Abstract
This study provides current evidence for efficacy and safety of treating COVID‐19 with combined traditional Chinese medicine (TCM) and conventional western medicine (CWM). Six databases were searched from January 1 to December 31, 2020. Randomized controlled trials (RCTs), case–control studies (CCTs), and cohort studies on TCM or TCM combined with CWM treatment for COVID‐19 were included. The quality of included RCTs was assessed by Cochrane risk of bias tool, and the Newcastle‐Ottawa Scale (NOS) was used to assess the quality of cohort studies and CCTs. Review Manager 5.4 software was used to perform meta‐analysis. The quality of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. A total of 35 studies (3,808 patients) composing 19 RCTs and 16 observational studies were included. The results of meta‐analysis revealed that comparing with CWM alone, integrated TCM and CWM had significant improvement in total effective rate, improvement rate of chest CT, the rate of disease progression, as well as improvement of fever, fatigue and cough. The overall quality of evidence was very low to moderate. In conclusion, TCM combined with CWM was a potential treatment option for increasing clinical effective rate, improving the clinical symptoms, and preventing disease progression in COVID‐19 patients. High‐quality clinical trials are required in the further.
Keywords: COVID‐19, integrative medicine, meta‐analysis, systematic review, traditional Chinese medicine
Abbreviations
- CCT
case–control study; CI, confidence intervals
- COVID‐19
coronavirus disease 2019
- CRP
C‐reactive protein
- CT
computed tomography
- CWM
conventional western medicine
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- LYM
lymphocyte count
- MD
mean difference
- NOS
Newcastle‐Ottawa Scale
- OR
odds ratio
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐analysis
- RCT
randomized controlled trial
- RR
risk ratio
- SMD
standardized mean difference
- TCM
traditional Chinese medicine
- WBC
white blood cell count
1. INTRODUCTION
Since December 2019, a novel positive strand RNA coronavirus named SARS‐COV‐2 was confirmed to be the pathogen causing an outbreak of unexplainable pneumonia (Zhu et al., 2020). The rapid human‐to‐human transmission and the lack of specific antiviral drugs and vaccines have resulted in an outbreak of coronavirus disease 2019 (COVID‐19) in 212 countries and regions around the world. By the end of 2020, so far, the cumulative number of confirmed cases has reached more than 70 billion, and the cumulative number of deaths has reached more than 1.6 million. Although the epidemic has subsided in China, the number of new confirmed cases overseas is on the rise. The COVID‐19 remains a major threat to global public health.
In China, traditional Chinese medicine (TCM) combined with conventional western medicine (CWM) has been a great success in the treatment of COVID‐19. During this COVID‐19 outbreak, over 90% of total confirmed COVID‐19 patients in China had been treated with TCM or TCM combined with CWM (News Conference of the State Council Information Office, 2020). Related reports including case reports, observational studies and randomized clinical trials had confirmed that integrated TCM and CWM could increase clinical effective rate, improve the clinical symptoms, shorten length of hospital stay, and reduce patient number of transferred to severe or critically ill cases (Hu et al., 2020; Tian et al., 2020; Xiao, Tian et al., 2020; Zhang, Tian et al., 2020). Moreover, a number of systematic reviews have assessed the efficacy and safety of TCM for treating COVID‐19 (M. Liu et al., 2020; Luo et al., 2020; Pang et al., 2020; Xiong, Wang et al., 2020). But the previous systematic reviews included a maximum of 11 RCTs (Pang et al., 2020). The limited number of literature included in these reviews provided limited TCM treatment schemes because TCM emphasizes perspective of harmonization between environment and human body, different provinces released different treatment guidance according to the disease, local climate characteristics, and different physical conditions. A summary at a time when the epidemic in China is leveling off was performed to provide complete guidance for clinical practice.
2. METHODS
2.1. Study registration
This review protocol has been registered in the International Prospective Register of Systematic Reviews (PROSPERO registration number: CRD42020201639). This study was based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) (Moher, Liberati, Tetzlaff, Altman, & PRISMA Group, 2009).
2.2. Inclusion criteria
2.2.1. Type of studies
Randomized controlled trials (RCTs), cohort studies and case–controlled studies (CCTs) were included in this systematic review. Publications in English and Chinese were included.
2.2.2. Type of participants
Participants confirmed with COVID‐19 were included regardless of their age, sex, race, and nationality. Diagnostic criteria consisted of the following: (a) historical epidemiology; (b) being symptomatic (either having fever, coughing, or fatigue) plus radiologic abnormalities consistent with pneumonia; (c) positive for SARS‐CoV‐2 nucleic acid tests; (d) the gene sequence of the virus was highly homologous to the known novel coronavirus. Patients who simultaneously met the (a) and (b), any one of (c), and (d) following criteria were included.
2.2.3. Type of interventions
TCM (Chinese herbal medicine and Chinese patent medicine) alone or TCM combined with CWM was included. There was no limitation on the number of herbs, administration methods, dosage, or duration of treatment for traditional Chinese medicine. Other TCM therapeutic methods including acupuncture, moxibustion, cupping, massage, qigong, Tai Chi, etc. were excluded. The control group was treated with CWM alone.
2.2.4. Type of outcome measurements
The primary outcome was rate of disease progression (proportion of patients who have progressed from mild or ordinary types to severe or critical types after treatment), negative conversion rate of nucleic acid (proportion of patients whose nucleic acid test become negative after treatment), and improvement rate of chest CT (number of patients whose CT lesions disappeared or decreased after treatment as a percentage of all patients treated). The secondary outcomes were total effective rate (number of patients cured plus the number in remission per group divided by the total number of patients in each group), incidence of adverse events (number of patients with side effects after treatment as a percentage of all patients treated), disappearance rate of main symptoms (number of patients whose symptoms [fever, cough, and fatigue] disappeared after treatment as a percentage of all patients treated), lymphocyte count, and length of hospital stay.
2.3. Exclusion criteria
The exclusion criteria were as follows: self‐control or lack of control, case report and case series, cross‐sectional study, experience summary, animal experiment research, systematic review and meta‐analysis, and full texts were not available.
2.4. Databases and search strategy
We searched 6 electronic databases as follows: PubMed, the Cochrane Central Register of Controlled Trials (CENTRAL), Chinese Biomedical Literature Database (CBM), China National Knowledge Infrastructure (CNKI), Chinese Science and Technology Periodical Database (VIP), and Wanfang database (Wanfang Data). The search time was limited from the January 1 to December 31, 2020. We would apply a combination of MeSH terms and free‐text to search and adjust the search strategy according to the characteristics of each database. Pubmed and Wanfang database detailed search strategy as examples were shown in Tables S1 and S2.
2.5. Data extraction
Two independent authors (F. Jiang and N.N. Xu) performed literature selection and data extraction independently and used NoteExpress software to remove duplicate literature and then excluded irrelevant literature by reading the title and abstract. In the remaining literature, full‐text assessment was carried out to determine whether to be included. Any disagreement between reviewers was resolved by discussion or consultation with a third reviewer (Y.X. Zhou).
2.6. Assessment of risk of bias
The RoB2 assessment form (revised tool for risk of bias in randomized trials) was used for assessing RCTs in following five fields: randomisation process, deviation from intended interventions, missing outcome data,measurement of the outcome, and selection of the reported result (Sterne et al., 2019). Each field was assessed to be “low risk”, “high risk”, or “some concerns”. The Newcastle‐Ottawa Scale (NOS) assesses the quality of cohort studies and CCTs with the following three broad categories: selection, comparability and exposure, or outcome (Wells et al., 2003). The total score is 9, the higher the score, the better the quality of the study. Two reviewers (F. Jiang and N.N. Xu) independently completed the data extraction and quality assessment. Any disagreement was resolved by discussion or consultation with a third reviewer (Y.X. Zhou).
2.7. Data analysis
Review Manager (version 5.4) was used to perform the statistical analysis. The effect measure of binary variable would be expressed as risk ratio (RR) or odds ratio (OR) and 95% confidence interval (CI). For continuous variables, 95% CI and mean difference (MD) or standardized mean difference (SMD) would be used. We assessed statistical heterogeneity in each pairwise comparison with I 2 test. If I 2 ≤ 50% and p > .05, the heterogeneity among included studies was considered low. If I2 > 50% or p ≤ .05, the heterogeneity among included studies was considered high. Considering the heterogeneity of TCM interventions, the random effects models would be used to calculate the effect size. If there was a significant level of heterogeneity and available data were enough, sensitivity analysis or subgroup analysis would be conducted. The publication bias was evaluated by funnel plot, and it is determined by observing whether the left and right were symmetrical. If the left and right were not symmetrical, there is a bias.
