Abbreviations
- ALD
alcohol‐related liver disease
- BMI
body mass index
- COVID‐19
coronavirus disease 2019
- HCV
hepatitis C virus
- IQR
interquartile range
- MELD‐Na
Model for End‐Stage Liver Disease–sodium
- NAFLD
nonalcoholic fatty liver disease
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- VHA
Veterans Health Administration
TO THE EDITOR:
Since the start of the coronavirus disease 2019 (COVID‐19) pandemic, patients with cirrhosis have demonstrated higher rates of hospitalization, decompensation, and death in the setting of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection.( 1 ) Given the higher risk for a severe COVID‐19 course in patients with cirrhosis,( 1 ) vaccination is of the utmost importance.
Per major national and international liver society guidelines, patients with chronic liver disease should be prioritized in receiving a SARS‐CoV‐2 vaccination.( 2 , 3 ) As vaccinations become more widely available, it is imperative for vaccination efforts to identify high‐risk individuals who remain unvaccinated and therefore unprotected.
To guide further vaccination efforts, we used national data from the Veterans Health Administration (VHA) to determine variables associated with the lack of SARS‐CoV‐2 vaccination among patients with cirrhosis.
Patients and Methods
We performed a retrospective cohort study using data from Veterans Outcomes and Costs Associated With Liver Disease, which contains detailed data from VHA patients with established cirrhosis. This database has been used for numerous COVID‐19–related natural history studies in cirrhosis.( 4 , 5 ) We identified patients age ≥18 years who were actively followed in the VHA with an index date of December 18, 2020, when the VHA vaccination campaign began. Patients with prior liver transplantations were excluded. We obtained covariates including demographics, body mass index (BMI), smoking status, etiology of liver disease, decompensated cirrhosis status, comorbidities, Model for End‐Stage Liver Disease–sodium (MELD‐Na) score, US region (Supporting Table 1), and rural versus urban location, which is consistent with prior methods.( 4 , 5 ) COVID‐19 vaccination data through maximum follow‐up (June 15, 2021) were obtained for each patient, including vaccine type (Pfizer‐BioNTech, Moderna, Janssen).
The proportion of patients receiving vaccination was presented at the US state level. Multivariable logistic regression was used to identify variables associated with vaccination (at least 1 dose). The modeling approach included a priori variables including age and major medical comorbidities, but also involved iterative clinician‐directed testing of potentially associated variables in univariable models followed by final model selection through the minimization of the Bayesian information criterion. To articulate patient characteristics associated with remaining unvaccinated, we generated predicted probabilities of vaccination and stratified patients into low (<50%), medium (50%‐70%), and high probability (>70%). Descriptive statistics were presented for stratified groups, and comparisons tested using Wilcoxon rank sum or chi‐square tests as indicated. Institutional review board study approval was obtained at the Corporal Michael J. Crescenz Veterans Affairs Medical Center. Data analyses were performed using STATA 16.1/IC (StataCorp, College Station, TX).
Results
After the exclusion of 1499 patients who previously received transplants, we identified 43,122 patients with cirrhosis in the analytic cohort. The cohort had a median age of 68 years (interquartile range [IQR], 62‐72), was 96% men and 64.7% White, and 31.3% had hepatitis C virus (HCV)–related liver disease, 33.0% had alcohol‐related liver disease (ALD), and 31.6% had nonalcoholic fatty liver disease (NAFLD). A total of 25,875 patients received a SARS‐CoV‐2 vaccination (60.0%) as of June 15, 2021, and 49.2% received the Pfizer‐BioNTech vaccine, 46.9% received the Moderna vaccine, and 4.8% received the Janssen vaccine. There was notable state‐level variation in the proportion of vaccinated patients with cirrhosis as shown in Fig. 1.
FIG. 1.
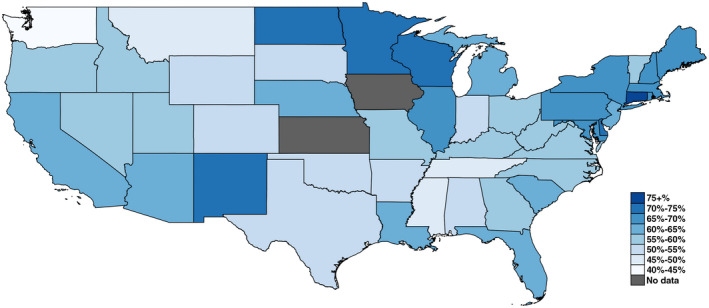
State‐level proportions of patients with cirrhosis receiving COVID‐19 vaccination through June 15, 2021, in the VHA.
