Abstract
Solid organ transplant recipients are at high risk of severe disease from COVID-19. We assessed the immunogenicity of mRNA-1273 vaccine using a combination of antibody testing, surrogate neutralization assays, and T cell assays. Patients were immunized with two doses of vaccine and immunogenicity assessed after each dose using the above tests. CD4+ and CD8+ T cell responses were assessed in a subset using flow-cytometry. A total of 127 patients were enrolled of which 110 provided serum at all time points. A positive anti-RBD antibody was seen in 5.0% after one dose and 34.5% after two doses. Neutralizing antibody was present in 26.9%. Of note, 28.5% of patients with anti-RBD did not have neutralizing antibody. T cell responses in a sub-cohort of 48 patients showed a positive CD4+ T cell response in 47.9%. Of note, in this sub-cohort, 46.2% of patients with a negative anti-RBD, still had a positive CD4+ T cell response. The vaccine was safe and well-tolerated. In summary, immunogenicity of mRNA-1273 COVID-19 vaccine was modest, but a subset of patients still develop neutralizing antibody and CD4+T- cell responses. Importantly polyfunctional CD4+T cell responses were observed in a significant portion who were antibody negative, further highlighting the importance of vaccination in this patient population.
IRB Statement: This study was approved by the University Health Network Research Ethics Board (CAPCR ID 20–6069).
KEYWORDS: clinical research/practice, infection and infectious agents - viral, infectious disease, vaccine
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range; LOQ, limit of quantitation; PBMC, peripheral blood mononuclear cells; RBD, receptor binding domain; SARS, severe acute respiratory syndrome; SOT, solid organ transplant
1. INTRODUCTION
Solid organ transplant (SOT) recipients are at high risk for complications from infection with the coronavirus disease 2019 (COVID-19).1 , 2 The use of exogenous immunosuppression in these patients hampers the response to most viral infections as well as diminishing the utility of preventative and therapeutic measures. Transplant patients may also have prolonged viral shedding potentially leading to increased infectivity and variant development.3 Public health strategies including the use of masks, physical distancing, testing of symptomatic individuals, contact tracing, and isolation have had variable success in preventing infections. In this context, multiple vaccine platforms have been investigated to try to impede the spread of COVID-19.4, 5, 6 While vaccines have proven extremely successful in the general population much less is known in immunocompromised patients.
The mRNA-1273 vaccine (Moderna), a lipid nanoparticle-encapsulated mRNA-based vaccine encoding the perfusion stabilized full-length spike protein of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was found to be highly effective and safe in a phase III, randomized, placebo-controlled trial involving 30,420 immunocompetent volunteers.4 Similar results have been reported for another mRNA vaccine, BNT162b2 (Pfizer-BioNTech).5
However, in immunocompromised populations, including SOT recipients, humoral vaccine immune response appears to be diminished or even absent in some individuals.7 Poor response to the vaccine has been associated with the use of anti-metabolite immunosuppression as well as other transplant-specific risk-factors.7 , 8 Currently published studies in transplant recipients have used different assays to measure humoral responses, but measurement of antibody against the receptor binding domain of the SARS-CoV-2 spike protein (anti-RBD) seems to be a relevant end-point. However, the neutralizing capacity of the antibody response is also important in that this measures the ability of detectable antibodies to block infection. Neutralizing antibody levels have recently been convincingly shown to be highly predictive of immune protection from symptomatic SARS-CoV-2 infection across multiple large studies.9 Another important aspect of the vaccine response is cellular immunity and specifically the CD4+ T cell response.10 There are limited data on both neutralizing antibody and T cell responses post-vaccine in SOT recipients. In this study, we performed a comprehensive assessment of antibody and cell-mediated immune responses to two doses of SARS-CoV-2 mRNA-1273 (Moderna) in SOT recipients.
2. METHODS
2.1. Patient population and study design
This was a prospective observational cohort study of SOT recipients recruited from a tertiary care transplant program, who were receiving the two scheduled doses of the mRNA-1273 vaccine (Moderna) 28 days apart. We enrolled adult patients (aged ≥18 years) who had received an organ transplant (kidney, liver, heart, lung and pancreas, or combined organs), had a functioning allograft, and were planning to receive the mRNA-1273 vaccine series. Those who completed the two-dose SARS-CoV-2 mRNA-1273 vaccine series between March 2, 2021–April 8, 2021 were included and followed to 6 weeks after the second dose. Exclusion criteria were as follows: less than 6-months post-transplant, previous confirmed COVID-19 infection, experienced a febrile illness within 1-week prior to vaccination, active cytomegalovirus infection (defined as viral load of >1000 IU/mI or a lower viral load accompanied by compatible symptoms), receipt of intravenous immunoglobulin in the past 30 days or planning to receive intravenous immunoglobulin in the next 4 weeks. The local institutional research ethics board approved the study and all patients provided written informed consent. A subset of patients also provided consent for additional blood for T cell immunity studies. Blood samples were obtained before dose one (V0), 4 weeks after the first dose (V1) and within 4–6 weeks after the second dose of vaccine (V2) for testing. A group of transplant patients with natural COVID-19 infection who had given informed consent to provide convalescent serum was also used for a comparator.