2.8. Quality of evidence
Considering that observational studies are of low‐quality evidence and that there are factors that reduce the quality of evidence, leading to a very low quality of evidence, we only evaluated the quality of evidence for RCTs. According to the GRADE approach, the quality of RCTs can be downgraded for five reasons (risk of bias, imprecision, inconsistency, indirectness, and publication bias). Summary of Findings table was created by GRADE online (https://gradepro.org/).
3. RESULTS
3.1. Study selection and characteristics
A total of 5,697 articles were retrieved, including 3,685 duplicates. According to the inclusion and exclusion criteria, 1967 articles were removed based on their titles and abstracts. Forty‐five full‐text articles were assessed for eligibility, and 10 articles were excluded due to following reasons: ① Duplicates (n = 5); ② Observation group did not meet the inclusion criteria (n = 2); ③ Including suspected case (n = 1); ④ Article retracted (n = 1); ⑤ Cross‐sectional study (n = 1). Thirty‐five clinical studies were included in this systematic review. The process of study screening is shown in Figure 1.
FIGURE 1.

Flow diagram of study screening
Basic characteristic of enrolled studies are summarized in Table 1. Five articles were published in English, and 30 studies were published in Chinese. Among the 35 clinical trials, 19 were RCTs (Ding et al., 2020; Duan et al., 2020; Fu, Lin, & Tan, 2020a, 2020b; Hu et al., 2020; Li & Zhang, 2020; Liao, 2020; Pan et al., 2020; Qiu et al., 2020; Sun et al., 2020; Wang, Wang, et al., 2020; Wang, Yang, et al., 2020; Wen, Zhou, Jiang, & Huang, 2020; Xiao, Tian et al., 2020; Xiong et al., 2020; Yu, Li, Wan, & Wang, 2020; Zhang, Lei, Xu, Wei, & Hu, 2020; Zheng et al., 2020; Zhou, Zhao, Li, & Tian, 2020), 14 were CCTs (Chen, Chen, et al., 2020; Chen, Liu, et al., 2020; Cheng et al., 2020; Huang et al., 2020; Ji, Feng, & Fei, 2020; Lian et al., 2020; F. Liu, 2020; Qu et al., 2020; Shi et al., 2020; Xiao, Jiang, et al., 2020; Yang, Dang, Huang, Li, & Guo, 2020; Yang, Sun, et al., 2020; Yao, Liu, Li, Huang, & Cai, 2020; Zhang, Huang et al., 2020) and 2 were retrospective cohort study (Tian et al., 2020; Xia et al., 2020). Totally, 3,808 patients including 2077 patients in the intervention group and 1731 patients in the control group were enrolled, with the sample size ranged from 12 to 721. In the intervention group, COVID‐19 patients were all treated by integrated TCM and CWM, while patients were treated by CWM alone in the control group. TCM used in these studies including Chinese patent medicine (n = 14) and Chinese herbal decoction (n = 20) and injection (n = 1). The compositions of TCM were summarized in Table S3. Treatment duration varied from 5 to 15 days. The outcome measure of concern is total effective rate, improvement of chest CT, adverse events, negative conversion of nucleic acid, relief rate and relief time of main clinical symptom, rate of disease progression and length of stay.
TABLE 1.
Basic characteristic of included literatures on the treatment of COVID‐19 with integrated Traditional Chinese and Western medicine
| First author | Time of publish ion | Type of study | Severity of disease (no.) | Sample size | Sex ratio (male/female) | Mean age(y) | Intervention characteristics | Duration (days) | Outcome measures a | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||
| Ding XJ 2020 | May, 2020 | RCT | Mild (21), Ordinary (70), Severe and Critical (9) | 51 | 49 | 39/12 | 39/10 | 54.7 ± 21.3 | 50.8 ± 23.5 | Qingfei Touxie Fuzheng recipe (150 ml, bid) + C | Antiviral therapy, antibacterial therapy, supportive therapy. | 10 | (2)(4)(6)(9) |
| Duan C 2020 | Mar 24, 2020 | RCT | Mild (123) | 82 | 41 | 39/43 | 23/18 | 51.99 ± 13.88 | 50.29 ± 13.17 | Jinhua Qinggan granule (10 g, tid) + C | Antiviral therapy, antibacterial therapy, supportive therapy. | 5 | (2)(3)(4)(7)(8) |
| Fu XX 2020a | Jun, 2020 | RCT | Mild (5), Ordinary (60) | 32 | 33 | 17/15 | 19/14 | 43. 26 ± 7. 15 | 43. 68 ± 6. 45 | Toujie Quwen granules+C | Antiviral therapy (abidol 0.2 g, tid), antibacterial therapy (Moxifloxacin hydrochloride 0.4 g, qd), supportive therapy (ambroxol hydrochloride 30 mg, tid), | 10 | (1)(6)(7)(8)(9) |
| Fu XX 2020b | May, 2020 | RCT | Ordinary (73) | 37 | 36 | 19/18 | 19/17 | 45.26 ± 7.25 | 44.68 ± 7.45 | Toujie Quwen granules+C | Antiviral therapy (arbidol tablets 0.2 g, tid), supportive therapy (ambroxol tablets 30 mg, tid) | 15 | (1)(7)(8)(9) |
| Hu K 2020 | May 8, 2020 | RCT | ‐ | 142 | 142 | 79/63 | 71/71 | 50.4 ± 15.2 | 51.8 ± 14.8 | Lianhua Qingwen capsule (4 capsules tid) + C | Antiviral therapy, antibacterial therapy, supportive therapy. | 14 | (1)(2)(3)(4)(5)(6)(7)(8) |
| Li YD 2020 | May, 2020 | RCT | Severe (12) | 6 | 6 | 4/2 | 3/3 | 52. 00 ± 6. 56 | 50. 00 ± 10. 00 | Qingfei Paidu decoction+C | Antiviral therapy, antibacterial therapy, supportive therapy. | 6 | (1)(5)(6)(8)(9) |
| Liao GR 2020 | Jun, 2020 | RCT | ‐ | 35 | 35 | 20/15 | 18/17 | 65.25 ± 7.42 | 67.16±8.64 | Chinese herbal medicine+C | Antiviral therapy, antibacterial therapy, supportive therapy. | 7 | (2)(3)(4)(8) |
| Pan GT 2020 | Apr, 2020 | RCT | Severe and Critical (40) | 26 | 14 | 14/12 | 8/6 | 57.31 ± 9.88 | 64.01 ± 16.00 | Chinese herbal medicine+C | Antiviral therapy (abidol), antibacterial therapy (moxifloxacin, levofloxacin, cephalosporins, meropenem), supportive therapy (asmeton, ambroxol, acetyl cysteamine acid, gamma globulin, methylprednisolone) | 7 | (1)(5)(6)(9) |
| Qiu M 2020 | May 7, 2020 | RCT | Ordinary (50) | 25 | 25 | 13/12 | 14/11 | 53.35 ± 18.35 | 51.32 ± 14.62 | Maxing Xuanfei Jiedu decoction (150 ml, tid) + C | Antiviral therapy (α‐interferon 50 μg bid, lopinavir/ritonavir 400 and 100 mg bid), supportive therapy. | 10 | (1)(2)(4)(6)(7) |
| Sun HM 2020 | Jul, 2020 | RCT | Ordinary (57) | 32 | 25 | 17/15 | 11/14 | 45.4 ± 14.10 | 42.0 ± 11.70 | Lianhua Qingke granule (50 mg, tid) + C | Antibacterial therapy (α‐interferon 50 μg bid, lopinavir/ritonavir 400 and 100 mg bid), supportive therapy. | 14 | (2)(3)(4)(6)(7) |
| Wang JB 2020 | Jun 26, 2020 | RCT | ‐ | 24 | 23 | 14/10 | 12/11 | 46.8 ± 14.4 | 51.4 ± 17.6 | Keguan‐1 (19.4 g bid) + C | Antibacterial therapy (α‐interferon 50 μg bid, lopinavir/ritonavir 400 and 100 mg bid). | 14 | (2)(5)(8) |
| Wang YL 2020 | Mar 23, 2020 | RCT | Ordinary (20) | 10 | 10 | 5/5 | 5/5 | 39.24±10.01 | 55.90 ± 3.71 | Chinese herbal medicine+C | Antiviral therapy, antibacterial therapy, supportive therapy. | 7 | (2)(3)(4)(5)(6) |
| Wen L 2020 | Apr, 2020 | RCT | Severe (60) | 40 | 20 | 12/8 | 9/11 | 47.1 ± 5.2 | 47.7 ± 5.7 | Xuebijing injection (50 or 100 ml, bid) + C | Antiviral therapy, antibacterial therapy, supportive therapy. | 7 | (5)(7)(8)(9) |
