Abstract
Objective
To study the effect of delivery on the pO2/FiO2 ratio (P/F ratio) in patients with COVID‐19‐related acute respiratory distress syndrome (ARDS) and to compare characteristics between delivered and undelivered pregnant patients with COVID‐19.
Design
Retrospective cohort.
Setting
Four hospitals in Houston, Texas.
Population
Pregnant patients admitted to the hospital for COVID‐19.
Methods
Among patients with ARDS who were delivered during their hospitalisation for COVID‐19, linear mixed models were used to investigate time trends before and after delivery of the P/F ratio. Patient characteristics were compared between patients delivered during their hospitalisation for COVID‐19 and those discharged undelivered.
Main outcome measures
The P/F ratio, age, gestational age, length of stay and severity of illness,
Results
Between 4 May 2020 and 26 July 2020, a total of 61 pregnant patients were admitted for COVID‐19. Baseline characteristics were similar between the study groups. Delivery occurred in 21 (34%) of patients during their hospitalisation for COVID‐19. Delivered patients had more severe disease and were admitted at a later gestational age than patients not delivered. Ten of these 21 patients (48%) were delivered preterm; of these, six were delivered due to complications of COVID‐19 and four were delivered for obstetric indications. In patients with ARDS who were delivered (n = 17), the P/F ratio had a negative slope that improved after delivery.
Conclusions
COVID‐19‐related ARDS in pregnancy requires multidisciplinary care and individualised decision‐making, but delivery slows the deterioration of the P/F ratio in these patients.
Tweetable abstract
Delivery improves the P/F ratio in COVID‐19‐related ARDS, though individualised delivery management is needed.
Keywords: ARDS, coronavirus, COVID‐19, critical care, delivery, pregnancy, respiratory distress, SARS‐CoV‐2
Tweetable abstract
Delivery improves the P/F ratio in COVID‐19‐related ARDS, though individualised delivery management is needed.
Introduction
Pregnant people with Coronavirus Disease 2019 (COVID‐19) are more likely to be hospitalised and admitted to the intensive care unit (ICU) than are their non‐pregnant counterparts, though rates of mechanical ventilation are similar. 1 , 2 Decisions regarding necessity and timing of delivery are a unique consideration of pregnant women with COVID‐19 infection. In several case series of pregnant patients with COVID‐19, 39–54% percent of patients were delivered during the initial hospitalisation, and 24–57% were delivered preterm. 3 , 4 , 5
One of the major causes of morbidity and mortality in COVID‐19 is acute respiratory distress syndrome (ARDS). 6 The case mortality for ARDS in pregnancy is between 9 and 14%. 7 Pregnant patients with COVID‐19‐related ARDS are likely to be delivered for maternal indications. 8 However, whether delivery improves maternal survival and reduces morbidity in ARDS is unknown.
Small case series show improvement in some, but not all, respiratory parameters with delivery in ARDS during pregnancy and no clear recommendation for delivery criteria has emerged. 9 , 10 , 11 , 12 The pO2/FiO2 ratio (P/F ratio) is a ratio of the arterial partial pressure of oxygen to the fraction of inspired oxygen, thus lower values represent worse gas exchange. It is both part of the definition of ARDS (P/F ratio < 300) and defines the severity of ARDS. The P/F ratio is slightly lower in pregnant people than in non‐pregnant people and has been used in this population. 13 A recent study examined respiratory parameters in ten pregnant patients with ARDS (n = 6), pulmonary oedema (n = 3), septic shock (n = 1) or neurological disease (n = 1) delivered while on mechanical ventilatory support and found that oxygenation index and PEEP showed significant improvements from antepartum to 12–15 hours post‐delivery. 14 Though the mean P/F ratio did not change, in both patients with P/F ratios < 100, the oxygenation index improved by greater than 40%. However, changes in the P/F ratio around the time of delivery have not been examined in COVID‐19‐related ARDS. The objective of this study is to examine whether delivery affects time to recovery in pregnant patients with COVID‐19 and COVID‐19‐related ARDS. Our hypothesis is that delivery improves time to recovery in this population.
