Abstract
Purpose
Conflicting information on potential benefits of drugs as well as reports on hypothetical harm of commonly used drugs in COVID‐19 treatment have challenged clinicians and healthcare systems. We analyzed the change in ambulatory drug utilization before, during, and after the first wave of the pandemic in 2020.
Methods
We explored dispensing data of nearly 19 000 pharmacies at the expense of the statutory health insurance funds covering 88% of Germany's population. We analyzed utilization of publicly discussed drugs with conflicting information. Drug utilization as number of packages dispensed per week from January to June 2020, reflecting 314 million claims, was compared with 2019.
Results
Utilization of hydroxychloroquine increased +110% during March 2020 and then slightly decreased until week April 13–19. Renin–angiotensin–aldosterone system inhibitors and simvastatin/atorvastatin increased, +78% and +74%, respectively, and subsequently decreased below 2019 levels. Utilization of azithromycin and all systemic antibiotics decreased continuously from March 2–8 until June to levels considerably lower compared to 2019 (June 22–28: azithromycin: −55%, all systemic antibiotics: −27%). Pneumococcal vaccines utilization initially increased +373%, followed by supply shortages. Paracetamol utilization showed an initial increase of +111%, mainly caused by an increase of over‐the‐counter dispensings.
Conclusions
Apart from the pandemic itself, the data suggest that dissemination of misinformation and unsound speculations as well as supply shortages influenced drug prescribing, utilization, and purchasing behavior. The findings can inform post‐pandemic policy to prevent unfounded over‐ and underprescribing and off‐label use as well as drug shortages during a public health crisis.
Keywords: COVID‐19, drug utilization, hydroxychloroquine, pandemic, pharmacy claims, renin–angiotensin–aldosterone system, statins
Abbreviations
- ACE2
angiotensin‐converting enzyme 2
- ACEi
angiotensin‐converting enzyme inhibitor
- ARB
angiotensin receptor blocker
- BfArM
Bundesinstitut für Arzneimittel und Medizinprodukte (Federal Institute for Drugs and Medical Devices)
- COVID‐19
coronavirus disease 2019
- OTC
over‐the‐counter (drugs)
- PHI
private health insurance
- RAASi
renin–angiotensin–aldosterone system inhibitor
- RAS
renin–angiotensin system
- SARS‐CoV‐2
severe acute respiratory coronavirus 2
- SHI
statutory health insurance (funds)
Key Points.
Drug utilization was significantly altered during the COVID‐19 pandemic, particularly during the first weeks.
Conflicting information on potential benefits of drugs in COVID‐19 and reports on hypothetical harm of commonly used drugs could have influenced utilization.
Course of utilization hints stockpiling at the beginning of the first pandemic wave, most probably caused by the anticipated intensification of nationwide restrictions for public life and concerns of continuous drug supply.
Findings can inform post‐pandemic policy to prevent unfounded over‐ and underprescribing, off‐label use and drug shortages during a public health crisis.
1. INTRODUCTION
Healthcare systems and clinicians around the world faced major challenges in drug supply during the coronavirus disease (COVID‐19) pandemic in 2020. The spreading of the severe acute respiratory coronavirus 2 (SARS‐CoV‐2) was accompanied by dissemination of misinformation and unsound speculations concerning potential treatment efficacy or harm of some drugs, primarily via public media and social networks. 1
A media analysis identified 2311 reports of rumors, stigma, and conspiracy theories in 25 languages from 87 countries. 19% of the claims were related to treatment and cure. 2
Several drugs were being tested for treatment or prevention of COVID‐19 and might have been used off‐label. 1 , 3 , 4 , 5 Consequently, patients may have been exposed to adverse effects of these drugs without proven benefits.
Hydroxychloroquine, approved for malaria prophylaxis and treatment as well as treatment of rheumatoid arthritis and systemic lupus erythematosus (SLE), is a potent in vitro replication inhibitor of most coronaviruses. It was therefore discussed for treating COVID‐19. 1 The human immunodeficiency virus therapeutics lopinavirs' and ritonavirs' protease inhibiting abilities were discussed to be effective against SARS‐CoV‐2. 6 , 7
For other drugs, an increasing risk for infection and critical outcomes of COVID‐19 was hypothesized. This has been mainly discussed for drugs inhibiting the renin–angiotensin–aldosterone system (RAAS), among them widely used angiotensin‐converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB). 8 The mechanism for SARS‐CoV‐2 infection is the requisite binding of the virus to the membrane‐bound form of the angiotensin‐converting enzyme 2 (ACE2) and internalization of the complex by the host cell. 8 , 9 ACE2, however, is a key enzymatic component of the RAAS. Experimental evidence suggests that RAAS blockade enhance ACE2, which, in part, contributes to the benefit of these regimens. 8
Statins were proposed as an adjunct therapy for COVID‐19 because of their anti‐inflammatory and other potential beneficial effects. 10 However, statins have been shown to upregulate the expression of ACE2, 11 with the potential for increasing viral entry into cells. 12 It was even suggested to cease cholesterol‐lowering therapy in patients with COVID‐19. 13
Fang et al. 14 suggested that ACE2 can also be increased by the non‐steroidal inflammatory drug ibuprofen. This theoretical concern led to a recommendation by the World Health Organization (WHO) 15 on March 17, 2020 that ibuprofen should not be used by patients who show symptoms of COVID‐19, but be replaced by paracetamol. 16
Pneumococci infections can lead to severe pneumonia and sepsis and can potentially require artificial ventilation of intensive care patients. It has been hypothesized that pneumococcal vaccinations could stimulate an immune response in older adults potentially lowering the severity of other infections. 17 However, clinical data supporting this hypothesis with regard to COVID‐19 are scarce. 18 , 19 , 20 , 21
Data on drug utilization during the pandemic is limited 4 but potentially helpful for future public health crises. We aimed, therefore, to investigate the change in ambulatory drug utilization before, during, and after the first wave of the COVID‐19 pandemic in 2020. We hypothesized an increase in utilization for drugs reported to be beneficial, such as hydroxychloroquine, and a decline in utilization of drugs, such as RAASi and ibuprofen, for which an increase of risk was speculated.
2. METHODS
We performed a descriptive drug utilization study. Drug prescriptions were analyzed using the database of the German Institute for Drug Use Evaluation (DAPI), which contains anonymous dispensing data of community pharmacies claimed to the statutory health insurance (SHI) funds and thus covers 88% of Germany's population, that is, approximately 73.3 million people. 22 All claims data from a representative sample of more than 80% (until June 2019) and more than 95% (from July 2019 onwards) of community pharmacies were available. The data were extrapolated by regional factors to 100% of the SHI‐insured population.
Dispensing data were linked to a database containing information on the name, composition, active ingredients, package size, dosage form, and route of administration using the specific product code (Pharmazentralnummer, an identification number for pharmaceutical products in Germany). Allocation of active ingredients was based on the official version of the Anatomical Therapeutic Chemical classification system published by the German Institute of Medical Documentation and Information. 23
We analyzed the time course of utilization for hydroxychloroquine, RAASi (ACEi and ARB), azithromycin, the two most frequent used statins (simvastatin and atorvastatin), pneumococcal vaccines, ibuprofen and lopinavir–ritonavir. We also analyzed the time course of paracetamol utilization.