| Xiao MZ 2020 | Aug 3, 2020 | RCT | ‐ | 61 | 63 | 33/28 | 35/28 | 56.07 ± 12.10 | 53.9 ± 13.92 | Huoxiang Zhengqi dropping pills/Lianhua Qingwen granules+C | Antiviral therapy (oseltamivir tablet 75 mg qd, arbidol 200 mg tid, ribavirin 150 mg tid), antibacterial therapy (penicillins, cephalosporins, ofloxacin) and macrolide, supportive therapy | 14 | (2)(3)(4)(8) |
| Xiong WZ | Jul, 2020 | RCT | Mild, ordinary, severe | 22 | 20 | ‐ | ‐ | 57.10 ± 14.00 | 62.40 ± 12.30 | Xuanfei Baidu decoction+C | Antiviral therapy, antibacterial therapy, supportive therapy. | 7 | (2)(3)(4)(9) |
| Yu P 2020 | Apr 22, 2020 | RCT | Mild (295) | 147 | 148 | 82/65 | 89/59 | 48.27 ± 9.56 | 47.25 ± 8.67 | Lianhua Qingwen granule (6 g. tid) + C | Antiviral therapy (abidol hydrochloride 0.2 g tid), antibacterial therapy (moxifloxacin 0.4 g qd), supportive therapy (ambroxol hydrochloride 30 mg tid. | 7 | (1)(6)(7)(8)(9) |
| Zhang YL 2020 | May 5, 2020 | RCT | Ordinary (120) | 80 | 40 | 30/50 | 23/17 | 53.40 ± 13.70 | 52.0 ± 14.10 | Oral honeysuckle (60 ml, tid) + C | α‐interferon (50 μg bid), lopinavir/ritonavir (400 and 100 mg bid), supportive therapy. | 10 | (2)(3)(4)(5)(6)(7)(8) |
| Zheng ZZ 2020 | Feb, 2020 | RCT | Ordinary (119), Severe (11) | 65 | 65 | 42/23 | 44/21 | ‐ | ‐ | Chinese herbal medicine +C | Antiviral therapy (α‐interferon, abidol, lopinavir/ritonavir), antibacterial therapy (moxifloxacin), supportive therapy (methylprednisolone). | 14 | (1) |
| Zhou WM 2020 | Feb 28, 2020 | RCT | Ordinary (104) | 52 | 52 | 32/20 | 28/24 | 52.47 ± 10.99 | 51.11 ± 9.87 | Diammonium glycyrrhizinate (150 mg,tid) + C | Antiviral therapy (lopinavir/ritonavir 400 and 100 mg bid), supportive therapy, oxygen therapy | 14 | (1)(8)(9) |
| Chen L 2020a | Jul 23, 2020 | CCT | Ordinary (230) | 115 | 115 | 55/60 | 47/68 | 63.02 ± 13.61 | 60.17 ± 16.02 | Ganlu Xiaodu decoction (100 ml, tid) + C | Antiviral therapy, antibacterial therapy, supportive therapy. | 7 | (1)(2)(3)(4)(6)(7)(8)(9)(10) |
| Chen L 2020b | Aug, 2020 | CCT | Ordinary (68) | 34 | 34 | 14/20 | 15/19 | 65.06 ± 10.63 | 64.35 ± 10.34 | Shufeng Jiedu Capsule (2.08 g, tid) + C | Antiviral therapy (abidol 0.2 g tid), antibacterial therapy (moxifloxacin 0.4 g qd), supportive therapy (ambroxol) | 7 | (1)(2)(3)(4)(6)(8)(9)(10) |
| Cheng DZ 2020 | May 2020 | CCT | Ordinary (102) | 51 | 51 | 26/25 | 27/24 | 55.5 ± 12.3 | 55.8 ± 11.6 | Lianhua Qingwen granule (6 g tid) + C | Antiviral therapy, antibacterial therapy, supportive therapy. | 7 | (1)(2)(3)(4)(6)(7) |
| Huang H 2020a | Aug, 2020 | CCT | ‐ | 30 | 15 | 13/17 | 9/6 | 58.4 ± 15.5 | 66.3 ± 14.1 | Chinese herbal medicine+C | Antiviral therapy, antibacterial therapy, supportive therapy | 10 | (2)(3)(4)(5)(6)(7)(9)(10) |
| Huang H 2020b | Aug, 2020 | CCT | ‐ | 28 | 15 | 16/12 | 9/6 | 61.9 ± 12.2 | 66.3 ± 14.1 | Chinese herbal medicine+C | Antiviral therapy, antibacterial therapy, supportive therapy | 10 | (2)(3)(4)(5)(6)(7)(9)(10) |
| Ji D 2020 | Jul, 2020 | CCT | Ordinary (50) | 28 | 22 | 16/12 | 12/10 | 45.3 ± 13.7 | 47.6 ± 14.1 | Chinese herbal medicine+C | Antiviral therapy (abidol, 0.2 g tid, ribavirin, recombinant human interferon a‐2b), antibacterial therapy (moxifloxacin), supportive therapy (ambroxol tablets 30 mg tid). | 10 | (2)(3)(4)(7) |
| Lian J 2020 | Jun 28, 2020 | CCT | Mild, ordinary, severe and critical, recovery | 38 | 26 | 15/23 | 10/16 | 61.3 ± 14.1 | 58.07 ± 11.9 | Chinese herbal medicine+C | Antiviral therapy (arbidol tablets 0.2 g tid, recombinant human interferon a‐2b, resochin), antibacterial therapy (moxifloxacin, 0.4 g qd, cefperazone‐Sulbactam), supportive therapy (human immunoglobulin). | 10 | (2)(3)(4)(6)(7)(8) |
| Liu F 2020 | 2020 | CCT | Ordinary (35), Severe (42), Critical (7) | 42 | 42 | 15/27 | 17/25 | 52.7 ± 16.8 | 49.5 ± 13.8 | Chinese herbal medicine+C | Antiviral therapy, antibacterial therapy, supportive therapy. | ‐ | (1)(2)(7)(8)(10) |
| Qu XK 2020 | Mar, 2020 | CCT | Ordinary (70) | 40 | 30 | 25/15 | 16/14 | 40.65 ± 8.23 | 39.82 ± 6.40 | Shufeng Jiedu capsule (2.08 g, bid) + C | Antiviral therapy (abidol hydrochloride 0.2 g tid), antibacterial therapy, supportive therapy, | 10 | (1)(2)(3)(4)(5)(6)(8) |
| Shi J 2020 | Apr, 2020 | CCT | Ordinary (67) | 49 | 18 | 26/23 | 10/8 | 47.94 ± 14.46 | 46.72 ± 17.40 | Chinese herbal medicine+C | Antiviral therapy (recombinant human interferon a‐2b, lopinavir/ritonavir, arbidol, darunavir corbita, interferon K, hydroxychloroquine), antibacterial therapy, methylprednisolone sodium succinate, gamma globulin, and supportive therapy | ‐ | (1)(2)(3)(4)(6)(7)(10) |
| Xiao Q 2020 | May, 2020 | CCT | Mild (200) | 100 | 100 | 64/36 | 66/34 | 60.90 ± 8.70 | 62.20 ± 7.50 | Shufeng Jiedu capsule (2.08 g, tid) + C | Antibacterial therapy (abidol 0.2 g tid) | 14 | (1)(2)(3)(4)(6)(8)(9) |
| Yang MB 2020 | Jul, 2020 | CCT | Ordinary (49) | 26 | 23 | 16/10 | 9/14 | 50.35 ± 13. 37 | 47.17 ± 16. 57 | Reyanning mixture (10–20 ml, 2 to 4 times daily) + C | α‐interferon (50 μg bid), lopinavir/ritonavir (400 and 100 mg bid), ribavirin (0.5 g, bid), Abidol hydrochloride(0.2 g, tid) | 7 | (2)(3)(4)(5)(6)(8)(9) |
| Yang Q 2020 | Apr, 2020 | CCT | Severe (103) | 51 | 52 | 28/23 | 24/28 | 61.57 ± 1.84 | 66.46 ± 2.29 | Chinese herbal medicine+C | Antiviral therapy, antibacterial therapy, supportive therapy. | ‐ | (1)(6)(8)(9)(10) |
| Yao KT 2020 | Jun, 2020 | CCT | Ordinary (42) | 21 | 21 | 16/5 | 12/9 | 57.1 ± 14.0 | 62.4 ± 12.3 | Lianhua Qingwen granules (6 g, tid) + C | Antiviral therapy, antibacterial therapy, supportive therapy. | 14 | (2)(3)(4) |
| Zhang HT 2020 | May, 2020 | CCT | Ordinary (22) | 11 | 11 | 4/7 | 4/7 | 43.4 ± 15.9 | 40.7 ± 13.3 | Chinese herbal medicine+C | Antiviral therapy (α‐interferon, lopinavir/ritonavir), antibacterial therapy, supportive therapy | ‐ | (2)(5)(6)(7)(8) |
| Tian JX 2020 | Aug, 2020 | RCS | Mild (721) | 430 | 291 | 201/229 | 146/145 | 43.79 ± 12.099 | 55.44 ± 14.641 | Hanshiyi formula | Conventional treatment | ‐ | (7) |
| Xia WG 2020 | Mar, 2020 | RCS | Ordinary (40), Severe (10), Critical (2) | 34 | 18 | 17/17 | 6/12 | 54. 18 ± 13. 08 | 53. 67 ± 12. 70 | Chinese herbal medicine+C | Antiviral therapy (abidol, ribavirin, interferon alpha, Lopinavir/ritonavir, oseltamivir), antibacterial therapy (moxifloxacin, levofloxacin, azithromycin, cephalosporins, penicillins), supportive therapy (gamma globulin, methylprednisolone). | 10 | (1)(2)(3)(4)(5)(6)(7)(8)(9)(10) |
Abbreviations: CCT, case‐control study; RCS, retrospective cohort study; RCT, randomized controlled trial; −, lack of data.
