Abstract
Expanded trinucleotide repeats underlie a growing number of human diseases. The human FMR1 (CGG)n array can exhibit genetic instability characterized by progressive expansion over several generations leading to gene silencing and the development of the fragile X syndrome. While expansion is dependent upon the length of uninterrupted (CGG)n, instability occurs in a limited germ line and early developmental window, suggesting that lineage-specific expression of other factors determines the cellular environment permissive for expansion. To identify these factors, we have established normal- and premutation-length human FMR1 (CGG)n arrays in the yeast Saccharomyces cerevisiae and assessed the frequency of length changes greater than 5 triplets in cells deficient in various DNA repair and replication functions. In contrast to previous studies with Escherichia coli, we observed a low frequency of orientation-dependent large expansions in arrays carrying long uninterrupted (CGG)n arrays in a wild-type background. This frequency was unaffected by deletion of several DNA mismatch repair genes or deletion of the EXO1 and DIN7 genes and was not enhanced through meiosis in a wild-type background. Array contraction occurred in an orientation-dependent manner in most mutant backgrounds, but loss of the Sgs1p resulted in a generalized increase in array stability in both orientations. In contrast, FMR1 arrays had a 10-fold-elevated frequency of expansion in a rad27 background, providing evidence for a role in lagging-strand Okazaki fragment processing in (CGG)n triplet repeat expansion.
Dynamic mutation, as exemplified by trinucleotide repeat expansions, is a novel pathway of DNA mutation that is known to underlie a number of major human disorders (51). In the fragile X syndrome, a common form of inherited mental retardation, instability arises in a (CGG)n trinucleotide repeat array which lies within the promoter and the 5′ untranslated region of the FMR1 gene (12, 45, 63, 71). Progressive expansion over several generations eventually leads to loss of FMR1 expression (47, 59), and this is associated with extensive de novo methylation (21) and the presence of a folate-sensitive fragile site at Xq27.3. These unstable FMR1 arrays exist in several states depending upon their length. In the range (CGG)54–200, arrays are termed premutations; they are nonpenetrant for mental impairment and are generally somatically stable, although some instability has been noted in arrays longer than (CGG)130 (70). Premutation arrays exhibit intergenerational instability through both the male and female germ lines, although the exact timing of expansion is uncertain. Arrays longer than (CGG)200, termed full mutations, are transmitted only through the female germ line; the male germ line appears to be protected against arrays of this length (49). Full mutations exhibit somatic instability in early embryogenesis (5, 68) but not at later stages (69), suggesting a window of expansion in early development. Recently, investigations have shown that expansion to full mutation can occur pre-zygotically, either in the germ-line or prior to germ-line segregation in the embryo (37, 44).
Interruptions within FMR1 arrays play a critical role in determining their instability. In the normal population, stable arrays of up to 54 triplets in length are regularly interspersed with single AGG triplets (25, 34, 56, 72). In contrast, fragile X premutations are either entirely uninterrupted or have long runs of (CGG)n at their 3′ end (7, 25, 56, 72). Expansion occurs within this uninterrupted region, and the overall degree of array instability is related to its length (7, 56). The risk of transition from premutation to full mutation in female transmission is length dependent, rising to 100% for arrays over 90 repeats (9, 12, 22). Other factors must govern the timing of expansion to account for the differential rates of triplet instability seen between male and female germ lines and for variable levels of somatic instability. In addition, attempts to model triplet repeat expansion by using transgenic mice carrying human (CAG)n and (CGG)n have found that arrays do not exhibit the same degree of instability as in humans (1, 2, 17, 19, 35, 38, 43). While the site of integration and transgene expression most probably contributes to the variable levels of instability observed, the overall low level of expansion suggests that there may be interspecies differences in triplet stability between humans and mice. Taken together, cell lineage or species-specific patterns of instability most probably reflect differences in the activity or expression of the trans-acting factors, which play a critical role in repeat expansion.
Parallels with microsatellite instability in bacteria and yeasts and with human tumors defective in DNA mismatch repair (MMR) have led to suggestions that replication slippage might play a role in triplet repeat expansion. Simple replication slippage events appear to be universally corrected by the mismatch repair system, which is primarily responsible for repairing small loops and base-pair mismatches (30). Failure to repair these leads to the accumulation of length changes in microsatellite arrays (8, 23, 58), and although these are most often decreases in repeat number, defects in MMR clearly play a role in the maintenance of genome integrity and therefore might also play a role in triplet repeat expansion. While multiple small slippage events could lead to triplet repeat length variation, dramatic expansions seen in long FMR1 (CGG)n arrays most probably occur through large slippage or exchange events (for a review, see reference 40).
Deletion of the Saccharomyces cerevisiae RAD27 gene, which encodes an endonuclease believed to be responsible for processing branched DNA structures such as those that arise during Okazaki fragment processing, was found to destabilize dinucleotide arrays, favoring repeat addition (28, 32) and also the accumulation of duplication mutations (60). It was suggested that RAD27 might therefore be involved in triplet repeat expansion (18, 33), and this has indeed been shown to be the case for the (CAG)n and (CTG)n repeats (10, 54). The mammalian RAD27 homologue, FEN-1, functions both in the processing of Okazaki fragments during lagging-strand synthesis and in the processing of branched structures which arise in long-patch base excision repair (31).
Central to most models of triplet repeat expansion is the formation of a stable secondary structure, nucleated within the triplet array, which forms in single-stranded triplet DNA during DNA synthesis. Several studies have shown that unusual structures can form within triplet arrays, including hairpins, triplexes, and quadraplexes (reviewed in reference 42). Primer extension studies have provided indirect evidence for structures which might interfere with replication (27, 29, 62), and recent in vivo studies indicate that stable structures formed in the lagging strand during (CGG)n array replication do indeed cause replication stalling (52). The ability of the cellular DNA repair pathways to detect and process these structures, should they arise, may well be critical in determining their instability.
We recently demonstrated that the stability of fragile X (CGG)n arrays cloned in Escherichia coli is dependent upon both array length and orientation with respect to replication (26). To extend this analysis further, we have now introduced several of these arrays into a single chromosomal site in S. cerevisiae and have studied the influence of various DNA repair and replication functions upon array stability.
MATERIALS AND METHODS
Isolation of recombinant human FMR1 arrays.