Methods
This was a retrospective cohort study of all hospitalised pregnant patients with laboratory‐confirmed SARS‐CoV‐2 infection and COVID‐19 admitted to one of four large metropolitan hospitals staffed by one university physician group between 4 May 2020 and 26 July 2020. Patients with asymptomatic SARS‐CoV‐2 infection were not included. The primary exposure was delivery during hospitalisation for COVID‐19. Criteria for admission and delivery were at the discretion of the attending physician. Patients were divided into four groups based on the severity of COVID‐19: mild, moderate, severe or critical. 15 Mild COVID‐19 was defined as symptomatic patients (fever, cough, fatigue, anorexia, shortness of breath, myalgias) without evidence of viral pneumonia or hypoxia. Moderate COVID‐19 was defined as clinical signs of pneumonia (fever, cough, dyspnoea, fast breathing) but no signs of severe pneumonia, including oxygen saturation (SpO2) ≥ 90% on room air. Severe COVID‐19 was defined as clinical signs of pneumonia plus one of the following: respiratory rate > 30 breaths/min; severe respiratory distress; or SpO2 < 90% on room air. Critical COVID‐19 was defined as ARDS (P/F ratio ≤ 300 mmHg), sepsis or septic shock. 15 When blood gases were unavailable, SpO2 was converted to pO2 using an oxyhaemoglobin dissociation curve. Among delivered people with ARDS, the lowest P/F ratio was computed for each day and for the 12 hours before and after delivery. Respiratory specimens were collected by nasopharyngeal swab and SARS‐CoV‐2 infection confirmed by reverse‐transcription polymerase chain reaction (RT‐PCR).
Demographic and clinical characteristics were compared between the group of patients delivered during their hospitalisation for COVID‐19 and those discharged undelivered using Chi‐square, Fisher exact and Wilcoxon tests as appropriate. Primary outcomes were length of stay and P/F ratios. Length of stay was compared between delivered and undelivered patients. Among patients with ARDS who were delivered during their hospitalisation for COVID‐19, linear mixed models were used to investigate time trends of the P/F ratio before and after delivery. For each hospital day, the lowest P/F ratio was used. The model included gestational age at delivery, a time variable (day), an indicator variable for before and after delivery, and their interaction as covariates. Random intercepts and slopes were included in the model to adjust for within patient correlation. An equal number of observations (days) was selected before and after delivery because most patients had more observations after delivery than before, and all but one were discharged home on room air. These models allow us to estimate the change (slope) in the outcome before delivery and after and evaluate whether these slopes are different.
Approval was obtained from the Committee for the Protection of Human Subjects at the University of Texas Health Science Center at Houston. The STROBE guidelines for reporting observational studies in epidemiology were followed in this manuscript. 16
Results
During the study period, 386 women with COVID 19 infection were treated at our four hospitals, of whom 61 (15.8%) met criteria for inclusion in our study. Twenty‐one (34.4%) patients were delivered during their hospitalisation for COVID‐19. Delivered patients had more severe disease and were admitted at a later gestational age than were patients not delivered (Table 1). Length of stay was significantly longer for patients delivered during their hospitalisation (12 days, interquartile range 8–39) compared with those not delivered (6 days, interquartile range 2–9, P = 0.029). Ten patients (48%) were delivered preterm: six delivered due to complications of COVID‐19 and four delivered for obstetric indications (Table 2).
Table 1.