For an overview on the general course of utilizations in 2020, we analyzed dispensings of all prescribed drugs, all systemic antibiotics, the most frequently used substance in the classes of penicillins (amoxicillin), cephalosporins (cefuroxime), and quinolones (ciprofloxacin).
We determined three periods: A, from January 2020 until week March 16–22 (on March 22, nationwide restrictions on public and social life were implemented); B, from week March 23–29 until week April 13–19 during nationwide restrictions, and C, the period after first relaxation of restrictions on April 20 until the end of June 2020.
We measured drug utilization as number of packages dispensed per week and determined the percentage change in number of utilized packages from January to June 2020 in comparison with 2019. For pneumococcal vaccines, we calculated dispensed vaccine doses. Dispensing data from 2020 and 2019 were matched by weeks in consideration of public holidays to account for seasonal fluctuations.
For ibuprofen und paracetamol, we included dispensing data from private health insurance (PHI)‐insured patients and self‐medication utilization from the INSIGHT Health database to portray full utilization since those drugs are not primarily prescription drugs but over‐the‐counter (OTC) products. This database includes extrapolated data from a representative sample of over 4500 community pharmacies. 24
We determined the distribution of the package sizes per analyzed drug in 2020 compared to 2019 to rule out possible bias due to different amounts of drugs per package in both evaluation periods. Further, we supplemented the data on dispensed packs of the study drugs with defined daily doses 23 per 1000 SHI‐insured persons per day (DID). The number of persons insured by the SHI system was obtained from the Federal Ministry of Health. 25
3. RESULTS
There were no relevant differences in the distribution of package sizes in the analyzed drugs in 2020 compared to 2019 (Table S1 in Data S1). There were no differences in the weekly time courses between DID and packages (Table S2 in Data S1).
3.1. All prescription drugs
Until week February 17–23, utilization of all prescription drugs remained at 2019 levels (+2%). During the remaining of period A, utilization increased continuously in comparison with 2019, peaked at 17.6 million packages per week (+43%) and subsequently decreased below the level of 2019 with 12.1 million packages (−18%) at the beginning of period C (Table 1, Figure 1A).
TABLE 1.
Weekly dispensed drug packages before (Period A), during (Period B), and after (Period C) the first COVID‐19 pandemic wave and relative change from 2019
| Drug | January 6–12 | … | February 24–March 1 | March 2–8 | March 9–15 | March 16–22 | March 23–29 | March 30–April 5 | April 6–12 | April 13–19 | April 20–26 | April 27–May 3 | May 4–10 | … | June 22–28 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period A | Period B | Period C | |||||||||||||
| All prescription drugs | |||||||||||||||
| Change from 2019, % | 1.0% | 7.8% | 12.3% | 18.8% | 42.9% | −14.9% | −18.1% | −16.1% | −7.4% | −18.0% | −9.7% | −10.3% | −17.6% | ||
| Packages dispensed | 14 331 957 | 12 878 475 | 15 360 267 | 15 297 938 | 17 612 702 | 12 062 613 | 11 734 236 | 9 737 995 | 8 992 123 | 12 093 385 | 10 205 599 | 11 897 790 | 11 647 779 | ||
| Hydroxychloroquine | |||||||||||||||
| Change from 2019, % | −4.9% | 32.2% | 24.7% | 36.5% | 109.9% | 38.7% | 13.2% | 8.7% | 22.1% | 3.5% | 10.7% | 5.1% | −10.6% | ||
| Packages dispensed | 5572 | 6123 | 7036 | 7344 | 10 726 | 7956 | 6710 | 5365 | 4732 | 6349 | 5309 | 6206 | 5592 | ||
| RAAS inhibitors | |||||||||||||||
| Change from 2019, % | 5.8% | 12.0% | 21.6% | 30.7% | 77.9% | −8.8% | −11.5% | −11.5% | −1.3% | −16.6% | −10.7% | −7.2% | −19.0% | ||
| Packages dispensed | 1 364 377 | 1 168 590 | 1 489 546 | 1 480 982 | 1 888 470 | 1 185 012 | 1 160 730 | 935 786 | 858 281 | 1 172 412 | 966 063 | 1 149 883 | 1 121 710 | ||
| Simvastatin, atorvastatin | |||||||||||||||
| Change from 2019, % | 5.7% | 10.5% | 17.6% | 27.3% | 73.9% | −11.2% | −10.3% | −9.6% | 0.7% | −15.0% | −9.4% | −6.1% | −20.5% | ||
| Packages dispensed | 467 600 | 390 065 | 494 644 | 493 013 | 622 989 | 398 868 | 402 012 | 324 179 | 298 297 | 405 450 | 330 941 | 397 744 | 382 767 | ||
| Lopinavir + ritonavir | |||||||||||||||
| Change from 2019, % | −22.9% | −19.0% | −25.5% | 16.0% | 43.0% | −9.4% | −25.5% | −22.2% | −15.7% | −29.9% | 2.2% | −36.1% | −38.8% | ||
| Packages dispensed | 121 | 124 | 108 | 116 | 143 | 125 | 108 | 98 | 75 | 101 | 79 | 99 | 85 | ||
| Systemic antibiotics | |||||||||||||||
| Change from 2019, % | −3.4% | −0.7% | −0.5% | 5.3% | −2.8% | −27.2% | −36.8% | −38.7% | −36.7% | −44.0% | −31.1% | −38.6% | −27.2% | ||
| Packages dispensed | 729 275 | 728 055 | 780 733 | 763 758 | 680 693 | 510 407 | 432 291 | 357 195 | 312 653 | 372 403 | 335 860 | 367 660 | 402 300 | ||
| Azithromycin | |||||||||||||||
| Change from 2019, % | 12.8% | 2.3% | 8.5% | 15.8% | 5.2% | −22.9% | −40.8% | −51.9% | −57.6% | −62.0% | −58.0% | −65.0% | −55.3% | ||
| Packages dispensed | 71 201 | 70 190 | 77 382 | 72 002 | 61 218 | 44 758 | 32 630 | 21 577 | 16 165 | 18 411 | 14 725 | 15 312 | 14 830 | ||
| Amoxicillin | |||||||||||||||
| Change from 2019, % | −1.2% | 6.4% | 5.9% | 11.2% | −2.1% | −31.2% | −45.6% | −50.0% | −47.5% | −53.2% | −40.5% | −49.7% | −32.3% | ||
| Packages dispensed | 138 658 | 151 755 | 159 660 | 154 216 | 132 104 | 90 030 | 70 135 | 55 803 | 48 302 | 55 306 | 50 344 | 53 725 | 62 032 | ||
| Cefuroxime | |||||||||||||||
| Change from 2019, % | −1.0% | −6.2% | −4.3% | 2.7% | −2.0% | −28.9% | −40.2% | −44.4% | −46.9% | −51.3% | −39.2% | −45.9% | −37.5% | ||
| Packages dispensed | 86 579 | 82 401 | 88 705 | 85 265 | 76 863 | 56 811 | 46 659 | 37 784 | 32 172 | 38 047 | 34 004 | 36 656 | 39 486 | ||
| Ciprofloxacin | |||||||||||||||
| Change from 2019, % | −40.9% | −35.3% | −35.7% | −31.5% | −31.6% | −40.8% | −30.9% | −9.6% | −1.8% | −17.2% | −2.0% | −6.1% | −10.0% | ||
| Packages dispensed | 29 813 | 28 164 | 30 384 | 30 610 | 29 575 | 26 027 | 25 232 | 22 754 | 20 896 | 25 370 | 22 830 | 26 098 | 25 172 | ||
| Pneumococcal vaccines | |||||||||||||||
| Change from 2019, % | −15.4% | 82.8% | 96.9% | 372.7% | 267.7% | 222.0% | 136.2% | 55.2% | 22.6% | 10.9% | 150.5% | 294.3% | 42.0% | ||
| Doses dispensed | 73 360 | 89 782 | 123 009 | 302 715 | 350 483 | 225 863 | 159 752 | 74 573 | 58 438 | 80 619 | 151 863 | 306 057 | 90 209 | ||
Abbreviation: RAAS, renin–angiotensin–aldosterone system.