(1) Total effective rate; (2) Fever improvement; (3) Fatigue improvement; (4) Cough improvement; (5) negative conversion rate of nucleic acid; (6) chest CT improvement; (7) rate of conversion to severe cases; (8) Adverse events; (9) Peripheral blood index; (10) length of stay.
3.2. Assessment of risk of bias
The risk of bias of the included RCTs was shown in Figure 2. Among the included 19 studies, nine studies had low risk of bias, eight studies had some concerns and two studies had high risk of bias.
FIGURE 2.

Risk of bias graph (left) and summary (right)
Sixteen observational studies including 14 CCTs and 2 retrospective cohort studies (RCSs) were assessed for quality by the NOS. The range of scores was 6 to 8. These studies showed a moderate quality sufficient to conduct a meta‐analysis (Table 2).
TABLE 2.
Quality of included observational studies
| Study | Selection | Comparability | Outcome | Total score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Case–control study | Is the case definition adequate? | Representativeness of the cases | Selection of controls | Definition of controls | Comparability of cases and controls on the basis of the design or analysis | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non‐response rate | |
| Chen L 2020a | ★ | ★ | ★★ | ★ | ★ | ★ | 7 | ||
| Chen L 2020b | ★ | ★ | ★★ | ★ | ★ | ★ | 7 | ||
| Cheng DZ 2020 | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| Huang H 2020 | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| Ji D 2020 | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| Lian J 2020 | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 | |
| Liu F 2020 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | |
| Qu XK 2020 | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| Shi J 2020 | ★ | ★ | ★★ | ★ | ★ | ★ | 7 | ||
| Xia WG 2020 | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 | |
| Xiao Q 2020 | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| Yang MB 2020 | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| Yang Q 2020 | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| Yao KT 2020 | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| Zhang HT 2020 | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| Retrospective cohort study | Representativeness of the exposed cohort | Selection of the non‐exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow‐up long enough for outcomes to occur | Adequacy of follow up of cohorts | |
| Tian JX 2020 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | 8 | |
3.3. Meta‐analysis
3.3.1. Rate of disease progression
Rate of disease progression was reported in 21 studies (Chen, Chen, et al., 2020; Chen, Liu, et al., 2020; Cheng et al., 2020; Duan et al., 2020; Fu et al., 2020b; Hu et al., 2020; Huang, Wang, et al., 2020; Huang, Tan, et al., 2020; Ji et al., 2020; Lian et al., 2020; F. Liu, 2020; Qiu et al., 2020; Shi et al., 2020; Sun et al., 2020; Tian et al., 2020; Wang, Wang, et al., 2020; Xia et al., 2020; Xiao, Tian, et al., 2020; Yang, Dang, et al., 2020; Yu et al., 2020; Zhang, Lei, et al., 2020). Compared with the CWM group, the TCM + CWM group significantly reduced the rate of conversion to severe cases, the difference was statistically significant (RR = 0.30, 95%CI = [0.20, 0.44], I 2 = 19%, p < .00001) (Figure 3).
FIGURE 3.

Rate of disease progression of patients with COVID‐19 between TCM + CWM group and CWM group
3.3.2. Negative conversion rate of nucleic acid
The negative conversion rate of nucleic acid after treatment was analyzed in 6 studies (Hu et al., 2020; Pan et al., 2020; Qu et al., 2020; Wen et al., 2020; Yang, Dang, et al., 2020; Zhang, Lei, et al., 2020). Meta‐analysis showed no significant difference between the TCM + CWM group and the CWM group (RR = 1.17, 95%CI = [0.99, 1.39], I2 = 57%, p = .07) (Figure 4).
FIGURE 4.

Negative conversion rate of nucleic acid of patients with COVID‐19 between TCM + CWM group and CWM group
3.3.3. Improvement rate of chest CT
Seventeen studies (Chen, Chen, et al., 2020; Chen, Liu, et al., 2020; Cheng et al., 2020; Ding et al., 2020; Fu et al., 2020b; Hu et al., 2020; Huang, Wang, et al., 2020; Huang, Tan, et al., 2020; Pan et al., 2020; Shi et al., 2020; Sun et al., 2020; Wang, Wang, et al., 2020; Xia et al., 2020; Xiao,Tian et al., 2020; Yang, Dang, et al., 2020; Yang, Sun, et al., 2020; Yu et al., 2020;) evaluated improvement rate of chest CT. Compared with the CWM group, a significant difference was identified (RR = 1.21, 95%CI = [1.13, 1.29], I 2 = 23%, p < .00001) (Figure 5).
FIGURE 5.

Improvement rate of chest CT of patients with COVID‐19 between TCM + CWM group and CWM group
3.3.4. Total effective rate
Fifteen studies (Chen, Chen, et al., 2020; Chen, Liu, et al., 2020; Cheng et al., 2020; Fu et al., 2020a, 2020b; Hu et al., 2020; Li & Zhang, 2020; F. Liu, 2020; Pan et al., 2020; Xia et al., 2020; Xiao, Tian et al., 2020; Yang, Sun, et al., 2020; Yu et al., 2020; Zheng et al., 2020; Zhou et al., 2020) evaluated the effects of TCM + CWM on total effective rate. The intervention group exhibited a significant improvement compared with the control group (RR = 1.20, 95%CI = [1.14, 1.26], I 2 = 12%, p < .00001) (Figure 6).
FIGURE 6.

Total effective rate of patients with COVID‐19 between TCM + CWM group and CWM group
3.3.5. Incidence of adverse events
Nineteen studies (Chen, Chen, et al., 2020; Chen, Liu, et al., 2020; Duan et al., 2020; Hu et al., 2020; Li & Zhang, 2020; Lian et al., 2020; Liao, 2020; F. Liu, 2020; Qu et al., 2020; Wang, Wang, et al., 2020; Wen et al., 2020; Xia et al., 2020; Xiao, Tian, et al., 2020; Xiong et al., 2020; Yang, Sun, et al., 2020; Yu et al., 2020; Zhang, Huang, Tan et al., 2020; Zhang, Lei, et al., 2020; Zhou et al., 2020) evaluated incidence of adverse events. Meta‐analysis revealed no significant difference between the TCM + CWM group and the CWM group (RR = 0.77, 95%CI = [0.53, 1.13], I 2 = 28%, p = .18) (Figure 7).
FIGURE 7.
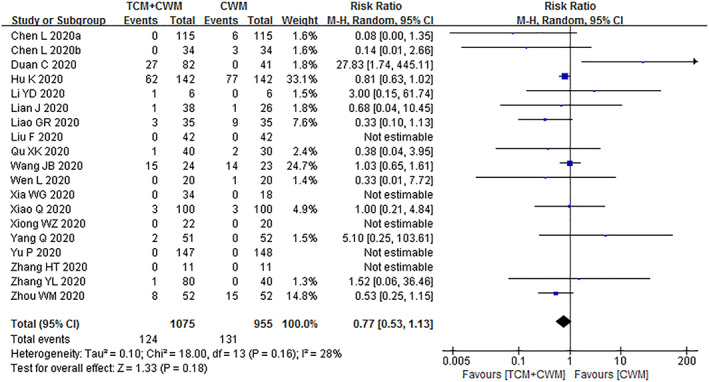
Incidence of adverse events of patients with COVID‐19 between TCM + CWM group and CWM group
3.3.6. Major symptom (fever, fatigue and cough) relief
The main clinical symptoms are summarized in Table 3. Thirteen studies (Chen, Chen, et al., 2020; Chen, Liu, et al., 2020; Cheng et al., 2020; Ding et al., 2020; Duan et al., 2020; Ji et al., 2020; Liao, 2020; Sun et al., 2020; Wang, Yang, et al., 2020; Xiao, Tian, et al., 2020; Xiong, Wang, Du, & Ai, 2020; Yao et al., 2020; Zhang, Lei, et al., 2020) reported relief rate of fever symptom, and eleven studies (Chen, Chen, et al., 2020; Chen, Liu, et al., 2020; Cheng et al., 2020; Huang, Tan, et al., 2020; Huang, Wang, et al., 2020; F. Liu, 2020; Qiu et al., 2020; Qu et al., 2020; Xia et al., 2020; Xiao, Jiang, et al., 2020; Zhang, Huang, Tan et al., 2020) reported fever relief time. Meta‐analysis revealed statistically significant between the TCM + CWM group and the CWM group in fever relief rate (RR = 1.20, 95%CI = [1.05, 1.37], I 2 = 86%, p = .008) and fever reduction time (MD = ‐1.54, 95%CI = [−1.91, −1.17], I 2 = 54%, p < .00001). A significant heterogeneity was identified in fever relief rate. We conducted a sensitivity analysis by removing studies and recalculated the combined estimate on remaining studies. The I 2 become 13% after removing “Zhang YL 2020” and “Xiao MZ 2020” at the same time, indicating that these two studies were the main sources of heterogeneity. For fever reduction time, the value of I 2 was 14% after removing “Xiao Q 2020”, varying that this study was the source of heterogeneity.