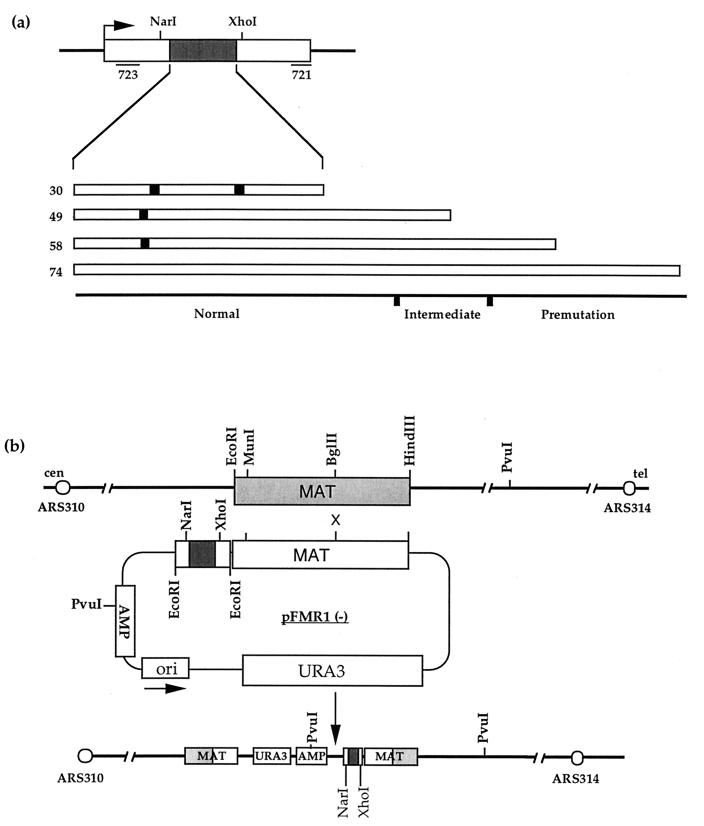
Human FMR1 arrays were amplified directly from genomic DNA of anonymous individuals of known genotype by using primers 721 to 723 as described by Hirst et al. (25) and cloned into the EcoRI site of pJH257 (3) (Fig. 1b) as described by Hirst and White (26). For the arrays FMR1-10A9A9, FMR1-9A39, and FMR1-9A48, plasmids were isolated with the array in both orientations, arbitrarily labelled as (+) or (−) as described by Hirst and White (26). For the 74-repeat array, only the (−) orientation can be stably propagated in bacteria, and so to achieve an opposite chromosomal orientation, the MAT-targeting locus was inverted by using the MunI and HindIII sites (Fig. 1b).
FIG. 1.
Integration of human FMR1 (CGG)n arrays at the S. cerevisiae MAT locus. (a) Schematic representation of human FMR1 exon 1 and the positions of the primers 721 to 723 used in the amplification of various arrays. The (CGG)n portions of the array are shown as open boxes, and the AGG interspersions are shown as solid boxes. The arrays studied have structures of 10A9A9, 9A39, 9A48 (where A represents an AGG variant repeat), and (CGG)74 and are shown above a scale indicating their status in human fragile X families. The overall length of the triplet array (including the variant AGG repeat) is indicated. (b) Schematic representation of the integration of human FMR1 (CGG)n arrays, carried in the vector pJH257, into S. cerevisiae chromosome III at the MAT locus. Integration of FMR1 arrays, shown here in the (−) orientation, results in a direct duplication of the targeted MAT locus. The positions of ARS314 and ARS310, relevant restriction sites, and the orientation with respect to the centromere and telomere are also shown.
Yeast methodologies.
All strains were isogenic with the Y55 background and are listed in Table 1. FMR1 arrays carried in the vector pJH257 (3) were transformed into yeast after linearization at the BglII site, targeting the construct for integration at the MATa locus. Transformation was performed by the standard polyethylene glycol-lithium acetate technique as described by Gietz et al. (14). Ura+ transformants were selected and analyzed by PvuI restriction analysis to confirm the correct site of integration and repeat array length. Approximately 5% of integrants were located at the HMR MATa locus as determined by Southern blot analysis. To study the degree of instability of each array, the original Ura+ transformant was dispersed to produce single colonies from which genomic DNA and a frozen glycerol stock of cells was prepared. Genomic DNA was prepared from overnight 30°C (wild type) or 2-day (rad27) 25°C 5-ml liquid cultures in yeast extract-peptone-dextrose (YPD) broth with Nucleon reagents (Nucleon Biosciences).
TABLE 1.
Genotypes of S. cerevisiae strains used in this study
| Strain | Pertinent genotype | Remaining genotype |
|---|---|---|
| Y55 2172 | MATa hoΔ his6-1 leu2Δ lys2-d ade1-1 ura3-n can1-R | |
| Y55 2203 | MATα Δho leu2Δ met13-4 cyh2-R ura3-n | |
| MH163 | mlh1::ura3::LYS2 | MATa Δho his3/4 leu2 met13-4 can1-R lys2 ura3-n |
| MH256 | msh2Δ::Kmx2 | MATa Δho his4 leu2 met13 lys2 ade1 ura3-n |
| MH274 | msh6Δ::Kmx2 | MATa Δho his4R1 leu2R lys2d ade1-1 thr4-a can1-R HML::ADE1.1 |
| MH314 | msh3Δ::Kmx2 | MATa Δho HML::ADE1.1 his4-r1 leu2-r1 lys2-d thr4-a met13-4 ura3n can1-R |
| MH275 | sgs::leu2::LYS2 | MATa Δho HML::ADE1.1 his4-r1 leu2-r1 thr4-a met13-4 ura3-n |
| MH196 | rad27Δ::Kmx2 | MATa hoΔ his6-1 leu2Δ lys2-d ade1-1 ura3-n can1-R |
| MH312 | din7Δ::Kmx2 | MATa hoΔ his6-1 leu2Δ lys2-d ade1-1 ura3-n can1-R |
| MH313 | exo1Δ::Kmx2 | MATa hoΔ his6-1 leu2Δ lys2-d ade1-1 ura3-n can1-R |
Hemizygous diploids were generated by mating with an isogenic MATα strain, strain 2203. For random spore analysis, sporulation was induced on plates containing 2% potassium acetate and spores were released from their asci by overnight incubation at 30°C with gentle shaking in water containing 2 mg of lyticase (Sigma) per ml and 300 mM 2-mercaptoethanol. After incubation, Nonidet P-40 was added to 0.75%, and the solution was chilled on ice and subjected to several rounds of sonication, centrifugation, resuspension in 1.5% Nonidet P-40, and vortexing until the spores were dispersed. The spores were then rinsed in water and plated on synthetic complete-uracil plates containing 10 μg of cycloheximide per ml to select for cells which had undergone meiosis. For tetrad dissection, asci were collected from sporulation plates containing 2% potassium acetate and subjected to glusulase digestion, and individual spores were dissected. Viability was scored, marker segregation was analyzed, and the length of the FMR1 array was determined.
Plasmid rescue.
FMR1 arrays were rescued as plasmids by excision, circularization, and transformation into bacteria. Briefly, 50 ng of genomic DNA was digested with HindIII at 37°C, heated to 65°C for 5 min, and purified with Qiaex II reagents (Qiagen Corp.). Then 2 ng of this DNA was circularized in a 20-μl ligation reaction mixture overnight at 16°C in the presence of T4 DNA ligase, after which the reaction was heat inactivated at 65°C and the products were extracted with an equal volume of phenol-chloroform, ethanol precipitated, and resuspended in 1 μl of water. This was then introduced into electrocompetent XL1-Blue MRF cells (Stratagene), plated onto selective Luria-Bertani plates containing 50 μg of ampicillin per ml, and incubated at 37°C overnight. Plasmid DNA was isolated by standard methods, and the array integrity was checked by restriction and/or sequence analysis. For fine-point triplet array restriction analysis, HinPI (which cuts 1 bp 5′ of the first CGG triplet and 12 bp 3′ of the last CGG triplet) was used to excise the array. Secondary digestion with MnlI was used to detect the position of AGG interspersions, and Fnu4HI (which recognizes the sequence 5′-GCGGC) was used to digest the FMR1 array to completion.
