Abstract
We have recently reported two different methodologies that improve sperm functionality. The first method involved transient exposure to the Ca2+ ionophore A23187, the second required sperm incubation in the absence of energy nutrients (starvation). Both methods were associated with an initial loss of motility followed by a rescue step involving ionophore removal or addition of energy metabolites, respectively. In the present work, we show that starvation is accompanied by an increase in intracellular Ca2+ ([Ca2+]i). Additionally, the starved cells acquire a significantly enhanced capacity to undergo a progesterone-induced acrosome reaction. Electrophysiological measurements show that CatSper remains active in starvation conditions. However, the increase in [Ca2+]i was also observed in sperm from CatSper null mice. Upon starvation, addition of energy nutrients reversed the effects on [Ca2+]i and decreased the effect of progesterone on the acrosome reaction to control levels. These data indicate that both methods have common molecular features.
Keywords: Intracellular calcium, starving, mice sperm, acrosome reaction, progesterone, CatSper
INTRODUCTION
To acquire fertilizing ability mammalian sperm must undergo capacitation in the female reproductive tract (1). Capacitation is associated with hyperactivated motility and with the ability of sperm to undergo the acrosome reaction (1, 2) At the molecular level, capacitation requires activation of several signaling pathways, including but not limited to cAMP-dependent pathways, increase in intracellular pH (pHi), changes in intracellular Ca2+ ([Ca2+]i) and hyperpolarization of the sperm plasma membrane potential (Em). Among these pathways, we focused on the study of [Ca2+]i due to its central role in sperm function: 1) in low external Ca2+ (1), neither hyperactivation nor the acrosome reaction are observed and fertilization does not occur; 2) sperm from knock-out (KO) mice lacking the Ca2+ channel complex CatSper cannot hyperactivate and are sterile (3); and 3) increasing sperm [Ca2+]i with reagents such as procaine and thimerosal hyperactivates sperm from different species (4). Overall, these experiments use loss- and gain-of-function approaches to investigate the necessity and sufficiency of Ca2+ regulated pathways.
Although the role of Ca2+ in the acrosome reaction is well-established, the mechanisms by which this ion induces this exocytotic event are still not understood. For many years, the favored hypothesis was that upon capacitation, sperm reached the egg and the acrosome reaction was induced by the zona pellucida. However, this sequence of events has been recently challenged (5, 6). Now it is believed that, at least in the mouse, the acrosome reaction takes place before reaching the egg. Another candidate to be a physiological agonist for the sperm acrosome reaction is progesterone. Sperm exposed to this steroid depict increased [Ca2+]i and higher percentages of acrosome reacted sperm (7, 8). Although in human sperm, progesterone is capable of activating CatSper at nM concentrations, in mouse sperm, similar progesterone concentrations failed to activate this channel (9).
In addition to signaling pathways, capacitation also requires energy in the form of ATP. In mammalian sperm, both, glycolysis and OXPHOS (10, 11, 12) contribute to energy production. However, the contribution of these pathways and their regulation is species-specific. While in bovine sperm, glucose blocks capacitation, in mouse sperm, changes in motility patterns associated with capacitation (i.e. hyperactivation) are based on ATP produced by glycolysis (11, 12). Supporting the role of glycolysis for sperm function, mice lacking sperm-specific glycolytic enzymes are essentially infertile (13, 14). Although a crosstalk between signaling and metabolic pathways has been predicted, how this communication occurs is not well-established.
We have recently presented evidence suggesting that manipulation of sperm signaling and metabolic pathways can be used to improve sperm functional parameters. First, we showed that a short incubation with the Ca2+ ionophore A23187 can overcome cAMP-dependent protein phosphorylation pathways which are a requisite for capacitation. In addition we showed that this treatment induce in vitro fertilizing capacity in sperm from sterile CatSper, KO SLO3 and and Adcy10 (aka sAC) knock-out (KO) genetic models (15). Second, we documented that sperm incubation in nutrient-free media (starvation) followed by rescue with the addition of energy metabolites (e.g. glucose and pyruvate) also increased their fertilizing capacity (16). In both cases, while the first step consisting in either A23187 exposure or starvation (STRV), renders the sperm completely immotile, the second step which entails Ca2+ ionophore removal or metabolite addition is followed by fast recovery of motility. More surprising was that in both cases, zygotes derived from A23187- or STRV-treated sperm reached blastocyst developmental stages in higher percentages.
Considering the similarities of the outcome in both treatments, in the present manuscript we evaluated whether exhaustion of energy resources was connected with a regulation of [Ca2+]i levels. For this purpose, sperm were loaded with the Ca2+ sensor Fluo 4, attached to laminin and the fluorescence followed for 30 min. During this period, the entire sperm population became immotile and depicted an increase in [Ca2+]i. This increase in [Ca2+]i was accompanied by acrosomal exocytosis in a fraction of the sperm population. Moreover, starvation also significantly potentiated the effect of progesterone on the acrosome reaction. Unexpectedly, the starvation-induced increase in [Ca2+]i as well as the potentiated progesterone-induced acrosome reaction was maintained in sperm from CatSper knock-out mice. On the other hand, the addition of glucose and/or pyruvate rescued sperm motility, decreased [Ca2+]i in a majority of the sperm, and, surprisingly, abolished the potentiation of the progesterone-induced acrosome reaction.
MATERIAL AND METHODS
Materials
In what follows the principal reagents and their sources are indicated: Progesterone, Carbonyl cyanide m-chlorophenyl hydrazone (CCCP), Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), Dimethyl sulfoxide (DMSO), Glucose and HEPES from Sigma Aldrich (St. Louis, MO, USA). Ionomycin and Valinomycin from Alomone Labs (Jerusalem, Israel). Fluo-4-AM, FM4–64, 5-(and-6)-Carboxy SNARF 1 AM, Em-sensitive dye 3,3′ dipropylthiacarbocyanine iodide (DiSC3(5)), laminin and pluronic acid from ThermoFisher Scientific (Waltham, MA, USA). Salts: NaCl, KCl, CaCl2.2H2O, KH2PO4 were from J.T. Baker laboratory (Phillipsburg, NJ. USA).
Sperm preparation
The experimental protocols used in this study were approved by the Instituto de Biotecnología/UNAM and the University of Massachusetts Animal Care Committees. In all experiments, cauda epididymal sperm were collected from euthanized mature males (10–12 weeks old) obtained from CD1 or KO mice genetic model CatSper1 KO (Ren et al., 2001) that were obtained from Dr. Clapham and Dr. Chung from Harvard University, Boston (MA, USA). CatSper KO mice and their corresponding wild-type siblings were on a C57BL/6 background. Epididymal motile mice sperm were collected by swim-out in modified Toyoda–Yokoyama–Hosi (TYH) medium (NaCl 119.4, KCl 4.7, CaCl2.2H2O 1.8 mM, KH2PO4 1.2 mM, MgSO4.7H2O 1.2 mM, Na-pyruvate 0.5 mM, glucose 2.5 mM, HEPES 20 mM, at pH 7.3) (16) at 37 °C for 15 minutes in non- capacitating conditions, when capacitating conditions were used media were supplemented with BSA 5 mg/ml and NaHCO3 25 mM. Starving medium (STRV) was prepared as TYH swim-out medium except that Na-pyruvate and glucose were not added.
[Ca2+]i imaging recordings
For [Ca2+]i imaging recordings, once obtained by swim-out, sperm were incubated with 2 μM Fluo 4 AM and 0.05% pluronic acid for loading during 40 minutes in TYH medium as described (17). Then, cells were washed in TYH and immobilized on mouse laminin (1 mg/ml) coated coverslips, unattached spermatozoa were removed by gentle washing and the chamber was filled with TYH medium. To change solutions the medium was completely removed and replaced using a pipette. To fix the external Ca2+ concentration ([Ca2+]e) at 100 nM the Ca-EGTA Calculator TS v1.3 program was used and 1 mM EGTA added for this purpose. For the imaging experiments a chamber recording model MS-508D (ALA Scientific; Farmingdale, NY) was used. The temperature during the recordings was controlled at 37°C using a heater controller model 202A (Harvard Apparatus, MA). Sperm were observed with an inverted microscope Nikon Eclipse Ti-U (Melville NY, USA) equipped with an oil immersion fluorescence objective (Nikon plan Apo TIRF DIC H/N2 60x/1.45 NA) and a Nikon Intensilight-CHGFI as light source for Fluo 4 fluorescence detection, the filter set GFP 96343, D: 495, Exc: 470/40, barrier 525/50 (Nikon) was used and images were acquired with an Andor Ixon 3 EMCCD camera model DU-8970-C00#B (Andor Technology; Belfast, Ireland) under protocols written in Andor iQ 1.10.2 software version 4.0. Images were acquired at one image per second or one image every 20 seconds (as indicated in the figure legend for each experiment) with a 50 msec exposure/illumination time for a period of 3 to 30 minutes depending on the experiment.
[Ca2+]i and acrosome reaction simultaneous imaging
To measure [Ca2+]i and acrosome reaction in the same experiment, alternate measurements were conducted as described (8). Briefly, Fluo 4 loaded mouse sperm were, washed and once attached to laminin, as described above, were bathed for 5 minutes in a recording medium containing 5 μM FM4–64. The dye was also added to all media used in each experiment to maintain its concentration in the chamber during the entire recording. A pair of alternated Fluo 4/FM4–64 images were acquired every 20 sec with an exposure/illumination time of 50 msec during 30 minutes. The interchange delay between Fluo 4/FM4–64 filter cubes was 400 msec. For fluorescence detection of FM4–64, the filter set Wide Green 11007v2, D: 565 dcxt, Exc: 535/50, Em: 590 lpv2 (Chroma Technology Corporation; Bellows Falls, VT, USA) was used.
For Ca2+/acrosome reaction measurements in CatSper knockout mice sperm the protocols for dye loading and experimental recordings were the same as described above. For imaging Fluo 4/FM4–64 acquisition, Catsper knockout loaded sperm were placed in a recording chamber filled with TYH medium at 37°C controlled by a heating stage Universal Heating System, model #10918 (Ibidi; Gräfelfing. Germany) Sperm were viewed with an inverted Nikon A1 Resonant Confocal with 6-color TIRF microscope. An oil immersion fluorescence objective Nikon Apo 60x/1.40 NA was used. Fluorescence illumination was supplied by a SOLA LED (Lumencor; Beaverton, OR, USA) (2% light power). For excitation and emission collection of Fluo 4, the filter set GFP HQ, D: 495, Exc: 470/40, barrier 525/50 (Nikon) was used. For excitation and emission collection of FM4–64, a filter set mCherry HQ, D: 600, Exc: 570/40, Em: 645/75 Nikon was used. Images were acquired every 20 sec with an exposure/illumination time of 40 msec (for Fluo-4) or 60 msec (for FM4–64) for periods of 30min. A ND8 filter was inserted in the excitation light path. Fluorescence images were recorded on an Andor sCMOS Zyla camera (VSC-01746) (Andor Technology; Belfast, Ireland).
pHi imaging recordings.