The final logistic regression for the outcome of receiving vaccination is shown in Supporting Table 2. When stratified by predicted probabilities of vaccination, individuals with low probabilities (<50%) were more likely to be younger (median age 57 versus 72 years for high probability; P < 0.001), White (80.7% versus 41.3% high probability; P < 0.001), current smokers (49.9% versus 11.8% for high probability; P < 0.001), in rural locations (7.7% versus 2.4% high probability; P < 0.001), and substantially more likely to reside in the southern United States (69.4% versus 4.1% for high probability; P < 0.001; Table 1). Patients with low probabilities of vaccination were also significantly less likely to have chronic comorbidities such as diabetes mellitus, coronary artery disease, or congestive heart failure.
TABLE 1.
Patient Characteristics Stratified by Probability of Receiving COVID‐19 Vaccination
| Factor | Predicted Probability of Receiving COVID‐19 Vaccination | P Value | ||
|---|---|---|---|---|
| <50% (n = 4890) | 50%‐70% (n = 34,018) | >70% (n = 3949) | ||
| Age, years | 57.0 (51.0‐61.0) | 68.0 (64.0‐72.0) | 72.0 (69.0‐75.0) | <0.001 |
| Age category | <0.001 | |||
| <60 years | 3491 (71.4) | 3713 (10.9) | 19 (0.5) | |
| 60‐70 years | 1229 (25.1) | 15,348 (45.1) | 992 (25.1) | |
| >70 years | 170 (3.5) | 14,957 (44.0) | 2938 (74.4) | |
| Sex | <0.001 | |||
| Female | 374 (7.6) | 1277 (3.8) | 80 (2.0) | |
| Male | 4516 (92.4) | 32,741 (96.2) | 3869 (98.0) | |
| Race/ethnicity | <0.001 | |||
| White | 3946 (80.7) | 22,189 (65.2) | 1631 (41.3) | |
| Black | 152 (3.1) | 6424 (18.9) | 1667 (42.2) | |
| Hispanic | 107 (2.2) | 2245 (6.6) | 520 (13.2) | |
| Asian | 55 (1.1) | 494 (1.5) | 43 (1.1) | |
| Other | 630 (12.9) | 2666 (7.8) | 88 (2.2) | |
| BMI kg/m2 | 29.2 (25.1‐33.9) | 29.6 (25.8‐34.1) | 29.6 (25.9‐34.0) | 0.005 |
| Smoking status | <0.001 | |||
| Never smoked | 1150 (23.5) | 12,788 (37.6) | 1672 (42.3) | |
| Former smoker | 1015 (20.8) | 11,858 (34.9) | 1775 (44.9) | |
| Current smoker | 2439 (49.9) | 8728 (25.7) | 465 (11.8) | |
| Unknown | 286 (5.8) | 644 (1.9) | 37 (0.9) | |
| Etiology of liver disease | <0.001 | |||
| HCV | 1264 (25.8) | 10,885 (32.0) | 1254 (31.8) | |
| ALD | 2286 (46.7) | 10,860 (31.9) | 995 (25.2) | |
| NAFLD | 856 (17.5) | 11,051 (32.5) | 1637 (41.5) | |
| Autoimmune | 26 (0.5) | 194 (0.6) | 24 (0.6) | |
| Other | 458 (9.4) | 1028 (3.0) | 39 (1.0) | |
| Decompensated cirrhosis | 1652 (33.8) | 10,348 (30.4) | 1381 (35.0) | <0.001 |
| Hypertension | 2608 (53.3) | 31,243 (91.8) | 3902 (98.8) | <0.001 |
| Diabetes mellitus | 1184 (24.2) | 19,309 (56.8) | 3007 (76.1) | <0.001 |
| Coronary artery disease | 378 (7.7) | 10,614 (31.2) | 1872 (47.4) | <0.001 |
| Heart failure | 312 (6.4) | 6565 (19.3) | 1024 (25.9) | <0.001 |
| MELD‐Na score | 8.0 (6.0‐13.0) | 8.0 (6.0‐12.0) | 7.0 (6.0‐12.0) | <0.001 |
| US region | <0.001 | |||
| West | 871 (17.8) | 8279 (24.3) | 431 (10.9) | |
| Midwest | 578 (11.8) | 6850 (20.1) | 667 (16.9) | |
| Northeast | 48 (1.0) | 2716 (8.0) | 2691 (68.1) | |
| South | 3393 (69.4) | 16,173 (47.5) | 160 (4.1) | |
| Rurality | <0.001 | |||
| Rural | 375 (7.7) | 1223 (3.6) | 96 (2.4) | |
| Urban | 4515 (92.3) | 32,795 (96.4) | 3853 (97.6) | |
| Received vaccination* | 2231 (45.6) | 20,589 (60.5) | 2898 (73.4) | <0.001 |
Data are provided as median (IQR) or n (%).