TABLE 3.
Comparison of some second outcomes between TCM group and CWM group
| Outcome measure | Number of study | Sample size | Statistical method | I2 | Effect value | p‐value | |
|---|---|---|---|---|---|---|---|
| TCM | CWM | ||||||
| Fever relief rate | 13 | 390 | 259 | RR, random, 95% | 86% | 1.20 [1.05, 1.37] | .008 |
| Fatigue relief rate | 11 | 323 | 247 | RR, random, 95% | 53% | 1.31 [1.13, 1.52] | .0004 |
| Cough relief rate | 11 | 432 | 366 | RR, random, 95% | 59% | 1.35 [1.14, 1.59] | .0003 |
| Time to fever relief | 11 | 383 | 343 | MD, random, 95%CI | 54% | −1.54 [−1.91, −1.17] | <.00001 |
| Time to fatigue relief | 8 | 262 | 219 | MD, random, 95%CI | 81% | −1.50 [−2.19, −0.81] | <.0001 |
| Time to cough relief | 8 | 354 | 313 | MD, random, 95%CI | 84% | −1.96 [−2.88, −1.04] | <.0001 |
| WBC | 10 | 549 | 522 | MD, random, 95%CI | 91% | 0.77 [0.47, 1.06] | <.00001 |
| LYM | 10 | 520 | 491 | MD, random, 95%CI | 93% | 0.22 [0.12, 0.33] | <.0001 |
| Length of stay | 9 | 389 | 315 | MD, random, 95%CI | 96% | −2.98 [−5.48, −0.48] | .02 |
Fatigue relief rate was reported in 11 studies (Chen, Chen, et al., 2020; Chen, Liu, et al., 2020; Cheng et al., 2020; Duan et al., 2020; Ji et al., 2020; Liao, 2020; Sun et al., 2020; Xiao, Tian, et al., 2020; Xiong et al., 2020; Yao et al., 2020; Zhang, Lei, et al., 2020), and fatigue relief time was reported in 7 studies (Chen, Chen, et al., 2020; Chen, Liu, et al., 2020; Cheng et al., 2020; Huang, Tan, et al., 2020; Huang, Wang, et al., 2020; Qu et al., 2020; Xiao, Jiang, et al., 2020). Meta‐analysis showed a significant improvement on number of fatigue relief cases (RR = 1.31, [1.13, 1.52], I 2 = 53%, p = .0004) and fatigue relief time (MD = −1.50, [−2.19, −0.81], I 2 = 81%, p = .0001). We investigated the influence of a single study on the overall risk estimate by excluding one study at a time. For fatigue relief rate and fatigue relief time, “Zhang YL 2020” and “Xiao Q 2020” were the source of heterogeneity. I 2 become 21 and 0%, respectively, after removing these two studies.
The effect of TCM on cough relief rate and cough relief time was evaluated in 11 studies (Chen, Chen, et al., 2020; Chen, Liu, et al., 2020; Cheng et al., 2020; Ding et al., 2020; Duan et al., 2020; Ji et al., 2020; Liao, 2020; Xiao, Tian, et al., 2020; Xiong et al., 2020; Yao et al., 2020; Zhang, Lei, et al., 2020) and 8 studies (Chen, Chen, et al., 2020; Chen, Liu, et al., 2020; Cheng et al., 2020; Huang, Tan, et al., 2020; Huang, Wang, et al., 2020; Qiu et al., 2020; Qu et al., 2020; Xiao, Jiang, et al., 2020) respectively. A significant improvement on cough relief rate (RR = 1.35, [1.14, 1.59], I 2 = 59%, p = .0003) and cough relief time (MD = −1.96, [−2.88, −1.04], I 2 = 84%, p < .0001) was observed by the TCM + CWM group. Removing “Xiao MZ 2020” and “Xiao Q 2020” separately in two outcome measures above, values of I 2 were all reduced to less than 50%.
For these studies considered to be the source of heterogeneity, we found that there was not statistically significant (p < .05) in relevant outcome measurements between the TCM + CWM group and the CWM group.
3.3.7. Main peripheral blood indexes
The main peripheral blood indexes are summarized in Table 3. The effect of TCM on white blood cell count and lymphocyte count was evaluated in 10 (Chen, Chen, et al., 2020; Chen, Liu, et al., 2020; Fu et al., 2020a, 2020b; Huang, Tan, et al., 2020; Huang, Wang, et al., 2020; Li & Zhang, 2020; Wen et al., 2020; Xiao, Jiang, et al., 2020; Yu et al., 2020) and 10 studies (Chen, Chen, et al., 2020; Chen, Liu, et al., 2020; Fu et al., 2020a, 2020b; Huang, Tan, et al., 2020; Huang, Wang, et al., 2020; Wen et al., 2020; Xiao, Tian, et al., 2020; Yang, Sun, et al., 2020; Yu et al., 2020), respectively. Meta‐analysis showed a significant improvement of WBC (MD = 0.77, 95%CI = [0.47, 1.06], I 2 = 91%, p < .0001) and LYM (MD = 0.22, 95%CI = [0.12, 0.33], I 2 = 93%, p < .0001) in TCM + CWM group. Sensitivity analysis and subgroup analysis of the main peripheral blood index based on study type and disease severity showed the stability of our results.
3.3.8. Length of stay
Nine studies (Chen, Chen, et al., 2020; Chen, Liu, et al., 2020; Huang, Tan, et al., 2020; Huang, Wang, et al., 2020; Li & Zhang, 2020; F. Liu, 2020; Shi et al., 2020; Xia et al., 2020; Yang, Sun, et al., 2020) reported length of stay. Comparing with the CWM group, patients in the TCM + CWM group had a shorter hospital stay with a statistical significance (MD = ‐2.98, 95%CI = [−5.48, 0.48], I 2 = 96%, p = .02). Sensitivity analysis showed the stability of our result (Table 3).
3.4. Publication bias
We assessed publication bias for primary outcome indicators which exceeded 10 articles. These outcome measures include total effective rate, improvement rate of chest CT, rate of disease progression and adverse events. The funnel plots suggested a mild publication bias in total effective rate, improvement rate of chest CT and incidence of adverse events. A severe publication bias was observed in rate of disease progression (Figure 8).
FIGURE 8.

Funnel plot of total effective rate (a), improvement rate of chest CT (b), rate of disease progression (c) and incidence of adverse events (d)
3.5. Subgroup analysis
We performed a subgroup analysis of the total effective rate based on study type and disease severity. The results of the subgroup analyses revealed more effective outcomes in the integrated medicine than the control group. Detailed results of the analyses are shown in Table 4.
TABLE 4.
Subgroup analyses of the total clinical effectiveness
| Subgroup | Number of study | Sample size | Statistical method | I 2 | Effect value | p‐value | |
|---|---|---|---|---|---|---|---|
| TCM | CWM | ||||||
| Study type | |||||||
| RCT | 8 | 507 | 496 | OR, random, 95% | 0 | 2.45 [1.80, 3.34] | <.00001 |
| CCT | 7 | 427 | 412 | OR, random, 95% | 0 | 3.09 [2.08, 4.59] | <.00001 |
| Disease severity | |||||||
| Mild or ordinary case | 9 | 710 | 711 | OR, random, 95% | 0 | 2.65 [2.04, 3.44] | <.00001 |
| Severe or critical case | 3 | 83 | 72 | OR, random, 95% | 31 | 2.37 [1.11, 5.04] | .03 |
| Mixed case | 3 | 141 | 125 | OR, random, 95% | 0 | 5.87 [2.08, 16.51] | .0008 |
3.6. Quality of evidence
The quality of evidence from RCTs was low (total effective rate, negative conversion rate of nucleic acid, rate of disease progression, and incidence of adverse events) to moderate (improvement rate of chest CT). The quality of evidence from observational studies was very low. The overall quality of evidence was low to moderate (Table 5).