FIGURE 1.
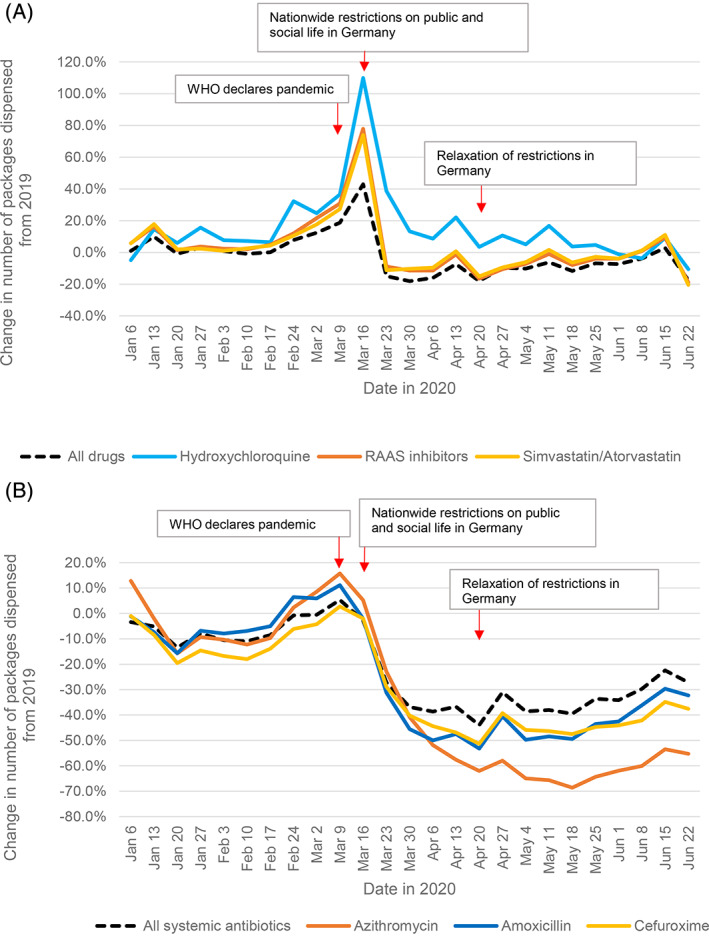
Dispensings of prescription drugs before, during, and after the first COVID‐19 pandemic wave. Relative change in weekly dispensings in 2020, compared to 2019. Each date on the x‐axis refers to the first day of the week. A, All prescription drugs, hydroxychloroquine, RAAS inhibitors, and simvastatin/atorvastatin. B, All systemic antibiotics, azithromycin, amoxicillin, and cefuroxime. Abbreviations: RAAS, renin–angiotensin–aldosterone system; WHO, World Health Organization
3.2. Hydroxychloroquine
Compared to 2019, utilization of hydroxychloroquine increased +110% to 10 700 packages per week at the end of period A, and then decreased to 4700 packages per week at the end of period B with still +22% packages compared to 2019 (Table 1, Figure 1A).
3.3. Renin–angiotensin–aldosterone system inhibitors (RAASi)
Until week February 17–23, utilization of RAASi remained at similar levels compared to 2019 (+5%). During the remaining of period A, utilization increased continuously and subsequently decreased in period B below the level of 2019 (Table 1, Figure 1A). Utilization peaked at 1.89 million packages (+78%) and then fell below 2019 levels with up to −17% during period C. RAASi utilization first reached similar prior‐year levels during week June 8–14 with 1.06 million dispensed packages.
3.4. Statins, lopinavir–ritonavir
Simvastatin and atorvastatin, similar to RAASi and all prescription drugs, peaked at 623 000 packages per week (+74%) at the end of period A and then dropped to 405 500 packages (−15%) at the beginning of period C. Utilization approximated to 2019 values until the end of the observation period (Table 1, Figure 1A).
The amount of ambulatory dispensed packages of lopinavir–ritonavir was low (approximately 102 packages per week) and did not show differences between 2020 and 2019 (Table 1).
3.5. Antibiotics
During period A, azithromycin use slightly increased to 77 400 packages (+9%) in week March 2–8 and 72 000 packages (+16%) in week March 9–15. Amoxicillin use increased to 159 700 packages (+6%) and 154 200 packages (+11%) in the weeks of March 2–8 and March 9–15 (Table 1, Figure 1B). After implementation of restrictions, dispensing levels of all analyzed antibiotics decreased to values substantially lower than in 2019. All systemic antibiotics dropped from 780 700 packages during week March 2–8 −37%, azithromycin −58%, amoxicillin −48%, and cefuroxime −47%, at the end of period B in week April 13–19. Ciprofloxacin use, which was markedly reduced in the first quarter of 2020 compared to 2019 (−37%), returned to slightly below 2019 levels during period C (average: −6%) (Table 1).
3.6. Pneumococcal vaccines
Until week February 17–23, utilization of pneumococcal vaccines hovered around 2019 levels, then substantially increased to 302 700 doses (+373%) and peaked at 350 500 doses (+268%) at the end of period A. After decreasing back to 58 400 doses with still +23% compared to 2019, utilization increased again to 306 000 doses (+294%) during week May 4–10, then decreased again (Table 1, Figure 2).
FIGURE 2.
