Abstract
Introduction
Dexmedetomidine has been suggested to be a promising sedative for patients with Covid‐19 infection (CV19). However, use of dexmedetomidine is limited by its heart rate (HR) and arterial blood pressure lowering effects. Moreover, CV19 is associated with cardiac manifestations including bradyarrythmias. The hemodynamic effects of dexmedetomidine have not been previously studied in CV19 patients. We evaluated the effects of dexmedetomidine on hemodynamic and respiratory parameters of CV19 patients.
Methods
In this single center study, all CV19 patients receiving dexmedetomidine for sedation during a one year period were included. Our primary outcomes included changes in HR, mean arterial pressure (MAP), respiratory rate (RR), partial oxygen pressure of arterial blood/fraction of inspired oxygen‐ratio (PF‐ratio), and Richmond Agitation and Sedation Score (RASS) during dexmedetomidine administration.
Results
We identified 39 patients with a mean (SD) age of 58.3 (12.7) years. After initiation of dexmedetomidine, HR decreased by 16.9 (3.3) beats/min (95% CI 9.5–22.4; p < 0.001). During the 12‐hour follow‐up period, HR decrease was significant at 2 to 12 h. Incident bradycardia (<45/min) was reported in 12 (30.8%) patients and it was associated with lower plasma C‐reactive protein, Pro‐calcitonin, and troponin T levels. There was no change in MAP compared to baseline. Dexmedetomidine administration was associated with improvement of PF‐ratio (p < 0.001) and with decrease of RASS (p = 0.004).
Conclusions
Dexmedetomidine is an effective sedative for CV19 patients and may improve their oxygenation. However, dexmedetomidine administration is associated with marked decline in HR and with a high incidence of bradycardia in patients with CV19.
Keywords: Covid 19, critical care, dexmedetomidine, sedation
Editorial Comment.
Dexmedetomidine is a frequently used sedative and anxiolytic in critically ill patients. This analysis shows that dexmedetomidine administration in critically ill patients with COVID‐19 was associated with improved oxygenation but a marked decline in heart rate and a high incidence of bradycardia, which was associated to lower plasma C‐reactive protein, pro‐calcitonin, and troponin T levels.
1. INTRODUCTION
Patients admitted to intensive care unit (ICU) for the Covid‐19 infection (CV19) usually require sedation to facilitate their respiratory work and ventilatory support. Most available sedative drugs carry a risk for adverse reactions including respiratory depression, confusion, and circulatory depression, which may be harmful or even life‐threatening to the patient. 1 , 2 , 3 , 4
Dexmedetomidine is a relatively recent alpha‐2‐adrenoceptor activating drug registered for sedation in adult ICU patients. It may be used as an anesthetic adjunct in intubated patients, but also for light sedation in awake, spontaneously breathing patients with or without noninvasive ventilation. 5 In addition to its sedative effect, dexmedetomidine has analgesic and antiemetic effects. 6 , 7 Compared to traditional sedatives and anesthetics, dexmedetomidine has very little effect on respiration, which makes it an ideal sedative for respiratory compromised spontaneously breathing patients. 8 Furthermore, dexmedetomidine has been shown to carry both cytoprotective and anti‐inflammatory properties. 9
Dexmedetomidine has been suggested to be a promising sedative for CV19 patients 10 and to potentially attenuate the uncontrolled CV19‐associated inflammatory response. 11 However, the use of dexmedetomidine is limited by its heart rate and blood pressure lowering effects, which preclude its use in many critically ill patients. 12 Moreover, patients with CV19 show a high incidence of cardiac manifestations including bradyarrhythmias. 13 , 14 Current data on the hemodynamic effects of dexmedetomidine in CV19 patients is scarce and limited to case reports. 15 , 16
The aim of this study was to evaluate hemodynamics and respiratory parameters in critically ill CV19 patients receiving dexmedetomidine sedation. Our hypothesis was that CV19 patients may benefit from dexmedetomidine use, but in some patients, a decrease in heart rate may prevent its use. Furthermore, we aimed to study the incidence of bradycardia and associated risk factors in CV19 patients receiving dexmedetomidine.
2. MATERIALS AND METHODS
2.1. Patient population and data collection
We retrospectively identified all patients admitted to the ICU of Turku University Hospital (Turku, Finland) between March 2020 and March 2021 due to CV19 infection (ICD‐10 codes U07.1 and U07.2). All patients with an age of 18 to 80 years, confirmed CV19 infection and requiring dexmedetomidine sedation for at least 12 consecutive hours during their ICU stay were included in the analysis. Patients in whom dexmedetomidine was stopped due to bradycardia within 12 h were also included. Patients with suspected but not proven CV19 infection and patients with ICU stay of less than 24 h were excluded from the analysis.
Eligible patients were identified, and patient data were retrieved from the patient database of Turku University Hospital, Finland. Patient material was collected by retrieving data from the GE Clinisoft and Uranus medical record databases of Turku University Hospital.
2.2. Drug administration
Dexmedetomidine hydrochloride (100 mcg/ml) was diluted in NaCl 0.9% and administered as an intravenous (4 mcg/kg) infusion with a rate of 0.2–1.4 mcg/kg/h according to a prescription by the attending physician. Patients received other sedatives and analgesic opioids according to standard clinical practice. The depth of sedation was defined depending on the clinical situation of each patient, severity of respiratory failure and potential presence of patient‐ventilator dyssynchrony. Norepinephrine was initiated in patients with hypotension (MAP <65 mmHg) and discontinuated if MAP remained above 65 mmHg over an hour with a norepinephrine infusion rate of 0.005 mcg/kg/min.