KanMX disruption of the RAD27 gene.
The rad27 deletion mutant strain was made by PCR-based gene disruption with the KanMX module as described by Wach et al. (64). Briefly, a disruption cassette was constructed by PCR amplification of the KanMX module by using primers with 40 bp of homology to the flanking region of the RAD27 open reading frame. The PCR product was purified and used directly for transformation of strain 2172. G418-resistant transformants were selected for 5 days at room temperature on YPD plates containing 400 μg of G418 per ml. Correct deletions were confirmed by PCR analysis with flanking and KanMX module primers. rad27 mutants were grown at room temperature (24°C) because they are temperature sensitive (4, 48, 57). The primers used were Rad27-5′ (5′-TGCCAAGGTGAAGGACCAAAAGAAGAAAGTGGAAAAAGAACCCCCatcgatgaattcgagctcg) and Rad27-3′ (5′-CGGTGGGCAGTTGACCAATGAAGCCGGTGAAACAACGTCACACTTGcgtagcctgcaggtcgac) (where capital letters indicate RAD27 flanking homology and lowercase letters indicate the KanMX module homology). Amplification conditions were as follows: an initial denaturation at 95°C for 5 min followed by 35 cycles of 95°C for 30 s, 55°C for 45 s, and 70°C for 5 min. Each 20-μl reaction mixture contained 20 mM Tris-HCl (pH 8.8), 10 mM KCl, 1.5 mM MgCl2, 10 μM (NH4)2SO4, 0.1% Triton, 100 μg of bovine serum albumin per ml, 0.5 μM each oligonucleotide, 200 μM each deoxynucleoside triphosphate, 5% dimethyl sulfoxide, and 1 U of wild-type Pfu polymerase (Stratagene). Transformation was carried out as described above, except that cells were incubated overnight in YPD medium at 4°C to allow expression of G418 resistance. Methyl methanesulfonate sensitivity was tested by plating in a dilution series onto YPD plates containing 0.03% methyl methanesulfonate. Temperature sensitivity was tested by comparing the growth of a serial dilution series on YPD plates at 24 and 37°C.
KanMX disruption of the EXO1 and DIN7 genes.
Disruption cassettes for the EXO1 and DIN7 genes were made from strains carrying KanMX module deletions of these genes. The deletion cassettes were amplified by PCR with primers homologous to flanking genomic DNA under the conditions described above. The primers used were Din7-5′ (5′-GCTCAACGGGATAGAAGTTGAatcgatgaattcgagctcg), Din7-3′ (5′-AGGTGAGTCCAGGATGTACGcgtagcctgcaggtcgac), Exo1-5′ (5′-AATAGTGATGTAACAGCGCCCatcgatgaattcgagctcg), and Exo1-3′ (5′-TTGATAGCGAATGTAGACCGCcgtagcctgcaggtcgac).
Genomic array analysis.
All FMR1 array lengths in genomic DNA were assessed by restriction analysis with EcoRI and/or XhoI-NarI. Genomic DNA fragments were resolved on 2% agarose (Nusieve GTG, normal agarose [50:50]), transferred onto Hybond-N+ (Amersham Int.) with 0.4 M NaOH, and detected by hybridization to radiolabelled human FMR1 exon 1 probe. Hybridization was performed at 65°C for 16 h in buffer containing 0.5 M sodium phosphate (pH 7.2), 7% sodium dodecyl sulfate, 1 mM EDTA, and 5% dextran sulfate, after which the filters were washed to a stringency of 0.5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at the same temperature and exposed to Hyperfilm (Amersham Int.) for 5 to 48 h. Length changes were estimated by comparison to DNA size markers, and where any mosaicism was present in the array length, colonies were scored as containing an array of altered length if the mosaicism was greater than 10%.
Statistical analysis of instability.
To test the significance of the differences between arrays in various replicative conformations or mutant backgrounds, the distributions of events (contractions, expansions, and no length changes) were compared in a standard G test. The G test is equivalent to a contingency chi-square test but allows for classes with zero events. For example, we observed 3 contractions, 1 expansion, and 67 no changes with the 9A48 (−) array in the wild-type background and 15 contractions, 0 expansions, and 55 no changes with the 9A48 (+) array. This gives a G value of 11.2945, which is significant at P < 0.005 with 2 degrees of freedom. We can therefore conclude that these arrays exhibit a significant orientation-dependent difference in their instability.
RESULTS
Establishment of human FMR1 arrays at the S. cerevisiae MAT locus.
Human FMR1 arrays with different internal structures and lengths (Fig. 1a), representative of normal [(CGG)10AGG(CGG)9AGG(CGG)9 (abbreviated as 10A9A9)], intermediate (9A39), and premutation [9A48 and (CGG)74] arrays, were introduced into the MAT locus in both replicative orientations on S. cerevisiae chromosome III (Fig. 1b). This experimental system has been used to study meiosis (3) and mitotic recombination (66). The arrays are flanked with 220 bp of human FMR1 exon 1, which was coamplified from normal individuals (10A9A9 and 9A39) or fragile X premutation carriers [9A48 and (CGG)74]. These arrays have been successfully maintained as plasmids in E. coli (26), with the exception of the FMR1-(CGG)74 array, which is extremely unstable in one replicative orientation. To study this particular array in both replicative directions, the MAT homologous fragment was inverted to drive integration in the (+) orientation. Integration of the arrays into the MAT locus results in the generation of a nontandem duplication. DNA from primary transformants was prepared and analyzed to establish the array integrity prior to any stability investigations.
PCR amplification of the FMR1 (CGG)n arrays is problematic, presumably due to the repetitive, GC-rich nature of the repeat arrays. We found that PCR analysis was unreliable for detecting expansion events due to the preferential amplification of shorter arrays, from DNA prepared directly from colonies and from liquid cultures (data not shown). Mixing experiments showed that in the presence of only a small percentage of the short array, the longer arrays failed to amplify, and that this was more pronounced with longer arrays (data not shown). Presumably, most colonies contain a small fraction of contracted arrays, similar to our observations with bacteria (26). We therefore chose to score all array length changes by Southern blot analysis of restriction fragments. Whole yeast genomic DNA was digested with EcoRI, and the length of the array was assessed by hybridization to a human FMR1 exon 1 DNA probe. Using this approach, we can reliably detect array length changes greater than ±5 repeat units.
