Abstract
Cytomegalovirus (CMV) is serious viral infection in allogeneic hematopoietic cell transplantation (allo HCT) recipients. November 2017, the novel CMV DNA terminase complex inhibitor letermovir was approved for prophylaxis of CMV infection in CMV-seropositive allo HCT recipients. Here, we sought to determine the effectiveness of letermovir in preventing CMV infection in CMV-seropositive patients undergoing haploidentical or mismatched adult unrelated donor allo HCT using post-transplant cyclophosphamide-based graft-versus host-disease prophylaxis. Sixty-four patients were transplanted between 2014 and 2019 of whom 32 received letermovir and 32 did not receive letermovir. The day 180 cumulative incidence of CMV infection requiring therapy was 45.3% (95% conf. interval 32.7% − 57.1%) in the entire cohort, 68.8% (48.9% - 82.2%) in the patients that did not receive letermovir, and 21.9% (9.5% − 37.6%, P < 0.001) in patients that received letermovir. Adjusting for regimen intensity, disease histology, and age, the hazard ratio for CMV infection was 0.19 (0.08 – 0.47, P < 0.001) in patients that received primary prophylaxis with letermovir. The one-year cumulative incidence of treatment related mortality was similar between patients with and without letermovir treatment (16.9% versus 18.9%) as was overall survival (64.0% versus 49.0%, respectively). Persistent CMV infection requiring >28 days of therapy was more common in patients that did not receive letermovir (31.2% versus 6.2%, P = 0.02). In summary, letermovir was effective at preventing CMV infection in this high-risk population of HLA mismatched allo HCT recipients.
Introduction
Cytomegalovirus (CMV) is a common viral infection in recipients of allogeneic hematopoietic cell transplantation (allo HCT).1,2 Untreated CMV infection results in significant morbidity and mortality in this population, necessitating the use of pre-emptive anti-viral therapies in patients with detectable viremia.3,4 CMV-active antiviral agents have significant adverse effects including myelosuppression (ganciclovir and valganciclovir), or renal injury (foscarnet), among others, leading to additive toxicities in allo HCT recipients requiring treatment for CMV.5 November 2017, the novel CMV DNA terminase inhibitor letermovir was approved for prophylactic use in CMV-seropositive allo HCT recipients on the basis of a phase 3 trial demonstrating a significant reduction in CMV infection through week 24 after allo HCT in patients taking letermovir versus placebo (18.9% vs 44.3%, P<0.001).6,7 The majority of subjects in this study received an allograft from an HLA matched donor and received standard, calcineurin inhibitor based, graft-versus-host disease (GVHD) prophylaxis.
Human leukocyte antigen mismatched adult donors, including haploidentical donors and mismatched unrelated donors (MMUD), are frequently used as allo HCT donors when combined with post-transplant cyclophosphamide (PT-Cy).8 PT-Cy results in an in vivo lymphodepletion of alloreactive donor lymphocytes, theoretically sparing quiescent, non-alloreactive donor cells.9 Clinical evidence suggests that PT-Cy results in some impact to the either the recipient or donor CMV-specific lymphocyte pool: CMV reactivation after PT-Cy based GVHD prophylaxis is approximately 10–20% more frequent when compared to recipients receiving methotrexate and calcineurin inhibitor based GVHD prophylaxis and HLA matched related or unrelated donor allografts.2,10,11
The clinical effectiveness of letermovir in higher risk populations, such as HLA mismatched donor with PT-Cy based GVHD prophylaxis, is not widely reported at this time.12 Here, we compared the CMV-specific outcomes in adult patients undergoing either related haploidentical or HLA-mismatched (≤ 7/8 matched at HLA-A, -B, -C, -DRB1) unrelated donor allo HCT with PT-Cy based GVHD prophylaxis who did and did not receive primary CMV prophylaxis with letermovir.
METHODS
Patient population, clinical CMV monitoring, and letermovir prophylaxis
Subjects were CMV-seropositive adult allo HCT recipients treated at Memorial Sloan Kettering Cancer center between 2014 – 2019 using either an HLA MMUD (≤ 7/8 matched at HLA-A, -B, -C, -DRB1) or a related haploidentical donor. All recipients received GVHD prophylaxis with PT-Cy as is previously described.13 The study was reviewed and approved by the Institutional Review Board and Privacy Board.
