG levels after the first jab. RT‐PCR‐positive residents who were seropositive for N‐protein IgG more often exhibited a high S‐RBD‐protein IgG level after vaccination (over 96% of them) than seronegative residents with a positive RT‐PCR from an older (80%) or newer (28.6%) infection. This result is in line with influenza for which pre‐existing immunity in older individuals, contrary to immune‐senescence or poor functional status, is a strong correlate of post‐vaccination humoral immune response. 30 , 31
It is reassuring to note that 80% of the RT‐PCR‐positive residents with an older infection but who do not have a persistent humoral immunity exhibit a high S‐RBD‐protein IgG level after the vaccine. This result suggests a long‐term persistence of previously‐generated memory B cells that can induce a rapid clonal expansion and terminal differentiation to produce high‐affinity anti‐S‐RBD‐protein IgG after the vaccine, 32 as observed for common viruses. 33 In line with this hypothesis, a robust boosting after the first mRNA jab was found to strongly correlate with levels of pre‐existing memory B cells in recovered individuals, identifying a key role for memory B cells in recall responses to SARS‐CoV‐2 antigens. 34 Taken together, these results suggest that both natural humoral immunity (that appears to decrease over time) and cellular immunity (that seems to be maintained over time) may modulate the high level of SARS‐CoV‐2 S‐RBD‐protein IgG following a single BNT162b2 jab in nursing home residents having recovered from COVID‐19. This highlights the utility of defining cellular responses in addition to serologies to predict the immune response to vaccine in this population. This result may be important since the role of pre‐existing humoral immune response was underscored during the 2009 A (H1N1) pandemic, when older adults were less severely affected than younger individuals. 35
The present study shows that NH residents with prior COVID‐19 achieve much higher S‐RBD‐protein IgG levels after one single jab than in younger COVID‐19 convalescents. 36 It remains to be determined whether vaccinated residents with prior COVID‐19 (with at least one jab) will be better protected against reinfection during outbreaks in NHs than unvaccinated residents with prior COVID‐19. 4
The persistence of N‐protein IgG in 85.7% of residents with a newer infection (in the past 3 to 7 months) and the increase in S‐RBD‐protein level observed with increasing time between COVID‐19 and vaccination both support the hypothesis that, in most residents, the vaccine should be administered at least 3 months after COVID‐19 infection, even if reinfection is possible within those 3 months. 37
4.2. Strengths and limitations of the study
This study has several strengths. Our sample is probably representative of the population of NH residents in France since it was conducted on a large sample of residents who were tested across 22 NHs facing a COVID‐19 outbreak. The ascertainment of positive and negative RT‐PCR results is probably almost complete since all studied NHs followed the same regional Health Agency guidance published in March 2020. 2 , 3 We used an automated quantitative assay to measure the RBD IgG level that correlates well with virus neutralisation. 38 , 39 N‐protein IgG measurement in all individuals allowed us to differentiate residents with SARS‐CoV‐2 immunisation while having repeated negative RT‐PCR tests.
The main limitation of the study is the lack of clinical outcome. It remains indeed to demonstrate that a single BNT162b2 jab in residents having recovered from COVID‐19 has the same efficacy in preventing reinfection as two jabs. If the N‐protein IgG level is associated with a substantially reduced risk of SARS‐CoV‐2 reinfection, 8 there is no available publication demonstrating a link between the S‐RBD‐protein IgG level obtained after the vaccine and the risk of incident SARS‐CoV‐2 infection and of symptomatic or severe COVID‐19. It remains to be determined whether the thresholds we have chosen to define a low level (≤1050 AU/ml) or a high S‐RBD‐protein IgG after the vaccine (≥4,160 AU/ml) are effectively associated with an increased or decreased risk of developing SARS‐CoV‐2 infection. 7 This is particularly important since serum neutralising activities against SARS‐CoV‐2 six months after COVID‐19 hospitalisation remain significant for ancestral strains and for the D614G and B.1.16 variants, but are weaker for the B.1.351 variant. 40 It is therefore plausible that the post‐vaccine S‐RBD‐protein IgG level necessary to obtain protection against new variants may be higher than previously defined. 41
4.3. Generalisability
Although this study has been carried out in one European region, its size allows generalisability in residents infected by the natural strain of the virus. New studies are needed to study the post‐vaccine antibody response in residents infected by different variants.