During initial establishment of the FMR1 premutation length 9A48 and (CGG)74 arrays, we observed several events associated with transformation where strains carried a mixture of expected and expanded arrays. These arose only in the (−) orientation and ranged from +5 to +30 repeats (data not shown). Similar events, but of much greater length, have been described with a (CGT)130 array by Freudenreich et al. (10), who were uncertain of their origins. It is most likely that they arose during the process of integration, since no expansions have been observed in DNA preparations from these plasmids (26). These expansion events were not studied further.
Human FMR1 arrays show an orientation-dependent expansion and contraction in a wild-type background.
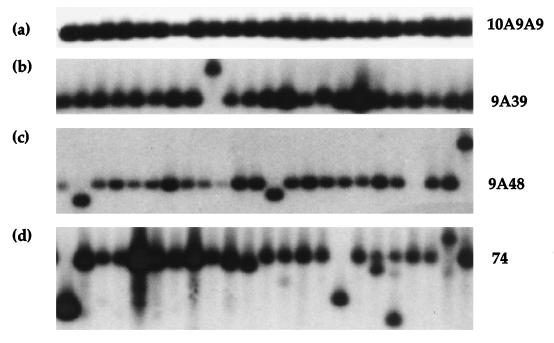
We assessed FMR1 array stability in a haploid state, initially in the wild-type Y55 background. Cells from strains carrying verified arrays were dispersed for single colonies, DNA was prepared from small overnight cultures of between 48 and 71 sib colonies, and the FMR1 array length was assessed by Southern blot analysis. A frozen glycerol stock was also made to allow repropagation. The data obtained is summarized in Table 2, and a typical hybridization analysis for each array in the (−) orientation is shown in Fig. 2. As can be seen, expansions occur at a low frequency in the (−) orientation 9A39, 9A48 and (CGG)74 arrays, with length changes of up to +40 repeats (Fig. 2b, 2c, and 2d). The overall pattern of length changes appears to be highly orientation dependent, with only contraction in the (+) orientation and both contraction and expansion in the (−) orientation.
TABLE 2.
Length changes in FMR1 (CGG)n arrays in mitotically dividing wild-type Y55-2172 cells
| Array | Orientation | No. of strains | No. (repeats) of expansions (%) | No. (%) of contractions |
|---|---|---|---|---|
| 10A9A9 | (+) | 48 | 0 | 0 |
| 10A9A9 | (−) | 48 | 0 | 0 |
| 9A39 | (+) | 60 | 0 | 7 (11.7) |
| 9A39 | (−) | 60 | 2 (+5, +30) (3.3) | 1 (1.7) |
| 9A48 | (+) | 70 | 0 | 15 (21.4) |
| 9A48 | (−) | 71 | 1 (+40) (1.4) | 3 (4.2) |
| 74 | (+) | 45 | 0 | 39 (86.7) |
| 74 | (−) | 50 | 2 (+25, +30) (4) | 10 (20) |
FIG. 2.
FMR1 (CGG)n length variation in mitotically dividing wild-type S. cerevisiae. FMR1 array length stability was assessed by EcoRI analysis of whole yeast genomic DNA made from sibling colonies carrying 10A9A9(−) (a), 9A39(−) (b), 9A48(−) (c), and (CGG)74(−) (d) arrays. In each panel, the expected fragment for the expected array length [10A9A9, 329 bp; 9A39, 386 bp; 9A48, 413 bp; and (CGG)74, 461 bp] is indicated.
The frequency of contraction increases in both orientations as the overall length of uninterrupted (CGG)n increases. The interrupted 10A9A9 array exhibited no length changes in 48 strains examined in either orientation. With the intermediate-sized 9A39(−) array, we observed two expansion events of +5 and +30 repeats (2 of 60 colonies; 3.3%), whereas with the 9A39(+) array, we saw 7 of 60 colonies (11.7%) carrying contractions ranging from −5 to −20 repeats. With the 9A48 array, we saw a significant effect of orientation upon stability (P < 0.005); 1 of 71 colonies (1.4%) carried an expansion of +40, and 3 of 71 colonies (4.2%) carried contractions in the (−) orientation, whereas we saw no colonies carrying expansions and 15 of 70 colonies (21.4%) carrying contractions with the 9A48(+) array. The levels of instability of the 9A39 and 9A48 arrays are not significantly different. The (CGG)74(+) array is significantly more unstable, with 39 of 45 colonies (86.7%) carrying contracted arrays. Again, in contrast, the opposite orientation appeared more stable (10 of 50 colonies [20%] with contractions) and prone to expansion (2 of 50 colonies [4%]).
To verify that these length changes were due to alterations in triplet repeat number, several strains carrying expansions were analyzed in more detail. Initially, fine-point restriction mapping of restriction sites in human FMR1 exon 1 (NarI and XhoI) which lie 25 bp proximal and 10 bp distal with respect to the (CGG)n array showed that length changes were restricted to the region between these two sites (data not shown), highly suggestive of alterations in repeat copy number. To confirm this, several integrated plasmids carrying (CGG)95 and (CGG)105 from the FMR1 (CGG)74(−) expansions were rescued by circularization and subjected to sequence analysis. By using primers at both ends, only CGG triplets were observed (data not shown). This confirms that array expansion in these cells is due to an increase in the number of triplet repeats.
Expanded triplet repeats in humans exhibit pronounced intergenerational length changes, and this is commonly attributed to instability during meiotic cell division, even though this represents only a single stage along the pathway of germ line and embryonic development. To address whether there may be a fundamental property of meiosis that destabilizes expanded arrays, we examined progeny from a single round of meiotic division. Initially, hemizygous diploid strains were made for each array in a wild-type background and the length of arrays in meiotic progeny carrying the URA3 marker was assessed by random spore analysis. As can be seen from Table 3, we detected no difference in the frequency of array length changes in random spore analysis; the frequency was similar to that in mitotically dividing cells. To ensure that we had not selected against cells in which array instability may have induced recombination and loss of the URA3 marker, we also performed array length analysis after tetrad dissection. We observed no increase in events leading to loss of this interval and no meiotic events, since all array length changes were found in both spores from a single meiosis. These are most likely to have been preexisting mitotic changes. Thus, there appears to be no fundamental property of premeiotic DNA replication, at least in yeast, that causes triplet array destabilization.
TABLE 3.
Length changes in meiosis in FMR1 (CGG)n arrays
| Diploid | Array | Orientation | No. of strains | No. (repeats) of expansions (%) | No. (%) of contractions |
|---|---|---|---|---|---|
| Y55-2172/Y55-2203 | 10A9A9 | (+) | 48 | 0 | 2 (4.2) |
| 10A9A9 | (−) | 48 | 0 | 0 | |
| 9A39 | (+) | 48 | 0 | 4 (8.3) | |
| 9A39 | (−) | 48 | 0 | 1 (2.1) | |
| 9A48 | (+) | 48 | 0 | 11 (22.9) | |
| 9A48 | (−) | 48 | 0 | 2 (4.2) | |
| 74 | (+) | NTa | |||
| 74 | (−) | 48 | 3 (+5, +10, +20) (6.3) | 12 (25) | |
| Tetrads | 9A48 | (+) | 96b | 0 | 24c (25) |
| 9A48 | (−) | 96b | 2c (2.1) | 8c (8.3) |
NT, not tested.