CMV viremia and disease were assigned according to standard guidelines.14 Plasma CMV viral load (VL) was monitored by quantitative CMV polymerase chain reaction (PCR) (COBAS® AmpliPrep /COBAS® TaqMan® CMV Test, Roche Diagnostics) according to the manufacturer’s guidelines.15 CMV blood PCR was monitored on day +5 and continued weekly until day +100, then every 1–2 weeks for months 3–6 post allo HCT. The limit of detection of this assay is 137 IU/mL and values below this limit (i.e. low-detected) were considered negative for the purposes of this analysis. Primary letermovir prophylaxis was instituted at 7 days post-allo HCT unless there were extenuating clinical circumstances. The goal duration of letermovir prophylaxis was 6 months in this and other high-risk patient populations. Each patient’s duration of letermovir prophylaxis was occasionally shorter if insurance coverage was limited to 100 days and/or for patient compliance and pill burden issues. Letermovir was discontinued and systemic anti-CMV therapy was instituted when there were 2 consecutive values of CMV VL > 300 IU/mL or a single CMV VL > 1000 IU/mL. The CMV treatment thresholds for initiation of pre-emptive therapy and monitoring frequency were the same before and after letermovir became available. Standard supportive care measures included chemoprophylaxis for herpes simplex virus, Pneumocystis jirovecii, and fungi as per institutional guidelines.4
Statistical considerations and study endpoints
The primary outcome was the proportion of patients who developed clinically significant CMV reactivation (CMV viremia requiring pre-emptive therapy or CMV disease; CS-CMVi). Persistent CMV infection was defined as detectable CMV viremia despite >28 days of anti-CMV specific therapy, excluding patients who had interrupted CMV specific therapy. The primary endpoint was evaluated by comparing the cumulative incidence of CS-CMVi in the study groups. Death or relapse were considered as competing events for this and the following endpoints as appropriate. We examined the role of clinically significant covariates using a competing risk regression framework. Overall survival (OS) was determined using the Kaplan-Meier method. Treatment-related mortality (TRM) and persistent CMV were evaluated using cumulative incidence. Wilcoxon rank sum and Fisher’s exact tests were used to compare the frequency of clinical covariates between the study groups. Analyses were performed using R version 3.6.1.
RESULTS
Patient characteristics
Baseline characteristics of the sixty-four study patients are summarized in Table 1. Complete 180-day follow-up was available for all subjects. Thirty-two CMV-seropositive patients received letermovir as primary prophylaxis. From January 2018 onward, all patients eligible to receive letermovir for primary prophylaxis were treated. The median age was 63 years (range, 26–75) and 66% (42/64) were men. Approximately half of the patients received a myeloablative preparative regimen and the majority (72%) were haploidentical T-cell replete transplants. Rates of grade II-IV acute GVHD were similar in patients who did and did not receive letermovir prophylaxis (56% vs. 66%, respectively, P = 0.387). Severe grade 3–4 acute GVHD was rare in this population. Additionally, glucocorticoid exposure ≥ 1 mg/kg prednisone or equivalent for acute GVHD treatment did not impact CS-CMVi. Clinical co-variates were similar between patients that did or did not receive letermovir prophylaxis (Table 1) except for graft source: Letermovir recipients were more likely to receive peripheral blood-derived allografts reflecting a trend towards increased use of that collection strategy over time at our center.
Table 1.