2.3. Pharmacodynamic assessment
Hemodynamic effects of dexmedetomidine were assessed by recording heart rate (HR), mean arterial pressure (MAP), vasoactive requirement, and cardiac rhythm. Respiratory effects of dexmedetomidine were assessed by recording the ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PF) and respiratory rate (RR). Restlessness and the depth of sedation were assessed by recording the Richmond Agitation and Sedation Scale (RASS). We recorded HR, MAP, PF, RR, RASS, vasoactive requirement, and heart rhythm before dexmedetomidine administration and at two‐hour intervals up to 12 h after the initiation of dexmedetomidine infusion. A heart rate below 45/min was considered as significant bradycardia.
2.4. Laboratory assessment
To assess the potential effects of laboratory values on the hemodynamic effects of dexmedetomidine, we recorded blood hemoglobin, plasma, creatinine, c‐reactive protein, procalcitonin, ferritin, sodium, potassium, and interleukin‐6 at start of dexmedetomidine, and peak troponin T within 5 days before and after initiation of dexmedetomidine sedation.
2.5. Statistics
Primary outcomes of this study were changes in heart rate, mean arterial pressure, norepinephrine requirement, Richmond Agitation and Sedation Score, and PaO2/FiO2 ratio. Secondary outcomes of the study were factors associated to incident bradycardia. Results are expressed as mean values with standard deviations (SD) for normally distributed variables, and as medians with interquartile ranges (IQR) when the normality assumption was not met. The Shapiro‐Wilks test (p > 0.05) was used to assess normality assumptions. Repeated measures analysis of variance, with to use of post hoc Bonferroni correction to allow for multiple pairwise comparisons was used to assess the significance of alterations in vital signs at six different time points compared to the baseline value. The correlation/clustering between repeated measurements within a subject was taken in account by using autoregressive or unstructured correlation structures, respectively, based on Akaike's and Bayesian information criterion examination. Student's t‐test was used to compare continuous normally distributed covariates and Chi‐square test or Fisher's exact test for categorical covariates in the study subgroups. For skewed variables, the comparisons between groups were done using a nonparametric Kruskall‐Wallis test. Associations between risk factors and incident bradycardia were assessed using logistic regression and receiver operating characteristics curve analyzes. p < 0.05 (two‐tailed) was considered statistically significant. The analyzes were performed with JMP Pro 13.0 for Mac (SAS Institute Inc.).
3. RESULTS
A total of 53 patients were treated due to CV19 infection in our ICU between March 2020 and March 2021. Of these 53 patients, 39 received dexmedetomidine during their treatment. Eligible patients were identified, and patient data were retrieved from the patient database of Turku University Hospital, Finland. The mean age of the patients was 58.3 ± 12.7 years and 30.8% were females. Mean BMI of the patients was 30.6 ± 6.5 and 19 (48.7%) of the patients were obese (BMI > 30 kg/m2). The patient characteristics and comorbidities are shown in Table 1. Apart from early discontinuation of dexmedetomidine due to severe bradycardia, total duration of dexmedetomidine sedation ranged in our study patients from 12 hours to three weeks.
TABLE 1.
Patient characteristics
| Age (years) a | 58.3 ± 12.7 |
| Weight (kg) a | 94.1 ± 18.5 |
| Height (cm) a | 175.6 ± 8.3 |
| BMI a | 30.6 ± 6.5 |
| Male/Female c | 27/69% / 12/21% |
| Mean dexmedetomidine infusion rate (mcg/kg/h) a | 0.39 ± 0.12 |
| Time to dexmedetomidine administration from ICU admission (h) b | 26 (6–68) |
| Peak SOFA b | 7 (6–8) |
| SAPS II b , d | 38 (31–41) |
| CCI b , d | 1 (0–2) |
| PF‐ratio at initiation of dexmedetomidine a , d | 17.2±5.6 |
| Hourly urine output (ml/kg/h) b , d | 1.0 (0.7–1.9) |
| Creatinine (umol/L) b , d | 58 (53–72) |
| BUN (mmol/L) b , d | 5.0 (3.6–7.2) |
| INR b , d | 1.0 (0.9–1.1) |
| Bilirubin (umol/L) b , d | 7 (5–11) |
| ALAT (U/L) b , d | 44 (25–61) |
| Norepinephrine infusion rate (mcg/kg/min) b , d | 0 (0–0.003) |
| Patients requiring norepinephrine c , d | 10/25.6 |
| Patients receiving propofol c , d | 15/38.4 |
| Patients receiving midazolam c , d | 3/7.7 |
| Patients receiving neuromuscular blocking agents c , d | 2/5.1 |
| Length of ICU stay (days) b | 8.5 (4.4–22.6) |
| 30 day mortality c | 2/5.1 |
| ICU mortality c | 3/7.7 |
| Hospital mortality c | 4/10.2 |
| Comorbidities c | |
| Hypertension | 22/56 |
| Diabetes | 10/26 |
| Obstructive sleep apnoea | 6/15 |
| Obstructive pulmonary disease | 4/10 |
| Coronary artery disease | 3/8 |
| Immunosuppression | 3/8 |
| Hypercholesterolemia |
Abbreviations: ALAT, alanine aspartase transferase; BMI, body mass index; BUN, blood urea nitrogen; CCI, charlson comorbidity index; ICU, intensive care unit; INR, international number ratio; PF‐ratio, SOFA, sequential organ failure assessment; SAPS‐II, simplified acute physiology score II.
Mean and standard deviation or median.
Interquartile range except for procedures and comorbidities.
Number and percentage of patients.