Arrays from Ura+ spores from 48 meioses analyzed.
Identical length changes were found in both sister spores from each meiosis.
In summary, human FMR1 (CGG)n arrays exhibit an orientation-dependent instability in mitotically dividing cells. We have observed a small number of expansion events in arrays carrying uninterrupted lengths of triplet repeats. These expansions are in the range of +5 to +40 repeats, similar to the length changes which occur in small human premutation arrays.
Effect of DNA MMR and Sgs1p helicase deficiency.
To test the effect of the yeast mismatch repair pathways upon FMR1 triplet stability, we studied various arrays in mlh1, msh2, msh3, and msh6 mutant backgrounds. We observed no increase in the frequency of large expansion events with any MMR mutant strain examined (data not shown). Due to limitations in our assays, however, we cannot exclude an alteration in the frequency of small incremental changes which are beyond the limit of detection by Southern blotting. For msh2 and mlh1 mutants, we did detect several large expansion events at the wild-type frequency in various arrays, indicating that events leading to such large expansions do not require the presence of these proteins (data not shown).
In the sgs1 mutant background, which is deficient in the Sgs1p helicase, we did observe a significant effect upon array instability (Table 4). The bias toward array contraction in the 9A48(+), (CGG)74(+), and (CGG)74(−) arrays was significantly diminished. The pattern of array length changes was significantly different to those in the wild-type background. With the 9A48(+) array, we detected significantly increased stability (P < 0.005), and for the (CGG)74 arrays, we detected a similar effect in both orientations (both significant at P < 0.005). To ensure that this effect was not due to alterations in the FMR1 array structure, arrays were rescued from the 9A48(+) and 9A48(−) strains and their structures were confirmed by MnlI and Fnu4HI restriction analysis. The sgs1 mutant background therefore appears to exert a generalized effect which stabilizes (CGG)n arrays.
TABLE 4.
Length changes in FMR1 (CGG)n arrays in sgs1, rad27, exo1, and din7 cells
| Mutation | Array | Orientation | No. of strains | No. (repeats) of expansions (%) | No. (%) of contractions |
|---|---|---|---|---|---|
| sgs1 | 9A48 | (+) | 69 | 0 | 1 (1.4) |
| 9A48 | (−) | 68 | 0 | 4 (5.9) | |
| 74 | (+) | 45 | 0 | 5 (11.1) | |
| 74 | (−) | 48 | 0 | 1 (2.1) | |
| rad27 | 10A9A9 | (+) | 46 | 0 | 9 (19.6) |
| 10A9A9 | (−) | 48 | 3 (+20, +20, +40) (6.25) | 2 (4.2) | |
| 9A48 | (+) | 45 | 2 (+5, +5) (4.4) | 11 (24.4) | |
| 9A48 | (−) | 92 | 16 (+5 to +50)a (17.4) | 18 (19.6) | |
| exo1 | 9A48 | (+) | 48 | 0 | 8 (16.7) |
| 9A48 | (−) | 48 | 0 | 2 (4.2) | |
| din7 | 9A48 | (+) | 48 | 0 | 5 (10.4) |
| 9A48 | (−) | 48 | 0 | 4 (8.3) |
In addition, a further 4 of 92 colonies showed evidence of expansion during sibling colony growth.
Loss of Rad27p leads to increased instability.
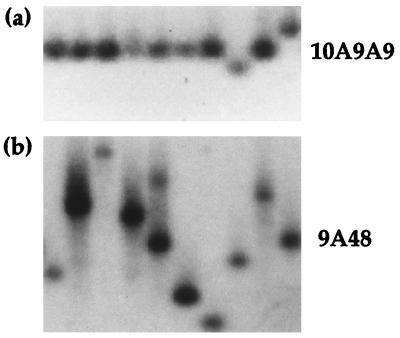
The Rad27 protein has been suggested to play a role in repeat instability and has recently been found to affect the stability of (CAG)n and (CTG)n arrays (10, 54). We therefore chose to test the stability of the premutation FMR1-9A48 and normal 10A9A9 arrays in a rad27 mutant background. The results of sibling colony analysis are summarized in Table 4, and a typical example is shown in Fig. 3. We observed significant destabilization of FMR1 arrays in the rad27 background, with increases in both array contraction and expansion. For example, with the 9A48(−) array, we found highly significant destabilization of the array compared to the wild type (P < 0.001). In this strain, we observed a 10-fold increase in the frequency of expansion of the 9A48(−) array (16 of 92 colonies [17.4%] carrying expanded arrays) with increases up to +50 repeats, as can be seen in Fig. 3b. We also observed a low frequency of expansion events in the 9A48(+) array for the first time (2 of 45 colonies [4.4%]), with length increases of +5 to +10 repeats, although the overall pattern of destabilization in this strain is not significant (P < 0.1). Further evidence for an elevated frequency of mitotic expansion was found in the presence of colonies mosaic for the expected and expanded array lengths (4 of 92 colonies). A number of DNA preparations contained two lengths of array, as shown in Fig. 3b (track 5), suggesting that the expansion event must have occurred during very early colony growth. In such mosaics, there is no relationship between the lengths of arrays present. For example, in Fig. 3b (track 5), the cells carry the normal 9A48 array and an expansion of +40 repeats. Thus, increases in array length do not appear to involve reciprocal exchanges of repeats between arrays. To confirm that these were triplet based, integrated plasmids from several strains carrying expanded arrays were rescued into E. coli and mapped by using fine-point restriction analysis with HinPI, MnlI, and Fnu4HI digestion. The arrays from strains shown in Fig. 3a tracks 2 (+30) and 4 (+25) have restriction maps consistent with array structures of 9A80 and 9A75, respectively (data not shown).
FIG. 3.
FMR1 array length instability in the rad27 mutant background. FMR1 array lengths for rad27 mutant strains carrying a 10A9A9(−) array showing contraction of 10 repeats in track 8 and an expansion of +10 repeats in track 10 (a) and a 9A48(−) array showing array expansions in tracks 2, 3, 4, and 9, a mosaic strain with an expansion which arose during colony growth (track 5), and array contractions in tracks 1, 6, 7, and 8 (b). In each panel, the position of the expected array length (as in Fig. 2) is indicated.
In addition to the increased number of observed expansion events, the frequency of array contraction in the rad27 mutant background increased in both orientations, with length changes ranging from −5 to −50 repeats. Attempts to establish the FMR1 (CGG)74 array in the rad27 background were unsuccessful since it was too unstable. With the interrupted 10A9A9(−) array, we observed a significantly increased level of instability (P < 0.001) compared to wild-type cells with both contractions (2 of 48 colonies [4.2%]) and expansions (3 of 48 colonies [6.25%]) (Fig. 3a and Table 4), the only expansion events observed for the 10A9A9(−) array in any of the genetic backgrounds tested. The 10A9A9(+) array also exhibited an elevated frequency of contraction (9 of 46 colonies [19.6%]), but no expansions were detected. Interestingly, expansion and contraction sizes appear to be in multiples of 10 repeat units, suggesting the loss or duplication of whole subunits of the array. The structure of these expansions is currently under investigation.