Characteristics of patients who received post-transplant cyclophosphamide as graft-versus-host disease prophylaxis
| Characteristic | Overall, N = 64 | No Letermovir, N = 32 | Letermovir, N = 32 | P value |
|---|---|---|---|---|
| Regimen | 0.6 | |||
|
| ||||
| Busulfan based | 3 (4.7%) | 1 (3.1%) | 2 (6.2%) | |
| Nonmyeloablative Fludarabine, Cyclophosphamide, TBI based |
20 (31%) | 12 (38%) | 8 (25%) | |
| Ablative – Fludarabine, TBI based | 1 (1.6%) | 0 (0%) | 1 (3.1%) | |
| Melphalan based | 40 (62%) | 19 (59%) | 21 (66%) | |
|
| ||||
| Disease histology | 0.12 | |||
|
| ||||
| Acute leukemia | 22 (34%) | 11 (34%) | 11 (34%) | |
| Lymphoid malignancy | 22 (34%) | 8 (25%) | 14 (44%) | |
| Myelodysplastic or proliferative syndrome | 16 (25%) | 9 (28%) | 7 (22%) | |
| Non-malignant diagnosis | 4 (6.2%) | 4 (12%) | 0 (0%) | |
|
| ||||
| Age | 63 (26, 75) | 65 (26, 75) | 61 (26, 75) | <0.001 |
|
| ||||
| Gender | 0.8 | |||
|
| ||||
| Female | 22 (34%) | 12 (38%) | 10 (31%) | |
| Male | 42 (66%) | 20 (62%) | 22 (69%) | |
|
| ||||
| HLA | 0.4 | |||
|
| ||||
| Related haploidentical | 46 (72%) | 25 (78%) | 21 (66%) | |
| Unrelated mismatched | 18 (28%) | 7 (22%) | 11 (34%) | |
|
| ||||
| Source | 0.024 | |||
|
| ||||
| Bone marrow derived | 34 (53%) | 22 (69%) | 12 (38%) | |
| Peripheral blood derived | 30 (47%) | 10 (31%) | 20 (62%) | |
|
| ||||
| Conditioning intensity | 0.2 | |||
|
| ||||
| Myeloablative | 35 (55%) | 20 (62%) | 15 (47%) | |
| Non-myeloablative | 18 (28%) | 6 (19%) | 12 (38%) | |
| Reduced intensity | 11 (17%) | 6 (19%) | 5 (16%) | |
|
| ||||
| CMV serostatus | 0.5 | |||
|
| ||||
| D-/R+ | 32 (50%) | 18 (56%) | 14 (44%) | |