This represents the actual number and proportion of patients who received a vaccination in this cohort.
Discussion
In this national database of VHA patients with cirrhosis, we identified variables associated with a low probability of SARS‐CoV‐2 vaccination, including younger age, White race, current tobacco use, fewer chronic comorbidities, and residence in the southern United States and rural locations. Understanding factors associated with lack of vaccination may guide vaccine campaign efforts. Of note, Black and Hispanic patients were more likely to be vaccinated, highlighting that integrated VHA care is well suited to achieve equitable vaccine distribution.
With initial efforts to prioritize vaccination for high‐risk individuals (ie, patients in nursing homes, those aged ≥65 years, or those with high‐risk comorbidities), patients with cirrhosis of a younger age or with fewer comorbidities were less likely to be offered early vaccination. It is also possible that such patients may underestimate the risk for severe infection and thus defer vaccination. However, all patients with cirrhosis are at an increased risk of severe infection as a result of immune dysregulation.( 2 , 3 ) Communication to patients with cirrhosis regarding SARS‐CoV‐2 vaccination must emphasize the significant risk for hepatic decompensation and death from active SARS‐CoV‐2 infection.( 1 ) With the increased availability of vaccines, additional efforts are needed to identify patients who deferred vaccination or were triaged because of younger age or fewer comorbidities.
The association of active tobacco use and lack of SARS‐CoV‐2 vaccination is also important to note given the primary cause of death among patients with cirrhosis and SARS‐CoV‐2 infection is respiratory failure.( 1 ) Regional differences in vaccination status may be explained by barriers to distribution in rural areas and state differences in vaccination promotion in addition to vaccine hesitancy stemming from personal or political beliefs. National efforts to increase public health efforts in the southern United States including increased funding for health care supplies and educational materials is needed.
Study limitations include possible misclassification of outcomes if patients received vaccination outside the VHA. However, given that the VHA was among the earliest and best‐supported systems to widely offer vaccination and that we only included patients actively followed in the VHA, this misclassification is likely minimal. In addition, as we focused on VHA patients with cirrhosis, it is unclear if similar risk factors would predict an unvaccinated status in other settings.
In conclusion, we demonstrated younger age, active tobacco use, fewer medical comorbidities, and residence in the southern United States or rural locations as factors associated with a lower probability of COVID‐19 vaccination among patients with cirrhosis. Future campaign efforts may use this information to increase vaccination rates.
Supporting information
Table S1‐S2
Nadim Mahmud is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (K08‐DK124577) and by an American College of Gastroenterology Junior Faculty Development Award (ACG‐JR‐010‐2020). Marina Serper is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (K23‐DK115897‐03).
Marina Serper consults for Gilead, Inc.
SEE EDITORIAL ON PAGE 1535
References
- 1. Marjot T, Moon AM, Cook JA, Abd‐Elsalam S, Aloman C, Armstrong MJ, et al. Outcomes following SARS‐CoV‐2 infection in patients with chronic liver disease: an international registry study. J Hepatol 2021;74:567‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fix OK, Blumberg EA, Chang K‐M, Chu J, Chung RT, Goacher EK, et al. American Association for the Study of Liver Diseases expert panel consensus statement: vaccines to prevent Coronavirus Disease 2019 infection in patients with liver disease. Hepatology 2021;74:1049‐1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cornberg M, Buti M, Eberhardt CS, Grossi PA, Shouval D. EASL position paper on the use of COVID‐19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol 2021;74:944‐951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mahmud N, Hubbard RA, Kaplan DE, Serper M. Declining cirrhosis hospitalizations in the wake of the COVID‐19 pandemic: a national cohort study. Gastroenterology 2020;159:1134‐1136.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mahmud N, Kaplan DE, Goldberg DS, Taddei TH, Serper M. Changes in hepatocellular carcinoma surveillance and risk factors for noncompletion in the Veterans Health Administration cohort during the coronavirus disease 2019 pandemic. Gastroenterology 2021;160:2162‐2164.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2