Comparison of patient characteristics by delivery status
|
Not delivered (n = 40) |
Delivered (n = 21) |
P‐value | |
|---|---|---|---|
| Maternal age | 29 (26–32) | 28 (24–35) | 0.982 |
| Race/ethnicity | |||
| Hispanic | 22 (55%) | 13 (62%) | 0.158 |
| Non‐Hispanic black | 9 (23%) | 1 (5%) | |
| Non‐Hispanic white | 5 (13%) | 3 (14%) | |
| Non‐Hispanic Asian | 0 | 2 (10%) | |
| Other or unknown | 4 (10%) | 2 (10%) | |
| Nulliparous | 7 (18%) | 6 (29%) | 0.316 |
| Twin pregnancy | 0 | 2 (10%) | 0.115 |
| Insurance | |||
| Government‐assisted | 26 (65%) | 2 (10%) | 0.555 |
| Private | 8 (20%) | 16 (76%) | |
| Self‐pay/uninsured | 6 (15%) | 3 (14%) | |
| Comorbidities | |||
| Obesity* | 30 (75%) | 18 (86%) | 0.332 |
| Hypertensive disease | 6 (15%) | 0 | 0.085 |
| Diabetes mellitus | 6 (15%) | 1 (5%) | 0.405 |
| Asthma | 6 (15%) | 3 (14%) | 0.940 |
| Gestational age at admission | 28 (25–29) | 37 (33–38) | <0.001 |
| Gestational age at delivery | – | 38 (34–39) | – |
| Severity of illness | |||
| Mild | 2 (5%) | 0 | 0.030 |
| Moderate | 15 (37%) | 3 (14%) | |
| Severe | 4 (10%) | 0 | |
| Critical | 19 (48%) | 18 (86%) | |
| Acute respiratory distress syndrome (ARDS) | 17 (43%) | 18 (86%) | 0.001 |
| Reason for delivery | |||
| COVID‐19 | — | 9 (43%) | — |
| Labour or scheduled caesarean | — | 6 (29%) | — |
| Pre‐eclampsia with severe features | — | 1 (5%) | — |
| Acute fatty liver of pregnancy | — | 1 (5%) | — |
| Transaminitis | — | 1 (5%) | — |
| Cholestasis of pregnancy | — | 1 (5%) | — |
| Oligohydramnios | — | 1 (5%) | — |
| Perimortem | — | 1 (5%) | — |
| Length of stay | 6 (2–9) | 12 (8–39) | 0.029 |
Data are presented as median (interquartile range) or n (%).
*Body mass index ≥ 30 m/kg2.
Bold indicates statistical significance between groups with P < 0.05.
Table 2.
Details of patients delivered during hospitalisation for COVID‐19
| Patient number | Severity of COVID‐19 | Age (years) | Gestational age at delivery (weeks + days) | Hospital day at delivery | Reason for delivery | Mode of delivery | Respiratory support at the time of delivery | Comorbidities | Complications | Length of postpartum hospital stay (days) | Lowest P/F ratio* |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1** | Moderate | 33 | 34+5 | 2 | Acute fatty liver of pregnancy, monochorionic‐diamniotic twins | Caesarean | None | Obesity | Postpartum endometritis, ileus | 5 | 310 |
| 2** | Moderate | 33 | 38+4 | 2 | Cholestasis of pregnancy | Vaginal | None | Obesity, asthma, late latent syphilis | None | 6 | 329 |
| 3** | Moderate | 31 | 28+5 | 1 | Pre‐eclampsia with severe features | Caesarean | None | Obesity | None | 5 | 307 |
| 4 | Critical | 33 | 37+2 | 3 | Labor, dichoronic‐diamniotic twins | Caesarean | None | Obesity | None | 13 | 60 |
| 5 | Critical | 27 | 38+0 | 5 | Labor | Caesarean | Nasal cannula | None | None | 2 | 164 |
| 6 | Critical | 36 | 38+0 | 2 | Labor | Vaginal | None | Obesity, lupus, bipolar disorder | Disseminated intravascular coagulopathy, postpartum hemorrhage | 5 | 96 |
| 7 | Critical | 27 | 38+1 | 2 | Labor, non‐reassuring fetal heart rate tracing | Caesarean | None | Obesity | None | 7 | 172 |
| 8 | Critical | 26 | 38+4 | 4 | Labor | Vaginal | Nasal cannula | None | None | 6 | 172 |
| 9 | Critical | 23 | 39+4 | 1 | Labor | Vaginal | Nasal cannula | Obesity | None | 2 | 246 |