| D+/R+ | 32 (50%) | 14 (44%) | 18 (56%) | |
Statistics presented: n (%); median (minimum, maximum)
Letermovir administration and incidence of CMV-specific outcomes
Primary letermovir prophylaxis was started at a median of 7 days (range, 5–12) after allo HCT. At initiation, 29 patients had an undetectable CMV VL, 2 patients had a detectable CMV VL <137 IU/mL, and one patient had a CMV VL of 151 IU/mL. The median duration of letermovir prophylaxis was 191 days (range, 16 to 796) with 7 patients continuing letermovir at last follow-up. Twenty-four of 32 patients (75%) continued letermovir prophylaxis beyond 14 weeks after allo HCT. Transplant characteristics did not influence the hazard for CMV infection requiring treatment in this smaller cohort (Table 2). Recipients with a diagnosis that required more intensive prior lymphodepletion, including lymphoma or non-malignant disorders, had a non-significant trend towards increased hazard for the primary endpoint (hazard ratio (HR): 2.1, 95% CI: 0.9 – 5.2, P= 0.26) when compared to recipients with a diagnosis of acute leukemia. Competing risk regressions were adjusted for covariates in this population regardless of their significance in univariate analysis.
Table 2:
Univariate associations of clinical covariates with CMV infection requiring pre-emptive therapy
| Variable | HR (95% CI) | P value |
|---|---|---|
| Age | 1.02 (0.98–1.05) | 0.35 |
|
| ||
| Letermovir | <.001 | |
|
| ||
| No | Reference | |
| Yes | 0.21 (0.09–0.49) | |
|
| ||
| Disease Histology | 0.26 | |
|
| ||
| Acute leukemia | Reference | |
| Lymphoid or non-malignant diagnosis | 2.13 (0.86–5.23) | |
| Myelodysplasia or myeloproliferative syndrome | 1.69 (0.64–4.44) | |