For pHi measurements, sperm were loaded with 20 μM 5-(and-6)-Carboxy SNARF 1 AM (Invitrogen) and 0.05% pluronic acid during 30 minutes in TYH basal medium as previously described (18, 19). Once loaded sperm were washed by centrifugation and immobilized in the recording chamber as was done for [Ca2+]i imaging. For excitation and emission collection of SNARF 1 AM the set Wide Green 11007v2, D: 565 dcxt, Exc: 535/50, Em: 590 lpv2 (Chroma Technology Corporation) was used. Images were obtained every 20 seconds during 30 minutes recording.
Image Analysis
In all experiments, 16-bit images were obtained, and movies were processed and analyzed with macros written in Image J (version 1.4.3.67, National Institutes of Health). Regions corresponding to the head of individual sperm were drawn in the movie for quantification. A plot of the fluorescence intensity of each spermatozoon versus time was generated in Origin 6.0 (Microcal Software). Fluorescence is expressed as (F − F0)/F0. When brightness and contrast were adjusted, this was done equally in all images or movies taken under the same conditions. Acquisitions were performed in the imaging set-up describe above.
Membrane potential (Em) measurements.
Em measurements were performed following the protocol described previously in detail (20, 21). Briefly, mature sperm from caudal epididymis were collected, diluted in STRV medium and washed twice in this buffer by gentle centrifugation. After these washes, sperm were resuspended in the same media, or in media containing 5 mM glucose and 0.5 mM pyruvate to a final concentration of 106 cells per ml. After incubation for different time periods (as explained in figure legends), sperm were exposed to a final concentration of 1 μM Em-sensitive dye DiSC3(5) for 5 min. Mitochondrial membrane potential was dissipated with 500 nM CCCP, and sperm were incubated for 2 additional minutes. After this period, 1.5 ml of the sperm suspension was transferred to a gently stirred cuvette at 37 °C and the fluorescence monitored using an Ocean Optics USB4000 spectrofluorometer operated by Spectra Suite (Ocean Optics, Largo, FL) at 620/670 nm excitation/emission wavelength pair (20). Cell hyperpolarization decreases dye fluorescence. Recordings were initiated after reaching steady-state fluorescence (1–3 min) and were converted to Em as described previously (20). Calibration was performed by adding 1 μM valinomycin and sequential additions of KCl. The equilibrium potential for K+ was calculated with the Nernst equation considering an intracellular mouse sperm K+ of 120 mM (22).
Electrophysiology recordings.
Cauda epididymal sperm obtained by swim-out were suspended in HS medium containing: NaCl 135 mM, KCl 5 mM, CaCl2 2 mM, MgSO4 1 mM, lactic acid 10 mM, pyruvic acid 1 mM, glucose 5 mM, HEPES 20 mM, pH 7.4 (NaOH). One hundred μl aliquots of the cell suspension were dispensed into a recording chamber (1 ml total volume) and subjected to electrophysiological recording. Whole-cell macroscopic CatSper monovalent currents (ICatSper) were obtained by patch clamping the sperm cytoplasmic droplet in cauda epididymal sperm and were analyzed as reported previously (23, 24). Seals between the patch pipette and the cytoplasmic droplet in sperm were formed in HS bath solution with (HS NUTR) or without nutrients (HS STRV). After establishing the whole-cell configuration, the bath solution was changed for divalent cation free solution with (DVF NUTR) or without nutrients respectively (DVF STRV), though containing gluconate as an impermeant anion, and currents measured. The DVF bath solution contained: Na-gluconate 150 mM, Na2EDTA 2 mM, EGTA 2 mM, HEPES 20 mM, pH 7.4, while the pipette solution contained: Met-Cs 135 mM, CsCl 5 mM, EGTA 10 mM, HEPES 10 mM, pH 7.0. Though gluconate metabolism is not well known (25), the fact that sperm stop swimming indicates this substrate is unable to supply energy for the flagella to beat. Recordings in DVF STV were performed once the cell had stopped moving. All recordings were performed using an Axopatch 200 (Molecular Devices, Sunnyvale, CA, USA) patch-clamp amplifier at room temperature (22°C) and pulse protocols, data capture and analysis were performed using pCLAMP6 software (all from Molecular Devices, San José CA). Data analysis was also carried out with Origin 7.5 (Microcal Software, Origin Lab, Corp, Northampton, MA, USA) and Sigma Plot 10 (SYSTAT Software, Inc, San Jose, CA, USA). Current records, unless indicated otherwise, were acquired at 20–100 kHz and filtered at 5–10 kHz (lowpass Bessel filter) using a computer attached to a DigiData 1200 (Molecular Devices). Patch pipettes were pulled from borosilicate glass (Kimble® Queretaro Qro, México) and had a final resistance between 15–20 MΩ. ICatSper currents were evoqued employing a conventional voltage-ramp protocol from −80 mV to +80 mV, with duration of 750 ms from a holding potential of 0 mV.
Statistical Analysis
All numerical data are presented as the mean ± standard error of the mean of at least 4 independent experiments. Statistical tests were performed using Student t test, when paired t test was applied it is stated. One-way analysis of variance (ANOVA), in combination with Bonferroni’s multiple comparisons test was used to analyze the dose-curve responses promoted by Progesterone in AR experiments. For all the significant differences p and t values are shown. P-values< 0.05 were considered significant.
RESULTS
Starving medium promotes [Ca2+]i increase in mice sperm
We have previously reported two incubation procedures that improve sperm functional parameters, including fertilization and embryo development rates (15, 16). These treatments are based on either a temporary increase in [Ca2+]i or on the incubation of sperm in energy depletion conditions followed by nutrient addition, named “Sperm Energy restriction and Recovery”. A common feature is that in both cases, the initial outcome is that sperm stopped moving. However, the extent to which these pathways are connected is not known. In this work, we examined if sperm exposure to starving (STRV) conditions induces changes in [Ca2+]i. To this end, sperm were loaded with Fluo 4 in standard TYH nutrient-containing medium (NUTR). After attachment to laminin, the standard nutrient-containing TYH (NUTR) medium was removed and replaced by the same medium (NUTR) or with TYH media lacking glucose and pyruvate (STRV) (see arrow in Fig. 1 A). [Ca2+]i increased slowly in all sperm exposed to STRV media (Fig. 1 A and supplementary movie 1) but not in NUTR controls (Fig. 1B). For the initial experiments, we used high illumination conditions (see Methods); however, we observed that the sperm viability was affected. Although low illumination conditions (see Methods) reduced overall resolution, they maintained sperm viability in almost 100 % of the sperm population. Therefore, we used this low illumination setting in the following experiments. Upon 30 min of incubation in STRV medium, all sperm manifested [Ca2+]i increases (Fig. 1 B). The number of sperm depicting higher [Ca2+]i levels increased over time (Fig. 1 D, upper panel) and was correlated with the number of individual sperm becoming immotile (Fig. 1 C, lower panel). Moreover, normalized [Ca2+]i levels from sperm incubated in STRV conditions that become immotile are significantly higher than those that remain motile (Fig. 1 D). In the presence of nutrients sperm remain motile throughout the experiment (30 min) (not shown). Two lines of evidence indicate that immotile sperm are still alive. First, ionomycin further elevates [Ca2+]i in almost all starved sperm; and second, upon addition of nutrients, most sperm recover their motility (see below).
Fig. 1.
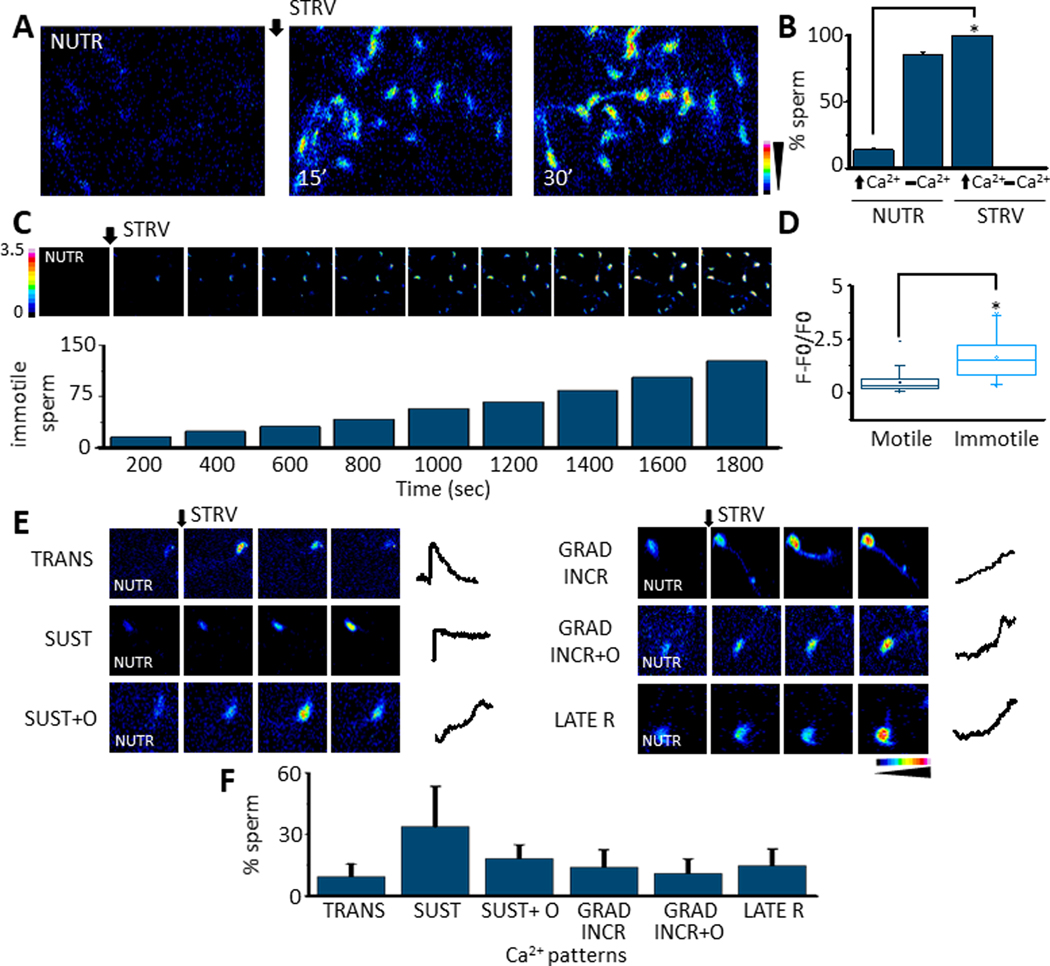
A. Fluorescence image sequence showing that changing mouse sperm from a medium containing nutrients (NUTR) to one without them, named starving (STRV), induces [Ca2+]i increases in mouse sperm (n=8) B. Quantification showing the % of cells that display [Ca2+]i increases (⬆Ca2+), or no [Ca2+]i responses (▂ Ca2+]i) after either NUTR or STRV medium application. STRV medium significantly increases the number of sperm that elevate their [Ca2+]i (p=5.5E-9, t= 14.5, n=8). C. Normalized Fluo 4 fluorescence (F-F0/F0) images sequence to illustrate that the STRV medium application promotes sperm [Ca2+]i increases that lead to sperm immotility which was measured every 200 seconds and expressed as accumulated number of immotile sperm in time (n=6). D. Box plot illustrating that immotile sperm display significantly higher [Ca2+]i levels compared to motile sperm (Boxes enclose 25%−75% the data. The internal horizontal lines show the median, the squares inside the boxes indicate the average, and upper and lower small horizontal lines represent the maximum and minimum values of the data, vertical lines are SD values. (Paired t-Test p= 7.5E-9, t= 6.4, n=6) E. STRV medium application induces different [Ca2+]i elevation patterns in sperm: transitory (TRANS), sustained (SUST), sustained and others (SUST+O), gradual increase (GRAD INCR), gradual increase and others (GRAD INCR+O) and late responses (LATE R) (n=6). F. Bars summarizing the proportion of each [Ca2+]i pattern observed in sperm after STRV protocol. Black arrows ⬇ indicates the time 0’ of STRV medium application in all experiments. All results are expressed as the mean ± S.E. t-Test was applied for statistical analysis * indicates statistically significant differences, p value < 0.05.