2.2. Antibody responses
The primary outcome was a measure of IgG antibody against the receptor binding domain of the spike protein (anti-RBD). This was measured by semi-quantitative anti-spike serologic testing using the Roche Elecsys anti– SARS-CoV-2 S enzyme immunoassay.11 Testing was performed as per manufacturer’s instructions in a certified biochemistry testing laboratory. This assay has a lower limit of detection (LOD) of 0.4 U/ml and as per test instructions, a positive response is defined as ≥0.8 U/ml. Neutralizing antibodies were assessed via the SARS-CoV-2 Surrogate Virus Neutralization Test (SVNT) assay (GenScript), according to the manufacturer’s specifications. This SVNT assay has received emergency use authorization from the FDA. This assay works by incubating serum with horseradish peroxidase (HRP)-conjugated spike-RBD and then transferring the mixture to ACE2 coated wells. If neutralizing antibodies are present in the serum, the RBD-ACE2 interactions are blocked. The SVNT measures total neutralizing antibodies in sera. The assay was originally described by Tan et al.12 and has been used in several peer-reviewed studies to assess neutralizing antibodies outside of biosafety level 3 (BSL3) facilities.13, 14, 15 According to the manufacturer’s instructions, a positive is defined based on a neutralizing antibody threshold of 30% neutralization/inhibition or greater. At this cut-off, the negative and positive percent agreement with conventional plaque reduction neutralization test (PRNT)50 and PRNT90 assays is approximately 100%. The manufacturer reported sensitivity and specificity for the assay is 93.80% and 99.4% respectively. As per kit specifications, patients with neutralization below 30% were considered negative for neutralizing antibodies.
2.3. Cell-mediated immunity assessment
In a subset of consenting participants, SARS-CoV-2 specific CD4+ and CD8+ T cell responses were assessed. Peripheral blood mononuclear cells (PBMCs) were isolated, and cryopreserved in liquid nitrogen using a validated protocol to ensure high cell viability. A total of 106 PBMCs were rested for 2 h and incubated with overlapping peptides encompassing the full SARS-CoV-2 spike protein. Peptides consisted mainly of 15-mer sequences with 11 amino acid overlaps (PepTivator®, Miltenyi Biotec) and were included at a final concentration of 5 µg/mI per peptide, based on preliminary studies to determine optimal concentration. Cells were incubated overnight with peptides, a CD28/CD49d co-stimulatory antibody cocktail (BD Biosciences) and a protein transport inhibitor to prevent cytokine release (ThermoFisher Scientific). Intracellular cytokine staining (ICS) was used to measure the frequency of spike-specific T cells, as has been commonly done in other SARS-CoV-2 vaccine studies.16, 17, 18, 19 The cytokine effectors used in this study were the principle Th1 effectors, IFN-γ and IL-2, which have been used to evaluate SARS-CoV-2-reactive T cells following immunization with mRNA-1273.10 , 20 PMA/ionomycin was used as a positive control and cells treated with media alone were used as a negative (media) control. Following incubation at 37°C, cells were stained with a viability dye (Zombie Aqua, Biolegend), Fc blocked (BD Biosciences) and incubated with a surface marker antibody cocktail (CD3, CD4, CD8). Cells were then fixed, permeabilized, and incubated with an antibody cocktail to detect intracellular markers (IFN-γ and IL-2). Table S1 lists the antibodies used in this study, their clones, suppliers, and associated fluorochromes. Flow cytometry was performed on an LSR II BGRV (BD Biosciences) at the SickKids-UHN Flow Cytometry Facility. A representative gating strategy is shown in Figure SA. As a robust, validated, yet conservative measure of vaccine-induced T cell responses, we specifically measured frequencies of CD4+ and CD8+ T cells that expressed two cytokines (IFN-γ and IL-2 positive). Polyfunctional T cells are commonly used to assess vaccine-induced immunogenicity.16, 17, 18 The frequency of vaccine-antigen specific T cells was determined by subtracting the frequency of polyfunctional T cells in untreated comparators from the frequency in peptide-stimulated samples. A positive T cell response was defined as minimum of 3-standard deviations above the mean of the background, plus a minimal polyfunctional T cell frequency of 0.01%, which was set as the limit of quantitation (LOQ). A minimum number of 100,000 live, CD3+ T cells were required for samples to be included in the flow analysis. These conditions and definitions were validated using samples from healthy controls (vaccinated and post-recovery from COVID-19), and represent a conservative measure of a positive T cell response to ensure robustness of the reported data.
2.4. Safety and adverse events
All patients were followed closely for the duration of the study. Safety assessments included monitoring through a participant-directed vaccine diary for local and systemic adverse events for 7 days after each injection. Adverse events were categorized by the Food and Drug Administration toxicity grading scale for volunteers in vaccine trials as follows; grade 1 (no interference in daily activities), grade 2 (some interference in daily activities), grade 3 (participants unable to perform daily activities), and grade 4 (potentially life threatening).19 In addition, study team members contacted all participants every 2 weeks by phone call and chart review for episodes of acute organ rejection, hospitalization, other adverse events, or COVID-19 infection for ≥60 days post first dose of vaccine or until May 21, 2021.
2.5. Statistical analysis
The immunogenicity analysis was performed in those who received both vaccine doses and returned for follow-up serum (per-protocol population). The safety analysis was performed in all patients who received the study vaccine regardless of whether they returned for follow-up serum. Demographics and safety analysis was performed using descriptive statistics. The primary outcome was vaccine immunogenicity by assessment of pre- and post-second dose of vaccine anti-RBD titer. A positive anti-RBD response was defined as >0.8 U/ml. Pre- and post-vaccination titers were compared using Wilcoxon rank-sum test (titers <0.4 were assigned a value of 0.2 for statistical analysis). The neutralization responses and T cell response were analyzed using descriptive statistics. Spearman’s test was used to correlate anti-RBD titers and neutralization. For the purposes of quantitative statistical analysis for each of the assays, where required, values below threshold (e.g., LOD) were coded as threshold/2. Univariate analyses were performed to determine significant factors affecting development of a positive anti-RBD titer using χ2 or Fisher’s exact test for categorical variables and Mann–Whitney U test for continuous variables. Anti-RBD titers (quantitative) between vaccinated and naturally infected transplant patients were compared using the Mann–Whitney U test. Statistical significance was defined as a p value < .05. All statistical analysis was done using SPSS version 29.0 (Chicago, Ill) and Prism GraphPad version 9.1.1.