|
| ||
| Regimen | 0.61 | |
|
| ||
| Non-myeloablative fludarabine, cyclophosphamide, TBI-200 | Reference | |
| All others | 0.82 (0.39–1.74) | |
|
| ||
| Donor | 0.684 | |
|
| ||
| Related haploidentical | Reference | |
| Unrelated mismatched adult | 1.17 (0.54–2.53) | |
|
| ||
| Graft Source | 0.479 | |
|
| ||
| Bone marrow | Reference | |
| Peripheral blood | 0.77 (0.37–1.59) | |
|
| ||
| CMV serostatus | 0.9 | |
|
| ||
| D-/R+ | Reference | |
| D+/R+ | 1.05 (0.51–2.14) | |
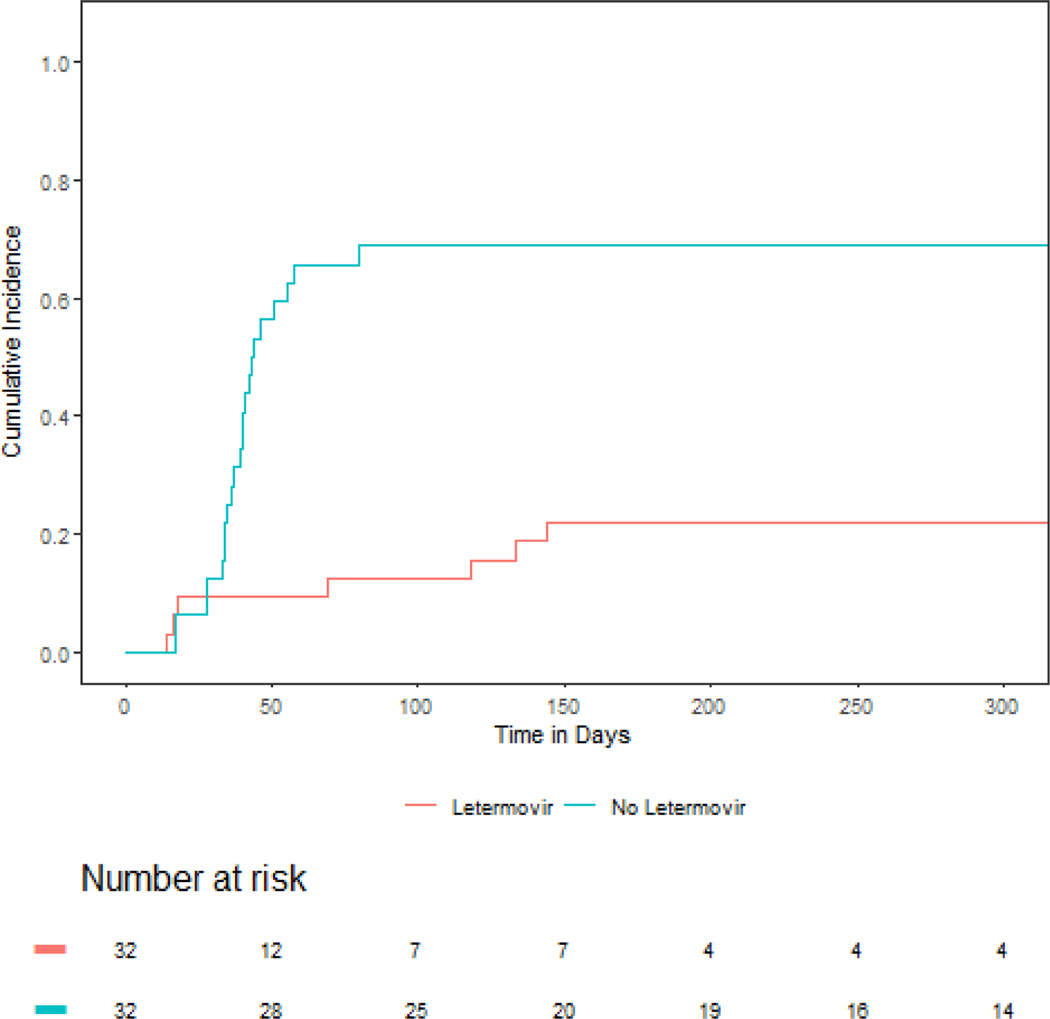
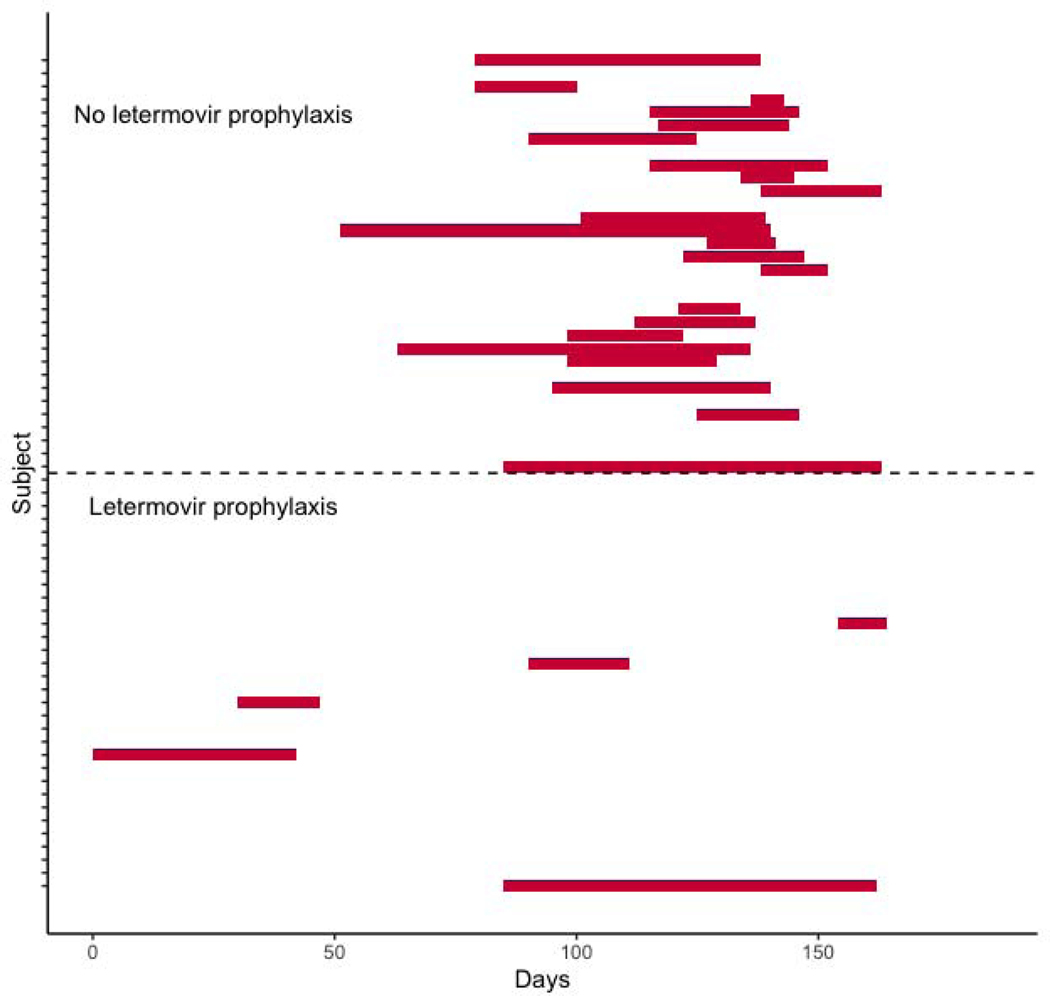
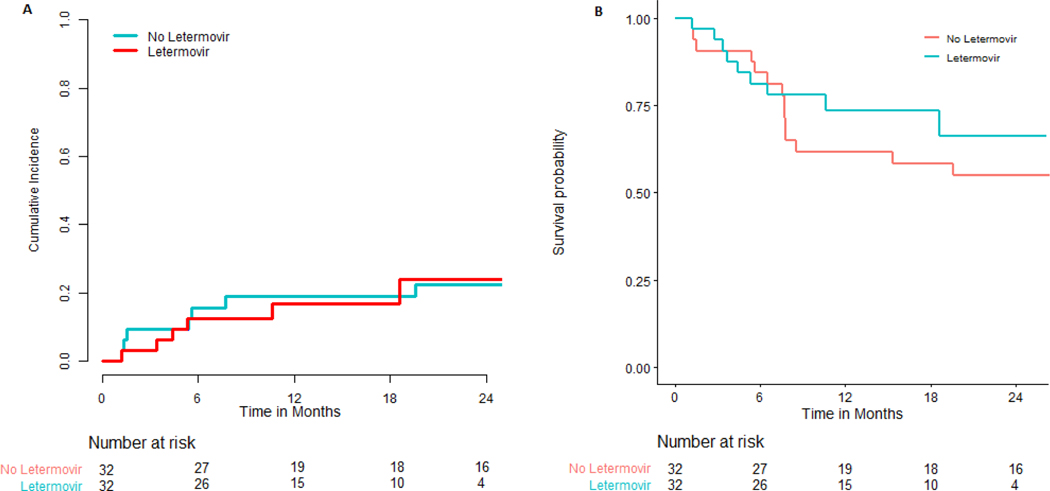
The primary endpoint of 180-day cumulative incidence of CMV infection requiring therapy in patients that did not receive letermovir prophylaxis was 68.8% (95% CI: 48.9% - 82.2%) compared with 21.9% (95% CI: 9.5% - 37.6%) in patients that did receive letermovir prophylaxis (P < 0.001), (Figure 1). Duration of CMV viremia in patients who did or did not receive letermovir is outlined in Figure 2. Overall, only seven patients who received letermovir developed CS-CMVi. Of this group, five of the patients had this occur while receiving letermovir prophylaxis. Three of these patients had additional CMV resistance testing via conventional PCR followed by genotypic sequencing (ViraCor-IBT Laboratories). There were no documented mutations (e.g. at UL56) that would have conferred letermovir resistance amongst letermovir recipients. Pre-emptive therapy was successfully administered to all seven patients with either valganciclovir or foscarnet (figure 2). There was no difference in overall-survival (OS) or TRM in patients that did or did not receive letermovir (Figure 3).
