Coronavirus disease 2019 (COVID‐19) following severe acute respiratory syndrome coronavirus 2 infection can cause severe complications in pregnancy, impacting neonatal outcome. There is evidence that, during pregnancy, women become more susceptible to cell‐mediated viral infections due to their physiological adaptation. Subsequently, they become more prone to cardiopulmonary decompensation caused by reduced pulmonary and cardiac reserves1. As a consequence, the rate of admission to the intensive care unit (ICU) is slightly higher in pregnant compared with non‐pregnant women with COVID‐19 (7% vs 4%)2. Hypoxemia as a result of maternal respiratory failure leading to inadequate oxygen supply to the placenta and the fetus can cause fetal distress3, 4. We report a case of rapidly progressing COVID‐19 in pregnancy.
The patient was a 36‐year‐old woman, gravida 6, para 4, who developed COVID‐19 symptoms at 25 + 5 weeks of gestation. One week after admission, her oxygen saturation (SpO2) dropped suddenly to 87%, while receiving 3 L/min of supplemental oxygen. She was then transferred to ICU, where her lung function deteriorated rapidly, requiring intubation and venovenous extracorporeal membrane oxygenation (ECMO) within 6 h after admission. During intubation, she suffered cardiogenic shock accompanied by acute renal failure, requiring a high dose of catecholamines and dialysis. In this critical state, the lowest SpO2 was 77% and cardiotocography (CTG) showed intermittent prolonged fetal bradycardia (heart rate of 86 bpm). Given the pathological CTG, a Cesarean section was considered. However, the maternal condition was critical and general anesthesia was not feasible. Antiviral therapy with remdesivir was initiated, and betamethasone was administered for fetal lung maturation. The catecholamine dosage was then reduced steadily, and the patient was weaned from ECMO at 27 + 2 weeks.
The first ultrasound scan was performed at 27 + 0 weeks during ECMO and showed a normal, appropriately developed male fetus with normal fetal Doppler values. Within the next few days, we observed localized cerebral hyperechogenicity, progressing to bleeding, followed by the formation of porencephalic cysts and disintegration of the cerebellar hemispheres (Figure 1a). We observed worsening ventriculomegaly, fading basal ganglia and a ruptured falx cerebri (Figure 1b, Videoclip S1). Middle cerebral artery peak systolic velocity increased to 85.3 cm/s (multiples of the median of 2.3), indicating bleeding and anemia. The mother was counseled regarding the unfavorable outcome of the child, with a high risk for severe disability. After consideration, she opted for termination of pregnancy. Feticide and labor induction were performed at 30 + 3 weeks (birth weight of 1591 g). Samples of amniotic fluid, fetal blood, chorionic villi and fetal cerebrospinal fluid were collected at the time of feticide for virological studies (Table 1) and genetic tests (no anomaly).
Figure 1.
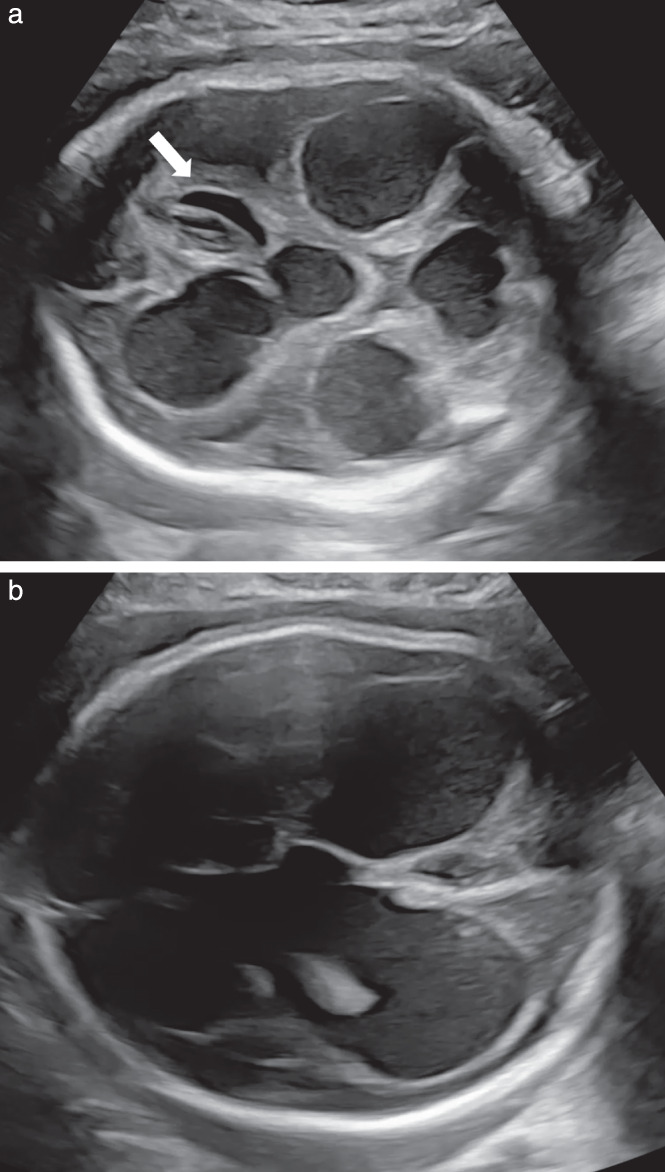
Grayscale ultrasound images of fetal head obtained at 29 + 4 weeks of gestation in a pregnancy with coronavirus disease 2019, showing progressive hydrocephalus with third and fourth ventricle dilatation, development of porencephalic cysts (arrow) and a disintegrating cerebellum with significant loss of the hemispheres (a) and progressive macrocephaly and hydrocephalus e vacuo (anterior ventricular diameter, 25.9 mm; posterior ventricular diameter 32.8 mm) (b).