3. RESULTS
3.1. Patient characteristics
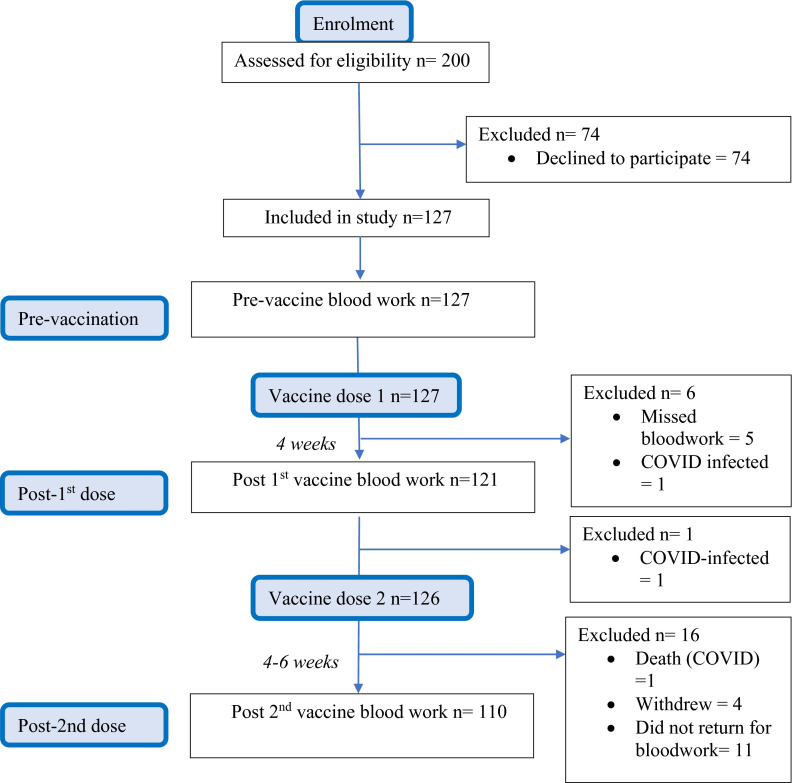
A total of 127 solid organ transplant recipients were enrolled and received mRNA-1273 (Moderna) vaccine. Figure 1 shows the study flow diagram. Of these, 126 (99.2%) received both doses of the vaccine at the recommended 1-month interval. One patient developed COVID-19 infection after the first dose and so did not receive a second dose. Baseline characteristics of the cohort are shown in Table 1. The median age was 66.2 years (interquartile range 63.4–70.6 years). Time from transplant was median 2.96 years (IQR 1.56–6.31 years). Current immunosuppression consisted primarily of a combination of a calcineurin inhibitor (cyclosporine or tacrolimus), prednisone, and an antiproliferative with 80/127 (63%) patients being on this triple regimen. A total of 97 participants (97/127, 76.4%) were on a mycophenolic acid compound. A relatively even mix of different organ types was included (kidney, liver, lung, heart, and kidney-pancreas; Table 1). “Other” in the table denotes one participant with a liver-kidney transplant and two participants with a pancreas transplant alone.
FIGURE 1.
Study flow [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 1.
Baseline characteristics of study participants
| Characteristic | All (n = 127) |
|---|---|
| Age, median (IQR) | 66.2 (63.4–70.6) |
| Male gender, n (%) | 88 (69.3) |
| Time from transplantation to first dose of vaccination (years), median (IQR) | 2.96 (1.56–6.31) |
| Within 1 year of transplantation, n (%) | 12 (9.45) |
| Rejection in preceding 3 months, n (%) | 3 (2.36) |
| Anti-thymocyte globulin in the preceding 6 months | 0 |
| Type of transplant (%) | |
| Lung | 33 (26) |
| Kidney | 30 (23.6) |
| Kidney-pancreas | 28 (22.04) |
| Heart | 18 (14.2) |
| Liver | 15 (11.8) |
| Other | 3 (2.36) |
| Immunosuppressiona | |
| Prednisone (%) | 102 (80.3) |
| Prednisone daily dose, mg; median (IQR) | 5 (5–5) |
| Calcineurin inhibitor (%) | 122 (96.1) |
| Tacrolimus | 92 (72.4) |
| Tacrolimus trough level, ng/ml (IQR) | 7.5 (5.8–9.5) |
| Cyclosporine | 30 (23.6) |
| Mycophenolate mofetil/ mycophenolate sodium (%) | 96 (75.6) |
| Azathioprine (%) | 11 (8.66) |
| Sirolimus (%) | 11 (8.66) |
No patient received Rituximab or Belatacept.