Figure 1:
Cumulative incidence of clinically significant CMV infection in patients that received letermovir primary prophylaxis (red) versus patients that did not receive primary prophylaxis (blue).
Figure 2:
Duration of anti-viral therapy in patients that received or did not receive letermovir. The red bars indicate the time of CMV specific pre-emptive or therapeutic therapy administration relative to the infusion of the allograft stem cell product on day 0.
Figure 3:
Cumulative incidence of treatment-related mortality (A) and Kaplan-Meier estimates of overall survival (B) in subjects.
Late CMV infection, persistent CMV viremia, and end-organ disease
Of the three patients who had a detectable or quantifiable CMV VL at the time of letermovir initiation, two patients required pre-emptive therapy for CMV viremia. Notably three patients had CS-CMVi occur in the period beyond 14-weeks after allo HCT but two of the patients had discontinued letermovir before CS-CMVi occurred. Overall, only 1 of 24 (4.2%) developed CS-CMVi if they remained on letermovir beyond the 14-week mark after allo HCT.
In this population, the cumulative incidence of persistent CMV in recipients that did not receive letermovir prophylaxis was 31.2% (95% CI: 16.1% − 47.7%) compared to 6.2% (95% CI: 1.1% − 18.4%, P = 0.02). Only two documented cases of CMV end-organ disease were described in this population (one patient treated with letermovir prophylaxis developed pneumonitis after discontinuing letermovir and one patient developed CMV colitis without letermovir prophylaxis).
DISCUSSION
In this study we demonstrate that primary letermovir prophylaxis was effective in preventing CS-CMVi in CMV-seropositive recipients of allo HCT from haploidentical or MMUD who received PT-Cy as GVHD prophylaxis. The cumulative incidence of CMV reactivation in recipients of haploidentical donor allo HCT with PT-Cy based GVHD prophylaxis is 50–80%.16–19 These results are similar to other high risk allo HCT recipients including those undergoing T-cell depletion or receiving HLA mismatched umbilical cord blood allografts.20,21 In this study, we observed a similar rate of CMV reactivation in patients that did not receive letermovir, whereas the cumulative incidence of CS-CMVi in letermovir recipients was significantly decreased. In this population, letermovir was well tolerated as there were no discontinuations due to treatment-emergent adverse events. Of note, persistent CMV infection was significantly reduced with utilization of letermovir prophylaxis. We and others have previously described persistent CMV infection (detectable CMV viremia for greater than 28 days despite optimal therapy) as a significant risk factor for TRM.22 Our findings are also in line with another large, 2020 report highlighting the efficacy of letermovir in high risk populations (e.g. umbilical cord blood and haploidentical allo HCT recipients).23 Overall, these results further suggest that letermovir reduces the incidence of CMV related complications in patients who are at high risk for CMV specific complications.
The optimal duration of letermovir prophylaxis, especially in higher risk allograft recipients, remains an unanswered question. This is of interest when considering high-risk populations such as presented here. At 14-weeks post allo HCT the patients who received letermovir prophylaxis had a similar incidence of CS-CMVi compared to the registration trial at 12.5% (4/32) vs. 7.7%, respectively. Marty et al. noted additional post-prophylactic CMV events starting around week 18, likely representing ongoing or new periods of CMV risk beyond day + 100.7 In this population, late CS-CMVi was rare if letermovir prophylaxis was continued beyond 14 weeks, occurring in only one patient (4.2%). These findings support the use of prolonged letermovir primary prophylaxis. There is some concern that letermovir prophylaxis of any duration only delays CS-CMVi until after prophylaxis is completed. An ongoing randomized clinical trial to evaluate efficacy and safety of letermovir prophylaxis when extended to 200 days after alloHCT (NCT03930615) should provide further data in this regard as there are key secondary endpoints evaluating the effect of extended prophylaxis at 38- and 48-weeks post allo HCT. Additionally, there is value in preventing CS-CMVi from occurring at all. CMV reactivation is associated with an increased risk of invasive fungal infections.24 Another report indicates letermovir may reduce mortality by preventing or delaying CS-CMVi in HCT recipients.25 Our study size may have been too small to capture this effect.