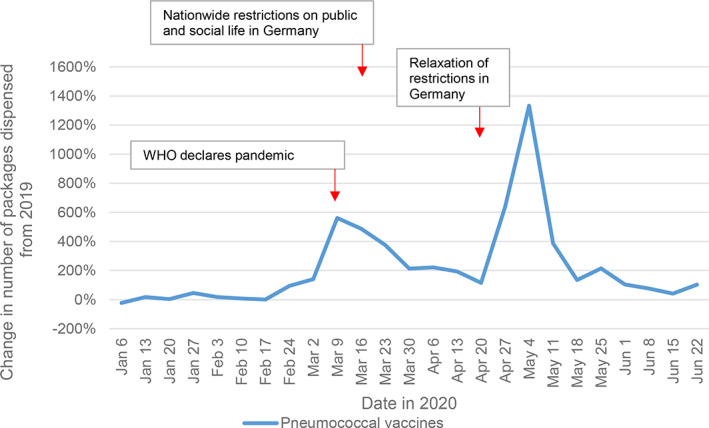
Dispensings of pneumococcal vaccine doses before, during, and after the first COVID‐19 pandemic wave. Relative change in weekly dispensings in 2020, compared to 2019. Each date on the x‐axis refers to the first day of the week
3.7. Ibuprofen and paracetamol
While the utilizations per month increased moderately for ibuprofen to 7.47 million packages in March 2020 with +19% compared to 2019, all paracetamol utilizations showed a huge increase with +111% to 8.04 million packages. These increases were mainly caused by the increase of OTC dispensings: +31% for ibuprofen with 4.66 million packages and +127% for paracetamol with 7.28 million packages.
In April 2020, all utilized packages for both drugs decreased markedly in comparison to 2019; ibuprofen: 3.73 million, −36%; paracetamol: 2.64 million, −19%, and slowly recovered by June with 4.37 million dispensed packages for ibuprofen (−15%) and 2.29 million (−15%) for paracetamol. Decreases were evenly distributed among OTC dispensings as well as SHI‐ and PHI prescriptions (Table 2, Figure 3).
TABLE 2.
Monthly dispensings of ibuprofen and paracetamol from January to June 2020 (OTC dispensings, SHI‐ and PHI prescriptions)
| January | February | March | April | May | June | |
|---|---|---|---|---|---|---|
| Ibuprofen | ||||||
| OTC | 3 597 354 | 4 022 403 | 4 657 881 | 2 188 182 | 2 272 510 | 2 521 809 |
| SHI | 2 530 391 | 2 506 373 | 2 440 847 | 1 370 304 | 1 431 638 | 1 641 885 |
| PHI | 379 558 | 403 379 | 373 825 | 168 396 | 176 477 | 202 889 |
| Total | 6 507 303 | 6 932 155 | 7 472 553 | 3 726 882 | 3 880 625 | 4 366 583 |
| Paracetamol | ||||||
| OTC | 3 298 893 | 3 607 558 | 7 280 035 | 2 298 097 | 1 871 681 | 1 957 676 |
| SHI | 400 980 | 413 921 | 410 980 | 200 989 | 191 492 | 211 746 |
| PHI | 261 388 | 275 553 | 344 126 | 142 123 | 117 067 | 124 222 |
| Total | 3 961 261 | 4 297 032 | 8 035 141 | 2 641 209 | 2 180 240 | 2 293 644 |
Abbreviations: OTC, over‐the‐counter (drug); PHI, private health insurance; SHI, statutory health insurance.
FIGURE 3.
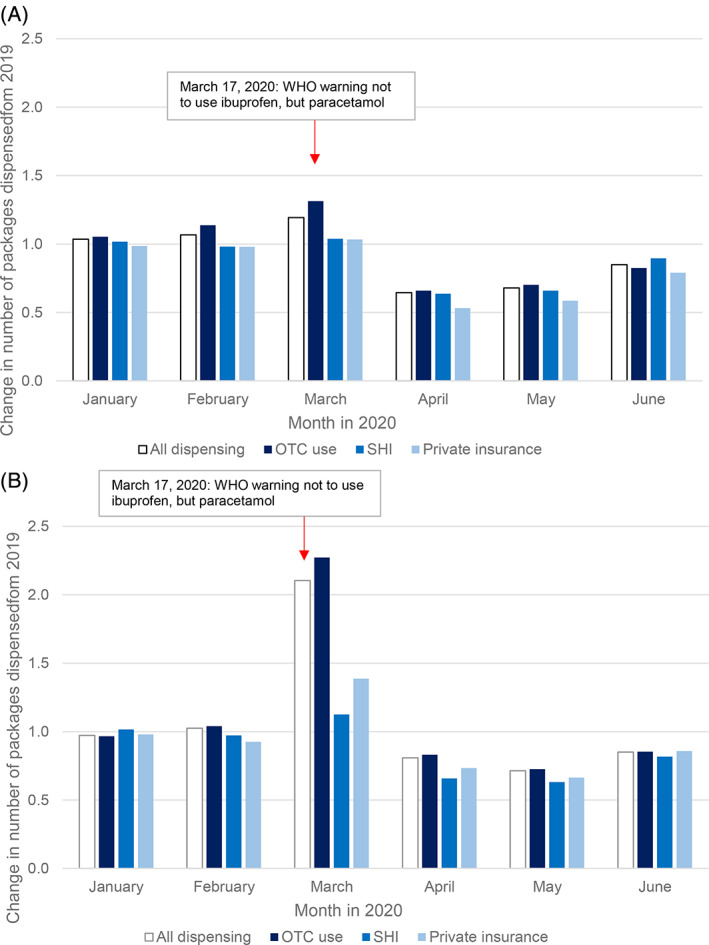
Dispensings of ibuprofen and paracetamol before, during, and after the first COVID‐19 pandemic wave. Relative change in monthly dispensings January to June 2020, compared to 2019. A, Ibuprofen. B, Paracetamol. Abbreviations: OTC, over‐the‐counter (drug); PHI, private health insurance; SHI, statutory health insurance
4. DISCUSSION
The main finding of our analyses is that drug prescribing, utilization, and purchasing behavior was significantly altered, particularly during the first weeks of the COVID‐19 pandemic in early 2020, possibly influenced by misinformation and speculations on potential treatment efficacy as well as hypothetical concerns on harmfulness of commonly used drugs. 2 , 9 , 13 , 14 , 15 , 16 , 26
4.1. Overall drug use and stockpiling
The observed peak of drug utilization of all prescription drugs at the end of period A, that is, March 16–22 indicates stockpiling and was most likely caused by the anticipated intensification of nationwide restrictions for public life and social interactions and, hence, concerns with regard to continuous drug supply.
We assume that patients contacted their prescribers prematurely to issue new prescriptions and physicians prescribed multiple packages with the intention to decrease the necessity for contacts in the near future. The latter is supported by analyzing the percentage of prescriptions with more than one package per drug prescribed in March 2020 compared to March 2019 (data not shown).
Reduced physician visits 27 and, subsequently, pharmacies under conditions of social interaction restriction correlate with the documented subsequent decrease of dispensings for all analyzed drugs. Other possible reasons for this subsequent decrease are drug shortages or the sufficient supply of patients due to previous stockpiling.