TABLE 5.
Summary of findings
| Traditional Chinese medicine compared to conventional western medicine for COVID‐19 | |||||
|---|---|---|---|---|---|
| Patient or population: Patients with COVID‐19; setting: Intervention: TCM; comparison: CWM | |||||
| Outcomes | No. of participants (studies) follow up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
| Risk with CWM | Risk difference with TCM | ||||
| Total effective rate | 1,003 (8 RCTs) | ⨁⨁◯◯ LOW a , b | RR 1.24 (1.15 to 1.33) | 667 per 1,000 | 160 more per 1,000 (100 more to 220 more) |
| Negative conversion rate of nucleic acid | 484 (4 RCTs) | ⨁⨁◯◯ LOW b , c | RR 1.07 (0.93 to 1.24) | 713 per 1,000 | 50 more per 1,000 (50 fewer to 171 more) |
| Improvement rate of chest CT | 888 (7 RCTs) | ⨁⨁⨁◯ MODERATE b | RR 1.27 (1.16 to 1.38) | 613 per 1,000 | 165 more per 1,000 (98 more to 233 more) |
| Rate of disease progression | 1,165 (9 RCTs) | ⨁⨁◯◯ LOW a , d | RR 0.45 (0.31 to 0.65) | 128 per 1,000 | 70 fewer per 1,000 (88 fewer to 45 fewer) |
| Incidence of adverse events | 1,137 (10 RCTs) | ⨁⨁◯◯ LOW a , b | RR 0.92 (0.75 to 1.13) | 220 per 1,000 | 18 fewer per 1,000 (55 fewer to 29 more) |
Note: The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI, Confidence interval; RR, Risk ratio.
GRADE Working Group grades of evidence.
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect.
Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Lack of blinding, lack of allocation concealment, unclear reporting bias.
Mild publication bias.
Small sample size.
Severe publication bias.
4. DISCUSSION
TCM had been used to treat and prevent infectious diseases for thousands of years in China. Since the outbreak of COVID‐19, TCM treatment has been widely used and achieved great success. RCTs and observational studies of TCM combined with CWM treatment for COVID‐19 were conducted throughout the country and had shown significant clinical effectiveness. This updated systematic review and meta‐analysis including 19 RCTs and 16 observational studies evaluated comprehensively the efficacy and safety of TCM combined with CWM for COVID‐19 treatment. Comparing with CWM alone, integrated TCM and CWM could improve overall efficiency without increasing the incidence of adverse events.
The commonly used dosage formulation was Chinese patent medicine and Chinese herbal decoction derived from medical plants. The main mechanisms of medical plants or their extract treatment for COVID‐19 were to inhibit viral replication and enhance the patient's immunity. For example, the aqueous extract of Houttuynia cordata, the major constituent of Lianhua Qingwen capsule, inhibits two key proteins of SARS‐CoV, namely chymotrypsin‐like protease (3CLpro) and RNA‐dependent RNA polymerase (RdRp). The extract also increased CD4+ and CD8+ cell count in test animals suggesting its immune‐stimulatory effect that can be an additional advantage on top of its role in slowing down viral replication (Lau et al., 2008). The extracts of Rheum officinale, which is one of the main components of Lianhua Qingke Granule, were found to inhibit the interaction of SARS‐CoV (S) protein and ACE2 (functional receptor to infect host cells) (Islam et al., 2020). In addition, many functional food plants with anti‐viral and immunomodulatory properties against SARS‐CoV‐2 including garlic, ginger, and tea and so on not only could inhibit viral replication but also enhance innate and adaptive immune responses (Upadhyay et al., 2020; Yang, Zhang, Huang et al., 2020). Concerns over the use of botanical drugs and supplements were immune‐stimulating herbs may initiate a cytokine storm, resulting in acute respiratory distress syndrome (ARDS), systemic coagulation and thrombus formation (coagulopathy) and sepsis‐related multipleorgan failure, because patients infected with SARS‐CoV‐2 had higher concentrations and circulating levels of various cytokine than those in healthy adults (Huang et al., 2020). But a great number of clinical studies showed that the immunomodulatory botanicals can improve parameters of the immune response, without evidence of risk of overstimulation, and even may have the potential to decrease the risk of a cytokine storm (Brendler et al., 2020).
In this review, there are several highlights deserved our attention. First, this review included more clinical trials, especially including 19 RCTs, which greatly enhanced the credibility of the evidence. Second, new studies included in this review provided new TCM formulations for the treatment of COVID‐19, including Huoxiang Zhengqi dropping pills, Xuanfei Baidu decoction, Oral honeysuckle, Diammonium glycyrrhizinate, Xuebijing injection, etc., which are not available in the previous literature. Third, though the CCTs and retrospective study may not contribute much to evidence compared with RCTs, the treatment regimen they used could provide an option for follow‐up of high‐quality RCTs.
Although our study can demonstrate the effectiveness of TCM combined with CWM on the treatment of COVID‐19, there were several limitations of this systematic review. First, patients infected with SARS‐CoV‐2 had higher concentrations and circulating levels of various cytokine than those in healthy adults (Huang et al., 2020). However, only four clinical trials detected cytokine levels, including IL‐6, IFN‐γ (Ding et al., 2020), CD4, CD8, CD4/CD8, CD45 (Fu et al., 2020b), IL‐6 (Huang et al., 2020), IL‐4, TNF‐α, CD3, CD4, CD8, CD4/CD8 (Zhou et al., 2020). The diversity of cytokines compromised the statistical significance of the data. Second, because of the time required to develop antibody detection kits, none of the clinical trials detected antibody levels in patients. In addition, most of the clinical trials had flaws in the methodological design, including randomization, concealment of allocation, and inadequate reports on blinding. The follow‐up in included studies was insufficient. Only studies published in Chinese and English were searched, and there may be language bias. And some ongoing or unpublished trials were not included, which may lead to potential publication bias.
5. CONCLUSIONS
In summary, the results of this review confirmed that integrated TCM and CWM was a potential treatment option to improve the clinical symptoms of COVID‐19 patients without increasing the incidence of adverse events. High‐quality RCTs are needed to further evaluate the effect of integrated medicine for COVID‐19.
CONFLICT OF INTEREST
The authors declared that there was no conflict of interest regarding the publication of this paper.
AUTHOR CONTRIBUTIONS
Fei Jiang: Searched the literature, conducted data analysis and drafted the manuscript. Nana Xu: Screened the articles and collected the data. Yanxi Zhou: Assessed the methodological quality of included studies. Jinxing Song, Jinjuan Liu, and Hong Zhu: Checked the data. Jihong Jiang and Rongpeng Li: Designed the study and participated in manuscript revision.
ETHICS STATEMENT
Ethical approval and patient consent are not required since this is an overview based on published studies.
Supporting information
Table S1. Details of the search strategy of PubMed.
Table S2. Details of the search strategy of Wanfang database.
Table S3. Components of Chinese herbal medicine used in the included studies.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (NSFC81870005); the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (18KJA180003); the Project of Xuzhou Applied and Basic Research (KC18009, KC20102); and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). We thank Dr. Yi Guo (Department of Laboratory Medicine, the Affiliated Hospital of Xuzhou Medical University) for his constant support and advice.
Jiang, F. , Xu, N. , Zhou, Y. , Song, J. , Liu, J. , Zhu, H. , Jiang, J. , Xu, Y. , & Li, R. (2021). Contribution of traditional Chinese medicine combined with conventional western medicine treatment for the novel coronavirus disease (COVID‐19), current evidence with systematic review and meta‐analysis. Phytotherapy Research, 35(11), 5992–6009. 10.1002/ptr.7209
Fei Jiang, Nana Xu, and Yanxi Zhou contributed equally to this work.
Funding information National Natural Science Foundation of China, Grant/Award Number: 81870005; Natural Science Foundation of the Jiangsu Higher Education Institutions of China, Grant/Award Number: 18KJA180003; Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD); Project of Xuzhou Applied and Basic Research, Grant/Award Numbers: KC20102, KC18009
Contributor Information
Yonghong Xu, Email: 6020150060@jsnu.edu.cn.