3.2. Vaccine immunogenicity by anti-RBD antibody
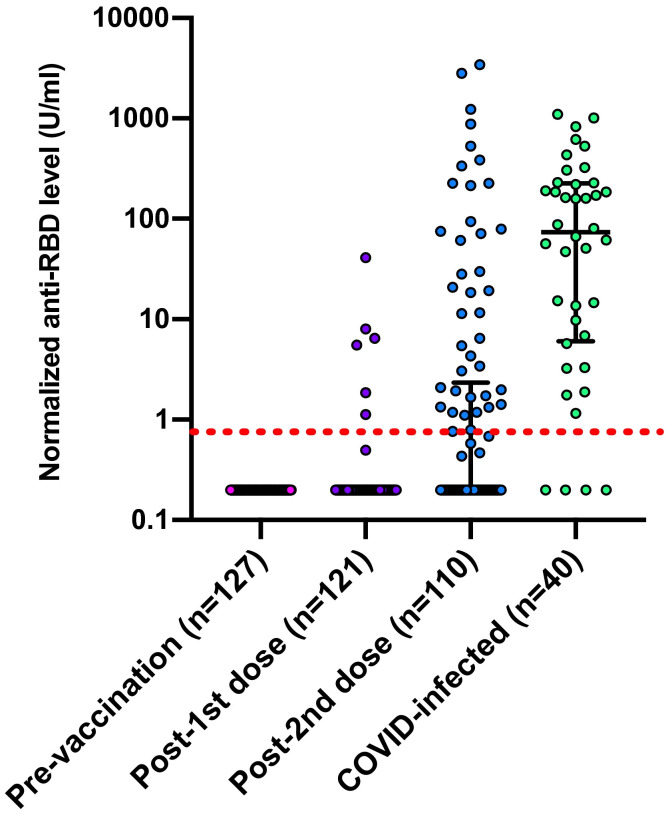
The primary outcome was incidence of positive serologic response based on measurement of anti-RBD antibody. Normalized anti-RBD levels pre-vaccine, after the first dose, and after the second dose are shown in Figure 2. After dose one, the response rate was 6/121 (5.0%). After two doses the response rate was 38/110 (34.5%) (no detectable response in 72/110 [65.5%]) and median antibody titer was 0.2 U/ml (IQR 0.2–2.10). In just those with a response (n = 38), the median antibody titer was 15.07 U/ml (IQR 1.68–214.2). We then analyzed factors associated with a positive anti-RBD response ( Table 2). The use of mycophenolate was associated with a significantly diminished response (64/72 [88.9%] seronegative patients were on mycophenolate versus 18/38 [47.4%] seropositive patients, p < .001). Liver transplant recipients were more likely to have a positive response (p = .002).
FIGURE 2.
Antibody titer in transplant recipients pre-/post-immunization with each dose of mRNA-1273 (Moderna) COVID-19 vaccine and convalescent titers of transplant recipients after COVID infection. Horizontal lines represent median and interquartile range. Dotted red line represents positive cut-off value of assay at 0.8 U/mI [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 2.
Factors associated with anti-RBD response after two doses of mRNA-1273 (Moderna) COVID-19 vaccine
| No. (%) antiRBD response at 2 doses |
Univariate p value | ||
|---|---|---|---|
| Negative = 72 | Positive = 38 | ||
| Age (years) | 65.9 (IQR 63.4–70.1) | 67.8 (54.3–71.8) | .273 |
| Time since transplant (years, IQR) | 2.83 (1.49–5.82) | 2.63 (1.37– 6.89) | .941 |
| Gender | |||
| Female | 21 (29.2) | 14 (36.8) | .29 |
| Male | 51 (70.8) | 24 (63.2) | |
| Transplant organ | |||
| Lung | 17 (23.6) | 12 (31.6) | .657 |
| Kidney | 20 (27.8) | 8 (21.1) | .366 |
| Kidney-pancreas | 18 (25.0) | 7 (18.4) | .641 |
| Heart | 14 (19.4) | 2 (5.26) | .16 |
| Liver | 3 (4.17) | 8 (21.1) | .002 |
| Immunosuppression | |||
| Prednisone | 59 (81.9) | 29 (76.3) | .084 |
| Prednisone daily dose; mg, median (IQR) | 5 (5–5) | 5 (5–5) | .432 |
| Calcineurin inhibitor | 71 (98.6) | 36 (94.7) | .554 |
| Tacrolimus | 57 (79.1) | 26 (68.4) | .213 |
| Tacrolimus trough; ng/mI, median (IQR) | 7.7 (6.4– 9.9) | 6.2 (5–8.9) | .039 |
| Cyclosporine | 15 (20.8) | 11 (28.9) | .341 |
| Cyclosporine trough; ng/ml, median (IQR) | 152 (117–241) | 154 (128–286) | .637 |
| Mycophenolate mofetil/ mycophenolate sodium | 64 (88.9) | 18 (47.4) | <.001 |
| Mycophenolate daily dose; mg, median (IQR) | 720 (360–1440) | 0 (0–720) | <.001 |
| Azathioprine | 3 (4.17) | 8 (21.1) | .018 |
| Sirolimus | 6 (8.33) | 4 (10.5) | .496 |
Bold denotes variables with p-values <.05
We also compared anti-RBD response in vaccinated patients with convalescent antibody response (>14 days post-infection) in transplant patients with natural COVID-19 infection (n = 40) (Figure 2). Demographics of this cohort were similar to the vaccine cohort in terms of type of transplant, age, and immunosuppression (Table S2). The median time post COVID-19 diagnosis at which blood collection was performed was 36.5 days (IQR 33.0–48.5) (Table S3). In this cohort 36/40 (90.0%) had a positive convalescent anti-RBD titer. The median overall titer was 73.5 U/mI (IQR 6.0–226.5) and the median titer in the anti-RBD-positive natural infection patients was 123.2 U/ml (IQR 13.9–229 U/ml). The rate of positivity was higher with natural infection versus vaccine (90% vs. 34.5% respectively, p < .001). In addition, the level of anti-RBD was also higher in natural infection versus vaccine both in the whole cohort (73.5 vs. 0.2 U/mI, p < .001) and in the subgroup of those that were positive (123.2 U/mI vs. 18.9 U/mI; p = .049, respectively).
3.3. Vaccine immunogenicity by neutralizing antibody
The presence of neutralizing antibody using the validated surrogate virus neutralization test (SVNT) was determined for all vaccine patients. The neutralizing antibody results are shown in Figure 3 and Figure SD. We found that after the first dose 7/119 (5.9%) had positive neutralization antibody and after the second dose, this increased to 29/108 (26.9%). There was no detectable neutralizing antibody in 79/108 (73.1%) patients. In those with positive neutralization post-second dose, the median level of percent inhibition was 51.7% (IQR 39.9–92.4%). Quantitative anti-RBD levels were highly correlated with percent neutralizing antibodies (Figure SB; Spearman r: 0.77, p < .001). However, several patients with positive anti-RBD had negative neutralization (28.5% of positive anti-RBD patients were negative by neutralization). These patients typically had very low levels of anti-RBD detected (median 1.83 U/ml, range 1.19–20.9 U/ml).