Effect of loss of EXO1 and DIN7.
To investigate the possible roles of other members of the RAD2 family of structure-specific nucleases in triplet expansion, deletions of the DIN7 (41) and EXO1 (61) genes were made and array stability was examined. As can be seen in Table 4, we observed no effect upon FMR1 array expansion or contraction in these mutant backgrounds.
DISCUSSION
We have described the first investigation into the stability of human fragile X (CGG)n arrays in the yeast S. cerevisiae. We have found that these triplet arrays exhibit an orientation-dependent expansion in a wild-type background, with increases of +5 to +40 repeats in small premutation and intermediate-sized FMR1 arrays, similar in size to those occurring in humans carrying alleles of similar lengths. The arrays also exhibit an orientation-dependent contraction, with shorter interrupted arrays exhibiting the highest level of stability and longer uninterrupted arrays exhibiting pronounced instability. Previous studies with arrays of similar length (CAG)n have not reported examples of expansion (39, 53) and this difference most probably reflects the greater instability of (CGG)n arrays, although there may also be an influence of flanking human FMR1 sequences included in our study. A higher degree of instability for (CGG)n arrays is further supported by our observations of transformation-associated expansion of the FMR1 9A48 and (CGG)74 arrays, similar to those observed with the longer (CGT)130 array by Freudenreich et al. (10), although the events we observed were smaller than those seen by Freudenreich et al. Since DNA secondary structure is most probably an important determinant of instability, this almost certainly reflects the differing structural potentials of the component triplet repeats (13, 52).
Expanded trinucleotide arrays exhibit a high degree of intergenerational length changes in human pedigrees, a feature which is frequently ascribed to meiosis-specific events, although meiotic division represents only a single stage in the generation of mature germ cells. To determine if the process of meiosis itself might induce increased rates of array length changes, we assessed the stability of our FMR1 arrays through meiosis in a wild-type background. We observed no length changes which could be attributed specifically to meiotic processes; all the length changes we observed were seen in both sister spores and were identical in size, suggestive of preexisting mitotic changes. In the sample size of 48 pairs of dissected spores, we would have been able to detect a meiotic event if the meiotic rate was 7% or greater. While this is the first study of meiotic changes of long (CGG)n arrays in yeast, previous studies on the rates of length changes in shorter dinucleotide arrays found no evidence for increased rates of instability in meiosis (58).
The DNA MMR system is known to influence the stability of tracts of dinucleotide repeat (8, 23, 58), and Schweitzer and Livingston (53) were recently able to show a similar influence on (CAG)n arrays, with pms1 and msh2 mutant cells having an increased frequency of 1 and 2 repeat unit changes, mostly deletions. Due to the difficulties with PCR amplification of our (CGG)n arrays, we were unable to study small changes in array length and have focused on larger changes of >5 triplets. We observed no effects on the frequency of these events in the mutant backgrounds discussed here.
We investigated the role that the yeast Sgs1 protein, the yeast homologue of the E. coli RecQ and the human BLM and WRN proteins, might play upon triplet array instability in light of reports which suggested tandem repeats instability within the cells of Bloom’s syndrome patients (20), although some studies found no instability (11). In addition, the Sgs1 protein is known to be involved in the maintenance of genome stability in yeast; its absence is characterized by an increase in mitotic recombination (66). Our results were surprising in that we observed a significant decrease in the frequency of array contractions in the 9A48(+) array and also in the (CGG)74(+) and (CGG)74(−) arrays in the sgs1 mutant background, suggesting that this helicase can influence triplet instability. While its molecular function is still unclear, the decreased frequency of array contraction might suggest that the secondary-structure formation underlying contraction is decreased. This might be due to a general cellular decrease in the replication rate, resulting in less single-stranded lagging-strand template, or to altered torsional stresses in locally unwound DNA in the absence of the Sgs1 helicase. This suggests that an additional influence upon array instability is local chromosome structure. Since both (CGG)n (15) and (CTG)n (16, 65) arrays induce unusual nucleosomal arrangements, the resulting chromatin environment surrounding these arrays may well also affect their propensity to form secondary structure and hence will affect their stability.
The overall pattern of array contraction which we observed with our integrated FMR1 (CGG)n arrays in yeast is very similar to that observed in bacteria with the same arrays, where contraction was more pronounced in one orientation and increased in proportion to array length (26). In these bacterial studies, the direction of replication fork passage through the triplet array was known. This led us (26) and others (46, 55) to suggest that differing structural propensities exist for the component strands of the triplet repeat when it is transiently single stranded as the lagging-strand template. In FMR1, with the (CGG)n as the lagging-strand template, we observed a dramatic tendency for array contraction (26). In addition, others have found that replication through plasmid-borne triplet repeats can also lead to replication stalling within the (CGG)n array (52). While we have been unable to directly examine the progression of replication forks through our integrated FMR1 (CGG)n arrays in yeast, we believe that the orientation bias in array contraction closely parallels their behavior in bacteria. Based upon this similarity, we suggest that replication progresses through the arrays from the telomeric side. An important difference between our observations in yeast and previous studies in bacteria is the occurrence of array expansions of between +5 and +40 triplets in the (−) orientation. If our conjecture about replicative orientation is correct, this suggests that expansion occurs when the (CGG)n strand is the newly synthesized lagging strand. Thus, as we discuss below, stable (CGG)n strand secondary structure may well account for both contraction and expansion. In the MAT region of chromosome III, several genomic elements which can act as replication origins have been identified; these include the ARS310 35-kb centromeric and ARS314 24-kb telomeric elements, both of which appear to be strong cellular replication origins (44a). Studies to confirm this and to study any effect upon replication stalling are under way.
Absence of Rad27p increases the frequency of (CGG)n expansion, suggesting a role for the processing of branched DNA structures in the process of triplet repeat expansion (Fig. 4). We saw a 10-fold increase in the frequency of expansion events in the 9A48(−) array and expansion in the punctuated 10A9A9 array. Analysis of several expanded 9A48 arrays showed that expansion had occurred within the long 3′ uninterrupted portion of the array. In addition, we observed a small number of expansions with the 9A48(+) array which were never seen in other backgrounds tested. Our observations with (CGG)n arrays are similar to those which have been described for both (CAG)n and (CTG)n arrays (10, 54). When taken together with these reports, these data provide strong evidence that expansion of all triplet repeats involved in human disease occurs through similar mechanisms. Expansion occurs as a result of errors in DNA synthesis, most probably during replication, although it might also occur during nonreplicative DNA repair, since human FEN1 has been shown to be required for long-patch base excision repair (31).
FIG. 4.