The total time course for utilization data of all prescription drugs in 2020 supports the hypothesis of initial stockpiling. However, the data reveal that the number of all packages dispensed from January to June 2020 differed from 2019 by only −2%, showing that the initial increase was compensated by the subsequent decrease.
Utilization data of RAASi, hydroxychloroquine, and simvastatin/atorvastatin show a similar course to all prescribed drugs, again indicating initial stockpiling and giving no signs of under‐prescribing within those drug groups.
4.2. Use of drugs with positive reports on COVID‐19
On March 17, hence immediately before the start of period B, a small clinical trial on COVID‐19 treatment showed a positive effect of hydroxychloroquine and additional benefit when adding azithromycin. 26 This report received high attention despite major methodological issues, including the design (open‐label, non‐randomized) and outcome measure (clearance of viremia alone as surrogate endpoint). 28 Several subsequent clinical trials falsified these reported beneficial effects of chloroquine/hydroxychloroquine in the context of COVID‐19 and even raised major safety concerns. 29 , 30 , 31
Moreover, the European Medicines Agency 32 and the Federal Institute for Drugs and Medical Devices (BfArM) 33 have warned of serious side effects, including cardiac arrhythmias and cardiac arrest due to prolongation of the QT interval (time from the beginning of the QRS complex to the end of the T wave in the electrocardiogram). In addition to myocardial effects, hydroxychloroquine may cause neuropsychiatric disorders. According to the warning, chloroquine/hydroxychloroquine is also known to affect the liver, cause neuronal damage that can lead to seizures, and hypoglycemia. 34 , 35
Our data show that the time‐course in prescription fills for hydroxychloroquine corresponded to the WHO declaring a global pandemic on March 11. 36 The BfArM reported a supply shortage for hydroxychloroquine sulfate 200 mg tablets from April to August 2020. 37 This drug shortage may have contributed to the observed subsequent decline in dispensings and may have affected hydroxychloroquine patients with SLE and rheumatoid arthritis. To counteract this limited availability of hydroxychloroquine for chronically ill patients, on April 4, the BfArM issued a “recommendation” that hydroxychloroquine should only be prescribed with an approved indication documented on the prescription and in a maximum supply of 100 tablets. 38 Misinformation on hydroxychloroquine may have provoked or sustained pre‐emptive stockpiling of packages, which ultimately were only used short‐term for (prophylactic) use, if any. Further, stock shifting from outpatient to clinic supply could have provoked and pinnacled drug shortages.
Despite the proposed beneficial effect of co‐treatment of hydroxychloroquine with azithromycin, 26 , 31 prescriptions of azithromycin rose only slightly and in contrast to the sharp increase in those of hydroxychloroquine, suggesting that off‐label co‐treatment was not prevalent in ambulatory care. Currently, there is no evidence of a beneficial use or effectiveness of hydroxychloroquine (in combination with azithromycin) at any disease stage of COVID‐19. 29 , 31 , 39 , 40 Of note, the antiviral effects of azithromycin remain questionable. 28
The combination of lopinavir–ritonavir was mainly administered to hospitalized patients with COVID‐19 infection. 6 , 41 We confirm this finding with only approximately 102 packages per week dispensed in German ambulatory care. Several randomized trials did not find significant clinical benefits or reduction of viral load in patients hospitalized for COVID‐19 and gastrointestinal adverse effects were more common in the lopinavir–ritonavir group. 6 , 7
4.3. Use of drugs with conflicting information regarding risks for COVID‐19
Our data indicate an inconsistent impact on utilization of drugs with conflicting information regarding risks for or critical outcomes of COVID‐19.
Our data do not suggest an insufficient supply of patients with RAASi or statins during or after the first pandemic wave. Pharmacological blockade of the RAAS 42 with ACEi or ARB as well as low‐density cholesterol lowering with statins 43 reduces morbidity and mortality in various cardiovascular diseases.
It was shown that RAASi may lead to upregulation of ACE2 expression/activity, and that, therefore, use of ACEi or ARB might be associated with an increased risk for and severity of COVID‐19 infection. 9 , 14 Various studies investigated the association of hypertension, treatment with RAASi and developing severe COVID‐19 disease progression. Although there was initial evidence for a difference in the severity of disease in a cohort in Wuhan, China, 44 several other studies concluded that data are insufficient to recommend discontinuation of RAASi. 42 , 45 Moreover, robust evidence is strongly encouraging patients to continue ACEi or ARB pharmacotherapy during the COVID‐19 pandemic. 45 , 46 , 47 Indeed, there is evidence suggesting that these medications might be rather protective against serious lung complications in patients with COVID‐19 infection. 46 , 48 Recently, it was shown that COVID‐19 patients are not characterized by major changes in RAS activity in plasma including ACE2 activity. 49
A study showed an association of lower risk of all‐cause mortality in in‐hospital COVID‐19 patients being treated with statins, compared to patients without statin therapy. 9 We speculate that this observation in a retrospective cohort study might have been influenced by confounding. Experts advise continuation of guideline‐based statin therapy, but do not recommend routine intake for COVID‐19 patients 50 , 51 without risk factors for atherosclerotic cardiovascular diseases. Our data indicate for the two most frequently used statins, simvastatin and atorvastatin, a sufficient supply during and after the first pandemic wave indicating that patients continued statin therapy despite public speculations. 52
A study in diabetic rats found upregulation of ACE2 by ibuprofen, however, lower ACE2‐levels were documented in the diabetic compared to healthy rats. 53 Other in vitro studies suggested ibuprofen may even facilitate cleavage of ACE2 from the membrane, preventing membrane‐dependent viral entry into the cell. 54 , 55 In a nationwide register‐based cohort study, there was no significant association between ibuprofen prescription claims and severe COVID‐19. 56 Recently, ibuprofen use in COVID‐19 patients was shown not to be associated with worsening clinical outcomes, compared with paracetamol or no antipyretic. 57 Hence, there is no experimental and clinical data demonstrating appropriate evidence to avoid ibuprofen in COVID‐19 patients. 58
Our data show that recommendations on the avoidance for ibuprofen had a marginal impact on utilization. Dispensings for SHI‐ and PHI prescriptions as well as OTC‐use increased only slightly in March but decreased in April, indicating similar stockpiling of ibuprofen to paracetamol, although not as pronounced. Though unconfirmed, recommendations to avoid intake of ibuprofen 15 and to prefer paracetamol led to a disproportional purchase of paracetamol drug products, as strongly supported by our data for March. Our data show that misinformation of ibuprofen only had a minor impact on patients and prescribers into choosing paracetamol over ibuprofen, with utilization of ibuprofen remaining higher than paracetamol utilization, except for OTC products in March and April.
The BfArM 33 reported several supply shortages of paracetamol since March 2020, partially estimated to last until March and June 2021. 37 In accordance with the Federal Ministry of Health, the Drug Commission of German Pharmacists in March 2020 asked pharmacists to dispense and physicians to prescribe paracetamol only if needed and to limit the number of tablets to treat the actual course of a disease. 59 This highlights the weakness of the distribution system and its vulnerability to sudden (justified) peaks in demands during pandemics.