FIGURE 3.
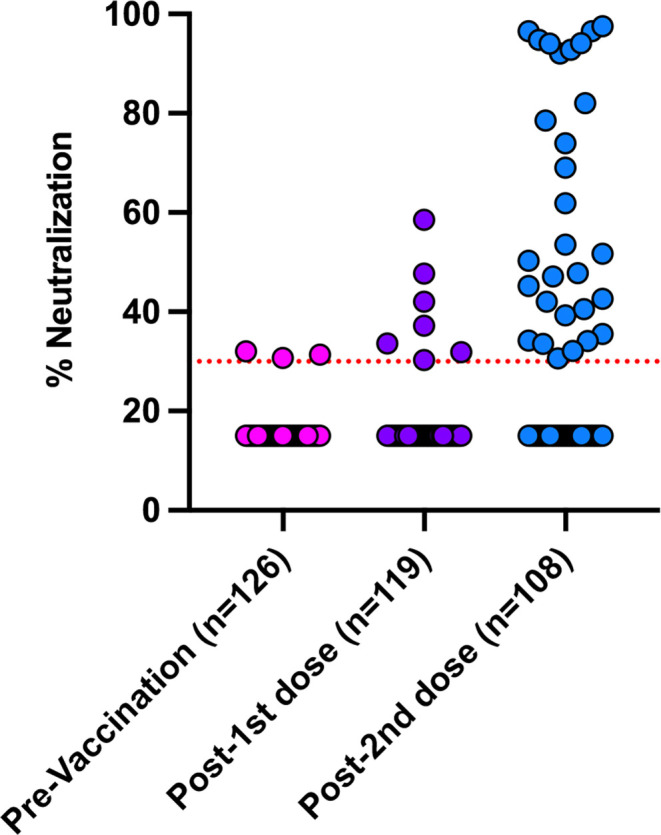
Surrogate Vaccine Neutralization Assay results in transplant recipients pre-/post-immunization with each dose of mRNA-1273 (Moderna) COVID-19 vaccine [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Cell-mediated vaccine immunogenicity
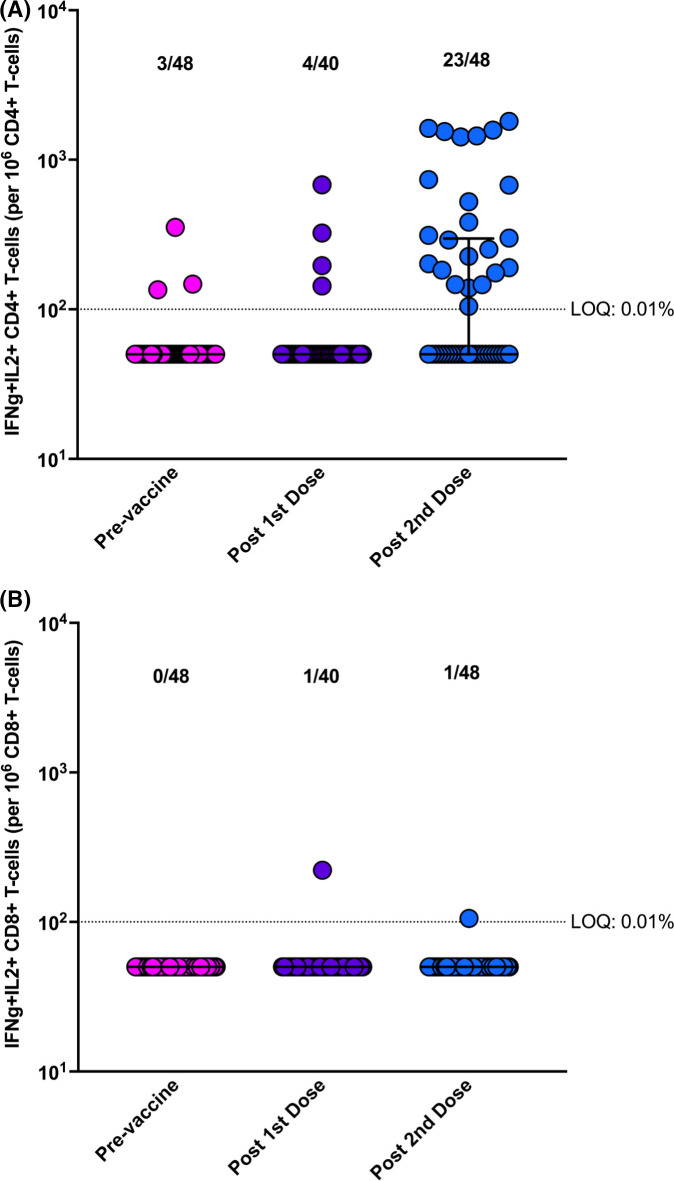
A summary of SARS-CoV-2 specific CD4+ and CD8+ T cell responses are shown in Figure 4. Representative cytokine plots for a “higher” and “lower” responding patient are shown in Figure SC. Using pre-specified and validated definitions for a positive versus negative cellular immune response (based on polyfunctional IFN-γ and IL-2 producing T cells; see Methods), we observed the following: 4/40 (10.0%) had a positive CD4+ T cell response after the first dose of vaccine, and 23/48 (47.9%) had a positive CD4+ T cell response after the second dose of vaccine. In those with a positive response the median number of polyfunctional CD4+ T cells was 300 cells/106 CD4+ T cells (range 182– 1420 cells/106). By univariate analysis, only female gender was found to be significantly associated with a positive CD4+ T cell response (Table S4). CD8+ T cell responses were generally not-detected in response to vaccination (Figure 4).