| 10 | Critical | 39 | 39+2 | 1 | Scheduled repeat caesarean | Caesarean | None | Obesity | None | 4 | 216 |
| 11 | Critical | 32 | 36+2 | 2 | Oligohydramnios | Vaginal | Nasal cannula | Obesity | None | 3 | 181 |
| 12 | Critical | 23 | 33+3 | 7 | Transaminitis, fetal growth restriction, oligohydramnios | Vaginal | Nasal cannula | Obesity, asthma | None | 3 | 112 |
| 13 | Critical | 31 | 27+2 | 4 | Respiratory failure | Caesarean | Mechanical ventilation | Obesity | Respiratory failure | 39 | 55 |
| 14 | Critical | 31 | 31+0 | 4 | Respiratory failure | Caesarean | Nasal cannula | Obesity | Respiratory failure | 6 | 69 |
| 15*** | Critical | 18 | 31+5 | 8 | Respiratory failure | Caesarean | Mechanical ventilation | Obesity | Respiratory failure, sepsis, renal failure, liver failure, trachea‐esophageal fistula | 293 | 27 |
| 16 | Critical | 23 | 34+3 | 4 | Respiratory failure | Caesarean | Nasal cannula | Obesity | Respiratory failure, deep vein thrombosis | 3 | 132 |
| 17 | Critical | 38 | 35+5 | 2 | Respiratory failure | Caesarean | Non‐invasive mechanical ventilation | Obesity, severe asthma | Respiratory failure | 38 | 37 |
| 18 | Critical | 23 | 37+0 | 3 | Respiratory failure | Caesarean | Nasal cannula | Obesity | Respiratory failure | 5 | 106 |
| 19 | Critical | 35 | 38+0 | 2 | COVID‐19 pneumonia | Vaginal | Non‐rebreather | Obesity | Respiratory failure | 11 | 73 |
| 20 | Critical | 22 | 40+1 | 2 | Respiratory failure, non‐reassuring fetal heart rate tracing | Caesarean | None | None | Respiratory failure | 8 | 78 |
| 21***,**** | Critical | 39 | 27+1 | 3 | Perimortem | Caesarean | None*** | Obesity, diabetes | Respiratory failure with self‐extubation and cardiopulmonary arrest | 0 | 133 |
*pO2/FiO2 ratio.
**Not included in P/F ratio analysis as there was no adult respiratory distress syndrome.
***No respiratory support at the time of delivery secondary to self‐extubation.
****Not included in P/F ratio analysis due to perimortem caesarean delivery with no postpartum P/F ratios.
Among the delivered patients, 17 had adequate data for inclusion in the P/F ratio analysis (Figure 1). The slope of the P/F ratio before delivery is negative (−28.7; 95% CI −44.8 to −12.6), indicating a decrease in the P/F ratio across time. The slope after delivery was also negative but less steep (−1.02; 95% CI −18.9 to 16.8), indicating less of a decrease in the P/F ratio across time after delivery (P < 0.0001 for difference in slopes).
Figure 1.
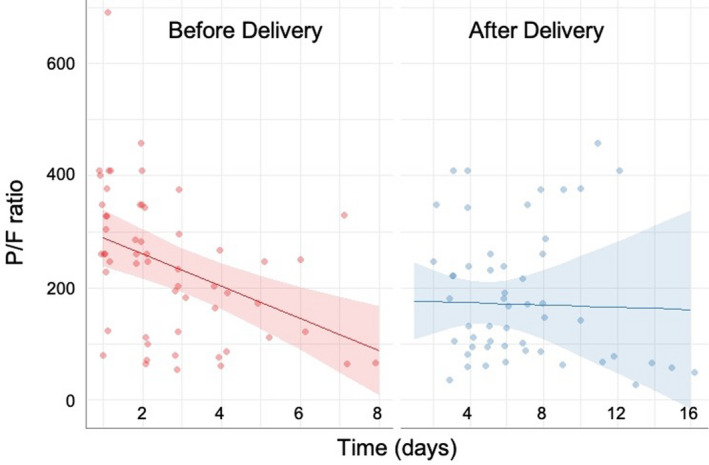
Change in pO2/FiO2 (P/F) ratio before and after delivery among patients with COVID‐19‐related acute respiratory distress syndrome (ARDS) (n = 17). One patient delivered with ARDS is not included due to perimortem delivery without postpartum data.
Two maternal deaths occurred. The first was in a 39‐year‐old patient at 27 weeks who self‐extubated and delivered via perimortem caesarean without return of spontaneous circulation due to inability to re‐intubate. Her medical history was complicated by obesity and type 2 diabetes (Table 2). A second 18‐year‐old patient with obesity died 9 months after preterm delivery for maternal respiratory failure on hospital day 8. She had multi‐organ failure and was never discharged home between her antepartum admission and death.