4.4. Use of antibiotics
Prescription fills for all systemic antibiotics, amoxicillin, cefuroxime as well as for azithromycin declined substantially (between −37% and −58%). These data were unexpected and in contrast to hydroxychloroquine.
The sharp fall in antibiotic prescriptions compared to 2019, and in particular, the decline in prescriptions for amoxicillin, azithromycin, and cefuroxime, suggests a corresponding decrease in the occurrence of respiratory tract infections. It is possible that this was due to the pandemic‐related measures of hygiene, such as the wearing of face masks, frequent hand washing, and social distancing. This observation is in line with data from the Netherlands, where general practitioners have also prescribed fewer antibiotics for respiratory tract infections within a similar time period. 60
The use of ciprofloxacin did not decrease considerably after the start of the pandemic, as in previous years. We assume that this fluoroquinolone antibiotic was only used for severe infections of the lower respiratory tract and for complicated urinary tract infections, but not for non‐serious respiratory tract infections, according to guidelines and recommendations. 61 , 62
4.5. Use of pneumococcal vaccines
The data show that the use of pneumococcal vaccines peaked after a recommendation by the German Federal Minister of Health on March 9, followed by drug shortages and increased again after imports of vaccines from England and Japan. 63 , 64 , 65 Our findings demonstrate that an unexpected rise in use of vaccines, for example, pneumococcal can result in drug shortages. These are difficult to counteract as vaccines have a long manufacturing time.
4.6. Limitations
Since pseudonymized data were unavailable we do not have patient level information including prescription indications and potential impact on patients' outcome. Whether intake of potentially beneficial drugs against COVID‐19 is associated with an increase in long‐term adverse events remains to be seen. We can only speculate that increase in utilization was connected to off‐label use for COVID‐19.
5. CONCLUSIONS
Apart from the pandemic itself, the data suggest that dissemination of misinformation and unsound speculations as well as supply shortages influenced drug prescribing, utilization, and purchasing behavior. The findings can inform post‐pandemic policy to prevent unfounded over‐ and underprescribing and off‐label use as well as drug shortages during a public health crisis.
CONFLICT OF INTEREST
All authors have completed the Pharmacoepidemiology and Drug Safety Conflict of Interest disclosure form and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could have influenced the submitted work.
ETHICS STATEMENT
This study used anonymized claims data, so no ethical approval was needed.
Supporting information
SUPPLEMENTAL TABLE S1 Distribution of package sizes of drugs from January to June 2020 compared to January to June 2019
SUPPLEMENTAL TABLE S2 Weekly dispensed drugs before (Period A), during (Period B), and after (Period C) the first COVID‐19 pandemic wave and relative change from 2019, expressed as DID
Enners S, Gradl G, Kieble M, Böhm M, Laufs U, Schulz M. Utilization of drugs with reports on potential efficacy or harm on COVID‐19 before, during, and after the first pandemic wave. Pharmacoepidemiol Drug Saf. 2021;30(11):1493‐1503. 10.1002/pds.5324
FUNDING INFORMATION: None
Contributor Information
Salka Enners, Email: s.enners@dapi.de.
Martin Schulz, Email: m.schulz@fu-berlin.de.
REFERENCES
- 1. Tuccori M, Convertino I, Ferraro S, et al. The impact of the COVID‐19 “Infodemic” on drug‐utilization behaviors: implications for pharmacovigilance. Drug Saf. 2020;43:699‐709. 10.1007/s40264-020-00965-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Islam MS, Sarkar T, Khan SH, et al. COVID‐19‐related Infodemic and its impact on public health: a global social media analysis. Am J Trop Med Hyg. 2020;103(4):1621‐1629. 10.4269/ajtmh.20-0812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ahn D‐G, Shin H‐J, Kim M‐H, et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID‐19). J Microbiol Biotechnol. 2020;30(3):313‐324. 10.4014/jmb.2003.03011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vaduganathan M, van Meijgaard J, Mehra MR, Joseph J, O'Donnell CJ, Warraich HJ. Prescription fill patterns for commonly used drugs during the COVID‐19 pandemic in the United States. JAMA. 2020;323(24):2524‐2526. 10.1001/jama.2020.9184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Institutes of Health . Considerations for Certain Concomitant Medications in Patients with COVID‐19; 2020. https://files.covid19treatmentguidelines.nih.gov/guidelines/section/section_13.pdf. Accessed October 16, 2020.
- 6. Cao B, Wang Y, Wen D, et al. A trial of Lopinavir‐ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med. 2020;382(19):1787‐1799. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Slomski A. No benefit for Lopinavir‐ritonavir in severe COVID‐19. JAMA. 2020;323(20):1999. 10.1001/jama.2020.6793 [DOI] [PubMed] [Google Scholar]
- 8. South AM, Diz DI, Chappell MC. COVID‐19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318(5):H1084‐H1090. 10.1152/ajpheart.00217.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang X‐J, Qin J‐J, Cheng X, et al. In‐hospital use of statins is associated with a reduced risk of mortality among individuals with COVID‐19. Cell Metab. 2020;32(2):176‐187.e4. 10.1016/j.cmet.2020.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodrigues‐Diez RR, Tejera‐Muñoz A, Marquez‐Exposito L, et al. Statins: could an old friend help in the fight against COVID‐19? Br J Pharmacol. 2020;177(21):4873‐4886. 10.1111/bph.15166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Y‐H, Wang Q‐X, Zhou J‐W, et al. Effects of rosuvastatin on expression of angiotensin‐converting enzyme 2 after vascular balloon injury in rats. J Geriatr Cardiol. 2013;10(2):151‐158. 10.3969/j.issn.1671-5411.2013.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Subir R, Jagat JM, Kalyan KG. Pros and cons for use of statins in people with coronavirus disease‐19 (COVID‐19). Diabetes Metab Syndr. 2020;14(5):1225‐1229. 10.1016/j.dsx.2020.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ravnskov U. Rapid response: cholesterol‐lowering treatment may worsen the outcome of a Covid‐19 infection. BMJ. 2020;368:m1182. https://www.bmj.com/content/368/bmj.m1182/rr-10 Accessed November 5, 2020.32213507 [Google Scholar]
- 14. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection? Lancet Respir Med. 2020;8(4):e21. 10.1016/S2213-2600(20)30116-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Updated: WHO Now Doesn't Recommend Avoiding Ibuprofen For COVID‐19 Symptoms; 2020. https://www.sciencealert.com/who‐recommends‐to‐avoid‐taking‐ibuprofen‐for‐covid‐19‐symptoms. Accessed October 16, 2020.
- 16. Schulz M, Schumacher PM, Schneider J, Said A, Laufs U. Ugly drug information: ibuprofen and COVID‐19; 2020. https://www.schulz‐martin.com/2020/03/24/ugly‐drug‐information‐ibuprofen‐and‐covid‐19/. Accessed November 5, 2020.