FIGURE 4.
Cell-mediated immune response in transplant recipients pre-/post-immunization with each dose of mRNA-1273 (Moderna) COVID-19 vaccine. Horizontal lines represent median and range. Dotted line is the limit of quantitation (LOQ; 0.01% or 100 per 106 cells). (A) Polyfunctional CD4+ T cell response. (B) Polyfunctional CD8+ T cell response [Color figure can be viewed at wileyonlinelibrary.com]
Only a modest overlap was observed between the anti-RBD antibody, neutralizing antibody, and CD4+ T cell response. In the 23/48 patients with a positive CD4+ response, 11/23 (47.8%) also had a positive anti-RBD response. Importantly, several patients with a negative anti-RBD response still had a positive CD4+ T cell response: of the 26/48 (54.2%) who had negative anti-RBD response, 12/26 (46.2%) still had a positive CD4+ T cell response. For example, the two patients in Figure SC both had negative anti-RBD but clear positive polyfunctional CD4-T cell responses to vaccine. Another way of analyzing this is to assess how many patients had either an anti-RBD antibody or a T cell response: this yielded a 33/48 (68.8%) total composite response rate. Similarly, in those with a positive neutralizing antibody, 57.1% also had a positive CD4+ T cell response.
3.5. Vaccine safety and other outcomes
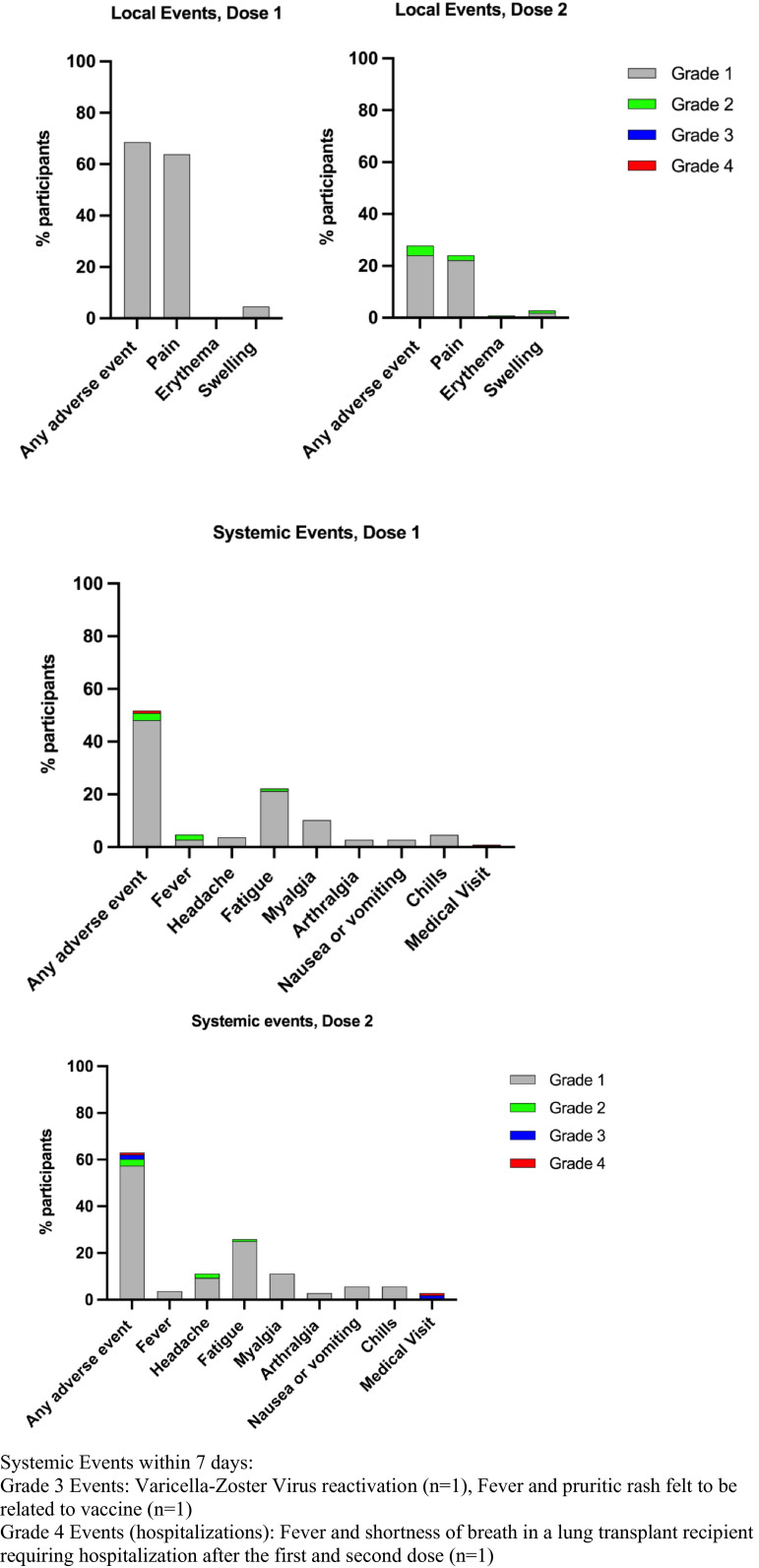
Safety analysis was completed for all participants out to the end of the study period for 108/127 (85%) patients who completed the vaccine diary after both doses of vaccine. Overall, the vaccines were well-tolerated. This is shown in Figure 5. Local events were most common and included pain and swelling at the injection site. Systemic events included fatigue, myalgia, and headache. There were no recorded episodes of organ rejection up to 6 weeks after the second vaccine dose. Two participants (2/127, 1.57%) developed COVID-19 infection, one after the first dose and another patient after two doses who died due to complications related to COVID-19. The latter patient was a lung transplant recipient who contracted COVID-19 within a week of the second dose of vaccine (negative anti-RBD and neutralizing antibody post-first vaccine dose). Eight participants (8/127, 6.3%) were hospitalized for any reason during the study period. Reason for hospitalization included: COVID-19 infection (n = 2), elective surgery (n = 1), acute cholangitis (n = 1), exacerbation of chronic allograft lung dysfunction not due to COVID-19 (n = 2), chemotherapy for multiple myeloma (n = 1), renal calculi and hydronephrosis in transplant graft (n = 1). No hospitalizations were directly as a result of vaccination.