Discussion
Main findings
Delivery for pregnant patients with COVID‐19‐related ARDS improved the trajectory of the P/F ratio.
Strengths and limitations
The strengths of this study include its relatively large number of very ill patients delivered and the detailed information available about each patient. In the P/F ratio analysis, the mixed‐models approach allowed us to isolate the effect of delivery from that of time by comparing the slope of the P/F ratio before delivery to after delivery. The weaknesses of the study include the overall small number of patients, which may have led to the inability to detect differences in baseline characteristics between groups. Additionally, our results do not apply to patients with COVID‐19 without ARDS. Additionally, as this is an observational study, causation cannot be inferred.
Interpretation
This study is consistent with prior evidence that suggests improvement of respiratory parameters after delivery in pregnant patients with ARDS. 14 However, much clinical controversy surrounds the topic, and there is no clear recommendation as to whether delivery improves the clinical course of pregnant patients with ARDS. 10 , 11 Multiple studies have described the clinical course of pregnant patients with COVID‐19 and critical COVID‐19 but this is the first to examine the effect of delivery in pregnant patients with COVID‐19‐related ARDS. Given the physiological changes of pregnancy that include decreases in total lung capacity, expiratory reserve volume, residual volume and functional residual capacity, delivery may improve patients' respiratory status. 17 Length of stay was longer in delivered patients than those discharged undelivered, which is likely due to the greater severity of COVID‐19 in delivered patients. Obstetric complications were rare and did not contribute significantly to length of stay.
These data provide information for clinicians caring for pregnant patients with COVID‐19‐related ARDS and demonstrate a small improvement in the P/F ratio. This finding is not generalisable to most pregnant patients with COVID‐19 because most do not have ARDS and thus are at a substantially lower risk of morbidity and mortality. Importantly, the deliveries in this analysis occurred at a median of 38 weeks (interquartile range 34–39; range 27–40). Consistent with other case series, 48% were delivered preterm. Gestational age is a critical factor in the decision to deliver and, in our analysis, patients not delivered were at significantly lower gestational age. Importantly, a higher P/F ratio allows weaning from respiratory support, which may have implications for long‐term health and reduction of lung injury. 18 This paper provides data on the response of the P/F ratio to delivery to assist multidisciplinary teams with the decision to deliver, which is complex and must be individualised.
Further studies are needed to strengthen the body of evidence surrounding delivery considerations in patients with COVID‐19. This study was limited to pregnant patients with ARDS, but patients with critical COVID‐19 due to other organ system failures may also benefit from delivery and research is needed in this population. Larger studies that can examine mortality and long‐term lung injury are sorely needed.
Conclusions
COVID‐19‐related ARDS in pregnancy requires multidisciplinary care and individualised decision‐making, but delivery slows the deterioration of the P/F ratio in these patients.
Disclosures of interests
None declared. Completed disclosure of interest forms are available to view online as supporting information.
Contribution to authorship
BP, AS and SB conceived and designed the analysis. LN, AT and CL collected the data. BP and CP analysed the data. BP wrote the manuscript. All authors contributed substantially to the revision of the manuscript for important intellectual content and approved the final version to be published. Beth L. Pineles, MD, PhD; Angela Stephens, MD; Leena M. Narendran, MBBS; Ms. Alyssa Tigner, BS; Christopher Leidlein, MD; Claudia Pedroza, PhD, Hector Mendez‐Figueroa, MD; Baha M. Sibai, MD.
Details of ethics approval
Approval was obtained on 13 April 2020 from the Committee for the Protection of Human Subjects at the University of Texas Health Science Center at Houston (Protocol number HSC‐MS‐20‐0336).
Funding
This study was not supported by any funding source.
Acknowledgements
The authors thank the patients represented in this series and their families.