Rongpeng Li, Email: lirongpeng@jsnu.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Brendler, T. , Al‐Harrasi, A. , Bauer, R. , Gafner, S. , Hardy, M. L. , Heinrich, M. , … Williamson, E. M. (2020). Botanical drugs and supplements affecting the immune response in the time of COVID‐19: Implications for research and clinical practice. Phytotherapy Research, 35, 3013–3031. 10.1002/ptr.7008 [DOI] [PubMed] [Google Scholar]
- Chen, L. , Chen, Y. G. , Cheng, Z. Q. , Liu, F. , Wu, J. H. , Xia, Y. , & Sheng, B. (2020). Retrospective analysis on clinical efficacy of Ganlu Xiaodu decoction combined with Western medicine in treatment of common COVID‐19 patients. Chinese Journal of Experimental Traditional Medical Formulae, 26(19), 60–67. 10.13422/j.cnki.syfjx.20202011 [DOI] [Google Scholar]
- Chen, L. , Liu, F. , Wu, J. H. , Song, H. Y. , Xia, J. S. , Sheng, B. , & Chen, Y. G. (2020). Clinical efficacy of Shufeng Jiedu capsule combined with western medicine in treatment of common COVID‐19 patients by retrospective analysis. Chinese Journal of Experimental Traditional Medical Formulae, 36(16), 16–20. 10.13422/j.cnki.syfjx.20201628 [DOI] [Google Scholar]
- Cheng, D. Z. , Wang, W. J. , Li, Y. , Wu, X. D. , Zhou, B. , & Song, Q. Y. (2020). Efficacy analysis on 51 cases of COVID‐19 treated with traditional Chinese medicine Lianhua Qingwen: A multi‐center retrospective study. Tianjin University of Traditional Chinese Medicine, 37(5), 509–516. 10.11656/j.issn.1672-1519.2020.05.06 [DOI] [Google Scholar]
- Ding, X. J. , Zhang, Y. , He, D. C. , Zhang, M. Y. , Tan, Y. J. , Yu, A. R. , … Liu, L. (2020). Clinical effect and mechanism of Qingfei Touxie Fuzheng recipe in the treatment of Novel Coronavirus pneumonia. Herald of Medicine, 39(5), 640–644. 10.3870/j.issn.1004-0781.2020.05.012 [DOI] [Google Scholar]
- Duan, C. , Xia, W. G. , Zhen, C. J. , Sun, G. B. , Li, Z. L. , Li, Q. L. , … Liu, Q. Q. (2020). Clinical observation on the treatment of mild COVID‐19 with Jinhua Qinggan Granules combined with conventional western medicine. Journal of Traditional Chinese Medicine 61(17), 1473–1477. 10.13422/j.cnki.syfjx.20201314 [DOI] [Google Scholar]
- Fu, X. X. , Lin, L. P. , & Tan, X. H. (2020a). Clinical observation on effect of Toujie Quwen granules in treatment of COVID‐19. Chinese Journal of Experimental Traditional Medical Formulae, 26(12), 44–48. 10.13422/j.cnki.syfjx.20201314 [DOI] [Google Scholar]
- Fu, X. X. , Lin, L. P. , & Tan, X. H. (2020b). Clinical study on 37 case of COVID‐19 treated with integrated traditional Chinese and Western Medicine. Traditional Chinese Drug Research & Clinical Pharmacology, 31(5), 600–604. 10.19378/j.issn.1003-9783.2020.05.016 [DOI] [Google Scholar]
- Hu, K. , Guan, W. J. , Bi, Y. , Zhang, W. , Li, L. J. , Zhang, B. L. , … Zhong, N. S. (2020). Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine, 85, 153242. 10.1016/j.phymed.2020.153242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. L. , Wang, Y. M. , Li, X. W. , Ren, L. L. , Zhao, J. P. , Hu, Y. , … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 395(10223), 497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. , Tan, S. C. , Zuo, X. H. , Jin, J. S. , Zhao, Y. , & Ding, Y. (2020). Clinical efficacy analysis and TCM syndrome characteristics of 72 patients with COVID‐19. Modern Journal of Integrated Traditional Chinese and Western Medicine, 29, 2395–2483. 10.3969/j.issn.1008-8849.2020.22.001 [DOI] [Google Scholar]
- Islam, M. T. , Sarkar, C. , El‐Kersh, D. M. , Jamaddar, S. , Uddin, S. J. , Shilpi, J. A. , & Mubarak, M. S. (2020). Natural products and their derivatives against coronavirus: A review of the non‐clinical and pre‐clinical data. Phytotherapy Research, 34(10), 2471–2492. 10.1002/ptr.6700 [DOI] [PubMed] [Google Scholar]
- Ji, D. , Feng, P. , & Fei, X. Y. (2020). Retrospective study of clinical efficacy of integrated Chinese and western medicine in treatment of COVID‐19. ShanDong ZhongYi ZaZhi, 39(7), 645–653. 10.16295/j.cnki.0257-358x.2020.07.001 [DOI] [Google Scholar]
- Lau, K. M. , Lee, K. M. , Koon, C. M. , Cheung, C. S. , Lau, C. P. , Ho, H. M. , & Fung, K. P. (2008). Immunomodulatory and anti‐SARS activities of Houttuynia cordata . Journal of Ethnopharmacology, 118, 79–85. 10.1016/j.jep.2008.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , & Zhang, W. (2020). Evaluation on the clinical effect of traditional Chinese medicine and western medicine regimens on COVID‐19. Guangming Journal of Chinese Medicine, 35(9), 1273–1275. 10.1016/j.biopha.2017.07.118 [DOI] [Google Scholar]
- Lian, J. , Zhang, S. J. , Li, G. L. , Shang, D. , Wang, Q. Y. , Xu, L. S. , … Liu, X. X. (2020). Retrospective analysis of 38 cases with COVID‐19 treated with integrated traditional Chinese and western medicine. Journal of Traditional Chinese Medicine, 61(24), 2126–2130. 10.13288/j.11-2166/r.2020.24.003 [DOI] [Google Scholar]
- Liao G. R. (2020). Study on efficacy and safety of TCM decoction in patients with COVID‐19. International Infectious Diseases, 9(2), 353. [Google Scholar]
- Liu, F. (2020). Clinical observation of 42 cases with COVID‐19 treated with integrated traditional Chinese and western medicine. Beijing, China: National Science and Technology Revitalization of urban Economic Research Association. [Google Scholar]
- Liu, M. , Gao, Y. , Yuan, Y. , Yang, K. , Shi, S. , Zhang, J. , & Tian, J. (2020). Efficacy and safety of integrated traditional Chinese and Western medicine for Corona Virus Disease 2019 (COVID‐19): A systematic review and meta‐analysis. Pharmacological Research, 158, 104896. 10.1016/j.phrs.2020.104896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, X. F. , Ni, X. J. , Lin, J. H. , Zhang, Y. D. , Wu, L. , Huang, D. H. , … Lin, L. (2020). The add‐on effect of Chinese herbal medicine on COVID‐19: A systematic review and meta‐analysis. Phytomedicine, 85, 153282. 10.1016/j.phymed.2020.153282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , Altman, D. G. , & PRISMA Group . (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. Annals of Internal Medicine, 151, 264–269. [DOI] [PubMed] [Google Scholar]
- News Conference of the State Council Information Office . (2020). Yu Yanhong briefing the important role of TCM in COVID‐19 containment and treatment. Beijing, China: State Council Information Office. [Google Scholar]
- Pan, G. T. , Du, C. , Liu, Y. H. , Liu, Y. , Cheng, Y. F. , Han, F. , … Yang, S. L. (2020). Integrated traditional Chinese and western medicine in the treatment of 40 cases of critical patients with COVID‐19. Acta Medicinae Universitatis Science of Technologiae Huazhong, 49(2), 202–207. 10.3870/j.issn.1672-0741.2020.02.016 [DOI] [Google Scholar]
- Pang, W. , Liu, Z. , Li, N. , Li, Y. , Yang, F. , Pang, B. , … Zhang, J. (2020). Chinese medical drugs for coronavirus disease 2019: A systematic review and meta‐analysis. Integrative Medicine Research, 9(3), 100477. 10.1016/j.imr.2020.100477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, M. , Li, Q. T. , Zhu, D. P. , Wang, C. H. , Sun, Q. Z. , Qian, C. F. , … Li, Y. P. (2020). Efficacy observation of maxing Xuanfei Jiedu decoction on moderate COVID‐19 patients. Journal of Emergency in Traditional Chinese Medicine, 39(7), 1129–1132. 10.3969/j.issn.1004-745X.2020.07.001 [DOI] [Google Scholar]
- Qu, X. K. , Hao, S. L. , Ma, J. H. , Wei, G. Y. , Song, K. Y. , Tang, C. , … Du, W. J. (2020). Observation on the clinical effect of Shufeng Jiedu capsule combined with Arbidol Hydrochloride Capsules in the treatment of COVID‐19. Zhong Cao Yao, 51, 1167–1170. 10.7501/j.issn.0253-2670.2020.05.011 [DOI] [Google Scholar]