Values given at initiation of dexmedetomidine infusion.
During the 12‐hour observation period after initiation of dexmedetomidine infusion, HR decreased by 15.9 (3.3) beats/min (95% CI 9.5–22.4 beats/min; p < 0.001) and was significantly lower at 2 to 12 hours (p ≤ 0.01 for all comparisons) compared to baseline values (Table 2, Figure 1). Dexmedetomidine was discontinued in two patients after 3‐ and 4‐hour infusion, respectively, due to severe bradyarrhythmia. None of the study patients developed a 2nd or 3rd degree atrioventricular block. All patients were in sinus rhythm throughout the study period. In the whole, cohort MAP remained unchanged compared to the baseline (Table 2, Figure 2). A total of 19 (49%) patients required norepinephrine during the 12‐hour follow‐up period, but there was no significant change in the norepinephrine requirement after initiation of dexmedetomidine (Table 2, Figure 3).
TABLE 2.
Primary outcomes
| Baseline | Time from dexmedetomidine administration | ||||||
|---|---|---|---|---|---|---|---|
| 2 h | 4 h | 6 h | 8 h | 10 h | 12 h | ||
| Heart rate (bpm) | 74 ± 14 | 63 ± 17** | 59 ± 13*** | 59 ± 14*** | 58 ± 12*** | 60 ± 15*** | 60 ± 14** |
| Mean arterial pressure (mmHg) | 85 ± 13 | 89 ± 12 | 91 ± 13 | 90 ± 15 | 88 ± 11 | 88 ± 12 | 88 ± 14 |
| Norepinephrine (mcg/kg/min) | 0 (0–0.00) | 0 (0–0.02) | 0 (0–0.02) | 0 (0–0.01) | 0 (0–0.01) | 0 (0–0.01) | 0 (0–0.02) |
| PaO2/FiO2‐ratio | 17 ± 6 | 19 ± 6 | 21 ± 7** | 21 ± 5*** | 20 ± 6** | 20 ± 6 | 20 ± 6* |
| Respiratory rate (1/min) | 22 ± 6 | 21 ± 7 | 20 ± 8 | 19 ± 6 | 19 ± 6 | 19 ± 5 | 18 ± 5 |
| RASS | 0 (−3–0) | −1 (−4–0) | −1 (−4–0) | −3 (−4–0)* | −3 (−4–0)** | −3 (−4–0)** | −3 (−4–0)** |
Data are shown as mean and standard deviation or as median and interquartile range.
Bpm, beats per minute; PaO2/FiO2, ratio of arterial oxygen partial pressure/fraction of inspired oxygen; RASS, richmond agitation and sedation score.
p < 0.05
p < 0.01
p < 0.001 compared to baseline.
FIGURE 1.
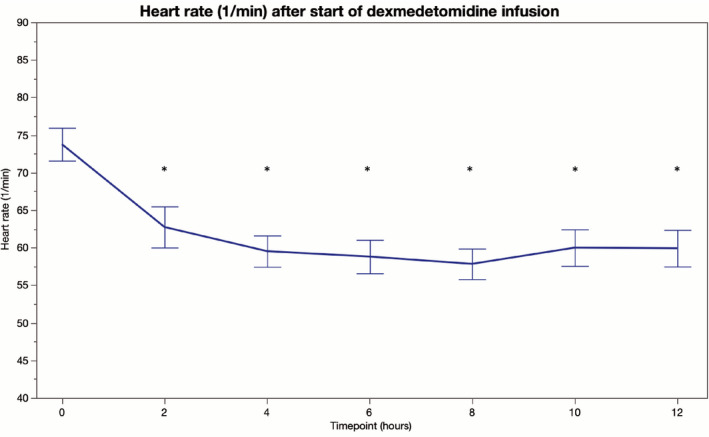
Heart rate (beats per min) before and 12 h after initiation of intravenous dexmedetomidine (*p < 0.05) [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 2.
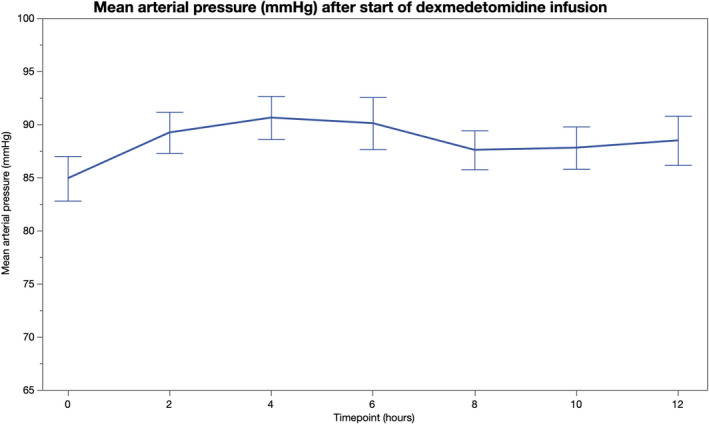
Mean arterial pressure before and 12 h after initiation of intravenous dexmedetomidine [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
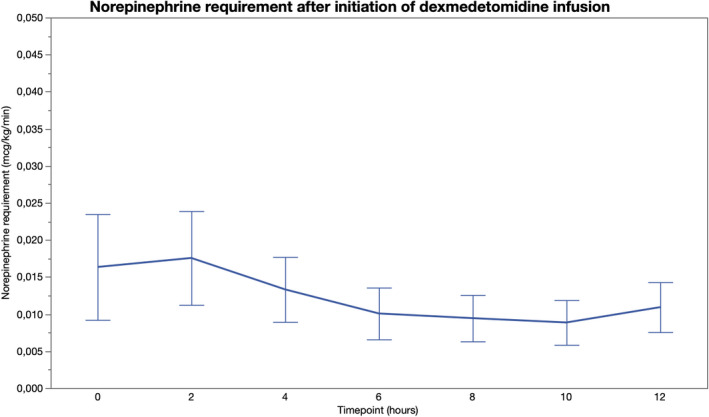
Norepinephrine requirement before and 12 h after initiation of intravenous dexmedetomidine [Colour figure can be viewed at wileyonlinelibrary.com]
Patients with incident bradycardia had lower plasma C‐reactive protein and Pro‐calcitonin at the start of dexmedetomidine infusion and lower peak Troponin T compared to others, whereas, demographic variables, use of beta blockers, severity of illness, norepinephrine requirement, peak norepinephrine requirement, mean norepinephrine infusion rate, dexmedetomidine infusion rate, and HR prior to dexmedetomidine were similar between the subgroups (Table 3). In univariate logistic regression, the only significant explanatory variables for bradycardia were C‐reactive protein [β = −0.02, OR 0.980 (95% Cl 0.963–0.998), AUC 0.77 (95% Cl 0.62–0.93), p = 0.03], Pro‐calcitonin [per 0.1 µg/l: β = −0.81, OR 0.446 (95% Cl 0.199–0.991), AUC 0.79 (95% Cl 0.64–0.94), p = 0.0498], and Troponin T [β = −0.19, OR 0.830 (95% Cl 0.694–0.992), AUC 0.83 (95% Cl 0.70–0.97), p = 0.04]. The limited number of patients with incident bradycardia did not allow for adjusted models.