- 17. Smith AM, Huber VC. The unexpected impact of vaccines on secondary bacterial infections following influenza. Viral Immunol. 2018;31(2):159‐173. 10.1089/vim.2017.0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zanettini C, Omar M, Dinalankara W, Imada EL, Colantuoni E, Parmigiani G, et al. Influenza vaccination and COVID19 mortality in the USA. medRxiv. 2020. 10.1101/2020.06.24.20129817 [DOI] [PMC free article] [PubMed]
- 19. Fink G, Orlova‐Fink N, Schindler T, Grisi S, Ferrer AP, Daubenberger C, et al. Inactivated trivalent influenza vaccine is associated with lower mortality among Covid‐19 patients in Brazil; 2020. [DOI] [PubMed]
- 20. Marín‐Hernández D, Schwartz RE, Nixon DF. Epidemiological evidence for association between higher influenza vaccine uptake in the elderly and lower COVID‐19 deaths in Italy. J Med Virol. 2020;93(5):2600‐2601. 10.1002/jmv.26120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Noale M, Trevisan C, Maggi S, et al. The association between influenza and pneumococcal vaccinations and SARS‐Cov‐2 infection: data from the EPICOVID19 web‐based survey. Vaccines. 2020;8(3):471. 10.3390/vaccines8030471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rudolph UM, Enners S, Kieble M, et al. Impact of angiotensin receptor blocker product recalls on antihypertensive prescribing in Germany. J Hum Hypertens. 2020. 10.1038/s41371-020-00425-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. German Institute of Medical Documentation and Information . German anatomical therapeutic chemical (ATC)‐classification with defined daily doses (DDD) for Germany. https://www.dimdi.de/dynamic/en/drugs/atc-classification/
- 24. Insight Health GmbH & Co. KG . APO Fusion Plus 2020. https://www.insight-health.de/
- 25. Federal Ministry of Health . KM6‐statistics; 2021. http://www.bmg.bund.de
- 26. Gautret P, Lagier J‐C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949. 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27. Alexander GC, Tajanlangit M, Heyward J, Mansour O, Qato DM, Stafford RS. Use and content of primary care office‐based vs telemedicine care visits during the COVID‐19 pandemic in the US. JAMA Netw Open. 2020;3(10):e2021476. 10.1001/jamanetworkopen.2020.21476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Machiels JD, Bleeker‐Rovers CP, ter Heine R, et al. Reply to Gautret et al: hydroxychloroquine sulfate and azithromycin for COVID‐19: what is the evidence and what are the risks? Int J Antimicrob Agents. 2020;56(1):106056. 10.1016/j.ijantimicag.2020.106056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosenberg ES, Dufort EM, Udo T, et al. Association of treatment with hydroxychloroquine or azithromycin with in‐hospital mortality in patients with COVID‐19 in New York state. JAMA. 2020;323(24):2493‐2502. 10.1001/jama.2020.8630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bai C, Chotirmall SH, Rello J, et al. Updated guidance on the management of COVID‐19: from an American Thoracic Society/European Respiratory Society coordinated international task force (29 July 2020). Eur Respir Rev. 2020;29(157):200287. 10.1183/16000617.0287-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abella BS, Jolkovsky EL, Biney BT, et al. Efficacy and safety of hydroxychloroquine vs placebo for pre‐exposure SARS‐CoV‐2 prophylaxis among health care workers: a randomized clinical trial. JAMA Intern Med. 2020;181(2):195‐202. 10.1001/jamainternmed.2020.6319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. European Medicines Agency . COVID‐19: reminder of risk of serious side effects with chloroquine and hydroxychloroquine; 2020. https://www.ema.europa.eu/en/news/covid‐19‐reminder‐risk‐serious‐side‐effects‐chloroquine‐hydroxychloroquine. Accessed November 5, 2020.
- 33. Bundesinstitut für Arzneimittel und Medizinprodukte . Hydroxychloroquin: Risiko für schwerwiegende Nebenwirkungen bei Anwendung zur Behandlung von COVID‐19; 2020. https://www.bfarm.de/SharedDocs/Risikoinformationen/Pharmakovigilanz/DE/RI/2020/RI-hydroxychloroquin.html. Accessed November 5, 2020.
- 34. European Medicines Agency . COVID‐19: Reminder of Risk of Serious Side Effects with Chloroquine and Hydroxychloroquine. Amsterdam: European Medicines Agency. 2020. https://www.ema.europa.eu/en/news/covid‐19‐reminder‐risk‐serious‐side‐effects‐chloroquine‐hydroxychloroquine. Accessed October 22, 2020 [Google Scholar]
- 35. Bundesinstitut für Arzneimittel und Medizinprodukte . Weitere Arzneimittelrisiken ‐ Hydroxychloroquin: Risiko für schwerwiegende Nebenwirkungen bei Anwendung zur Behandlung von COVID‐19; 2020. https://www.bfarm.de/SharedDocs/Risikoinformationen/Pharmakovigilanz/DE/RI/2020/RI‐hydroxychloroquin.html. Accessed October 22, 2020
- 36. Cucinotta D, Vanelli M. WHO declares COVID‐19 a pandemic. Acta Biomed. 2020;91(1):157‐160. 10.23750/abm.v91i1.9397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bundesinstitut für Arzneimittel und Medizinprodukte . Lieferengpässe für Humanarzneimittel in Deutschland (ohne Impfstoffe). https://lieferengpass.bfarm.de/ords/f?p=30274:3:6633884200576:NO
- 38. Bundesinstitut für Arzneimittel und Medizinprodukte . Hydroxychloroquin ‐ Sicherstellung der Versorgung von chronisch kranken Patientinnen und Patienten in den zugelassenen Indikationen. https://www.bfarm.de/DE/Service/Presse/Themendossiers/Coronavirus/Anlagen/Off_Label_Use_Hydroxychloroquin.pdf?__blob=publicationFile&v=1. Accessed October 31, 2020.
- 39. Boulware DR, Pullen MF, Bangdiwala AS, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid‐19. N Engl J Med. 2020;383(6):517‐525. 10.1056/NEJMoa2016638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID‐19: a randomized trial. Ann Intern Med. 2020;173(8):623‐631. 10.7326/M20-4207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Horby PW, Mafham M, Bell JL, et al. Lopinavir–ritonavir in patients admitted to hospital with COVID‐19 (RECOVERY): a randomised, controlled, open‐label, platform trial. The Lancet. 2020;396(10259):1345‐1352. 10.1016/S0140-6736(20)32013-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vaduganathan M, Michel T, McMurray J, Pfeffer M, Solomon S. Renin–angiotensin–aldosterone system inhibitors in patients with Covid‐19. N Engl J Med. 2020;382(17):1653‐1659. 10.1056/NEJMsr2005760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mach F, Baigent C, Catapano A, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140‐205. 10.1016/j.atherosclerosis.2019.08.014 [DOI] [PubMed] [Google Scholar]
- 44. Feng Y, Ling Y, Bai T, et al. COVID‐19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201(11):1380‐1388. 10.1164/rccm.202002-0445OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baral R, White M, Vassiliou VS. Effect of renin‐angiotensin‐aldosterone system inhibitors in patients with COVID‐19: a systematic review and meta‐analysis of 28,872 patients. Curr Atheroscler Rep. 2020;22(10):61. 10.1007/s11883-020-00880-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lopes RD, Macedo AVS, Moll‐Bernardes RJ, et al. Continuing versus suspending angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers: impact on adverse outcomes in hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)—the BRACE CORONA trial. Am Heart J. 2020;226:49‐59. 10.1016/j.ahj.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de Simone G. Position statement of the ESC Council on hypertension on ACE‐inhibitors and angiotensin receptor blockers; 2020. https://www.escardio.org/Councils/Council‐on‐Hypertension‐(CHT)/News/position‐statement‐of‐the‐esc‐council‐on‐hypertension‐on‐ace‐inhibitors‐and‐ang. Accessed July 21, 2020.
