CONFLICT OF INTEREST
The authors declare no conflict of interest.
To the Editor
The high demand for COVID‐19 vaccines, combined with a significant lack of supply, leaves smaller and developing countries behind in mass immunization. This prompts the question whether administering a single vaccine dose in SARS‐CoV‐2 seropositive individuals could be a method for rationing available vaccine doses.
We report results from a prospective study on Macedonian healthcare workers who received two doses of the Pfizer/BioNTech BNT162b2 mRNA vaccine, comparing antibody titres and frequency of side effects after vaccine administration between individuals who were SARS‐CoV‐2 seropositive (SeroPOS group) and seronegative (SeroNEG group) prior to immunization. The study included 226 participants recruited through convenience sampling, of whom 41 were SeroPOS (73.17% female; mean age 43 years, SD: 10.571), and 185 were SeroNEG (68.11% female, mean age 46 years, SD: 10.523). Baseline patients’ characteristics are provided in the Supplementary Appendix (Table S1). Blood samples were collected 18–21 days after the first vaccine dose and 25–28 days after the second dose. Baseline antibody levels were obtained from patient records. All participants gave blood samples after the first dose and filed a questionnaire for side effects, and 189 participants (83.63%) returned for assessment four weeks after the second dose. Serological testing was performed using the commercially available quantitative CLIA anti‐SARS‐CoV‐2 RBD kit (Snibe, Shenzhen, China), 1 which targets the S1 subunit of the viral spike protein. More details on methods are available in the Supplementary Appendix.
Anti‐SARS‐CoV‐2 RBD IgG antibody levels after the first dose of BNT162b2 were on average 11.7‐times higher in SeroPOS individuals (mean: 923.40 AU/ml, SD: 948.119, range 15.04–5034.70) compared to SeroNEG individuals (mean: 79.06 AU/ml, SD: 253.243, range 0.912–1867.30; Wilcoxon rank‐sum test, p < 2e‐16, Figure 1A). After the second dose, anti‐SARS‐CoV‐2 RBD IgG antibody levels were still higher in SeroPOS individuals (mean: 602.59 AU/ml, SD: 511.545, range 25.41–1986.00) compared to SeroNEG individuals (mean: 375.567 AU/ml, SD: 437.088, range 9.617–3704.40; Wilcoxon rank‐sum test, p = 0.006, Figure 1B). SeroNEG individuals had on average a 5.35‐fold increase in anti‐SARS‐CoV‐2 RBD IgG antibody levels after the second dose (Wilcoxon signed‐rank test, p < 2e‐16, Figure 1B), whereas SeroPOS individuals had no benefit of increased antibody levels after the second dose (Wilcoxon signed‐rank test, p = 0.529, Figure 1B). SeroPOS individuals had higher antibody levels after the first dose than SeroNEG individuals after the second dose (Wilcoxon rank‐sum test, p = 0.0039, Figure 1B). Exploratory analysis of the influence of sex and age on antibody response showed that older age had a reducing effect on antibody levels after the first and second vaccine dose (Supplementary Appendix, Table S2). The vast majority of the study participants reported at least one side effect after the first dose (91.15%, Figure 1C), mostly minor local pain (69.47%). A higher proportion of study participants reported at least one side effect after the second dose (97.35%, Figure 1D), again mostly minor local pain (53.97%).
FIGURE 1.
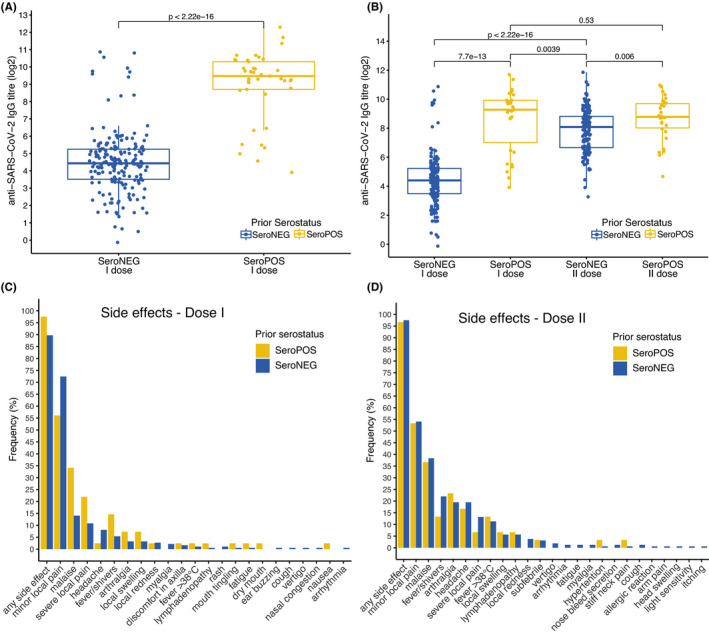
Antibody titres (log2‐transformed) and side effects after the first and second dose of the Pfizer/BioNTech BNT162b2 vaccine in patients with (SeroPOS) and without (SeroNEG) anti‐SARS‐CoV‐2 antibodies before vaccination. (A) Anti‐SARS‐CoV‐2 RBD antibody titres after the first dose of BNT162b2 in SeroPOS individuals (n = 41) versus SeroNEG individuals (n = 185), full cohort. (B) Anti‐SARS‐CoV‐2 RBD antibody titres after the first and second dose of BNT162b2 in SeroPOS individuals (n = 30) versus SeroNEG individuals (n = 159), participants who returned for analysis after the second dose. (C and D) Frequency of self‐reported side effects in SeroPOS and SeroNEG individuals after the first and second dose of BNT162b2
Our findings are in line with previous reports of higher antibody levels in SeroPOS individuals after a single dose of BNT162b2 compared to SeroNEG individuals 2 , 3 , 4 , 5 and support the hypothesis that a single dose of BNT162b2 in SARS‐CoV‐2 seropositive individuals might provide sufficient humoral immunity towards SARS‐CoV‐2. These findings should be validated in a clinical trial setting as soon as possible, due to direct implications for public health policy in developing countries with limited access to vaccines. Future investigations should incorporate analyses of the cellular immunity and take into consideration the duration of the immune response, which have not been evaluated in this study. The more rational use of vaccines could accelerate the attainment of collective immunity at reduced costs.
Supporting information
Supplementary Material
REFERENCES
- 1. Plebani M, Padoan A, Fedeli U, et al. SARS‐CoV‐2 serosurvey in health care workers of the Veneto region. Clin Chem Lab Med 2020;58(12):2107‐2111. [DOI] [PubMed] [Google Scholar]
- 2. Ebinger JE, Fert‐Bober J, Printsev I, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS‐CoV‐2. Nat Med 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krammer F, Srivastava K, Alshammary H, et al. Antibody responses in seropositive persons after a single dose of SARS‐CoV‐2 mRNA vaccine. N Engl J Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saadat S, Tehrani ZR, Logue J, et al. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS‐CoV‐2. JAMA 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prendecki M, Clarke C, Brown J, et al. Effect of previous SARS‐CoV‐2 infection on humoral and T‐cell responses to single‐dose BNT162b2 vaccine. Lancet (London, England). 2021;397(10280):1178‐1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