We have previously shown that individual sperm exposed to progesterone displayed several distinct patterns of [Ca2+]i elevation and that their distribution was different when, instead of progesterone, sperm were challenged with ionomycin (8). Similarly, the STRV-induced increase in [Ca2+]i depicted different patterns (Fig. 1 E). The percentage distribution of the STRV-induced (Fig. 1 F) [Ca2+]i patterns has similarities to the progesterone-induced ones and both qualitatively differed significantly from the ionomycin-induced changes (8).
Starvation potentiates the progesterone-induced acrosome reaction
One of the consequences of progesterone and ionomycin-induced increase in sperm [Ca2+]i is the stimulation of acrosomal exocytosis (8, 26). Starvation conditions also increased [Ca2+]i with patterns comparable to those described for progesterone. Therefore, to evaluate the effect of nutrient deprivation on the acrosome reaction, we used FM4–64 as a reporter of exocytosis. This methodology was previously validated in mice and human sperm (8, 26), where it was shown that as the multiple fenestrations that accompany acrosome reaction allow FM4–64 into the sperm head, its binding to inner membranes increases fluorescence. This strategy allows simultaneous evaluation of [Ca2+]i and acrosome reaction (8), by exposing Fluo 4-loaded and attached sperm to media containing FM4–64 (Fig. 2 A, B and C). Compared to sperm incubated in the presence of nutrients (NUTR), starvation conditions induced a significant increase in the percentage of sperm undergoing the acrosome reaction (Fig. 2 D). Similarly to the previously described progesterone effect (8, 26), almost all sperm undergoing the acrosome reaction in starvation conditions depicted a transitory pattern (Fig. 2 E and F). It is not clear at present why the Fluo 4 fluorescence decreases when STRV induces the acrosome reaction since [Ca2+]i remains high in ~ 75 % of sperm undergoing the ionomycin induced reaction (8, 26).
Fig. 2.
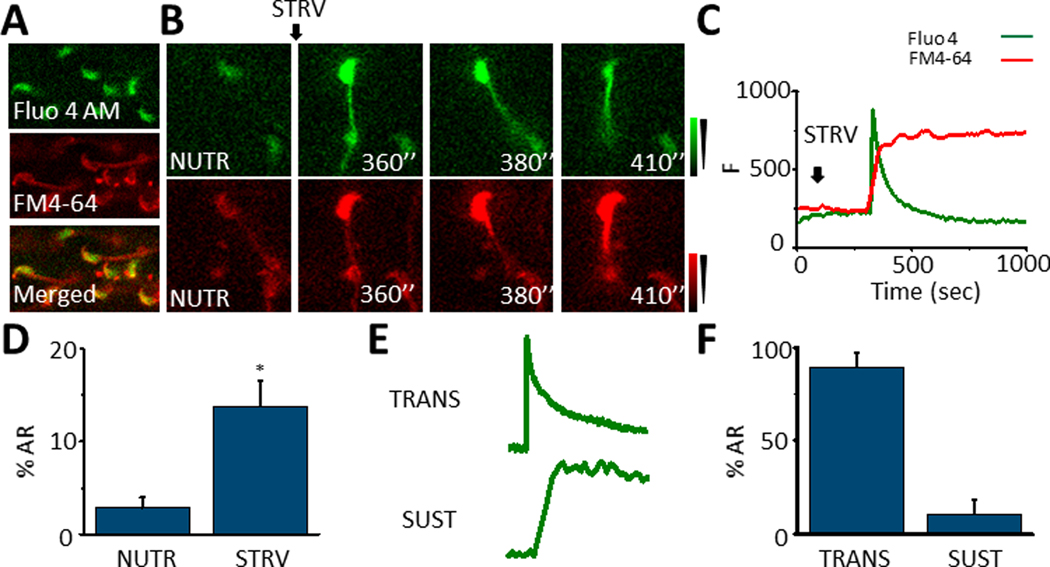
A. Loading of sperm with Fluo 4 and FM4–64 to monitor [Ca2+]i and AR in the same cell in real time. B. Representative sperm displaying a [Ca2+]i increase and undergoing AR reported by FM4–64 fluorescence increase. C. Fluo 4 and FM4–64 fluorescence traces showing a [Ca2+]i increase and AR almost simultaneously in time after STRV medium application. D. Quantification bars showing that the STRV condition induces higher AR in comparison to NUTR medium (p= 0.005, t= 3.9, n=5). E. [Ca2+]i traces illustrating the two patterns related to AR promoted by STRV medium: transitory (TRANS) and sustained (SUST). F. Percentage of each [Ca2+]i pattern related to AR induced by STRV medium. Black arrows indicate the time 0’ of STRV medium application for the experiments. All results are expressed as the mean ± S.E. A t-Test was applied for statistical analysis * indicates statistically significant differences, p value < 0.05.
These results indicate that a fraction of the sperm population undergoes spontaneous acrosome reaction when incubated in the absence of nutrients. However, these experiments are silent regarding the action of an agonist such as progesterone on the acrosome reaction in starvation conditions. To examine how STRV affects the progesterone response, we measured [Ca2+]i and acrosome reaction in the same individual sperm incubated in NUTR or STRV media for 15 min and then exposed or not to Progesterone (100 μM) or ionomycin (20 μM) as described in the figure text (Fig. 3 A). It is worth noting that similar to the experiments described above, neither the control (NUTR) nor the nutrient-free media (STRV) contain HCO3- or BSA (non-capacitating conditions). In the NUTR media non-capacitating conditions, progesterone produced a small increase in [Ca2+]i levels and barely induced acrosomal exocytosis (Supplementary movie 2). In contrast, when 100 μM progesterone was added to sperm pre-incubated for 15 min in STRV media that elevates [Ca2+]i above basal levels, a further significative and steady [Ca2+]i increase was observed (Fig. 3 A and C). Remarkably, pre-incubation in starved conditions, significantly potentiated the effect of progesterone on the acrosome reaction (Fig. 3 A and B and supplementary movie 3). As a positive control, we used ionomycin, which induced the acrosome reaction in NUTR and STRV conditions (Fig. 3 A and B). Since pre-incubation in STRV media significantly enhanced progesterone’s ability to trigger the acrosome reaction, we performed concentration-response experiments. Control experiments using 100 and 200 μM progesterone in NUTR medium resulted in small percentages of acrosome reacted sperm (Fig. 3 D). When the same concentrations were tested in capacitated sperm a significant increase in acrosome reaction was observed (Figs.3 D). Interestingly, starvation decreased significantly the progesterone concentration needed to induce AR (Figs. 3C, 3D).
Fig. 3.
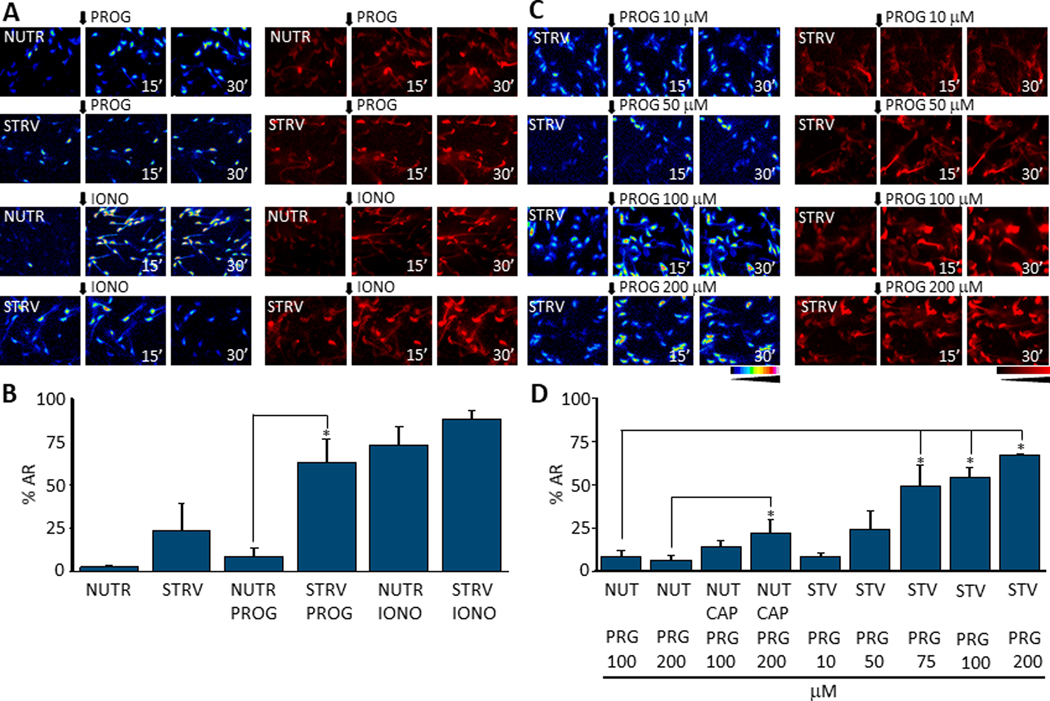
A. Fluorescence images showing [Ca2+]i levels and AR after adding progesterone (PROG) or ionomycin (IONO) to sperm previously exposed to NUTR or STRV media during 15 minutes (n=6). B. AR quantification measured after 30 minutes of agonist treatment shows that progesterone stimulation after 15 minutes of pre-incubation in STRV conditions significantly elevates AR in comparison to NUTR conditions. A t-Test was applied for statistical analysis (p = 0.007, t = 3.4 n=6). C. Fluorescence images illustrating [Ca2+]i and AR increases that are progesterone dose-dependent after STRV medium pre-incubation. D. Quantifications of experiments in C show that STRV medium pre-incubation significantly increases AR percentage at 75 μM or higher progesterone doses under STRV conditions. One way ANOVA with Bonferroni multiple comparisons test was performed (for 75 μM p = 0.012, t=3.3, n=4; for 100 μM p = 0.007, t= 4.5, n=6; for 200 μM p = 0.002, t=4.9, n=4). Black arrows indicate the time 0’ of agonist application for all experiments. All results are expressed as mean ± S.E. * indicates statistically significant differences, p value < 0.05.).