FIGURE 5.
Local and systemic adverse effects within 7 days of mRNA-1273 COVID-19 vaccine in organ transplant recipients after the first and second doses (n = 108) [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
We performed a study of solid organ transplant recipients receiving two doses of Moderna mRNA vaccine. The main findings of the study were as follows: (a) in the primary analysis, the immunogenicity as measured by anti-RBD was 5.0% after the first dose and 34.5% after the second dose; (b) many patients developed neutralizing antibody, primarily after the second dose (26.9%). However, a significant subset of anti-RBD positive patients did not show significant neutralization (28.5%); (c) the vaccine did elicit positive SARS-CoV-2 specific CD4+ T cell responses in a significant portion of the evaluated cohort (47.9%). Specifically, there were several patients, who although they did not have detectable anti-RBD, they still had detectable and often robust T cell responses (46.2%; discussed more below). Finally, we showed that the vaccine was relatively well-tolerated.
The measurement of T cell responses and neutralizing antibody are unique aspects of our study and provide important insight into how transplant patients respond to COVID-19 vaccine. One of the major concerns in transplant patients has been low immunogenicity of vaccines as measured by routine antibody testing. However, it is completely unknown how this translates into vaccine efficacy. A key finding of ours is that a SARS-CoV-2 specific CD4+ T cell response was observed in close to 50% of patients, and critically almost half of patients who did not have an antibody response still had evidence of a T cell response, which was polyfunctional and often quite robust (e.g., Figure SC). The less robust CD8+ T cell response observed in our study is consistent with the literature in both transplant and non-transplant populations, that is, CD8+ responses are weaker than CD4+ responses to vaccination.10 , 21 , 22 This may be the nature of mRNA vaccination and immunosuppression may amplify this difference in transplant patients. We used a conservative and clear definition of positive that required both polyfunctionality and a threshold level of cells. Based on these data, it may be invalid for patients to be labeled as vaccine “non-responders” in the absence of T cell data. It also suggests that transplant patients should be strongly encouraged to still receive vaccine despite data showing poor immunogenicity by antibody testing alone. The CD4+ T cell responses we observed are in the same frequency range as has been reported in healthy volunteers.18 , 23 It was also encouraging that many patients had evidence of neutralizing antibody. Neutralization levels have recently been robustly shown to be highly predictive of immune protection in immunocompetent cohorts.9 Of note, though, is that approximately 28.5% of patients in our study with a positive anti-RBD response did not have neutralizing antibody. These patients typically had very low levels of anti-RBD detected (median 1.83 U/ml, range 1.19–20.9 U/mI).
In a study of 80 liver transplant recipients, vaccinated with Pfizer-BioNTech COVID vaccine, the response rate was 47.5% using an S1/S2 chemiluminescent assay. Age, triple immunosuppression, and mycophenolate use were associated with poor response.24 In a study of 136 kidney transplant recipients receiving Pfizer-BioNTech, response rate was 37.5% using the same assay. Similar risk factors were associated with poor response.8 In a study of 168 lung transplant recipients, again receiving the Pfizer-BioNTech vaccine, response rates were only 18% after two doses (and 4% after one dose) based on measurement of spike IgG antibody.25 Age and use of antiproliferative agents and mTOR immunosuppression were associated with poor response. Finally, Boyarsky et al. assessed response to either Moderna or Pfizer-BioNTech in a cohort of 658 transplant recipients of varying organ types, using the enzyme immunoassay for IgG against S1.7 The response rate after two doses was 54%.7 Again use of antimetabolite immunosuppression was associated with poor response. None of the mentioned studies assessed neutralizing antibody and none assessed cellular immune responses. Our findings related to anti-RBD response and risk factors related to response are generally in agreement with this. However, an interesting finding in our study was that overall anti-RBD responses with vaccination were significantly lower post-vaccine versus post-natural COVID-19 infection. This is contrary with what has been generally reported in immunocompetent patients where vaccines generally elicit stronger antibody responses.9 , 26 , 27
Our study had several limitations. First, we did not have a control group of immunocompetent patients. However, immune responses have been extensively studied in immunocompetent people and have been consistently shown to be >95% after two doses of either of the available mRNA vaccines.20 , 28 Second, we did not assess cellular immune responses in the entire cohort. This type of testing requires substantially more volume of blood collection than with serology and not all patients were willing. In addition, cell mediated testing is significantly more labor intensive and time consuming compared with serology. We feel, however, that the results we obtained would unlikely be changed substantially with further samples from this cohort. The relatively older median age of our cohort may also have contributed to the lower immune response. We acknowledge that the antibody response in the infected cohort may be slightly biased toward a subgroup that survived the infection. Finally, we did not do HLA alloantibody testing. However, no episodes of acute rejection occurred in the follow-up period. The main strengths of our study are indeed the assessment of cellular immune responses and, also the assessment of neutralizing antibody using a validated surrogate assay as outlined above. In addition, we were able to compare anti-RBD responses to transplant patients with natural COVID-19 infection, another unique feature of our study.
