To the Editor,
A 45‐year‐old woman, without any past medical history or allergy presented in our clinic with a rapid onset of diffuse skin eruptions. Five days earlier, she received the first injection of the SARS‐CoV‐2 Pfizer‐BioNTech mRNA. Concomitantly, she took 1000mg paracetamol to prevent any post‐vaccination syndrome. She well tolerated the preceding vaccines (influenza every year) before this one.
The eruption started 24 hours after vaccine injection and was composed at time of the clinical examination of erythematous infiltrated papulosis located all over the body, without face involvement (Figure 1). No other extracutaneous symptoms were noted. Blood examinations showed increased blood count levels with increased neutrophils count (8.77 G/L), hepatic cytolysis (AST 67 UI/L and ALT 116 UI/L) with high level of PCR (115 mg/L). SARS‐CoV‐2 PCR test and serology were negative. Viral tests for EBV, CMV, parvovirus B19, and Herpes simplex/Herpes zoster showed only a slight EBV reactivation. Additional examinations ruled out infectious disease, neoplastic lesion, autoimmune, or inflammatory disease. Histopathological examination of the skin biopsy showed a hyperplastic epidermis with an edematous papillary dermis. A superficial and deep dermal perivascular, periadnexal and interstitial dense infiltrate composed of neutrophils, eosinophils and lymphocytes was also a feature. Leukocytoclastic vasculitis was also seen (Figure 2A‐B). Clinical and pathological examinations were compatible with the diagnosis of SS induced by SARS‐CoV‐2 Pfizer‐BioNTech mRNA vaccine. Systemic steroid therapy (prednisone 0.5 mg/kg/d) for five days was started and led to rapid improvement of the skin condition without any recurrence after treatment discontinuation. She did not receive the second vaccine injection.
FIGURE 1.
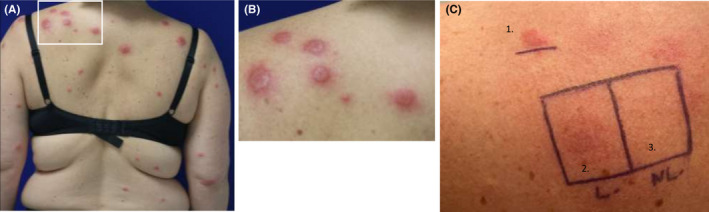
Sweet syndrome lesions (A) First localization appeared 24 h after the vaccine injection on the back. (B) Erythematous papulosis on the left shoulder. (C) (1) Cutaneous tests: Positive IDR with SARS‐CoV‐2 Pfizer‐BioNTech mRNA vaccine diluted to 1/10e at day 5. (2) Patch test with pur SARS‐CoV‐2 Pfizer‐BioNTech mRNA vaccine in healed skin. (3) Patch test with pur SARS‐CoV‐2 Pfizer‐BioNTech mRNA vaccine in normal skin
FIGURE 2.
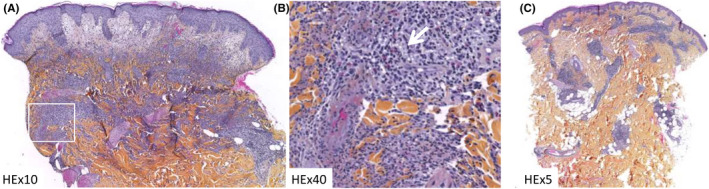
A‐ HEx5: papillary dermal edema, interstitial, perivascular and periadnexal superficial and deep dermal polymorphous inflammatory infiltrate. (B) HEx20: leucocytoclastic vasculitis, eosinophils (white arrow), perivascular lymphocytes, and interstitial neutrophils. (C) HEx3: perivascular and periadnexal superficial and deep lymphocytic infiltrate
Patch tests performed, 14 days after discontinuation of steroid treatment and one month after SS, both on healed and normal skin with pure SARS‐CoV‐2 Pfizer‐BioNTech mRNA vaccine prepared less than 4 hours in 0.9% saline before, were negative at 72 hours and 5 days reading (Figure 1C, 2–3). Prick tests with polyethylene glycol (PEG) 3350 and SARS‐CoV‐2 Pfizer‐BioNTech mRNA vaccine were negative at immediate and delayed readings (24 hours, 72 hours, and 5 days). Then, intradermal test (IDT) with vaccine diluted at 1/10 in 0.9% saline on normal skin was considered positive in delayed reading (Figure 1C, 1) according to the guidelines of delayed reading of IDT in cutaneous reaction. 1 The immediate reading at 20 minutes was negative, the delayed readings were all positive. Cutaneous biopsy was realized on the positive IDT reaction, at day 5, showing an abundant inflammatory infiltrate predominantly with lymphocytes (Figure 2C).