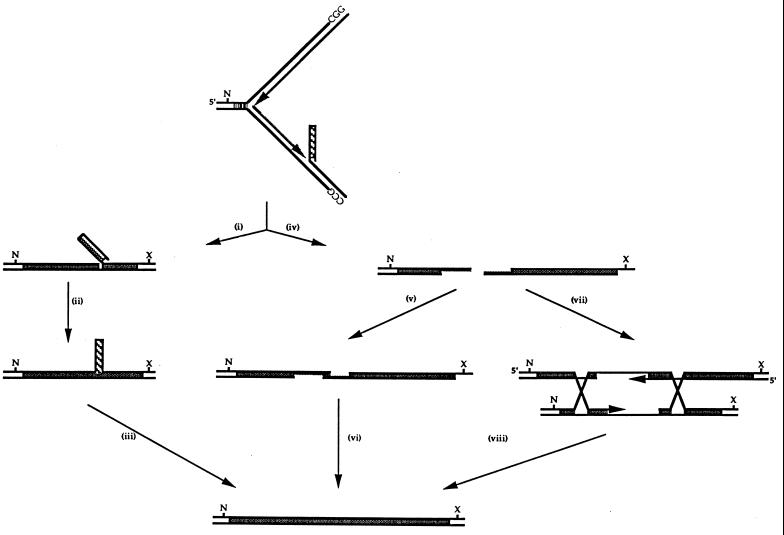
Model of FMR1 array contraction and expansion during in DNA synthesis. FMR1 (CGG)n expansion occurs predominantly in the (−) orientation, where replication proceeds from ARS314 and the lagging strand is G rich. The rare expansion events in the (+) orientation seen in the rad27 background could arise by the same mechanism upon replication from the opposite direction. We suggest that expansions in wild-type cells occur due to the propensity for stable secondary-structure formation of the G-rich strand to drive the formation of a Rad27p-resistant flap and that subsequent displacement synthesis occurs. After RNA primer removal by RNase H, displacement is probably required for the removal of the last ribonucleotide by Rad27p, since it requires single-stranded template for its activity. Resolution of this incompletely replicated region may occur through several pathways, including bypass replication (i to iii) by several possible routes following strand breakage and 5′-to-3′ excision (iv). Single-strand annealing of the repetitive ends (v) would be followed by DNA synthesis and ligation (vi). Alternatively, recombination with the sister chromatid as template could allow expansion (or contraction) if invasion and annealing occurred out of register (vii and viii). In a rad27 mutant, absence of this protein allows single-stranded-DNA secondary-structure formation to proceed unhindered as displacement synthesis from the 5′ Okazaki fragment progresses through the unprocessed flap region. Similar events could take place during DNA repair processing.
We propose that FMR1 array expansion occurs when two requirements are met, i.e., that the newly synthesized lagging strand is G rich and that an Okazaki fragment is initiated within the (CGG)n array. The process of expansion is initiated when continued polymerization of the lagging strand displaces the (CGG)n Okazaki fragment as a flap, leading to the formation of a stable G-rich secondary structure (Fig. 4). The length dependency of expansion most probably reflects the likelihood that an Okazaki fragment is synthesized within the (CGG)n array itself, as suggested by Richards and Sutherland (50). Since we see expansion in a wild-type background, formation of secondary structure within the displaced strand must occur rapidly enough to compete with its processing. In the absence of Rad27p, the rate of expansion increases as flap processing, presumably being performed by a compensating protein, becomes much less efficient. Furthermore, since G-rich structures appear to go undetected in the lagging-strand template, where they give rise to contractions, similar structures in the displaced flap will also go undetected.
To account for array expansion, the displaced lagging strand must be processed or repaired to allow the addition of triplet repeats. Tishkoff et al. (60) suggested that most stalled and displaced structures give rise to double-strand breaks (DSBs) and that repair occurs via single-strand annealing or recombination-based pathways (Fig. 4, steps iv to viii). Single-strand annealing would incorporate the additional triplets generated during displacement synthesis. To generate an expanded array by recombination, strand alignment must occur with the triplet repeats out of register, so that extension synthesis adds triplet repeats to the broken ends. An alternative pathway (Fig. 4, steps i to iii), proposed by Tishkoff et al. (60) and proposed to account for small length changes in microsatellites by Kokoska et al. (32), suggests that displaced repeats persist and are processed in the next round of replication as expansions. To account for the large length increases of up to +40 repeats, this would mean the persistence of large looped structures. In support of the occurrence of DSBs, we have also observed length-dependent elevated rates of intrastrand recombination with our integrated FMR1 (CGG)n arrays (26a). Similarly, Freudenreich et al. (10) observed DSBs in long (CTG)n arrays and suggested that they were repaired predominantly by a single-strand-annealing pathway. The occurrence of expansions in the (+) orientation suggests that the C-rich strand also has a propensity for expansion, albeit at a much lower rate than with the G-rich strand. This most probably reflects the differing structural potentials of the G-rich and C-rich strands (13, 52), since expansion in the C-rich strand occurs only when the efficiency of flap processing is diminished in the rad27 mutant background. In addition, a differential ability of the cell to recognize strand-specific secondary structures might play a role in expansion. It is of interest that it is the same CGG strand which has the highest propensity for expansion (as a displaced Okazaki fragment) and contraction (as a lagging-strand template).
Along with RAD27, the EXO1, DIN7, and YEN1 genes are members of the S. cerevisiae RAD2 gene family (36). Our studies suggest that since the loss of Exo1p and Din7p does not result in an increased expansion rate, the wild-type level of Rad27p is the critical determinant of triplet stability. Since Exo1p can compensate for some Rad27p functions, the levels of expression of this and other members of this family of structure-specific nucleases might become critical determinants of expansion when Rad27p becomes limiting. The S. cerevisiae RAD27 and EXO1 genes are both highly conserved in mammals (24, 67), and these may be critical genes involved in triplet repeat expansion in humans. While little is known about the expression of the human RAD27 homologue, FEN1, the Drosophila homologue of the S. cerevisiae EXO1 gene, Tosca, is differentially expressed between male and female germ lines (6).
In summary, we have described a model system in which the influence of repair and replication machinery on the expansion of human FMR1 (CGG)n repeat arrays can be assessed. This should provide valuable insights into the process of triplet repeat expansion and help identify other critical genes which govern triplet repeat expansion in humans. Intercellular variations in the expression of these genes, in combination with factors that influence structure formation, detection, and repair, are all likely to be critical elements determining the cellular environment that is permissive for expansion.
ACKNOWLEDGMENTS
This work was supported by grants from The Wellcome Trust to M.C.H. and R.H.B.
We thank Alex Bishop for help in initiating this study, Ed Louis for his assistance, and David Weatherall for his continued support. We also thank the reviewers for their helpful comments.