TABLE 3.
Parameters during 12 h dexmedetomidine infusion by bradycardia (Heart rate <45/min)
| Variable | No bradycardia (n = 27) | Bradycardia (n = 12) | p‐value |
|---|---|---|---|
| Women | 8 (30%) | 4 (33%) | 0.82 |
| Age (years) | 58.7 ± 13.9 | 57.4 ± 10.0 | 0.77 |
| BMI (kg/m2) | 29.1 (24.6–32.1) | 31.0 (28.1–35.7) | 0.13 |
| CCI | 1 (0–2) | 0 (0–1) | 0.15 |
| Oxygen therapy/NIV/MV | 4/9/14 | 4/5/3 | 0.23 |
| Dexmedetomidine only sedative | 14 (52%) | 9 (75%) | 0.29 |
| Administration of beta blockers | 3 (11%) | 0 (0%) | 0.11 |
| Use of beta blocker before ICU admission | 3 (11%) | 1 (8%) | 0.79 |
| Maximum SOFA score | 7 (6–8) | 7 (6–7) | 0.67 |
| SAPS‐II score | 37 (29–42) | 39 (33–40) | 0.63 |
| Need for NE during first 12 hours | 14 (52%) | 5 (42%) | 0.55 |
| Mean NE dose (mcg/kg/min) a | 0.003 (0.000–0.017) | 0.001 (0.00–0.003) | 0.15 |
| NE initiated after dexmedetomidine | 6 (22%) | 3 (25%) | 0.85 |
| PaO2/FiO2 | 17.8 ± 6.1 | 15.9 ± 3.9 | 0.32 |
| Heart rate (bpm) | 76 ± 14 | 69 ± 12 | 0.11 |
| Decrease in heart rate (bpm) a | 19 ± 9 | 27 ± 11 | 0.02 |
| Mean arterial pressure (mmHg) | 85 ± 14 | 84 ± 10 | 0.73 |
| Mean dexmedetomidine rate a (mcg/kg/h) | 0.32 ± 0.13 | 0.27 ± 0.11 | 0.21 |
| Peak dexmedetomidine rate a (mcg/kg/h) | 0.64 ± 0.22 | 0.51 ± 0.22 | 0.12 |
| Hemoglobin (mg/L) | 122 ± 15 | 130 ± 14 | 0.14 |
| C‐reactive protein (mg/L) | 91 (60–135) | 37 (19–66) | 0.007 |
| Pro‐calcitonin (µg/L) | 0.27 (0.13–0.55) | 0.10 (0.07–0.18) | 0.005 |
| Troponin T (ng/L) | 18 (11–38) | 8 (6–11) | 0.001 |
| IL−6 (ng/L) | 64 (27–85) | 65 (28–85) | 0.73 |
| Ferritin (µg/L) | 1099 (583–1476) | 1240 (545–2364) | 0.47 |
| Creatinine (µmol/L) | 60 (56–76) | 60 (51–70) | 0.52 |
| BUN (mmol/L) | 5.5 (3.4–7.3) | 4.2 (3.9–6.4) | 0.70 |
| Diuresis (ml/kg/h) | 0.9 (0.7–1.4) | 1.3 (0.7–2.0) | 0.44 |
| Sodium (mmol/L) | 140 ± 4 | 140 ± 3 | 0.70 |
| Potassium (mmol/L) | 4.1 ± 0.3 | 4.2 ± 0.4 | 0.20 |
| INR | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) | 0.12 |
| Bilirubin (µmol/L) | 7 (5–11) | 7 (4–11) | 0.90 |
| ALAT (U/L) | 40 (23–61) | 55 (26–69) | 0.23 |
Values are mean ± SD or median (IQR) and given at initiation of dexmedetomidine infusion, unless stated otherwise.
