We report two patients with slowly progressive amyotrophic lateral sclerosis (ALS) who experienced rapid functional decline after contracting severe acute respiratory virus–coronavirus‐2 (SARS‐CoV‐2).
A 78‐year‐old man developed proximal leg weakness in 2014 and was diagnosed with probable ALS based on El Escorial criteria1 in 2016. His revised ALS Functional Rating Scale (ALSFRS‐R) assessment, administered by physicians, showed scoring that progressed at an unusually slow rate since that time (Figure 1A). In December 2020, the patient developed a fever, and SARS‐CoV‐2 viral polymerase chain reaction of the nasopharyngeal swab was positive. One week later, he presented to an emergency department with fever, shortness of breath, and delirium. Computed tomography scan of the chest revealed signs of pneumonia. He also developed deep venous thrombosis in the left femoral vein but did not have a pulmonary embolism. The patient was hospitalized and treated with dexamethasone, remdesivir, oxygen supplement, and anticoagulation. He did not require mechanical ventilation. The patient was discharged 5 days later when he became afebrile and his peripheral capillary oxygen saturation (SpO2) was 93% on room air. Despite prompt treatment and recovery from pneumonia, his functional status declined rapidly. His ALSFRS‐R score that the physician administered during follow‐up telemedicine visits decreased by 16 points within 4 months, 11 points in the final month before he died of hypercarbic respiratory failure.
FIGURE 1.
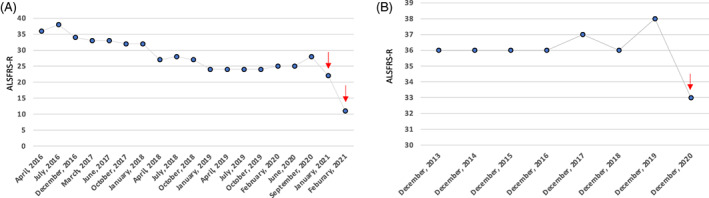
Rapid decline of revised ALSFRS‐R in two amyotrophic lateral sclerosis patients post–COVID‐19 infection. A, Slow decline of ALSFRS‐R in patient 1, which plateaued from January 2019 to September 2020, but declined rapidly after COVID‐19 infection. B, Stable ALSFRS‐R between 2013 and 2019 in patient 2, followed by a rapid decline in 2020 after COVID‐19 infection. ↓ indicates postinfection ALSFRS‐R. Abbreviation: ALSFRS‐R, revised Amyotropic Lateral Sclerosis Functional Rating Scale
A 66‐year‐old man developed his first sign of weakness in his legs in 2004 and was diagnosed with laboratory‐supported probable ALS in 2006. His disease had an unusually slow progression. Between 2014 and 2019, the ALSFRS‐R, administered by physicians, remained stable at 36 to 38. He had bilateral foot dorsiflexion and plantarflexion weakness as well as mild hand weakness during his clinic visit in December 2019. His bulbar and respiratory function were both normal. In April 2020, he contracted the virus after several family members had COVID‐19 infection. His infection was confirmed by a positive SARS‐CoV‐2 viral polymerase chain reaction of a nasopharyngeal swab sample. His symptoms included a low‐grade fever for 1 day, and fatigue, loss of smell, and loss of taste for 1 week. He was never hospitalized. After the COVID infection, he noticed fast progression of arm and leg weakness. He is now wheelchair‐dependent. He also developed mild‐to‐moderate speech and swallowing difficulty 4 months after the COVID‐19 infection. ALSFRS‐R score administered during a telemedicine visit decreased by 5 points when compared with December 2019 (Figure 1B).
Although some groups have warned about the potential for bad outcomes from COVID‐19 in patients with ALS (unpublished data), we are only aware of one published study supporting this position.2 That study compared ALSFRS‐R progression in a cohort of 84 patients before and during the pandemic lockdown in France. Progression appeared faster during the lockdown. It is not clear whether any of the 84 patients in the study actually had COVID‐19 infection. The authors postulated decreased contact with clinicians as an explanation for the faster decline. This explanation does not seem likely in our first patient, who had increased contact with clinicians during and after his infection. We theorize that the ability of COVID‐19 to trigger neuroinflammation3 may have played a role in our patients' accelerated declines. Although it is possible that the functional decline in these two patients was related to the known post‐COVID syndrome, there is strong evidence that suggests it was due to worsening of ALS. For example, instead of nonspecific feelings of fatigue, unwellness, and lingering respiratory symptoms, the first patient reported a significant decline of hand fine motor skills at 1 month post‐COVID infection, despite respiratory status that was the same as that pre‐COVID. The second patient, who had only mild COVID symptoms, reported a significant decline of leg strength and new bulbar weakness without respiratory decline.
We use these two examples to alert the medical community that SARS‐CoV‐2 infection can lead to more rapid progression of ALS. We emphasize the need for prompt testing and close monitoring of ALS patients who have contracted COVID‐19. It is also imperative that health‐care providers counsel patients about the safety and efficacy of COVID‐19 vaccine and encourage all ALS patients to be vaccinated in the absence of any clear medical contraindications.
CONFLICT OF INTEREST
R.B. has received research support from ALS Association, MediciNova, and Orion, and served as a paid consultant for Alexion, ALS Association, Amylyx, Biogen, Brainstorm Cell, Mallinkrodt, MT Pharma, New Biotic, Orphazyme, and Woolsey Pharma. X.L. declares no conflicts of interest.
ETHICAL PUBLICATION STATEMENT
We confirmed that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Brooks BR, Miller RG, Swash M. World Federation of Neurology Research Group on Motor Neuron Diseases El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293‐299. [DOI] [PubMed] [Google Scholar]
- 2.Esselin F, De La Cruz E, Pageot N, Juntas‐Moralès R, Alphandéry S, Camu W. Increased worsening of amyotrophic lateral sclerosis patients during Covid‐19‐related lockdown in France. Amyotroph Lateral Scler Frontotemporal Degener. 2021;1‐3. [DOI] [PubMed] [Google Scholar]
- 3.Pilotto A, Odolini S, Masciocchi S, et al. Steroid‐responsive encephalitis in coronavirus disease 2019. Ann Neurol. 2020;88:423‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