Cutaneous reactions after vaccine injection are rare and heterogenous. 2 They could be related to the vaccine or the adjuvant. In addition, vaccine could trigger flares of chronic inflammatory conditions as it was previously reported. 1 At that time, minor local side effects are reported with SARS‐CoV‐2 vaccines such as pain, swelling, or redness; hypersensitivity reactions were anaphylactic reaction but no severe delayed hypersensitivity is reported. 3 , 4 Three cases of acute febrile neutrophilic dermatosis are reported in the international bank of WHO, one in the United Kingdom, one in the United States of America, and our case. Under SARS‐CoV‐2 Pfizer‐BioNTech mRNA vaccine, four cases of vasculitis had been reported after injection. In France, one case of relapse of neutrophilic disorder was reported one day after SARS‐CoV‐2 Pfizer‐BioNTech mRNA vaccine. The adjuvant associated with the SARS‐CoV‐2 Pfizer‐BioNTech mRNA vaccine is polyethylene glycol (PEG) 2000. 4 However, our patient never received infusion containing PEG or polysorbate before. Patch tests with PEG or polysorbate alone were not performed because of the negativity of the patch test with the SARS‐CoV‐2 Pfizer‐BioNTech mRNA vaccine. Recently, it has been suggested that IDT at 1/1000 and 1/100 dilutions with the SARS‐Cov‐2 Pfizer‐BioNtech mRNA vaccine could be positive in healthy volunteers having received this vaccine while these tests remain negative in non‐immunized patients. 5 Therefore, we cannot rule out the possibility that our positive reaction is the consequence of a local immune response to the vaccine in this already immunized patient. IDT with PEG alone could not be performed because it was not a sterile compound so prick tests were undertaken with PEG 3350 and the SARS‐CoV‐2 Pfizer‐BioNTech mRNA vaccine. Only 10 cases of SS induced by vaccine are published so far including 3 with seasonal influenza, 1 with influenza A, 2 with pneumococcal, 2 with tuberculosis, and 2 with small pox. 6 SS is an acute inflammatory skin disease associated with important infiltration of neutrophils. Leukocytoclastic vasculitis could be present in SS. 7 One case of SS in a patient receiving pneumococcal vaccine showed the presence of dermal vasculitis associated with infiltration of neutrophils. 6
Most cases of cutaneous reaction under SARS‐CoV‐2 Pfizer‐BioNTech mRNA vaccine do not constitute a contraindication to a second injection. 3 To our knowledge, any case of SS induced by vaccine was re‐challenged with the same vaccine. To date, the second SARS‐CoV‐2 Pfizer‐BioNtech mRNA vaccine injection was not performed because of patient's refusal.
To conclude, we report the first case of SS induced by SARS‐CoV‐2 Pfizer‐BioNTech mRNA vaccine.
ACKNOWLEDGEMENT
The authors acknowledge the Uppsala Monitoring Centre (UMC) which provided some data presented in the present study. The opinions and conclusions in this study are not necessarily those of the WHO.
Funding information
No external funding for this manuscript.
REFERENCES
- 1. Barbaud A, Gonçalo M, Bruynzeel D, Bircher A. European Society of Contact Dermatitis. Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions. Contact Dermatitis. 2001;45(6):321‐328. [DOI] [PubMed] [Google Scholar]
- 2. Rosenblatt AE, Stein SL. Cutaneous reactions to vaccinations. Clin Dermatol. 2015;33(3):327‐332. [DOI] [PubMed] [Google Scholar]
- 3. Corbeddu M, Diociaiuti A, Vinci MR, et al. Transient cutaneous manifestations after administration of Pfizer‐BioNTech COVID‐19 Vaccine: an Italian single centre case series. J Eur Acad Dermatol Venereol. 2021. 10.1111/jdv.17268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Banerji A, Wickner PG, Saff R, et al. mRNA Vaccines to Prevent COVID‐19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach. J Allergy Clin Immunol Pract. 2020;S2213–2198(20):31411‐31412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bianchi L, Biondi F, Hansel K, Murgia N, Tramontana M, Stingeni L. Skin tests in urticaria/angioedema and flushing to Pfizer‐BioNTech SARS‐CoV‐2 vaccine: Limits of intradermal testing. Allergy. 2021;76:2605‐2607. 10.1111/all.14839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pedrosa AF, Morais P, Nogueira A, Pardal J, Azevedo F. Sweet's syndrome triggered by pneumococcal vaccination. Cutan Ocul Toxicol. 2013;32(3):260‐261. [DOI] [PubMed] [Google Scholar]
- 7. Ratzinger G, Burgdorf W, Zelger BG, Zelger B. Acute febrile neutrophilic dermatosis: a histopathologic study of 31 cases with review of literature. Am J Dermatopathol. 2007;29(2):125‐133. [DOI] [PubMed] [Google Scholar]