- 48. Braude P, Carter B, Short R, et al. The influence of ACE inhibitors and ARBs on hospital length of stay and survival in people with COVID‐19. Int J Cardiol Heart Vasc. 2020;31:100660. 10.1016/j.ijcha.2020.100660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kintscher U, Slagman A, Domenig O, et al. Plasma angiotensin peptide profiling and ACE (angiotensin‐converting enzyme)‐2 activity in COVID‐19 patients treated with pharmacological blockers of the renin‐angiotensin system. Hypertension. 2020;76(5):e34‐e36. 10.1161/HYPERTENSIONAHA.120.15841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dashti‐Khavidaki S, Khalili H. Considerations for statin therapy in patients with COVID‐19. Pharmacotherapy. 2020;40(5):484‐486. 10.1002/phar.2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Deutsches Ärzteblatt . Statine könnten Sterberisiko bei COVID‐19 senken. https://www.aerzteblatt.de/nachrichten/114149/Statine‐koennten‐Sterberisiko‐bei‐COVID‐19‐senken. Accessed November 5, 2020.
- 52. Nielsen SF, Nordestgaard BG. Negative statin‐related news stories decrease statin persistence and increase myocardial infarction and cardiovascular mortality: a nationwide prospective cohort study. Eur Heart J. 2016;37(11):908‐916. 10.1093/eurheartj/ehv641 [DOI] [PubMed] [Google Scholar]
- 53. Qiao W, Wang C, Chen B, et al. Ibuprofen attenuates cardiac fibrosis in streptozotocin‐induced diabetic rats. Cardiology. 2015;131(2):97‐106. 10.1159/000375362 [DOI] [PubMed] [Google Scholar]
- 54. Moore N, Carleton B, Blin P, Bosco‐Levy P, Droz C. Does ibuprofen worsen COVID‐19? Drug Saf. 2020;43(7):611‐614. 10.1007/s40264-020-00953-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smart L, Fawkes N, Goggin P, et al. A narrative review of the potential pharmacological influence and safety of ibuprofen on coronavirus disease 19 (COVID‐19), ACE2, and the immune system: a dichotomy of expectation and reality. Inflammopharmacology. 2020;28(5):1141‐1152. 10.1007/s10787-020-00745-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kragholm K, Gerds TA, Fosbøl E, et al. Association between prescribed ibuprofen and severe COVID‐19 infection: a Nationwide register‐based cohort study. Clin Transl Sci. 2020;13(6):1103‐1107. 10.1111/cts.12904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rinott E, Kozer E, Shapira Y, Bar‐Haim A, Youngster I. Ibuprofen use and clinical outcomes in COVID‐19 patients. Clin Microbiol Infect. 2020;26(9):1259.e5‐1259.e7. 10.1016/j.cmi.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kelleni MT. ACEIs, ARBs, ibuprofen originally linked to COVID‐19: the other side of the mirror. Inflammopharmacology. 2020;28(6):1477‐1480. 10.1007/s10787-020-00755-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Drug Commission of German Pharmacists . 14/20 Informationen der Institutionen und Behörden: BMG: Mengenbeschränkte Belieferung bzw. Abgabe von Paracetamol‐haltigen Arzneimitteln durch Hersteller, pharmazeutische Großhändler und Apotheken [Federal Ministry of Health: Delivery or dispensing of paracetamol‐containing drugs by manufacturers, pharmaceutical wholesalers and pharmacies]; 2020. https://www.abda.de/fuer‐apotheker/arzneimittelkommission/amk‐nachrichten/detail/14‐20‐informationen‐der‐institutionen‐und‐behoerden‐bmg‐mengenbeschraenkte‐belieferung‐bzw‐abgabe‐von‐paracetamol‐haltigen‐arzneimitteln‐durch‐hersteller‐pharmazeutische‐grosshaendler‐und‐apotheken/. Accessed June 2, 2021.
- 60. Hek K. Voorschrijven van antibiotica door de huisarts tijdens de coronapandemie. https://nivel.nl/sites/default/files/bestanden/1003882_0.pdf. Accessed October 20, 2020.
- 61. German College of General Practitioners and Family Physicians . [S2k Leitlinie: Ohrenschmerzen, AWMF‐Reg.‐Nr. 053/009], [S2k guideline: Earache]; 2014. https://www.awmf.org/uploads/tx_szleitlinien/053-009l_S2k_Ohrenschmerzen_2014-12-abgelaufen.pdf. Accessed November 4, 2020.
- 62. German Society of Oto‐Rhino‐Laryngology, Head and Neck Surgery , German College of General Practitioners and Family Physicians. [S2k Leitlinie: Rhinosinusitis, AWMF‐Reg.‐Nr. 017/049 (HNO) und 053/012 (DEGAM)], [S2k guideline: Rhinosinusitis]; 2017. http://www.awmf.org/uploads/tx_szleitlinien/017-049_und_053-012l_S2k_Rhinosinusitis_2017-07_01.pdf. Accessed May 31, 2018.
- 63. BMG . Bekanntmachung nach § 79 Absatz 5 des Arzneimittelgesetzes; 2020. https://www.bundesanzeiger.de/pub/publication/TRupGZewRRS6lTjtg0G;wwwsid=A1C9D39D6BDFD75E8267B8069B0C9EA6.web06‐pub?0. Accessed October 22, 2020.
- 64. Wallenfels M. Pneumokokken‐Vakzine: Corona führt zu hoher Impfbereitschaft. Deutsches Ärzteblatt. 2020;17(9):29. 10.1007/s11298-020-8043-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Robert Koch‐Institut . COVID‐19 und Impfen: FAQ. https://www.rki.de/SharedDocs/FAQ/COVID‐Impfen/COVID‐19‐Impfen.html. Accessed November 5, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL TABLE S1 Distribution of package sizes of drugs from January to June 2020 compared to January to June 2019
SUPPLEMENTAL TABLE S2 Weekly dispensed drugs before (Period A), during (Period B), and after (Period C) the first COVID‐19 pandemic wave and relative change from 2019, expressed as DID