There are some imitations inherent to the retrospective and observational nature of our study. The sample size was relatively small. Since this was a real‐world study, we relied on patient reports of adherence. While acknowledging these limitations, our data provide real‐world data of the effectiveness of letermovir in a population at high-risk of CMV reactivation and complications. Haploidentical allo HCT recipients only comprised 16% of the registry trial, but this study triples the number of patients in this high-risk group.20 Additional studies are needed to quantify the impact of letermovir on the reduction of days and toxicity of pre-emptive therapy, readmissions, hospital length-of-stay, and overall long‐term survival in these and other high‐risk patients.
In summary we found that letermovir resulted in a significant reduction in the need for CMV specific therapy but did not impact TRM or OS in this population. These results support the efficacy of letermovir for CMV prevention for the first 14 weeks in CMV-seropositive adult recipients of allo HCT and continued efficacy when given beyond 14 weeks in HLA mismatched allograft recipients using PT-Cy based GVHD prophylaxis. Results of a larger trial using prolonged letermovir use in this population are necessary to determine the standard of care in HLA mismatched allo HCT recipients.
Acknowledgements
The authors are grateful for the following funding support: Shaffer: NIH/NHLBI K23 HL140134–01A1, NIH/NCATS UL1-TR-002384; all authors: NIH/NCI Cancer Center Support Grant P30 CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial Disclosures:
SAG serves as a member of Advisory Board for Amgen, Actinuum, Celgene, Johnson &Johnson, Jazz Pharmaceutical, Takeda, Novartis, KITE, and SPECTRUM Pharma. He has received research funding from Amgen, Actinuum, Celgene, Johnson & Johnson, Miltenyi Biotec, and Takeda.
MAP reports honoraria from Abbvie, Bellicum, Bristol-Myers Squibb, Incyte, Merck, Novartis, Nektar Therapeutics, and Takeda. He serves on DSMBs for Servier and Medigene, and the scientific advisory boards of MolMed and NexImmune. He has received research support for clinical trials from Incyte, Kite (Gilead), and Miltenyi Biotec. He serves in a volunteer capacity as a member of the Board of Directors of American Society of Transplantation and Cellular Therapy (ASTCT) and Be the Match (National Marrow Donor Program, NMDP), as well as on the CIBMTR Advisory Committee and Cellular Immunotherapy Data Resource (CIDR) Oversight Committee.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Green ML, Leisenring WM, Xie H, et al. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood. 2013;122(7):1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teira P, Battiwalla M, Ramanathan M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127(20):2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ljungman P, Brand R, Hoek J, et al. Donor cytomegalovirus status influences the outcome of allogeneic stem cell transplant: a study by the European group for blood and marrow transplantation. Clin Infect Dis. 2014;59(4):473–481. [DOI] [PubMed] [Google Scholar]
- 4.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen K, Cheng MP, Hammond SP, Einsele H, Marty FM. Antiviral prophylaxis for cytomegalovirus infection in allogeneic hematopoietic cell transplantation. Blood Advances. 2018;2(16):2159–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Databases USFDADAa . PREVYMIS (letermovir) Tablets and PREVYMIS (letermovir) Injection. [Google Scholar]
- 7.Marty FM, Ljungman P, Chemaly RF, et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N Engl J Med. 2017;377(25):2433–2444. [DOI] [PubMed] [Google Scholar]