5. CONCLUSIONS
Recent studies show that even if the vaccine is very effective in reducing severe forms of COVID‐19 in nursing home residents, it may not, even when complete, totally prevent the risk of SARS‐CoV‐2 outbreaks in nursing homes (Blain et al., submitted). 42
This present study supports the hypothesis that (a) Most of the residents having recovered from COVID‐19 (with a diagnosis using RT‐PCR or N‐protein IgG) may only require one single jab to achieve peak antibody and memory B cell responses. (b) The second jab reduces however the prevalence of residents with low S‐RBD‐protein IgG levels (≤1050 AU/ml) (from 11.5% to 2.7%). (c) For individuals whose infection history is unknown, measuring S‐RBD‐protein IgG antibody levels just before the second jab could be useful in determining whether a second jab is required. (d) Most of the individuals without prior COVID‐19 have a low level of S‐RBD‐protein IgG 3 weeks after the first jab, further confirming the benefit to administer the second jab without delay to boost the antibody response. After two jabs, around 30% of residents without prior COVID‐19 have a low level of S‐RBD‐protein IgG. Whether these residents with low antibody response after the complete vaccine regimen are at higher risk of symptomatic COVID‐19 and whether they may benefit from a third jab remains to be further investigated.
Additional studies are required to demonstrate whether measuring S‐RBD‐protein IgG after the vaccination can help to personalise the vaccine schedules and reduce secondary effects related to possible reactogenicity. 43 Further studies are necessary also to determine whether the present results obtained in residents infected by the natural strain of the virus may be replicated in residents infected by the new variants. 44 , 45
CONFLICT OF INTEREST
The authors declare no conflicts of interest/competing interests.
Supporting information
Table S1
Table S2
Table S3
Table S4
Table S5
ACKNOWLEDGMENTS
The authors would like to thank (i) Anna Bedbrook, Fabienne Portejoie (MACVIA France), and Eva Pons (Master Métiers de l’Enseignement, de l'Education et de la Formation; éducation nationale française, Lyon) for editorial assistance, (ii) Joy Martin, Isabelle Bussereau, and Marie‐Suzanne Léglise, Secours Infirmiers (Department of Geriatrics, Montpellier University Hospital), Véronique Vera and Florence Biblocque (admission office, Montpellier University Hospital) for material support, as well as (iii) the residents and staff members of the nursing homes involved in the study. None of these contributors received any compensation for their help in carrying out the study.
Blain H, Tuaillon E, Gamon L, et al. Antibody response after one and two jabs of the BNT162b2 vaccine in nursing home residents: The CONsort‐19 study. Allergy. 2022;77:271–281. 10.1111/all.15007
Funding information
This research did not receive any funding from agencies in the public, commercial, or not‐for‐profit sectors
Contributor Information
Hubert Blain, Email: h-blain@chu-montpellier.fr.
Edouard Tuaillon, Email: e-tuaillon@chu-montpellier.fr.