In sperm, up-regulation of [Ca2+]i can be due to intracellular as well as to extracellular Ca2+ sources. To dissect between these possibilities, we compared the effect of starvation on [Ca2+]i in sperm incubated in standard (Fig. 4 A; 2 mM) or in low (Fig. 4 B; 100 nM) [Ca2+]e (see supplementary movie 4) [Ca2+]e. While the starvation-induced increase in [Ca2+]i was observed in standard conditions, it was completely obliterated in low [Ca2+]e media, suggesting that the primary source of the increase in [Ca2+]i during starvation is extracellular. As shown below, ionomycin increased [Ca2+]i in sperm incubated in low [Ca2+]e, indicating that intracellular Ca2+ stores were not depleted in these conditions (Fig. 4 C).
Fig. 4.
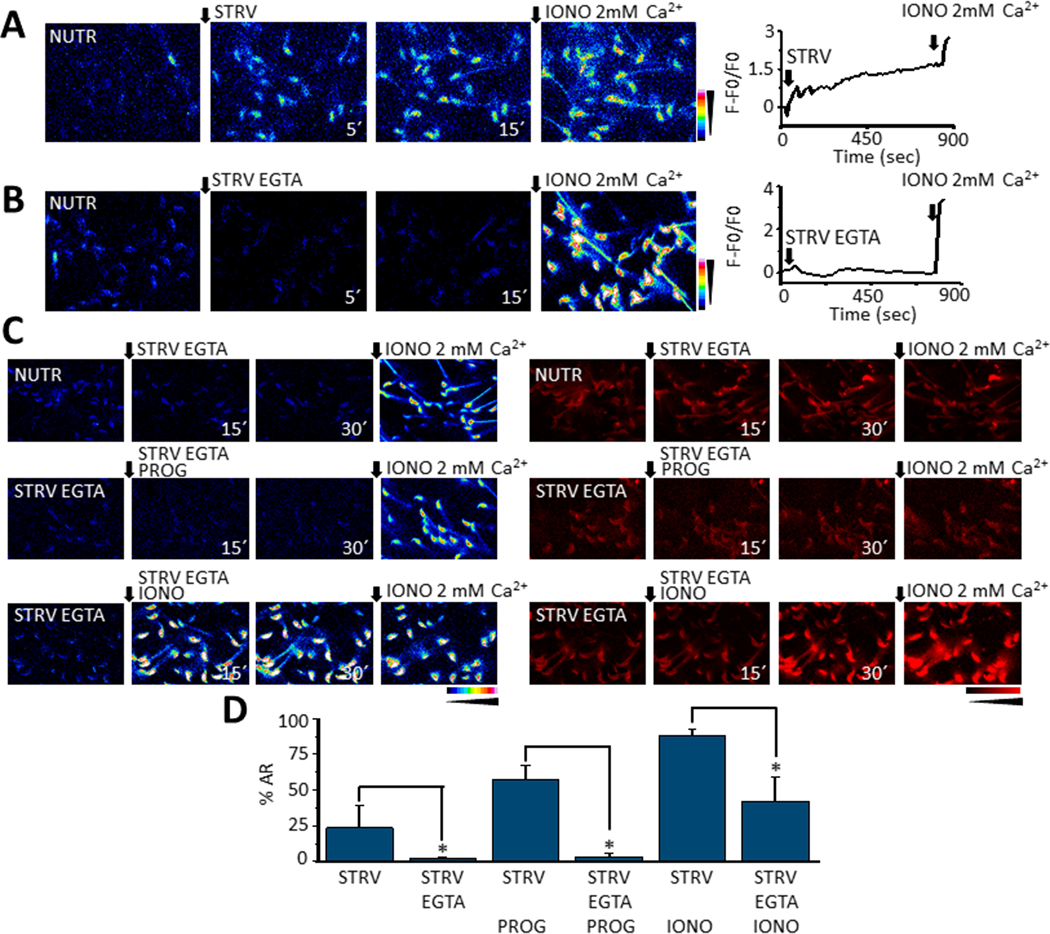
Time fluorescence images showing sperm [Ca2+]i increases promoted by STRV medium either containing normal 2 mM [Ca2+]e (A) or 100 nM [Ca2+]e (STRV EGTA) (B). Ionomycin in 2 mM [Ca2+]e (IONO 2 mM) was added as a control at the end of all recordings. In the right panels representative traces showing the [Ca2+]i changes observed in the two media containing the corresponding [Ca2+]e. Notice the STRV EGTA medium did not induce sperm [Ca2+]i responses. C. [Ca2+]i and AR time images of sperm exposed to STRV-EGTA medium after NUTR (top panel) or STRV EGTA pre-incubation (middle and lower panels) and then exposed or not to either progesterone (PROG) or ionomycin (IONO). D. Quantifications show the presence of EGTA in the STRV medium preincubation significantly reduces AR occurrence (for PROG-STRV p = 0.007, t = −3.5, n=4; for IONO-STRV p = 0.0335, t = −2.7 n=5) unless ionomycin is added at 15 sec (lower sequence). In all recordings black arrows indicate time 0’ of either STRV medium or agonists application, and the numbers at the bottom right of images are the time in seconds it was taken. t-Test was applied for statistical analysis, * indicates statistically significant differences, p value < 0.05.
Considering the role of Ca2+ on the acrosome reaction (for review see (27), we also tested the effect of reducing [Ca2+]e on the starvation-induced acrosome reaction as well as on the potentiation of the progesterone-induced exocytosis. As shown above (Fig. 2 D), in media with standard [Ca2+]e, starvation induces a significant percentage of acrosome reacted sperm. On the other hand, the starvation-induced increases in [Ca2+]i and the acrosome reaction were completely blocked in low [Ca2+]e conditions (Fig. 4 C & D). Similarly, the progesterone-induced acrosome reaction was completely obliterated in these conditions (Fig. 4 C & D). As shown earlier (Fig. 3 B), ionomycin added to starved sperm in standard [Ca2+]e induced the acrosome reaction. Interestingly, in both low and high [Ca2+]e, ionomycin also induced the acrosome reaction in starvation conditions (Fig. 4 C & D). These results can be explained by the release of Ca2+ from intracellular stores and the diminished capacity of sperm to regulate [Ca2+]i in STRV media. In sperm exposed to 100 nM [Ca2+]e in the presence of nutrients, ionomycin is only able to induce ~ 20 % of acrosome reaction (not shown). These results are summarized in Fig. 4D.
Starvation also increases [Ca2+]i and potentiates the progesterone-induced acrosome reaction in CatSper−/− sperm
Altogether, our findings suggest that the major contribution to the starvation-induced increase in [Ca2+]i and the acrosome reaction is external. Considering the relevance of the CatSper Ca2+ channel complex for sperm function, we evaluated if starvation affected CatSper activity using whole-cell patch clamp (23, 24). Currents were recorded using a voltage ramp protocol (Fig. 5 A) using NUTR and STRV conditions. In both cases, the protocol used was similar. Sperm were recovered in HS buffer containing (Fig. 5 B) or not (Fig. 5 C) nutrients. After the initial recording, the external HS buffer was exchanged for divalent-free (DVF) media containing (Fig. 5 B; red line) or not (Fig. 5 C; red line) nutrients and a new recording was conducted. To determine the response to internal alkalinization, 10 mM NH4Cl was added externally to sperm bathed in DVF media with NUTR (Fig. 5 B; blue line) or without nutrients (STRV) (Fig. 5 C; blue line). The current amplitude at −80 mV and +80 mV was found to be similar in NUTR (Fig. 5 D) and STRV (Fig. 5 E) conditions.
Fig. 5.