- Shi, J. , Yang, Z. G. , Ye, C. , Chen, S. S. , Lu, Y. F. , Lv, Y. , … Chen, X. R. (2020). Clinical observation of 49 cases of non‐critical COVID‐19 treated by integrated traditional Chinese and western medicine in Shanghai. ShangHai ZhongYi ZaZhi, 54(4), 30–35. 10.16305/j.1007-1334.2020.04.095 [DOI] [Google Scholar]
- Sterne, J. A. C. , Savović, J. , Page, M. J. , Elbers, R. G. , Blencowe, N. S. , & Boutron, I. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ, 366, l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- Sun, H. M. , Xu, F. , Zhang, L. , Wei, C. , Chen, J. Y. , Wang, Q. X. , & Jia, Z. H. (2020). Study on clinical efficacy of Lianhua Qingke granule in treatment of mild and ordinary COVID‐19. Chinese Journal of Experimental Traditional Medical Formulae, 26(14), 29–34. 10.13422/j.cnki.syfjx.20201438j [DOI] [Google Scholar]
- Tian, J. X. , Yan, S. Y. , Wang, H. , Zhang, Y. , Zheng, Y. J. , Wu, H. R. , … Tong, X. L. (2020). Hanshiyi formula, a medicine for Sars‐CoV2 infection in China, reduced the proportion of mild and moderate COVID‐19 patients turning to severe status: A cohort study. Pharmacological Research, 161, 105127. 10.1016/j.phrs.2020.105127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay, S. , Tripathi, P. K. , Singh, M. , Raghavendhar, S. , Bhardwaj, M. , & Patel, A. K. (2020). Evaluation of medicinal herbs as a potential therapeutic option against SARS‐CoV‐2 targeting its main protease. Phytotherapy Research, 34(12), 3411–3419. 10.1002/ptr.6802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. B. , Wang, Z. X. , Jing, J. , Zhao, P. , Dong, J. H. , Zhou, Y. F. , … Xiao, X. H. (2020). Exploring an integrative therapy for treating COVID‐19: A Randomized Controlled Trial. Chinese Journal of Integrative Medicine, 26(9), 648–655. 10.1007/s11655-020-3426-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. L. , Yang, X. D. , Liu, Y. P. , Zhang, J. , Feng, Y. F. , Shang, L. , … He, X. J. (2020). Preliminary clinical effect analysis of the treatment of novel coronavirus pneumonia by internal administration of traditional Chinese medicine plus fumigation and absorption combined with super dose of vitamin C in treating NOVID‐19. Journal of Xi'an Jiaotong University (Medical Science), 41(6), 931–935. 10.7652/jdyxb202006023 [DOI] [Google Scholar]
- Wells, G.A. , Shea, B. , O'Connell, D. , Peterson, J. , Welch, V. , Losos, M. , & Tugwell, P. (2003). The Newcastle Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta‐analyses. Retrieved from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Wen, L. , Zhou, Z. G. , Jiang, D. X. , & Huang, K. (2020). Effect of Xuebijing injection on inflammatory markers and disease outcome of coronavirus disease 2019. Chin Crit Care Med, 32(4), 426–429. 10.3760/cma.j.cn121430-20200406-00386 [DOI] [PubMed] [Google Scholar]
- Xia, W. G. , An, C. Q. , Zhen, C. J. , Zhang, J. X. , Huang, M. , Wang, Y. , … Zhang, B. L. (2020). Clinical study on 34 case of COVID‐19 treated with integrated traditional Chinese and Western Medicine. Journal of Traditional Chinese Medicine, 61(5), 375–382. 10.13288/j.11-2166/r.2020.05.002 [DOI] [Google Scholar]
- Xiao, M. Z. , Tian, J. X. , Zhou, Y. N. , Xu, X. , Min, X. J. , Lv, Y. , … Tong, X. L. (2020). Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID‐19: A randomized controlled trial. Pharmacological Research, 161, 105126. 10.1016/j.phrs.2020.105126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, Q. , Jiang, Y. J. , Wu, S. S. , Wang, Y. , An, J. , Xu, W. P. , & Wu, J. J. (2020). Value analysis of Shufeng Jiedu Capsule combined with arbidol hydrochloride capsules in the treatment of mild cases of COVID‐19. Journal of Emergency in Traditional Chinese Medicine, 29(5), 756–758. 10.3969/j.issn.1004-745X.2020.05.002 [DOI] [Google Scholar]
- Xiong, W. Z. , Wang, G. , Du, J. , & Ai, W. (2020). Efficacy of herbal medicine (Xuanfei Baidu decoction) combined with conventional drug in treating COVID‐19: A pilot randomized clinical trial. Integrative Medicine Research, 9(3), 100489. 10.1016/j.imr.2020.100489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, X. , Wang, P. , Su, K. , Cho, W. C. , & Xing, Y. (2020). Chinese herbal medicine for coronavirus disease 2019: A systematic review and meta‐analysis. Pharmacological Research, 160, 105056. 10.1016/j.phrs.2020.105056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, F. , Zhang, Y. , Tariq, A. , Jiang, X. L. , Ahmed, Z. , Zhang, Z. H. , … Bussmann, R. W. (2020). Food as medicine: A possible preventive measure against coronavirus disease (COVID‐19). Phytotherapy Research, 34(12), 3124–3136. 10.1002/ptr.6770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, M. B. , Dang, S. S. , Huang, S. , Li, Y. J. , & Guo, Y. L. (2020). Multi‐center clinical observation of Reyanning mixture in treatment of novel coronavirus pneumonia. Chinese Journal of Experimental Traditional Medical Formulae, 26(14), 7–14. 10.13422/j.cnki.syfjx.20201321 [DOI] [Google Scholar]
- Yang, Q. , Sun, Q. G. , Jiang, B. , Xu, H. J. , Luo, M. , Xie, P. , … Cong, Z. W. (2020). Retrospective clinical study on treatment of COVID‐2019 patients with integrated traditional Chinese and Western medicine. Zhong Cao Yao, 51(8), 2050–2054. 10.7501/j.issn.0253-2670.2020.08.009 [DOI] [Google Scholar]
- Yao, K. T. , Liu, M. Y. , Li, X. , Huang, J. H. , & Cai, H. B. (2020). Retrospective clinical analysis on treatment of novel coronavirus‐infected pneumonia with traditional Chinese medicine Lianhua Qingwen. Chinese Journal of Experimental Traditional Medical Formulae, 26(11), 8–12. 10.13422/j.cnki.syfjx.20201099 [DOI] [Google Scholar]
- Yu, P. , Li, Y. Z. , Wan, S. B. , & Wang, Y. (2020). Clinical observation of Lianhua Qingwen granule combined with Abidor in the treatment of mild COVID‐19. Chinese Pharmaceutical Journal, 55(12), 1042–1045. 10.11669/cpj.2020.12.014 [DOI] [Google Scholar]
- Zhang, H. T. , Huang, M. X. , Liu, X. , Zheng, X. C. , Li, X. H. , Chen, G. Q. , … Hong, Z. S. (2020). Evaluation of the adjuvant efficacy of natural herbal medicine on COVID‐19: A retrospective matched case‐control study. The American Journal of Chinese Medicine, 48(4), 779–792. 10.1142/S0192415X20500391 [DOI] [PubMed] [Google Scholar]
- Zhang, K. , Tian, M. L. , Zeng, Y. , Wang, L. W. , Luo, S. , Xia, W. , … Zha, Y. (2020). The combined therapy of a traditional Chinese medicine formula and Western medicine for a critically ill case infected with COVID‐19. Complementary Therapies in Medicine, 52, 102473. 10.1016/j.ctim.2020.102473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. L. , Lei, L. , Xu, Y. , Wei, D. R. , & Hu, F. (2020). Clinical efficacy of Jinyinhua oral liquid in the treatment of 80 patients with coronavirus disease 2019. China Pharmaceuticals, 29(9), 23–26. 10.3969/j.issn.1006-4931.2020.09.006 [DOI] [Google Scholar]
- Zheng, Z. Z. , Bai, Z. G. , Li, C. J. , Ge, S. P. , Luo, Y. , & He, G. D. (2020). Observation on the effect of TCM syndrome differentiation on COVID‐19. Medical Journal of Communication, 34(2), 117–118. 10.19767/j.cnki.32-1412.2020.02.005 [DOI] [Google Scholar]
- Zhou, W. M. , Zhao, F. M. , Li, B. L. , & Tian, Z. Q. (2020). Clinical value of glycyrrhizinate in the treatment of patients with common new coronavirus pneumonia. BingDu XueBao, 36(2), 160–164. 10.13242/j.cnki.bingduxuebao.003679 [DOI] [Google Scholar]
- Zhu, N. , Zhang, D. , Wang, W. , Li, X. , Yang, B. , Song, J. , … Tan, W. (2020). A novel coronavirus from patients with pneumonia in China, 2019. The New England Journal of Medicine, 382(8), 727–733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Details of the search strategy of PubMed.
Table S2. Details of the search strategy of Wanfang database.
Table S3. Components of Chinese herbal medicine used in the included studies.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