Abbreviations: ALAT, alanine amino transferase; BMI, body mass index; bpm, beats per minute; BUN, blood urea nitrogen; IL‐6, interleukin‐6; INR, international number ratio; MV, mechanical ventilation; NE, norepinephrine; NIV, noninvasive ventilation; PaO2/FiO2, ratio of arterial oxygen partial pressure/fraction of inspired oxygen; SAPS‐II, simplified acute physiology score II; SOFA, sequential organ failure assessment.
During the 12 h period following initiation of dexmedetomidine infusion.
PF increased after initiation of dexmedetomidine and was significantly higher at 4, 6, 8, and 12 h compared to baseline (Table 2, Figure 4). During dexmedetomidine initiation, 17 patients were mechanically ventilated, 14 received noninvasive ventilatory support, and eight were treated with a conventional oxygen mask. Out of the 14 patients receiving noninvasive ventilation, three required intubation during the 12‐hour follow‐up. After excluding these patients from the analysis of PF‐ratio, the results remained essentially the same for all time points (p < 0.01 for 4, 6, and 8 hours and p = 0.06 for 12 h). All other patients were treated with the same respiratory support and with same sedative agents throughout the 12‐hour follow‐up. Arterial oxygen partial pressure was increased at 4, 8, 10, and 12 hours from dexmedetomidine initiation compared to the baseline, whereas, no statistically significant changes were observed in the partial pressure of arterial carbon dioxide after initiation of dexmedetomidine (Table S1). RR remained unchanged from the baseline during the five‐hour observation period (p > 0.20 for all comparisons). (Table 2, Figure 5) RASS was significantly lower at 6 to 12 h compared to the baseline. (Table 2, Figure 6).
FIGURE 4.
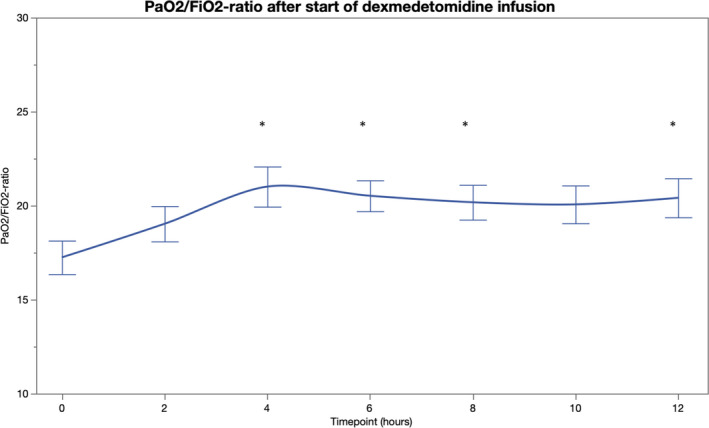
PaO2/FiO2‐ratio before and 12 h after initiation of intravenous dexmedetomidine (*p < 0.05) [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 5.
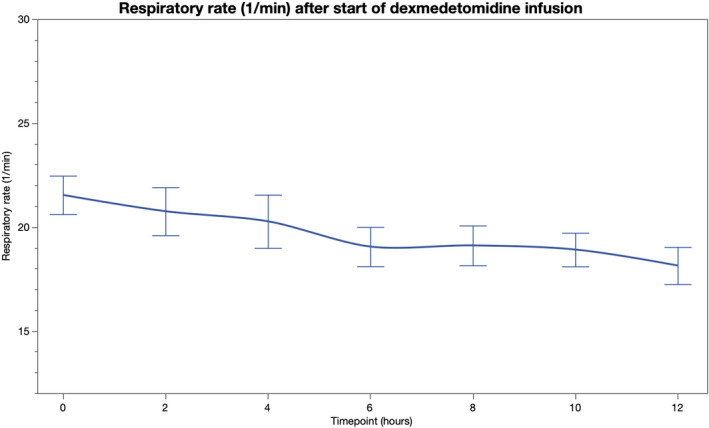
Respiratory rate (1/min) before and 12 h after initiation of intravenous dexmedetomidine [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 6.
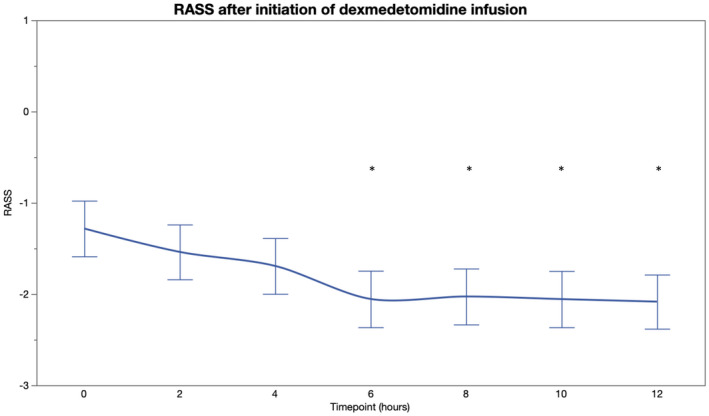
Richmond Agitation and Sedation Score (RASS) before and 12 h after initiation of intravenous dexmedetomidine (*p < 0.05) [Colour figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
This study is the first to examine the effects of dexmedetomidine sedation on hemodynamics and respiration of critically ill CV19 patients. Our results show that dexmedetomidine administration is associated with ameliorated oxygenation in critically ill patients with CV19. However, a concurrent marked decline in heart rate and a high incidence of bradycardia were observed during dexmedetomidine infusion. Incident bradycardia was associated with lower plasma C‐reactive protein, Pro‐calcitonin, and troponin T levels.