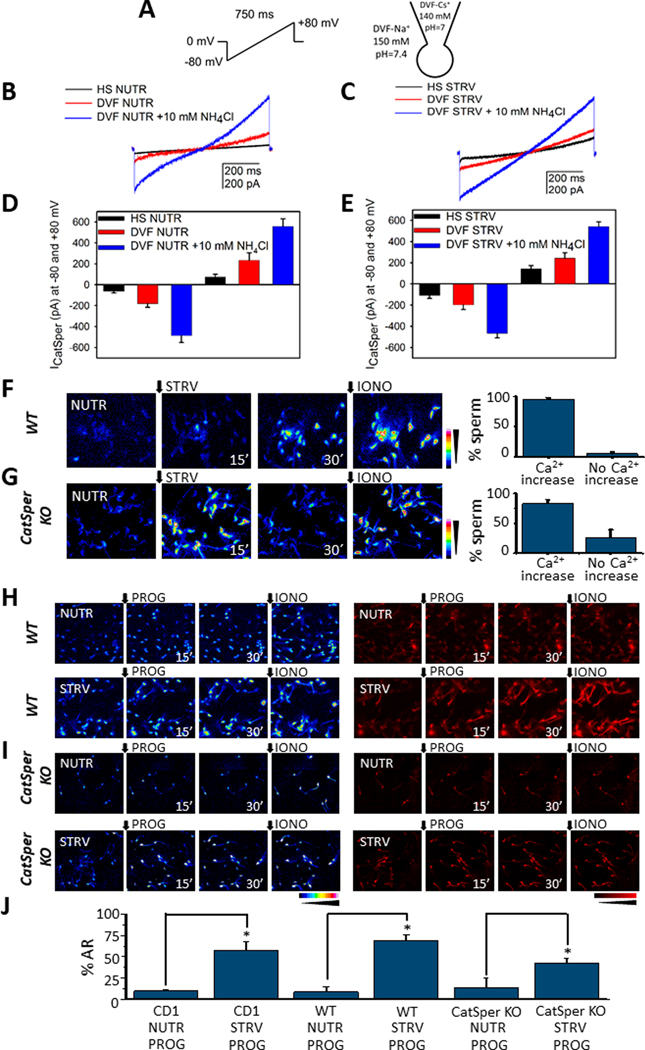
A. Voltage-ramp protocol applied to generate the CatSper currents (left panel) in the whole-cell mode and ionic conditions used to record monovalent cationic current (right panel). B. Representative CatSper currents obtained in medium containing (NUTR) or not nutrients (STRV) (C), and typical increases induced by NH4Cl induced alkalization. C. Family of CatSper currents obtained as in B but without nutrients. D, E. Summary of the measurements obtained in −80 mV and +80 mV from experiments shown in B and C, respectively, (n=4). F. Fluorescence time [Ca2+]i images of sperm from WT mice in response to STRV medium (left panel). Percentage of sperm from WT mice that manifest [Ca2+]i increases after STRV medium application (right panel). G. [Ca2+]i responses promoted by STRV medium in sperm from CatSper KO mice (left panel). Right panel summarizes the percentage of CatSper KO sperm responsive to STRV media. H. [Ca2+]i and AR measurements of sperm from WT mice pre-incubated in NUTR or STRV media and then exposed to progesterone (PROG) for 30 minutes. In all single cell fluorescence experiments ionomycin was applied at the end of the recording as a control (IONO). I. [Ca2+]i and AR recordings from sperm of CatSper KO mice pre-incubated with either NUTR or STRV media and then exposed to progesterone for 30 minutes. J. Quantifications for comparison of AR promoted by progesterone after either NUTR or STRV media pre-incubation obtained in CD1, WT and CatSper KO mice. STRV medium pre-incubation significantly induces AR improvement in all the cases (WT sperm NUTR progesterone vs WT sperm STRV progesterone p= 2.06E-4, t= 5.9, n=5; KO CatSper sperm NUTR progesterone vs KO CatSper sperm STRV progesterone p = 0.03, t = 2.4, n=4. The black arrows indicate the time 0’ for either STRV medium or agonist application. t-Test was applied for statistical analysis * indicates statistically significant differences, p value < 0.05.
The above findings confirm that CatSper channels are functional under starvation conditions and indicate that they can contribute to the [Ca2+]i increase observed in these conditions. To further investigate this possibility, we evaluated the effects of nutrient-free media in sperm from CatSper knock-out mice. Because previous experiments were done using outbred CD1 mice and the CatSper−/− has an inbred C57Bl6 background, controls were repeated using sperm from C57Bl6 wild type mice. Similar to the CD1 strain, starvation conditions increase [Ca2+]i in almost all C57Bl6 wild type sperm (Fig. 5F). Unexpectedly, nutrient-free conditions also induced an increase [Ca2+]i in most of the CatSper−/− sperm population (Fig. 5 G and supplementary movie 5). To test the effect of progesterone in wild type (Fig. 5 H) and CatSper−/− (Fig. 5 I), sperm were pre-incubated for 30 min in either NUTR (Fig. 5 H and I; upper panels) or STRV media (Fig. 5 H and I; lower panels) and then challenged with 100 μM progesterone (Fig. 5H & I). As in CD1 sperm, progesterone increased [Ca2+]i in wild type cells (Fig. 5 H) both in NUTR and in STRV conditions. Remarkably, progesterone also induced an increase in [Ca2+]i in CatSper−/− sperm incubated in both conditions (Fig. 5 I). Regarding exocytosis, when added to sperm incubated in STRV media, progesterone induced the acrosome reaction in over 50 % of the wild type CD1 and C57Bl6 population (Fig. H & J). Interestingly, a somehow lower but significant increase in acrosome reaction was also observed in CatSper−/− sperm (Fig. 5 I & J). Altogether, these results indicate that the CatSper channel complex is active in sperm incubated in nutrient-free media. In addition, these results also suggest that, in addition to CatSper, other Ca2+ transport systems are active during starvation.
STRV media activates similar mechanisms as capacitation
As shown above, starvation increases the percentage of acrosome reacted sperm (Fig. 2), and, also potentiates the progesterone-induced acrosome reaction (Fig. 3). We have previously shown that hyperpolarization of the sperm Em is necessary and sufficient to prepare mouse sperm for an agonist-induced acrosome reaction (28). Considering this conclusion, we were not surprised that in contrast to sperm incubated in NUTR media these starved sperm are somehow depolarized and underwent Em hyperpolarization upon reducing their motility (Fig. 6 A). Noteworthy, this effect was observed in non-capacitating media devoid of BSA and HCO3-, conditions that are not conducive to Em hyperpolarization when nutrients are present. In NUTR medium, when hyperpolarization was induced pharmacologically using valinomycin (VAL), an increase in [Ca2+]i was observed (Fig. 6 B). On the other hand, the addition of KCl, which is known to depolarize sperm did not elicit changes in [Ca2+]i (Fig. 6 C) and blocked the starvation-induced increase in [Ca2+]i (Fig. 6 D).
Fig. 6.
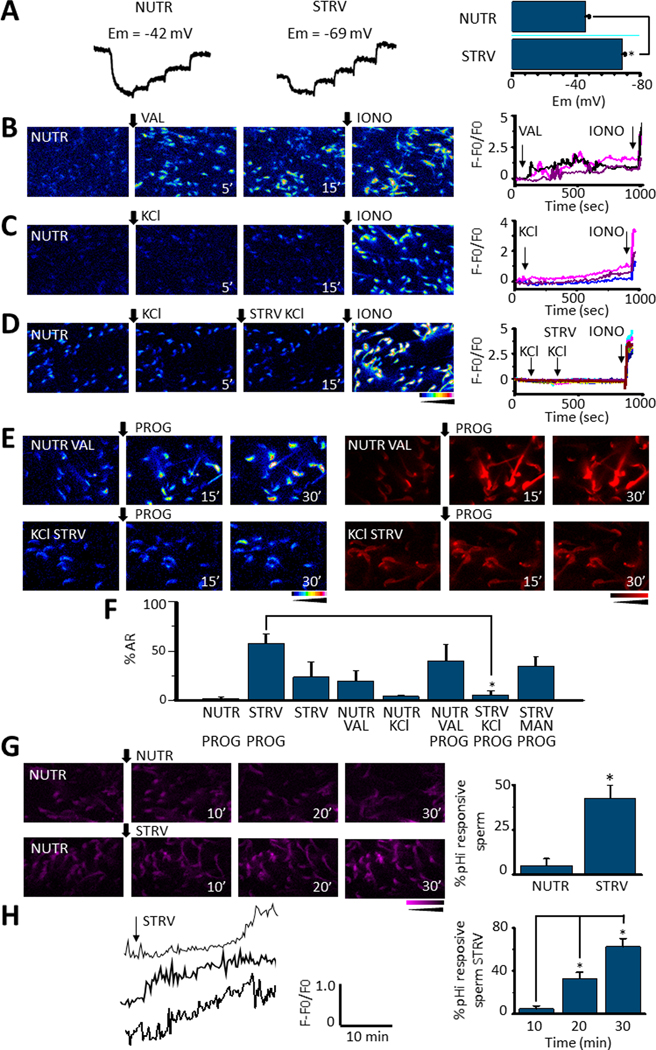
A. Representative DiSC3(5) fluorescence traces corresponding to sperm Em measurements during NUTR or STRV media incubation. Calibration was performed by adding 1 μM valinomycin followed by incremental KCl concentration additions (left and middle panels). Bars summarize Em values of the sperm population obtained after each condition. They show that STRV medium application significantly hyperpolarizes Em (p = 2.4E-6, t = −9.5, n=6) (right panel) B. [Ca2+]i responses to 1 μM valinomycin (VAL) addition to sperm incubated in NUTR medium. In B, C and D ionomycin (IONO) was added at the end of the experiment as a positive control and traces on the right panels correspond to individual sperm in the respective field. [Ca2+]i traces corresponding to some cells in the left panel show that valinomycin induces sperm [Ca2+]i increases (right panel). C. In contrast, 80 mM KCl only promotes a slow and small [Ca2+]i response in sperm in NUTR medium. D. [Ca2+]i responses 5 minutes after a KCl addition to sperm incubated in NUTR medium. Thereafter, cells were changed to STRV conditions and KCl applied; the image was recorded 15 minutes later. Neither in NUTR medium nor in STRV conditions was a KCl addition able to induce [Ca2+]i increases in non-capacitated sperm. However, ionomycin application promoted a clear [Ca2+]i response (right panel). E. [Ca2+]i and AR measurements of sperm pre-incubated with either valinomycin in NUTR medium or KCL under STRV conditions and then stimulated by progesterone (PROG), recording images at 15 and 30 minutes. F. Quantification of AR of experiments in E plus an osmotic control of the KCl addition in response to PROG. The results show that sperm pre-incubation in STRV medium in the presence of KCl significantly reduced AR promoted by progesterone, in comparison to cells pre-incubated in STRV medium alone (p = 1.2E-4, t = −6.8, n=5). G. Fluorescence images showing pHi changes after NUTR or STRV medium application (left panel). Percentage of sperm that display pHi increases under NUTR or STRV conditions. STRV medium application significantly elevated pHi in sperm (p = 5.0E-4, t = 6.7, n=5) (right panel). H. Representative single-cell pHi responses observed after STRV medium application (left panel). The right panel summarizes the percentage of sperm that manifest pHi increases at different times after STRV medium addition (paired t-test 10 vs 20min p = 0.012, t = −5.38; 20 vs 30 min, p = 0.03, t = −3.65, n=5 (right panel). Black arrows indicate the time 0’ for all applications and the time on the bottom when the image was recorded. All quantified results are expressed as the mean ± S.E. A t-Test was applied for statistical analysis * indicates statistically significant differences, p value < 0.05
To test the extent by which Em hyperpolarization mediates starvation effects on the progesterone-induced acrosome reaction, sperm incubated in nutrient containing medium were pharmacologically hyperpolarized using valinomycin before progesterone addition (Fig. 6 E, upper panel). In this experimental setting, progesterone further increased [Ca2+]i and induced acrosomal exocytosis (Fig. 6 E, upper right panel and F). On the contrary, progesterone had no effects on [Ca2+]i or the acrosome reaction when starved sperm were incubated in high K+-containing media (Fig. 6 E bottom panel and F). When instead of KCl, similar osmolarity conditions were obtained with 80 mM mannitol, the effect of progesterone was still observed (Fig. 6F). Altogether, these results suggest that Em hyperpolarization is required and mediates the starvation effects on [Ca2+]i.