Table 1.
Findings of severe acute respiratory syndrome coronavirus 1/2 screening tests in the mother and fetus
| Specimen | RT‐PCR | qPCR | IgG |
|---|---|---|---|
| Amniotic fluid | Positive (37 Ct) | — | — |
| Chorionic villi (native) | Positive (38 Ct) | — | — |
| Fetal cerebrospinal fluid | Negative | — | — |
| Fetal blood | — | Negative | Positive |
| Fetal nasopharyngeal swabs | Negative | — | — |
| Fetal anal swabs | Negative | — | — |
| Maternal plasma | — | — | Positive |
Ct, cycle threshold; IgG, immunoglobulin G; qPCR, quantitative polymerase chain reaction; RT‐PCR, reverse transcription polymerase chain reaction.
The autopsy showed an atrophic cerebral cortex with dilated ventricles and hydrocephalus in addition to intraventricular bleeding. Changes caused by acute hypoxia were discerned throughout the entire central nervous system. No evidence of microangiopathy, thromboembolism or systemic fetoplacental inflammation was found. In the placental tissue, there were minor regressive changes compatible with prolonged hypoxia without signs of villitis or intervillitis.
The observation of massive fetal brain damage secondary to critical cardiopulmonary deterioration and acute maternal hypoxia highlights the importance of close monitoring and sufficient oxygenation (SpO2 > 95%)5 of women with COVID‐19 during pregnancy.
Supporting information
Videoclip S1 Neurosonography at 29 weeks of gestation showing macrocephaly and hydrocephalus with porencephalic cysts, dilated lateral, third and fourth ventricles, a ruptured falx cerebri and fading basal ganglia. The corpus callosum was not detectable and the cerebellum had undergone cystic transformation with disintegration of the hemispheres.
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
References
- 1.Schnettler WT, Al Ahwel Y, Suhag A. Severe acute respiratory distress syndrome in coronavirus disease 2019‐infected pregnancy: obstetric and intensive care considerations. Am J Obstet Gynecol MFM 2020; 2: 100120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar R, Yeni CM, Utami NA, Masand R, Asrani RK, Patel SK, Kumar A, Yatoo MI, Tiwari R, Natesan S, Vora KS, Nainu F, Bilal M, Dhawan M, Emran TB, Ahmad T, Harapan H, Dhama K. SARS‐CoV‐2 infection during pregnancy and pregnancy‐related conditions: concerns, challenges, management and mitigation strategies – a narrative review. J Infect Public Health 2021; 14: 863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dang D, Wang L, Zhang C, Li Z, Wu H. Potential effects of SARS‐CoV‐2 infection during pregnancy on fetuses and newborns are worthy of attention. J Obstet Gynaecol Res 2020; 46: 1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Putbrese B, Kennedy A. Findings and differential diagnosis of fetal intracranial haemorrhage and fetal ischaemic brain injury: what is the role of fetal MRI? Br J Radiol 2017; 90: 20160253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oxford‐Horrey C, Savage M, Prabhu M, Abramovitz S, Griffin K, LaFond E, Riley L, Easter SR. Putting It All Together: Clinical Considerations in the Care of Critically Ill Obstetric Patients with COVID‐19. Am J Perinatol 2020; 37: 1044–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Videoclip S1 Neurosonography at 29 weeks of gestation showing macrocephaly and hydrocephalus with porencephalic cysts, dilated lateral, third and fourth ventricles, a ruptured falx cerebri and fading basal ganglia. The corpus callosum was not detectable and the cerebellum had undergone cystic transformation with disintegration of the hemispheres.
Data Availability Statement
Data available on request from the authors.