REFERENCES
- 1. McMichael TM, Currie DW, Clark S, et al. Epidemiology of covid‐19 in a long‐term care facility in king county, Washington. N Engl J Med. 2020;382(21):2005‐2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blain H, Rolland Y, Tuaillon E, et al. Efficacy of a test‐retest strategy in residents and health care personnel of a nursing home facing a COVID‐19 outbreak. J Am Med Dir Assoc.. 2020;21(7):933‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blain H, Rolland Y, Schols J, et al. August 2020 Interim EuGMS guidance to prepare European long‐term care facilities for COVID‐19. Eur Geriatr Med.. 2020;11(6):899‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jeffery‐Smith A, Iyanger N, Williams SV, et al. Antibodies to SARS‐CoV‐2 protect against re‐infection during outbreaks in care homes, September and October 2020. Euro Surveill. 2021;26(5):2100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid‐19 Vaccine. N Engl J Med. 2020;383(27):2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saadat S, Rikhtegaran Tehrani Z, Logue J, et al. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS‐CoV‐2. JAMA. 2021;325(14):1467‐1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ebinger JE, Fert‐Bober J, Printsev I, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS‐CoV‐2. Nat Med. 2021;27(6):981‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lumley SF, O'Donnell D, Stoesser NE, et al. Antibody status and incidence of SARS‐CoV‐2 infection in health care workers. N Engl J Med. 2021;384(6):533‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Padoan A, Dall'Olmo L, Rocca FD, et al. Antibody response to first and second dose of BNT162b2 in a cohort of characterized healthcare workers. Clin Chim Acta. 2021;519:60‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hansen CH, Michlmayr D, Gubbels SM, Molbak K, Ethelberg S. Assessment of protection against reinfection with SARS‐CoV‐2 among 4 million PCR‐tested individuals in Denmark in 2020: a population‐level observational study. Lancet. 2021;397(10280):1204‐1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rabinowich L, Grupper A, Baruch R, et al. Low immunogenicity to SARS‐CoV‐2 vaccination among liver transplant recipients. J Hepatol. 2021;75(2):435‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Korth J, Jahn M, Dorsch O, et al. Impaired Humoral response in renal transplant recipients to SARS‐CoV‐2 vaccination with BNT162b2 (Pfizer‐BioNTech). Viruses. 2021;13(5):756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165‐3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jerome B, Emmanuel C, Zoubir A, et al. Impaired immunogenicity of BNT162b2 anti SARS‐CoV‐2 vaccine in patients treated for solid tumors. Ann Oncol. 2021;32(8):1053‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jahn M, Korth J, Dorsch O, et al. Humoral response to SARS‐CoV‐2‐vaccination with BNT162b2 (Pfizer‐BioNTech) in patients on hemodialysis. Vaccines (Basel). 2021;9(4):360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blain H, Tuaillon E, Gamon L, et al. Spike Antibody levels of nursing home residents with or without prior COVID‐19 3 weeks after a single BNT162b2 vaccine dose. JAMA. 2021;325(18):1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brockman MA, Mwimanzi F, Sang Y, et al. Weak humoral immune reactivity among residents of long‐term care facilities following one dose of the BNT162b2 mRNA COVID‐19 vaccine. medRxiv. 2021. 10.1101/2021.03.17.21253773. Preprint. [DOI] [Google Scholar]
- 18. Muller L, Andree M, Moskorz W, et al. Age‐dependent immune response to the Biontech/Pfizer BNT162b2 COVID‐19 vaccination. Clin Infect Dis. 2021;ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blain H, Rolland Y, Benetos A, et al. Atypical clinical presentation of COVID‐19 infection in residents of a long‐term care facility. Eur Geriatr Med.. 2020;11(6):1085‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blain H, Gamon L, Tuaillon E, et al. Atypical symptoms, SARS‐CoV‐2 test results, and immunization rates in 456 residents from eight nursing homes facing a COVID‐19 outbreak. Age Ageing. 2021;50(3):641‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Van Praet JT, Vandecasteele S, De Roo A, De Vriese AS, Reynders M. Humoral and cellular immunogenicity of the BNT162b2 mRNA Covid‐19 vaccine in nursing home residents. Clin Infect Dis. 2021;ciab300. 10.1093/cid/ciab300. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tuaillon E, Bollore K, Pisoni A, et al. Detection of SARS‐CoV‐2 antibodies using commercial assays and seroconversion patterns in hospitalized patients. J Infect. 