The starvation effects on the sperm Em, [Ca2+]i and the progesterone-induced acrosome reaction are reminiscent to those observed in sperm incubated in conditions that support capacitation. Capacitation is also associated with an increase in pHi and phosphorylation pathways (e.g. activation of PKA-dependent and tyrosine phosphorylation). To test the effect of starvation on pHi, sperm were loaded with the pH dye SNARF, attached to laminin and fluorescence recorded using a protocol identical to the one applied for [Ca2+]i measurements. While no changes in pHi occurred in the presence of nutrients (Fig. 6G; NUTR), starvation induced a steady increase in pHi with different kinetic profiles in most sperm cells (Figs. 6G & 6H; STRV).
Effect of rescue on [Ca2+]i and other sperm parameters
We have previously shown that upon starvation, sperm motility can be rescued by adding back energy metabolites (e.g. glucose and pyruvate) (16). However, it was not known how this rescue would affect [Ca2+]i and the potentiation of the progesterone-induced acrosome reaction. To answer this question, after starvation for 30 min., nutrients were added back and [Ca2+]i was measured for 10 min. during this recovery (Fig. 7 A). We observed three types of [Ca2+]i behaviors in response to nutrient replenishment: a pattern without changes (NO INCR), an increasing pattern (INCR) and a decreasing pattern (DECR) (Fig. 7 B). Notably, quantifications showed that more than half of the cells decreased their [Ca2+]i (Fig. 7 C) and recovered their motility (Fig. 7 D), which is clearly observed in supplementary movie 6. Moreover, when we analyzed how the normalized levels of [Ca2+]i distribute in these sperm populations, three different normal distributions were observed with NUTR media (Fig. 7E; left panel), higher in STRV conditions (Fig 7E; middle panel) and intermediate in sperm that were subjected to starvation and then rescued (Fig. 7E; right panel), RESCUE). In addition to an overall reduction in [Ca2+]i, the potentiation of the progesterone-induced acrosome reaction was abolished upon reintroduction of glucose and pyruvate, (Fig. 7F). It is worth pointing out that upon rescue, the sperm Em depolarized to values similar to those observed when sperm are persistently incubated in nutrient-containing media (Fig. 7G). Altogether, these results suggest a connection between [Ca2+]i, energy levels, Em and the progesterone effects on the acrosome reaction.
Fig. 7.
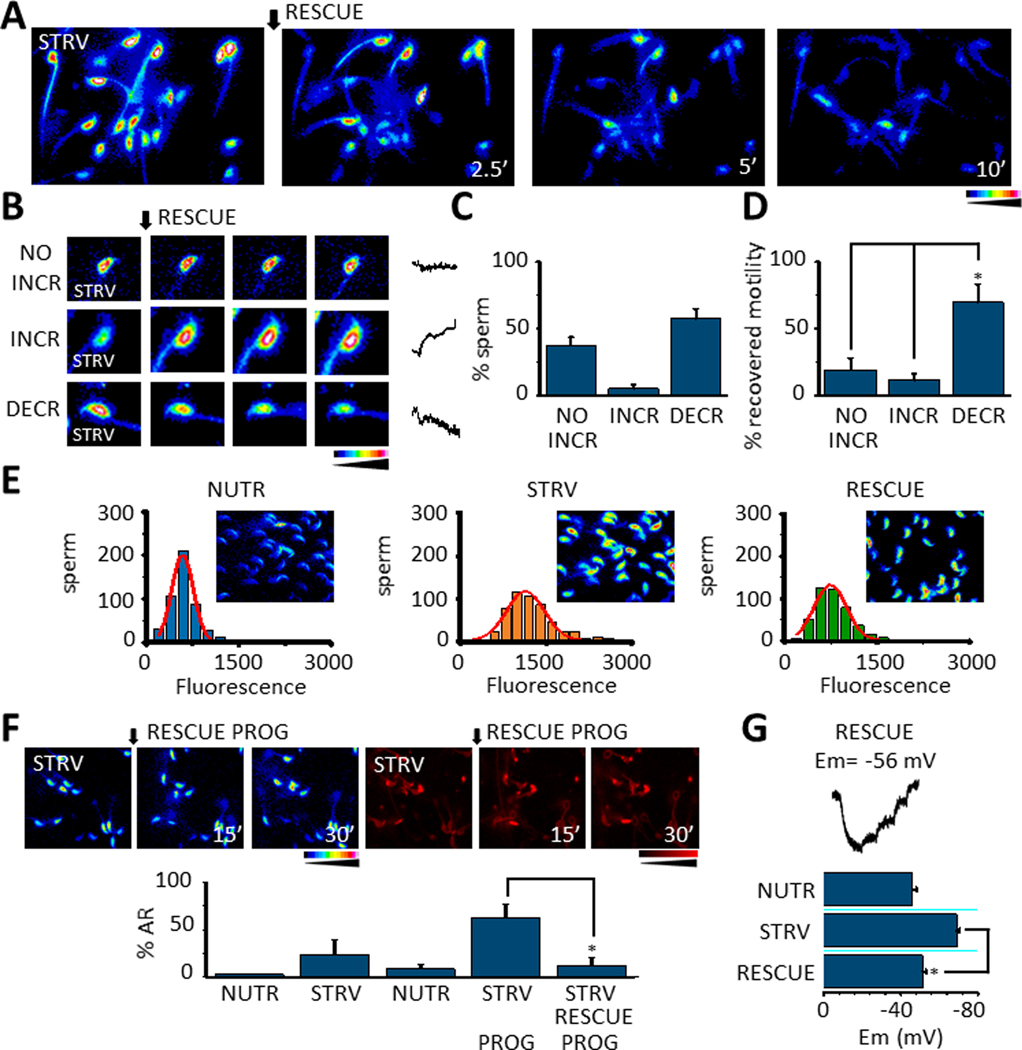
A. [Ca2+]i recordings during the application of RESCUE conditions (adding back glucose and pyruvate, see Methods) in sperm pre-incubated with STRV medium. B. Three types of [Ca2+]i behavior were observed in sperm after RESCUE conditions: NO INCREASE (NO INCR), increase (INCR) or decrease (DECR). Example traces are shown on the right side of the figure. C. Bars summarizing experiments as in B indicate that most sperm exposed to RESCUE conditions manifest a [Ca2+]i decrease. D. Quantification bars show that a statistically significant number of sperm that recover motility decrease their [Ca2+]i (No INCR vs INCR p = 0.007, t = 3.1, n=8; NO INCR vs DECR p = 0.001, t= 4.0, n=8). E. Distribution of the number of sperm displaying a certain Fluo 4 fluorescence corresponding to [Ca2+]i levels observed in sperm under NUTR conditions, while in STRV conditions and after RESCUE treatment. F. [Ca2+]i and AR measurements during 30 minutes of progesterone (PROG) treatment under RESCUE conditions after sperm were exposed to STRV medium during 15 minutes (top panel). Quantifications show that progesterone applied under RESCUE conditions significantly reduces its potential to induce AR (p = 0.01, t = −3.22, n= 5) (low panel). G. Representative Em trace of sperm exposed to STRV media and then to RESCUE conditions (top panel). Bars summarizing Em values of sperm recorded under NUTR, STRV and then RESCUE conditions showing that RESCUE conditions significantly hyperpolarize sperm Em in comparison to STRV conditions (p = 3.5E-6, t = 9.1, n=6) (lower panel). In all the experiments the quantified data plotted are mean ± S.E. A t-Test was applied for statistical analysis * indicates statistically significant differences when p value < 0.05.
DISCUSSION
Investigations using KO mice models revealed that loss of function of a variety of genes results in infertility. Several of these models present normal sperm counts and their main deficiency is found in capacitation-associated processes such as hyperactivation (3, 29) and acrosome reaction triggered by agonists. In a previous work, considering the role of Ca2+ ions in hyperactivation, we hypothesized that strategies designed to elevate [Ca2+]i should overcome the need for upstream signaling pathways, including but not limited to PKA activation. To test this hypothesis, we incubated mouse sperm with A23187; this Ca2+ ionophore increased [Ca2+]i and completely blocked sperm motility (30). Although immotile, A23187-treated sperm were alive and once A23187 was removed, [Ca2+]i decreased (17), sperm started moving, undergoing hyperactivation and could fertilize metaphase II eggs (15). Most interestingly, this transient increase in [Ca2+]i induced fertilizing potential in sperm from sterile mice KO models (15). Also, when wild type C57Bl6 sperm were incubated with A23187, higher embryo development rates were observed upon fertilization, suggesting that manipulation of sperm incubation conditions can affect post-fertilization events.
In addition to Ca2+ and other second messengers sperm movement requires energy (11). Among metabolic pathways, both, glycolysis and oxidative phosphorylation contribute to sperm energy production. At least in mouse sperm, glycolysis is essential for the sperm to hyperactivate (11). Supporting the role of glycolysis, mice lacking sperm-specific glycolytic enzymes are essentially infertile (13,14). Although both Ca2+ and energy are required for sperm function, the crosstalk between these pathways is not well understood. In our previous work, to study individual contributions of glycolysis and oxidative phosphorylation, we developed a technique in which sperm are incubated in media depleted of all nutrients (starvation, STRV) until they became motionless, and thereafter individual nutrients are added back (recovery, RESCUE). This method was successful to rescue sperm motility indicating that starvation maintains cell viability. However, unexpectedly, sperm recovered from starvation have functional improvements (16) including increased hyperactivation and fertilization rates. Moreover, zygotes derived from starved and rescued sperm reached the blastocyst stage in higher numbers than those derived from sperm persistently incubated with nutrient metabolites. These works revealed that increasing [Ca2+]i and removing nutrients from the sperm incubation media stopped sperm movement. In each of these cases, motility could be rescued by either removing A23187 or by adding metabolites to the incubation media, respectively. In the present work, we show that, in addition to rendering the sperm immotile, starvation significantly increases [Ca2+]i. Considering that [Ca2+]i is a balance between Ca2+ uptake and extrusion, these results can be explained by enhanced Ca2+ uptake, by reduced Ca2+ extrusion or by a combination of these possibilities. In the absence of nutrients, sperm will not be able to replenish their energy supplies, as a consequence, certain enzymes and ATPases will become inactive. In this regard, outward Ca2+ transport is mediated by two main mechanisms: the Ca2+ pump (PMCA4) (31) and the Na+/Ca2+ antiporter (32). Although from these possibilities, only PMCA4 is directly ATP-dependent, in low ATP conditions the Na+/K+ ATPase will also have much less activity. Therefore, the higher intracellular Na+ will not favor Ca2+ efflux through the Na+/Ca2+ antiporter NCX (Fig. 8).