- 8.Passweg JR, Baldomero H, Basak GW, et al. The EBMT activity survey report 2017: a focus on allogeneic HCT for nonmalignant indications and on the use of non-HCT cell therapies. Bone Marrow Transplantation. 2019;54(10):1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wachsmuth LP, Patterson MT, Eckhaus MA, Venzon DJ, Gress RE, Kanakry CG. Posttransplantation cyclophosphamide prevents graft-versus-host disease by inducing alloreactive T cell dysfunction and suppression. The Journal of Clinical Investigation. 2019;129(6):2357–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldsmith SR, Slade M, DiPersio JF, et al. Cytomegalovirus viremia, disease, and impact on relapse in T-cell replete peripheral blood haploidentical hematopoietic cell transplantation with post-transplant cyclophosphamide. Haematologica. 2016;101(11):e465–e468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.. Crocchiolo R, Bramanti S, Vai A, et al. Infections after T-replete haploidentical transplantation and high-dose cyclophosphamide as graft-versus-host disease prophylaxis. Transpl Infect Dis. 2015;17(2):242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel OS, Thomas E, Ganguly S, et al. Letermovir for Primary Cytomegalovirus Prevention in Haploidentical Stem Cell Transplant Recipients. Biol Blood Marrow Transplant. 2020;26(3):S336. [Google Scholar]
- 13.McCurdy SR, Luznik L. How we perform haploidentical stem cell transplantation with posttransplant cyclophosphamide. Blood. 2019;134(21):1802–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34(8):1094–1097. [DOI] [PubMed] [Google Scholar]
- 15.Babady NE, Cheng C, Cumberbatch E, Stiles J, Papanicolaou G, Tang YW. Monitoring of cytomegalovirus viral loads by two molecular assays in whole-blood and plasma samples from hematopoietic stem cell transplant recipients. J Clin Microbiol. 2015;53(4):1252–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huntley D, Giménez E, Pascual MJ, et al. Incidence, features, and outcomes of cytomegalovirus DNAemia in unmanipulated haploidentical allogeneic hematopoietic stem cell transplantation with post-transplantation cyclophosphamide. Transpl Infect Dis. 2020;22(1):e13206. [DOI] [PubMed] [Google Scholar]
- 17.Hammerstrom AE, Lombardi LR, Pingali SR, et al. Prevention of Cytomegalovirus Reactivation in Haploidentical Stem Cell Transplantation. Biol Blood Marrow Transplant. 2018;24(2):353–358. [DOI] [PubMed] [Google Scholar]
- 18.Crocchiolo R, Bramanti S, Vai A, et al. Infections after T-replete haploidentical transplantation and high-dose cyclophosphamide as graft-versus-host disease prophylaxis. Transpl Infect Dis. 2015;17(2):242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Law AD, Salas MQ, Lam W, et al. Reduced-Intensity Conditioning and Dual T Lymphocyte Suppression with Antithymocyte Globulin and Post-Transplant Cyclophosphamide as Graft-versus-Host Disease Prophylaxis in Haploidentical Hematopoietic Stem Cell Transplants for Hematological Malignancies. Biol Blood Marrow Transplant. 2018;24(11):2259–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin A, Maloy M, Su Y, et al. Letermovir for primary and secondary cytomegalovirus prevention in allogeneic hematopoietic cell transplant recipients: Real-world experience. Transpl Infect Dis. 2019;21(6):e13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma P, Gakhar N, MacDonald J, et al. Letermovir prophylaxis through day 100 post transplant is safe and effective compared with alternative CMV prophylaxis strategies following adult cord blood and haploidentical cord blood transplantation. Bone Marrow Transplant. 2020;55(4):780–786. [DOI] [PubMed] [Google Scholar]
- 22.Huang YT, Neofytos D, Foldi J, et al. Cytomegalovirus Infection after CD34(+)-Selected Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2016;22(8):1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnsrud JJ, Ngyuen IT, Domingo W, et al. Letermovir Prophylaxis Decreases Burden of Cytomegalovirus (CMV) in Patients at High Risk for CMV Disease Following Hematopoietic Cell Transplant. Biol Blood Marrow Transplant. 2020; S1083–8791(20):30411–0. [DOI] [PubMed] [Google Scholar]
- 24.Yong MK, Ananda-Rajah M, Cameron PU, et al. Cytomegalovirus Reactivation Is Associated with Increased Risk of Late-Onset Invasive Fungal Disease after Allogeneic Hematopoietic Stem Cell Transplantation: A Multicenter Study in the Current Era of Viral Load Monitoring. Biol Blood Marrow Transplant. 2017;23(11):1961–1967. [DOI] [PubMed] [Google Scholar]
- 25.Ljungman P, Schmitt M, Marty FM, et al. A Mortality Analysis of Letermovir Prophylaxis for Cytomegalovirus (CMV) in CMV-seropositive Recipients of Allogeneic Hematopoietic Cell Transplantation. Clin Infect Dis. 2020;70(8):1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]