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Pineles BL, Stephens A, Narendran LM, Tigner MA, Leidlein C, Pedroza C, Mendez‐Figueroa H, Sibai BM. The relationship between delivery and the PaO2/FiO2 ratio in COVID‐19: a cohort study. BJOG 2022;129:493–499.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.▪
References
- 1. Ellington S, Strid P, Tong VT, Woodworth K, Galang RR, Zambrano LD, et al. Characteristics of women of reproductive age with laboratory‐confirmed SARS‐CoV‐2 infection by pregnancy status—United States, January 22‐June 7, 2020. MMWR Morb Mortal Wkly Rep 2020;69:769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pineles BL, Goodman KE, Pineles L, O'Hara LM, Nadimpalli G, Magder LS, et al. In‐hospital mortality in a cohort of hospitalized pregnant and nonpregnant patients with COVID‐19. Ann Intern Med 2021;174:1186–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sentilhes L, De Marcillac F, Jouffrieau C, Kuhn P, Thuet V, Hansmann Y, et al. COVID‐19 in pregnancy was associated with maternal morbidity and preterm birth. Am J Obstet Gynecol 2020;223:914.e1–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pierce‐Williams RAM, Burd J, Felder L, Khoury R, Bernstein PS, Avila K, et al. Clinical course of severe and critical COVID‐19 in hospitalized pregnancies: a US cohort study. Am J Obstet Gynecol MFM 2020;2:100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blitz MJ, Rochelson B, Minkoff H, Meirowitz N, Prasannan L, London V, et al. Maternal mortality among women with coronavirus disease 2019 admitted to the intensive care unit. Am J Obstet Gynecol 2020;223:595–9.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu J, Yang X, Yang L, et al. Clinical course and predictors of 60‐day mortality in 239 critically ill patients with COVID‐19: a multicenter retrospective study from Wuhan, China. Crit Care 2020;24:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rush B, Martinka P, Kilb B, McDermid RC, Boyd JH, Celi LA. Acute respiratory distress syndrome in pregnant women. Obstet Gynecol 2017;129:530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Collop NA, Sahn SA. Critical illness in pregnancy. An analysis of 20 patients admitted to a medical intensive care unit. Chest. 1993;103:1548–52. [DOI] [PubMed] [Google Scholar]
- 9. Mabie WC, Barton JR, Sibai BM. Adult respiratory distress syndrome in pregnancy. Am J Obstet Gynecol 1992;167:950–7. [DOI] [PubMed] [Google Scholar]
- 10. Tomlinson MW, Caruthers TJ, Whitty JE, Gonik B. Does delivery improve maternal condition in the respiratory‐compromised gravida? Obstet Gynecol 1998;91:108–11. [DOI] [PubMed] [Google Scholar]
- 11. ACOG Practice Bulletin No. 211 . Critical care in pregnancy. Obstet Gynecol 2019;133:e303–19. [DOI] [PubMed] [Google Scholar]
- 12. Halscott T, Vaught J. Management Considerations for Pregnant Patients with COVID‐19. Washington, DC: Society for Maternal‐Fetal Medicine; 2021. [https://s3.amazonaws.com/cdn.smfm.org/media/2734/SMFM_COVID_Management_of_COVID_pos_preg_patients_2‐2‐21_(final).pdf]. Accessed 2/23/21. [Google Scholar]
- 13. Muthu V, Agarwal R, Dhooria S, Prasad KT, Aggarwal AN, Suri V, et al. Epidemiology, lung mechanics and outcomes of ARDS: a comparison between pregnant and non‐pregnant subjects. J Crit Care 2019;50:207–12. [DOI] [PubMed] [Google Scholar]
- 14. Lapinsky SE, Rojas‐Suarez JA, Crozier TM, et al. Mechanical ventilation in critically‐ill pregnant women: a case series. Int J Obstet Anesth 2015;24:323–8. [DOI] [PubMed] [Google Scholar]
- 15. Clinical Management of COVID‐19: Interim Guidance. Geneva: WHO Global; 2020. [Google Scholar]
- 16. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573–7. [DOI] [PubMed] [Google Scholar]
- 17. Ie S, Rubio ER, Alper B, Szerlip HM. Respiratory complications of pregnancy. Obstet Gynecol Surv 2002;57:39–46. [DOI] [PubMed] [Google Scholar]
- 18. Chiumello D, Coppola S, Froio S, Gotti M. What's next after ARDS: long‐term outcomes. Respir Care 2016;61:689–99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.▪