Fig. 8.
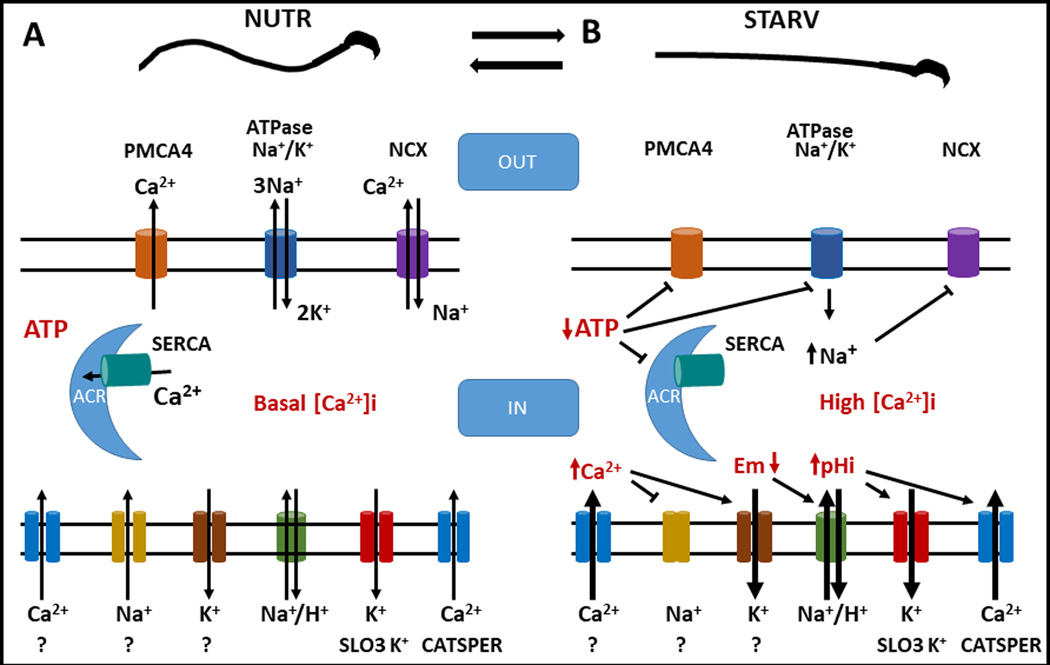
A. Model proposing that under NUTR conditions a basal Ca2+ entry occurs, the presence of intracellular ATP concentration allows the proper function of intracellular Ca2+ clearance mechanisms such as PMCA4 and SERCA pumps. During NUTR incubation also the Na+/K+ ATPase and the NCX exchanger work normally and all mechanisms functioning together maintain low levels of [Ca2+]I, and Em and pHi are maintained stable. B. Once sperm are exposed to STRV conditions, the intracellular ATP concentrations decrease and inhibit the normal operation of pumps and transporters, increasing the [Ca2+]i in sperm. [Ca2+]i elevation in sperm somehow activates K+ channels and/or inhibits Na+ permeable channels that contribute to Em hyperpolarization, which in turn activate the Na+/H+ transporter that would increase pHi and stimulate SLO3 and CatSper channels increasing, even more the [Ca2+]i accumulation.
Regarding inward Ca2+ transport, activation of CatSper channels is the best described mechanism by which Ca2+ enters sperm during capacitation (3, 33, 34). Because the CatSper complex is not ATP dependent, we hypothesized that this channel would remain active in starvation conditions. To test this hypothesis, we used electrophysiological measurements of sperm incubated in nutrient-containing media or in starvation conditions. These results, obtained in divalent-free media, showed the standard response of ICatSper in both conditions. Moreover, as would be expected, CatSper currents increased when pHi was alkalinized with NH4Cl. Although these data indicate that CatSper channels are not inhibited in starving conditions, the starvation-induced increase in [Ca2+]i was still observed in sperm from CatSper KO mice. These results suggest that, in addition to CatSper, Ca2+ can enter the sperm using alternative transporters, as recently proposed by our group (12, 35). Alternatively, the increase in [Ca2+]i could be due to release from intracellular Ca2+ stores. It has been proposed that Ca2+ in the sperm can be stored inside the acrosome (36) or, in some species, in a structure named redundant nuclear envelope (37). Although this is a logical explanation for the observed increase in [Ca2+]i, chelating extracellular Ca2+ with EGTA to low nM concentrations did block the starvation-induced increase in [Ca2+]i suggesting that [Ca2+]e is needed. Importantly, in low [Ca2+]e, addition of ionomycin increased [Ca2+]i in starved and control incubating conditions indicating that the intracellular Ca2+ stores were not depleted.
An increase in [Ca2+]i is a necessary step for acrosomal exocytosis (1, 38). Consistently, those sperm incubated in nutrient-free media underwent acrosomal exocytosis in higher numbers than those persistently incubated with nutrients. Moreover, starvation conditions also potentiated the effect of progesterone on the acrosome reaction. The starvation effects on both, the spontaneous and the progesterone-induced acrosome reaction were blocked in low [Ca2+]e suggesting that this cation mediates the effect of starvation on this exocytotic process. Interestingly, the potentiation of the progesterone-induced acrosome reaction was also observed in sperm from CatSper KO mice incubated in the absence of nutrients. Importantly, the effects of starvation on acrosomal exocytosis were observed in sperm incubated without HCO3- and BSA, two molecules essential for capacitation. In standard, nutrient-containing media, capacitation conditions are required for the progesterone-induced acrosome reaction. Therefore, in addition to the increase in [Ca2+]i, we hypothesized that lack of energy also affects other capacitation-associated processes.
In particular, capacitation conditions induce hyperpolarization of the sperm Em and alkalinization of the pHi. Although the mechanisms involved in these events are not fully understood, our group and others have shown that hyperpolarization changes in Em are necessary and sufficient to prepare the sperm for an agonist-induced acrosome reaction (28). This conclusion is based on the following experiments: 1) when hyperpolarization is induced pharmacologically with valinomycin, there is an increase in [Ca2+]i, and, sperm become responsive to agonists such as the zona pellucida (28) or progesterone (this work); 2) clamping the sperm Em in a depolarized state prevents an agonist-induced acrosome reaction in capacitated sperm (28); and 3) in the absence of the sperm-specific K+ channel SLO3, the acrosome reaction is inhibited (29).
Similar to capacitation, sperm incubated in starvation conditions undergo Em hyperpolarization and are alkalinized in higher proportion than those incubated in the presence of nutrients. These changes, in particular the ones related to the sperm Em, are compatible with the effect of progesterone. Moreover, when the sperm Em is clamped in a depolarized state using high external K+ concentrations, the starvation-induced potentiation of the progesterone effect on the acrosome reaction was blocked. Starvation also produced pHi alkalinization which could be explained by activation of membrane potential-dependent Na+/H+ exchangers present in sperm (39,40,41). The mechanisms by which starvation induced sperm Em hyperpolarization remain to be elucidated. Because of the low energetical environment, a role for the Na+/K+ or Ca2+ ATPases can be discarded. On the other hand, the starvation-induced hyperpolarization can be explained by activation of a K+ channel or, by inhibition of a Na+ electrogenic permeability. Electrophysiologically, SLO3 is the best described K+ channel in mouse sperm and it cannot be discarded that starvation conditions drive the opening of this K+ channel for instance by pHi or mechanisms not yet understood. Alternatively, hyperpolarization could be produced by the inhibition of the inward movement of Na+ ions (Fig. 8).
Sperm incubation in the presence of A23187 or in the absence of nutrients renders these cells immotile. In each of these cases, sperm motility can be rescued by the Ca2+ ionophore removal or by adding energy metabolites, respectively. We have shown that upon removal of A23187, sperm movement is accompanied by a decrease in [Ca2+]i to a threshold level (17). Similarly, we observed that the addition of glucose and pyruvate to the starved sperm reduce [Ca2+]i. Moreover, once rescued, sperm do not react when challenged with progesterone. It is possible to hypothesize that, once ATP starts to be synthesized, Ca2+ ATPases are activated with the consequent decrease in [Ca2+]i. However, the extent by which this decrease is responsible for the lack of progesterone action has yet to be established. Interestingly, we also observed that rescued sperm depolarized to similar Em than those persistently incubated in the presence of nutrients.
As mentioned, in previous works we described two alternative protocols that significantly improved mouse sperm functional parameters. One of the most surprising results is the finding that these treatments affect post-fertilization events. How sperm transmit information to the embryo is not well-established. Recent work suggests that the sperm contributes paternal epigenetic information to the offspring (42, 43). In this regard, the possibility that the female tract during in vivo capacitation or the sperm incubation conditions in vitro can affect early embryo development warrants investigation. The present study was aimed to investigate whether these methods were related at the molecular level. Our observations indicate that in both cases, there is an initial increase in [Ca2+]i which is reversed upon rescue. Whether this increase in [Ca2+]i is essential for the sperm to gain a functional advantage will be the subject of future studies. Because all these investigations were conducted using mouse models, it is too early to know whether they will have translational implications. We expect that understanding the molecular basis of these events will increase the likelihood that similar methods can be applied in the clinical setting.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by NIH grants HD-038082 (to PEV) and HD088571 (to JB, LL and PEV); 314839 (CONACyT) and IG200119 (PAPIIT-DGAPA) to AH-C and Fronteras 71 (CONACyT) and IN200919 to AD. David M. Hidalgo was recipient of a post-doctoral Grant from the Government of Extremadura (Spain) and by Fondo Social Europeo (PO14005). We are grateful for the excellent technical assistance of Yoloxochitl Sánchez and Paulina Torres from IBT UNAM and Diana Millán-Aldaco from the IFC UNAM. We thank also to the staff of the Animal Facilities Elizabeth Mata, Graciela Cabeza, Oswaldo López, and Sergio González from IBT UNAM and Claudia Rivera Cerecedo and Héctor Malagón from the IFC UNAM. We also appreciate the fine computational support of Omar Arriaga from IBT UNAM and Ana Maria Escalante and Francisco Pérez from the Computing Unit of IFC UNAM.