Apart from early discontinuation of dexmedetomidine in two patients with severe bradycardia, duration of dexmedetomidine sedation ranged in our study from 12 h to three weeks. A 12‐h follow‐up period was chosen, since hemodynamic and respiratory effects of dexmedetomidine typically emerge at the latest during this timeframe. Furthermore, intravenous dexmedetomidine may be used for intermittent sedation of critically ill patients to achieve a normal circadian rhythm. Thus, examining effects of dexmedetomidine during a 12‐h infusion is considered clinically relevant.
Dexmedetomidine is a centrally acting sedative and anxiolytic, which may improve oxygenation of patients with respiratory failure by reducing anxiety and discomfort. A recent meta‐analysis found that compared to other sedatives, dexmedetomidine decreased the need for mechanical ventilation in acutely ill adults requiring noninvasive ventilation. 5 In our study, oxygenation of the patients improved within 12 h after initiation of dexmedetomidine sedation. Compared to traditional analgosedatives, dexmedetomidine has little effects on spontaneous respiration, which is usually ideal for respiratory compromised patients. However, CV19 patients requiring invasive ventilation may have a high requirement for sedatives. 1 When patients are fighting the ventilator or require fully controlled ventilation to achieve adequate oxygenation, use of sedatives causing respiratory depression may be beneficial compared to sedatives with minimal effects on respiration (e.g., dexmedetomidine and ketamine). Due to the limited number of CV19 patients in our cohort, we were not able to compare the effects of dexmedetomidine to other sedatives. However, our study cohort included only critically ill patients with severe CV19, with or without the need for noninvasive or invasive mechanical ventilation corresponding the real‐world scenario of CV19 patients treated in ICUs. Interestingly, we also found that our patients had unusually low median severity of illness scores (SOFA, SAPS II, CCI; Table 1), despite the critical CV19 illness. This phenomenon has been reported also with other studies suggesting that the traditional severity of illness scoring systems do not always work properly in CV19 and may underestimate the severity and mortality of CV19 disease. 17 , 18
Dexmedetomidine may be used in deeply sedated ICU patients as well as for sedation of awake spontaneously breathing patients treated with oxygen therapy or noninvasive ventilation. In addition to ICUs, dexmedetomidine is commonly administered to patients in intermediate care units with adequate monitoring capabilities. However, hemodynamic effects of dexmedetomidine may limit its use outside the ICU. With clinically used concentrations, heart rate typically decreases 15–20% from the baseline value in healthy volunteers. 8 , 19 While hemodynamic effects of dexmedetomidine are usually well tolerated in healthy volunteers and in patients undergoing surgery, they may be detrimental to critically ill patients. Our study showed that initiation of dexmedetomidine infusion did not have a significant effect on MAP or vasoactive requirement, but heart rate decreased over 20% from the baseline. MAP even appeared to increase slightly after initiation of dexmedetomidine, despite there was no significant change compared to the baseline. In healthy volunteers, dexmedetomidine has a biphasic effect on blood pressure, since it decreases heart rate and cardiac output by centrally mediated sympatholysis, but in the same time increases vascular resistance by peripherally mediated vasoconstriction. 8 It has also been shown in critically ill that compared to propofol, sedation with dexmedetomidine decreases vasopressor requirement inspite of the decrease in HR in patients with septic shock. 20 Thus, the vasoconstrictive effect of dexmedetomidine may induce hemodynamic stability despite bradycardic effect. Considering previous reports regarding bradyarrhythmias in critically ill CV19 patients, dexmedetomidine should be used with caution in this patient population. However, bradycardia of 35–45 bpm did not decrease blood pressure or require any interventions (apart from decreasing the infusion rate or discontinuation of dexmedetomidine) in our study cohort. If dexmedetomidine appears to otherwise benefit CV19 patients, the associated bradycardia may be tolerated as long as it will be closely monitored and is not related to incident 2nd or 3rd degree atrioventricular block.
Dexmedetomidine has been shown to possess anti‐inflammatory properties. A recent correspondence suggested that dexmedetomidine could have organ protective effects in patients with CV19 infection. 11 Preclinical studies have shown that dexmedetomidine exerts renoprotective and neuroprotective effects via increasing parasympathetic tone, dampening of the inflammatory response, prevention of cell death, and inhibition of oxidative stress. 21 , 22 , 23 Thus, by up‐regulating protective proteins and attenuating cell death and systemic inflammation, dexmedetomidine could protect patients from organ failure, including pulmonary damage in critically ill CV19 patients who require sedation. 11 However, studies on the anti‐inflammatory effect of dexmedetomidine in CV19 patients are lacking and the potential anti‐inflammatory benefit of dexmedetomidine in CV19 remains to be evaluated. In this study, patients with incident bradycardia had lower levels of inflammatory markers and Troponin T and these biochemical markers were also inversely associated with bradycardia in logistic regression analyzes. The potential mechanism(s) responsible for this finding remain unclear, but it seems evident that dexmedetomidine‐associated bradycardia is not a result of the upregulated inflammation response or cardiac involvement in CV19. It has been shown that dexmedetomidine reduces sympathetic tone and catecholamine levels in plasma. 24 In subjects with severe infection, plasma catecholamine levels are often increased, 25 which may partly explain the lower incidence of bradycardia in patients with higher CRP. Similarly, high sympathetic tone may increase cardiac work and myocardial oxygen consumption leading to elevations in Troponin T. 26
A recent study evaluated the effectiveness of dexmedetomidine combined with high flow nasal oxygen and long periods of awake prone positioning in ICU patients with moderate or severe CV19 pneumonia. 27 The study found that dexmedetomidine was satisfactory for CV19 patients with moderate or severe ARDS treated with high flow nasal cannula facilitating the acceptance of long periods of awake prone positioning. However, the study lacked a control intervention. Comparative studies on the use of dexmedetomidine and other sedatives in treatment of CV19 patients are warranted.