2020;81(2):e39‐e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alter G, Seder R. The power of antibody‐based surveillance. N Engl J Med. 2020;383(18):1782‐1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gobbi F, Buonfrate D, Moro L, et al. Antibody response to the BNT162b2 mRNA COVID‐19 vaccine in subjects with prior SARS‐CoV‐2 infection. Viruses. 2021;13(3):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bolotin S, Tran V, Osman S, et al. SARS‐CoV‐2 seroprevalence survey estimates are affected by anti‐nucleocapsid antibody decline. J Infect Dis. 2021;223(8):1334‐1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruopp MD, Strymish J, Dryjowicz‐Burek J, Creedon K, Gupta K. Durability of SARS‐CoV‐2 IgG antibody among residents in a long‐term care community. J Am Med Dir Assoc.. 2021;22(3):510‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ibarrondo FJ, Fulcher JA, Goodman‐Meza D, et al. Rapid Decay of anti‐SARS‐CoV‐2 antibodies in persons with mild Covid‐19. N Engl J Med. 2020;383(11):1085‐1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS‐CoV‐2 infection persist for months. Science. 2020;370(6521):1227‐1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS‐CoV‐2 infection in humans. Nat Microbiol.. 2020;5(12):1598‐1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reber AJ, Kim JH, Biber R, et al. Preexisting immunity, more than aging, influences influenza vaccine responses. Open Forum Infect Dis. 2015;2(2):ofv052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van Epps P, Tumpey T, Pearce MB, et al. Preexisting immunity, not frailty phenotype, predicts influenza postvaccination titers among older veterans. Clin Vaccine Immunol. 2017;24(3):e00498‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS‐CoV‐2. Nature. 2021;591(7851):639‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357(19):1903‐1915. [DOI] [PubMed] [Google Scholar]
- 34. Goel RR, Apostolidis SA, Painter MM, et al. Distinct antibody and memory B cell responses in SARS‐CoV‐2 naive and recovered individuals following mRNA vaccination. Sci Immunol. 2021;6(58):eabi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gaglani M, Spencer S, Ball S, et al. Antibody response to influenza A(H1N1)pdm09 among healthcare personnel receiving trivalent inactivated vaccine: effect of prior monovalent inactivated vaccine. J Infect Dis. 2014;209(11):1705‐1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Azzi L, Focosi D, Dentali F, Baj A, Maggi F. Anti‐SARS‐CoV‐2 RBD IgG responses in convalescent versus naive BNT162b2 vaccine recipients. Vaccine. 2021;39(18):2489‐2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tillett RL, Sevinsky JR, Hartley PD, et al. Genomic evidence for reinfection with SARS‐CoV‐2: a case study. Lancet Infect Dis. 2021;21(1):52‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Salazar E, Kuchipudi SV, Christensen PA, et al. Convalescent plasma anti‐SARS‐CoV‐2 spike protein ectodomain and receptor‐binding domain IgG correlate with virus neutralization. J Clin Invest. 2020;130(12):6728‐6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang Z, Schmidt F, Weisblum Y, et al. mRNA vaccine‐elicited antibodies to SARS‐CoV‐2 and circulating variants. Nature. 2021;592(7855):616‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Betton M, Livrozet M, Planas D, et al. Sera neutralizing activities against SARS‐CoV‐2 and multiple variants six month after hospitalization for COVID‐19. Clin Infect Dis. 2021;ciab308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hoffmann M, Arora P, Gross R, et al. SARS‐CoV‐2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell. 2021;184(9):2384‐2393.e12. 10.1016/j.cell.2021.03.036. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cavanaugh AM, Fortier S, Lewis P, et al. COVID‐19 outbreak associated with a SARS‐CoV‐2 R.1 lineage variant in a skilled nursing facility after vaccination program–Kentucky, March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(17):639‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID‐19 RNA vaccine BNT162b1 in adults. Nature. 2020;586(7830):589‐593. [DOI] [PubMed] [Google Scholar]
- 44. Lustig Y, Nemet I, Kliker L, et al. Neutralizing response against variants after SARS‐CoV‐2 infection and one dose of BNT162b2. N Engl J Med. 2021;384(25):2453‐2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chang X, Augusto GS, Liu X, et al. BNT162b2 mRNA COVID‐19 vaccine induces antibodies of broader cross‐reactivity than natural infection but recognition of mutant viruses is up to 10‐fold reduced. Allergy. 2021;76(9):2895‐2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4
Table S5