Non standard abbreviations:
- AR
acrosome reaction
- [Ca2+]i
intracellular calcium concentration CAP capacitated
- CCCP
carbonyl cyanide m-chlorophenyl hydrazine
- DECR
decrease
- DiSC3(5)
Em-sensitive dye 3,3′ dipropylthiacarbocyanine iodide
- DMSO
dimethyl sulfoxide
- DVF medium
divalent free medium
- EGTA
ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid
- Em
membrane potential
- GRAD INCR
gradual increase
- GRAD INCR+O
gradual increase + other pattern
- INCR
increase
- IONO
ionomycin
- LATE R
late response
- NCX
Na/Ca2+ antiporter
- NO INCR
no increase
- NUTR
nutrients
- PMCA4
plasma membrane calcium ATPase 4
- pHi
intracellular pH
- PROG
progesterone
- sAC
soluble adenylate cyclase
- SERCA
sarco/endoplasmic reticulum Ca2+ATPase
- STRV
starving
- SUST
sustained
- SUST+O
sustained+other pattern
- TRANS
transitory
- TYH medium
Toyoda–Yokoyama–Hosi medium
- VAL
valinomycin
Footnotes
CONFLICT OF INTERESTS STATEMENT
Dr. Visconti owns equity interest in Sperm Capacitation Technologies Inc. a company with goals in improving assisted reproductive technologies. The others authors have not conflict of interest to declare.
REFERENCES
- 1.Yanagimachi R The Physiology of Reproduction New York: Knobil; & Neill JD (Eds.);1994. 317 pp. [Google Scholar]
- 2.Gervasi MG, & Visconti PE Chang’s meaning of capacitation: A molecular perspective. Mol Reprod Dev 2016;83:860–874. [DOI] [PubMed] [Google Scholar]
- 3.Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, Clapham DE A sperm ion channel required for sperm motility and male fertility. Nature 2001;413;603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang H, & Suarez SS Two distinct Ca(2+) signaling pathways modulate sperm flagellar beating patterns in mice. Biol Reprod 2011;85:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inoue N, Satouh Y, Ikawa M, Okabe M, & Yanagimachi R. Acrosome-reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proc Natl Acad Sci U S A 2011;108:20008–20011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Hirohashi N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci U S A 2011;108; 4892–4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meizel S, & Turner KO Progesterone acts at the plasma membrane of human sperm. Mol Cell Endocrinol 1991;77:R1–5. [DOI] [PubMed] [Google Scholar]
- 8.Romarowski A, Sanchez-Cardenas C, Ramirez-Gomez HV, Puga Molina L del C, Trevino CL, Hernandez-Cruz A, Buffone MG A Specific Transitory Increase in Intracellular Calcium Induced by Progesterone Promotes Acrosomal Exocytosis in Mouse Sperm. Biol Reprod 2016;94:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lishko PV, Botchkina IL, & Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 2011;471:387–391. [DOI] [PubMed] [Google Scholar]
- 10.Balbach M, Gervasi MG, Hidalgo DM, Visconti PE, Levin LR, & Buck J. Metabolic changes in mouse during capacitation dagger. Biol Reprod 2020;103:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodson SG, Qiu Y, Sutton KA, Xie G, Jia W, & O’Brien DA Metabolic substrates exhibit differential effects on functional parameters of mouse sperm capacitation. Biol Reprod 2012;87:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hidalgo DM, Romaroswski A, Gervasi MG, Navarrete F, Balbach M, Salicioni AM, Visconti PE Capacitation increases glucose comsumption in murine sperm. Mol Reprod Dev 2020;87:1037–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danshina PV, Geyer CB, Dai Q, Goulding EH, Willis WD, Kitto GB, O’Brien DA Phosphoglycerate kinase 2 (PGK2) is essential for sperm function and male fertility in mice. Biol Reprod 2010;82:136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, O’Brien DA Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci U S A 2004;101:16501–16506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navarrete FA, Alvau A, Lee HC, Levin LR, Buck J, Leon PM, Visconti PE Transient exposure to calcium ionophore enables in vitro fertilization in sterile mouse models. Sci Rep 2016;6:33589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navarrete FA, Aguila L, Martin-Hidalgo D, Tourzani DA, Luque GM, Ardestani G, Visconti PE Transient Sperm Starvation Improves the Outcome of Assisted Reproductive Technologies. Front Cell Dev Biol 2019;7:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez-Cardenas C, Montoya F, Navarrete FA, Hernandez-Cruz A, Corkidi G, Visconti PE, & Darszon A (2018). Intracellular Ca2+ threshold reversibly switches flagellar beat off and on. Biol Reprod 2018;99:1010–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chavez JC, Darszon A, Trevino CL, & Nishigaki T. Quantitative Intracellular pH Determinations in Single Live Mammalian Spermatozoa Using the Ratiometric Dye SNARF-5F. Front Cell Dev Biol 2019;7:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakai H, Li G, Hino Y, Moriura Y, Kawawaki J, Sawada M, & Kuno M. Increases in intracellular pH facilitate endocytosis and decrease availability of voltage-gated proton channels in osteoclasts and microglia. J Physiol 2013;591:5851–5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demarco IA, Espinosa F, Edwards J, Sosnik J, De La Vega-Beltran JL, Hockensmith JW, Visconti PE Involvement of a Na+/HCO-3 Cotransporter in Mouse Sperm Capacitation. J Biol Chem, 2003;278:7001–7009. [DOI] [PubMed] [Google Scholar]
- 21.Espinosa F, & Darszon A. Mouse sperm membrane potential: changes induced by Ca2+. FEBS Lett 1995;372:119–125. [DOI] [PubMed] [Google Scholar]
- 22.Babcock DF, Rufo GA Jr., & Lardy HA Potassium-dependent increases in cytosolic pH stimulate metabolism and motility of mammalian sperm. Proc Natl Acad Sci U S A 1983;80:1327–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirichok Y, Navarro B, & Clapham DE Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 2006;439: 737–740. [DOI] [PubMed] [Google Scholar]
- 24.Orta G, de la Vega-Beltran JL, Martin-Hidalgo D, Santi CM, Visconti PE, & Darszon A. CatSper channels are regulated by protein kinase A. J Biol Chem 2018;293:16830–16841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rohatgi N, Nielsen TK, Bjorn SP, Axelsson I, Paglia G, Voldborg BG, Rolfsson O. Biochemical characterization of human gluconokinase and the proposed metabolic impact of gluconic acid as determined by constraint based metabolic network analysis. PLoS One 2014; 9:e98760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez-Cardenas C, Servin-Vences MR, Jose O, Trevino CL, Hernandez-Cruz A, & Darszon A. Acrosome reaction and Ca(2)(+) imaging in single human spermatozoa: new regulatory roles of [Ca(2)(+)]i. Biol Reprod 2014;91:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beltran C, Trevino CL, Mata-Martinez E, Chavez JC, Sanchez-Cardenas C, Baker M, & Darszon A. Role of Ion Channels in the Sperm Acrosome Reaction. Adv Anat Embryol Cell Biol 2016;220:35–69. [DOI] [PubMed] [Google Scholar]
- 28.De La Vega-Beltran JL, Sanchez-Cardenas C, Krapf D, Hernandez-Gonzalez EO, Wertheimer E, Trevino CL, Darszon A. Mouse sperm membrane potential hyperpolarization is necessary and sufficient to prepare sperm for the acrosome reaction. J Biol Chem 2012;287:44384–44393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santi CM, Martinez-Lopez P, de la Vega-Beltran JL, Butler A, Alisio A, Darszon A, & Salkoff L. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett 2010;584:1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tateno H, Krapf D, Hino T, Sanchez-Cardenas C, Darszon A, Yanagimachi R, & Visconti PE Ca2+ ionophore A23187 can make mouse spermatozoa capable of fertilizing in vitro without activation of cAMP-dependent phosphorylation pathways. Proc Natl Acad Sci U S A 2013;110:18543–18548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okunade GW, Miller ML, Pyne GJ, Sutliff RL, O’Connor KT, Neumann JC, Shull GE Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J Biol Chem 2004;279:33742–33750. [DOI] [PubMed] [Google Scholar]
- 32.Wennemuth G, Babcock DF, & Hille B. Calcium clearance mechanisms of mouse sperm. J Gen Physiol 2003;122:115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlson AE, Westenbroek RE, Quill T, Ren D, Clapham DE, Hille B, Babcock DF CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc Natl Acad Sci U S A 2003;100:14864–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navarrete FA, Garcia-Vazquez FA, Alvau A, Escoffier J, Krapf D, Sanchez-Cardenas C, Visconti PE Biphasic role of calcium in mouse sperm capacitation signaling pathways. J Cell Physiol 2015;230:1758–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luque GM, Dalotto-Moreno T, Martin-Hidalgo D, Ritagliati C, Puga Molina LC, Romarowski A, Buffone MG Only a subpopulation of mouse sperm displays a rapid increase in intracellular calcium during capacitation. J Cell Physiol 2018;233:9685–9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Blas G, Michaut M, Trevino CL, Tomes CN, Yunes R, Darszon A, & Mayorga LS The intraacrosomal calcium pool plays a direct role in acrosomal exocytosis. J Biol Chem 2002;277:49326–49331. [DOI] [PubMed] [Google Scholar]
- 37.Ho HC, & Suarez SS Characterization of the intracellular calcium store at the base of the sperm flagellum that regulates hyperactivated motility. Biol Reprod 2003;68:1590–1596. [DOI] [PubMed] [Google Scholar]
- 38.Darszon A, Nishigaki T, Beltran C, & Trevino CL Calcium channels in the development, maturation, and function of spermatozoa. Physiol Rev 2011;9:1305–1355. [DOI] [PubMed] [Google Scholar]
- 39.Balbach M, Hamzeh H, Jikeli JF, Brenker C, Schiffer C, Hansen JN,Neugebauer P, Trötschel C, Jovine L, Han L, Florman HM, Kaupp UB, Strünker T, Watchen D. Molecular mechanisms underlying the action of zona-pellucida glycoproteins on mouse sperm. Front Cell Dev Biol 2020;8:572735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chávez JC, Ferreira JJ, Butler A, De La Vega Beltrán JL, Treviño CL, Darszon A, Salkoff L, Santi CM SLO3 K+ channels control calcium entry through CATSPER channels in sperm. J Biol Chem 2014; 289: 32266–32275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang D, Hu J, Bobulescu IA, Quill TA, McLeroy P, Moe OW, Garbers DL. A sperm specific Na+/H+ exchanger (sNHE) is critical for expression and in vivo bicarbonate regulation of the soluble adenylyl cyclase (sAC). Proc Natl Acad Sci USA 2007; 104:9325–9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conine CC, Sun F, Song L, Rivera-Perez JA, & Rando OJ Small RNAs Gained during Epididymal Transit of Sperm Are Essential for Embryonic Development in Mice. Dev Cell 2018;46: 470–480 e473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jung YH, Sauria MEG, Lyu X, Cheema MS, Ausio J, Taylor J, & Corces VG Chromatin States in Mouse Sperm Correlate with Embryonic and Adult Regulatory Landscapes. Cell Rep 2017;18:1366–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