Delirium has been shown to be common and associated with mortality in patients hospitalized with CV19. 28 , 29 Effects of dexmedetomidine on delirium in ICU patients has been studied extensively and many studies have shown that dexmedetomidine may reduce the risk of delirium compared to other sedatives. 5 , 30 There is also evidence that dexmedetomidine may reduce the duration of mechanical ventilation and ICU stay, but on the other hand hemodynamic effects of dexmedetomidine limit its use and may even increase the risk for adverse events. 28 The antidelirium effect of dexmedetomidine in CV19 patients remains to be evaluated.
Strengths of this study include reliable and highly available data. Patients treated in the ICU are monitored continuously and all vital parameters are recorded to the electronic patient database. The study included all critically ill CV19 patients receiving dexmedetomidine during a one‐year period at our ICU. Limitations of this study include its retrospective design and relatively limited number of CV19 patients treated during a one‐year period compared to large centers in countries with higher incidence of CV19. Due to the relatively low number of patients and heterogeneity of the study population, we were not able to compare dexmedetomidine to other sedatives administered. There was heterogeneity in the target for RASS due to the variation in respiratory failure severity and overall clinical state of the cohort patients. Due to the retrospective nature of the study, it is not possible to exactly know why a certain RASS target was chosen for each patient. Our current results, however, clearly indicate that dexmedetomidine infusion is associated with ameliorated oxygenation in critically ill CV19 patients but with a relatively high risk for bradycardia. However, due to the retrospective study design causality cannot be determined. Larger studies on the effects of dexmedetomidine on vital parameters of CV19 patients are warranted to justify its liberal use in this patient population.
To conclude, dexmedetomidine is an effective sedative agent for critically ill CV19 patients and may improve their oxygenation. However, use of dexmedetomidine is associated with a marked decline in heart rate and a high incidence of bradycardia in patients with CV19. Bradycardia appears to be associated to lower levels of inflammation markers and Troponin T levels.
CONSENT TO PARTICIPATE
For this retrospective, register‐based study, the regulatory review board waived the need for informed consent in terms of data collection and analysis and publication of results.
CONSENT FOR PUBLICATION
For this retrospective, register‐based study, the regulatory review board waived the need for informed consent in terms of data collection and analysis and publication of results.
CONFLICTS OF INTEREST
Panu Uusalo (PU), Mika Valtonen (MV), or Mikko Järvisalo (MJ) have no conflicts of interest that are directly relevant to the content of this article.
AUTHORS’ CONTRIBUTIONS
PU, MV, and MJ contributed to the study conception and design. Data collection and analysis were performed by PU and MJ. The first draft of the manuscript was written by PU. All authors read and approved the final manuscript.
ETHICS APPROVAL
The study protocol was approved by the Turku University Clinical Research Center scientific ethics review board and the Hospital district of Southwest Finland (T95/2021).
Supporting information
Table S1
Uusalo P, Valtonen M, Järvisalo MJ. Hemodynamic and respiratory effects of dexmedetomidine sedation in critically ill Covid‐19 patients: A retrospective cohort study. Acta Anaesthesiol Scand. 2021;65:1447–1456. 10.1111/aas.13970
DATA AVAILABILITY STATEMENT
Data that supported the findings of this study are available from the data sets of the Department of Anesthesiology and Intensive Care and the Informatics Department of Turku University Hospital on reasonable request and after permission from the Turku University Clinical Research Center scientific review board and the Hospital district of Southwest Finland.
REFERENCES
- 1. Karamchandani K, Dalal R, Patel J, Modgil P, Quintili A. Challenges in sedation management in critically Ill patients with COVID‐19: a brief review. Curr Anesthesiol Rep. 2021;11(2):107‐115. 10.1007/s40140-021-00440-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pun BT, Badenes R, Heras La Calle G, et al. Prevalence and risk factors for delirium in critically ill patients with COVID‐19 (COVID‐D): a multicentre cohort study. Lancet. Respir Med. 2021;9:239‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buckley MS, Agarwal SK, MacLaren R, Kane‐Gill SL. Adverse hemodynamic events associated with concomitant dexmedetomidine and propofol for sedation in mechanically ventilated ICU patients. J Intensive Care Med. 2020;35:1536‐1545. [DOI] [PubMed] [Google Scholar]
- 4. Aantaa R, Tonner P, Conti G, Longrois D, Mantz J, Mulier JP. Sedation options for the morbidly obese intensive care unit patient: a concise survey and an agenda for development. Multidiscip Respir Med. 2015;10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lewis K, Piticaru J, Chaudhuri D, et al. Safety and efficacy of dexmedetomidine in acutely Ill adults requiring noninvasive ventilation: a systematic review and meta‐analysis of randomized trials. Chest. 2021;159(6):2274‐2288. 10.1016/j.chest.2020.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blaudszun G, Lysakowski C, Elia N, Tramèr MR. Effect of perioperative systemic alpha2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta‐analysis of randomized controlled trials. Anesthesiology. 2012;116:1312‐1322. [DOI] [PubMed] [Google Scholar]