In summary, we assessed Moderna vaccine responses in a cohort of solid organ transplant recipients. We show that the vaccine response rate by anti-RBD was 34.5% and 26.9% had evidence of neutralizing antibodies. However, we also show that a significant number of patients had a positive SARS-CoV-2 specific CD4+ T cell response often in the absence of a detectable antibody response. The latter highlights the importance of vaccinating all transplant recipients and not assuming poor immunogenicity based on antibody response alone. Despite vaccination or antibody status, we encourage ongoing vigilance from SOT recipients, who are still at risk for breakthrough COVID-19 infection, in particular in the context of the circulating variants of concern including Alpha and Delta variants. Our data also suggest that it is likely these patients could still benefit from further strategies to enhance immunogenicity; such as booster dose regimens to provide additional antigen or reduction of immunosuppression around the time of vaccination (i.e., mycophenolate dose reduction). These strategies should be balanced with the risk of organ rejection and require further assessment as part of a clinical study. A subset of participants from this cohort are currently enrolled in a double-blind, randomized controlled trial of a third dose of Moderna vaccine versus placebo.
ACKNOWLEDGMENT
The study was funded by the Ajmera Transplant Centre, Toronto and the Public Health Agency of Canada through the COVID-19 Immunity Task Force and Vaccine Surveillance Reference Group. We are grateful to the Canadian Donation and Transplantation Research Program for their partnership.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. DK has received a clinical trials grant and advisory fees from Roche. The other authors have no conflicts to disclose.
AUTHOR CONTRIBUTIONS
Study Design: DK, AH, VGH, VHF; Data Analysis: VGH, VHF, VK; Performance of Study: VGH, VHF, TK, MI, BM-K, VK, AY; Manuscript Writing: DK, AH, VGH, VHF.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding information Ajmera Transplant Centre, Toronto; Public Health Agency of Canada through the COVID-19 Immunity Task Force and Vaccine Surveillance Reference Group
Footnotes
Victoria G. Hall and Victor H. Ferreira share joint first authorship.
Atul Humar and Deepali Kumar share joint senior authorship.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
Supplementary Material
Table S2-S4
REFERENCES
- 1.Kates OS, Haydel BM, Florman SS, et al. COVID-19 in solid organ transplant: a multi-center cohort study. Clin Infect Dis. 2020. 10.1093/cid/ciaa1097 [DOI]
- 2.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benotmane I, Risch S, Doderer-Lang C, Caillard S, Fafi-Kremer S. Long-term shedding of viable SARS-CoV-2 in kidney transplant recipients with COVID-19. Am J Transplant. 2021. 10.1111/ajt.16636 [DOI] [PMC free article] [PubMed]
- 4.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021. 10.1111/ajt.16615 [DOI] [PMC free article] [PubMed]
- 9.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 10.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel EU, Bloch EM, Clarke W, et al. Comparative performance of five commercially available serologic assays to detect antibodies to SARS-CoV-2 and identify individuals with high neutralizing titers. J Clin Microbiol. 2021;59(2):e02257-20. doi: 10.1128/JCM.02257-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan CW, Chia WN, Qin X, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38(9):1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 13.Le Bert N, Clapham HE, Tan AT, et al. Highly functional virus-specific cellular immune response in asymptomatic SARS-CoV-2 infection. J Exp Med. 2021;218(5):e20202617. doi: 10.1084/jem.20202617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584(7821):457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 15.Taylor SC, Hurst B, Charlton CL, et al. A new SARS-CoV-2 dual-purpose serology test: highly accurate infection tracing and neutralizing antibody response detection. J Clin Microbiol. 2021;59(4):e02438-20. doi: 10.1128/JCM.02438-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirzel C, L’Huillier AG, Ferreira VH, et al. Safety and immunogenicity of adjuvanted recombinant subunit herpes zoster vaccine in lung transplant recipients. Am J Transplant. 2021;21(6):2246–2253. doi: 10.1111/ajt.16534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.L’Huillier AG, Ferreira VH, Hirzel C, et al. T-cell responses following natural influenza infection or vaccination in solid organ transplant recipients. Sci Rep. 2020;10(1):10104. doi: 10.1038/s41598-020-67172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 19.Guidance for Industry - Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials. Food and Drug Administration. Published 2007; Accessed May 22.
- 20.Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sattler A, Schrezenmeier E, Weber UA, et al. Impaired humoral and cellular immunity after SARS-CoV2 BNT162b2 (Tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Investig. 2021;131(14):e150175. doi: 10.1172/JCI150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Havlin J, Svorcova M, Dvorackova E, et al. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J Heart Lung Transplant. 2021;40(8):754–758. doi: 10.1016/j.healun.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ewer KJ, Barrett JR, Belij-Rammerstorfer S, et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med. 2021;27(2):270–278. doi: 10.1038/s41591-020-01194-5. [DOI] [PubMed] [Google Scholar]
- 24.Rabinowich L, Grupper A, Baruch R, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75(2):435–438. doi: 10.1016/j.jhep.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shostak Y, Shafran N, Heching M, et al. Early humoral response among lung transplant recipients vaccinated with BNT162b2 vaccine. Lancet Respir Med. 2021;9(6):e52–e53. doi: 10.1016/S2213-2600(21)00184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Assis RJA, Nakajima R, Jasinskas A, et al. Substantial differences in SARS-CoV-2 antibody responses elicited by natural infection and mRNA vaccination. bioRxiv. 2021. 10.1101/2021.04.15.440089 [DOI] [PMC free article] [PubMed]
- 28.Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 VACCINATION. N Engl J Med. 2021;384(1):80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Table S2-S4
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.