- 7. Liang X, Zhou M, Feng JJ, et al. Efficacy of dexmedetomidine on postoperative nausea and vomiting: a meta‐analysis of randomized controlled trials. Int J Clin Exp Med. 2015;8:8450‐8471. [PMC free article] [PubMed] [Google Scholar]
- 8. Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382‐394. [DOI] [PubMed] [Google Scholar]
- 9. Wang K, Wu M, Xu J, et al. Effects of dexmedetomidine on perioperative stress, inflammation, and immune function: systematic review and meta‐analysis. Br J Anaesth. 2019;123:777‐794. [DOI] [PubMed] [Google Scholar]
- 10. Jain A, Lamperti M, Doyle DJ. Dexmedetomidine: another arrow in the quiver to fight COVID‐19 in intensive care units. Br J Anaesth. 2021;126:e35‐e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao H, Davies R, Ma D. Potential therapeutic value of dexmedetomidine in COVID‐19 patients admitted to ICU. Br J Anaesth. 2021;126:e33‐e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ice CJ, Personett HA, Frazee EN, Dierkhising RA, Kashyap R, Oeckler RA. Risk factors for dexmedetomidine‐associated hemodynamic instability in noncardiac intensive care unit patients. Anesth Analg. 2016;122:462‐469. [DOI] [PubMed] [Google Scholar]
- 13. Hu L, Gong L, Jiang Z, Wang Q, Zou Y, Zhu L. Clinical analysis of sinus bradycardia in patients with severe COVID‐19 pneumonia. Crit Care. 2020;24:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amaratunga EA, Corwin DS, Moran L, Snyder R. Bradycardia in patients with COVID‐19: a calm before the storm? Cureus. 2020;12:e8599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsimploulis A, Rashba EJ, Rahman T, Almasry IO, Singh A, Fan R. Medication unmasked Brugada syndrome and cardiac arrest in a COVID‐19 patient. Heart Rhythm Case Rep. 2020;6:554‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xie W, Zhong Z, Li G, Hou G, Huang K, Yu Z. A comparative study on clinical effects of dexmedetomidine and midazolam on patients with severe coronavirus disease 2019 on non‐invasive ventilation. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;32:677‐680. [DOI] [PubMed] [Google Scholar]
- 17. Zou X, Li S, Fang S, et al. Acute physiology and chronic health evaluation II score as a predictor of hospital mortality in patients of coronavirus disease 2019. Crit Care Med. 2020;48:e657‐e665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stephens JR, Stümpfle R, Patel P, et al. Analysis of Critical care severity of illness scoring systems in patients with coronavirus disease 2019: a retrospective analysis of three U.K. ICUs. Crit Care Med. 2021;49:e105‐e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Colin PJ, Hannivoort LN, Eleveld DJ, et al. Dexmedetomidine pharmacodynamics in healthy volunteers: 2. Haemodynamic profile. Br J Anaesth. 2017;119:211‐220. [DOI] [PubMed] [Google Scholar]
- 20. Morelli A, Sanfilippo F, Arnemann P, et al. The effect of propofol and dexmedetomidine sedation on norepinephrine requirements in septic shock patients: a crossover trial. Crit Care Med. 2019;47:e89‐e95. [DOI] [PubMed] [Google Scholar]
- 21. Sun Y‐B, Zhao H, Mu D‐L, et al. Dexmedetomidine inhibits astrocyte pyroptosis and subsequently protects the brain in in vitro and in vivo models of sepsis. Cell Death Dis. 2019;10:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gu J, Sun P, Zhao H, et al. Dexmedetomidine provides renoprotection against ischemia‐reperfusion injury in mice. Crit Care. 2011;15:R153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma J, Chen Q, Li J, et al. Dexmedetomidine‐mediated prevention of renal ischemia‐reperfusion injury depends in part on cholinergic anti‐inflammatory mechanisms. Anesth Analg. 2020;130:1054‐1062. [DOI] [PubMed] [Google Scholar]
- 24. Uusalo P, Al‐Ramahi D, Tilli I, Aantaa RA, Scheinin M, Saari TI. Subcutaneously administered dexmedetomidine is efficiently absorbed and is associated with attenuated cardiovascular effects in healthy volunteers. Eur J Clin Pharmacol. 2018;74:1047‐1054. [DOI] [PubMed] [Google Scholar]
- 25. Benedict CR, Rose JA. Arterial norepinephrine changes in patients with septic shock. Circ Shock. 1992;38:165‐172. [PubMed] [Google Scholar]
- 26. Agewall S, Giannitsis E, Jernberg T, Katus H. Troponin elevation in coronary vs. non‐coronary disease. Eur Heart J. 2011;32:404‐411. [DOI] [PubMed] [Google Scholar]
- 27. Taboada M, Baluja A, Dos Santos L, et al. Effectiveness of dexmedetomidine combined with high flow nasal oxygen and long periods of awake prone positioning in moderate or severe COVID‐19 pneumonia. J Clin Anesth. 2021;72:110261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Watne LO, Tonby K, Holten AR, Olasveengen TM, Romundstad LG, Neerland BE. Delirium is common in patients hospitalized with COVID‐19. Intern Emerg Med. 2021. 10.1007/s11739-021-02715-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pranata R, Huang I, Lim MA, Yonas E, Vania R, Kuswardhani RAT. Delirium and mortality in coronavirus disease 2019 (COVID‐19) ‐ a systematic review and meta‐analysis. Arch Gerontol Geriatr. 2021;95:104388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang S, Hong Y, Li S, et al. Effect of dexmedetomidine on delirium during sedation in adult patients in intensive care units: a systematic review and meta‐analysis. J Clin Anesth. 2021;69:110157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
Data that supported the findings of this study are available from the data sets of the Department of Anesthesiology and Intensive Care and the Informatics Department of Turku University Hospital on reasonable request and after permission from the Turku University Clinical Research Center scientific review board and the Hospital district of Southwest Finland.