REFERENCES
- 1.Bingham P M, Scott M O, Wang S, McPhaul M J, Wilson E M, Garbern J Y, Merry D E, Fischbeck K H. Stability of an expanded trinucleotide repeat in the androgen receptor gene in transgenic mice. Nat Genet. 1995;9:191–196. doi: 10.1038/ng0295-191. [DOI] [PubMed] [Google Scholar]
- 2.Bontekoe C J, de Graaff E, Nieuwenhuizen I M, Willemsen R, Oostra B A. FMR1 premutation allele (CGG)81 is stable in mice. Eur J Hum Genet. 1997;5:293–298. [PubMed] [Google Scholar]
- 3.Borts R H, Leung W Y, Kramer W, Kramer B, Williamson M, Fogel S, Haber J E. Mismatch repair-induced meiotic recombination requires the pms1 gene product. Genetics. 1990;124:573–584. doi: 10.1093/genetics/124.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budd M E, Campbell J L. A yeast replicative helicase, Dna2 helicase, interacts with yeast FEN-1 nuclease in carrying out its function. Mol Cell Biol. 1997;17:2136–2142. doi: 10.1128/mcb.17.4.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devys D, Biancalana V, Rousseau F, Boue J, Mandel J L, Oberle I. Analysis of full fragile X mutations in fetal tissues and monozygotic twins indicate that abnormal methylation and somatic heterogeneity are established early in development. Am J Med Genet. 1992;43:208–216. doi: 10.1002/ajmg.1320430134. [DOI] [PubMed] [Google Scholar]
- 6.Digilio F A, Pannuti A, Lucchesi J C, Furia M, Polito L C. Tosca: a Drosophila gene encoding a nuclease specifically expressed in the female germline. Dev Biol. 1996;178:90–100. doi: 10.1006/dbio.1996.0200. [DOI] [PubMed] [Google Scholar]
- 7.Eichler E, Holden J, Popovich B, Reiss A, Snow K, Thibodeau S, Richards C, Ward P, Nelson D. Length of uninterrupted CGG repeats determines instability in the FMR1 gene. Nat Genet. 1994;8:88–94. doi: 10.1038/ng0994-88. [DOI] [PubMed] [Google Scholar]
- 8.Fedorova I V, Gracheva L M, Kovaltzova S V, Evstuhina T A, Alekseev S Y, Korolev V G. The yeast HSM3 gene acts in one of the mismatch repair pathways. Genetics. 1998;148:963–973. doi: 10.1093/genetics/148.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisch G S, Snow K, Thibodeau S N, Chalifaux M, Holden J J, Nelson D L, Howard-Peebles P N, Maddalena A. The fragile X premutation in carriers and its effect on mutation size in offspring. Am J Hum Genet. 1995;56:1147–1155. [PMC free article] [PubMed] [Google Scholar]
- 10.Freudenreich C H, Kantrow S M, Zakian V A. Expansion and length dependent fragility of CTG repeats in yeast. Science. 1998;279:853–856. doi: 10.1126/science.279.5352.853. [DOI] [PubMed] [Google Scholar]
- 11.Foucault F, Buard J, Praz F, Jaulin C, Stoppa-Lyonnet D, Vergnaud G, Amor-Gueret M. Stability of microsatellites and minisatellites in Bloom syndrome, a human syndrome of genetic instability. Mutat Res. 1996;362:227–236. doi: 10.1016/0921-8777(95)00047-x. [DOI] [PubMed] [Google Scholar]
- 12.Fu Y, Kuhl D, Pizzuti A, Piereti M, Sutcliffe J, Richards S, Verkerk A, Holden J, Fenwick R, Warren S, Oostra B, Nelson D, Caskey C. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;61:1–20. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- 13.Gacy A M, Goellner G, Juranic N, Macura S, McMurray C T. Trinucleotide repeats that expand in human disease from hairpin structures in vitro. Cell. 1995;81:533–540. doi: 10.1016/0092-8674(95)90074-8. [DOI] [PubMed] [Google Scholar]
- 14.Gietz R D, Schiestl R H, Willems A R, Woods R A. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- 15.Godde J S, Kass S U, Hirst M C, Wolffe A P. Nucleosome assembly on methylated CGG triplet repeats in the fragile X mental retardation gene 1 promoter. J Biol Chem. 1996;271:24325–24328. doi: 10.1074/jbc.271.40.24325. [DOI] [PubMed] [Google Scholar]
- 16.Godde J S, Wolffe A P. Nucleosome assembly on CTG triplet repeats. J Biol Chem. 1996;271:15222–15229. doi: 10.1074/jbc.271.25.15222. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg Y P, Kalchman M A, Metzler M, Nasir J, Zeisler J, Graham R, Koide H B, O’Kusky J, Sharp A H, Ross C A, Jirik F, Hayden M R. Absence of disease phenotype and intergenerational stability of the CAG repeat in transgenic mice expressing the human Huntington disease transcript. Hum Mol Genet. 1996;5:177–185. doi: 10.1093/hmg/5.2.177. [DOI] [PubMed] [Google Scholar]
- 18.Gordenin D A, Kunkel T A, Resnick M A. Repeat expansion—all in a flap? Nat Genet. 1997;16:116–118. doi: 10.1038/ng0697-116. [DOI] [PubMed] [Google Scholar]
- 19.Gourdon G, Radvanyi F, Lia A S, Duros C, Blanche M, Abitbol M, Junien C, Hofmann-Radvanyi H. Moderate integenerational and somatic instability of a 55-CTG repeat in transgenic mice. Nat Genet. 1997;15:190–192. doi: 10.1038/ng0297-190. [DOI] [PubMed] [Google Scholar]
- 20.Groden J, German J. Bloom’s syndrome. XVIII. Hypermutability at a tandem repeat locus. Hum Genet. 1992;90:360–367. doi: 10.1007/BF00220459. [DOI] [PubMed] [Google Scholar]
- 21.Hansen R S, Gartler S M, Scott C R, Chen S H, Laird C D. Methylation analysis of CGG sites in the CpG island of the human FMR1 gene. Hum Mol Genet. 1992;1:571–578. doi: 10.1093/hmg/1.8.571. [DOI] [PubMed] [Google Scholar]
- 22.Heitz D, Devys D, Imbert G, Kretz C, Mandel J L. Inheritance of the fragile X syndrome: size of the fragile X premutation is a major determinant of the transition to full mutation. J Med Genet. 1992;29:794–801. doi: 10.1136/jmg.29.11.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henderson S T, Petes T D. Instability of simple sequence DNA in Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:2749–2757. doi: 10.1128/mcb.12.6.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiraoka L, Harrington J J, Gerhard D S, Lieber M R, Hseih C-L. Sequence of human FEN-1, a structure specific endonuclease, and chromosomal localisation of the gene (FEN1) in mouse and human. Genomics. 1995;25:220–225. doi: 10.1016/0888-7543(95)80129-a. [DOI] [PubMed] [Google Scholar]
- 25.Hirst M, Grewal P, Davies K. Precursor arrays for triplet repeat expansion at the fragile X locus. Hum Mol Genet. 1994;3:1553–1560. doi: 10.1093/hmg/3.9.1553. [DOI] [PubMed] [Google Scholar]
- 26.Hirst M C, White P J. Cloned human FMR1 trinucleotide repeats exhibit a length- and orientation-dependent instability suggestive of in vivo lagging strand secondary structure. Nucleic Acids Res. 1998;26:2353–2358. doi: 10.1093/nar/26.10.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Hirst, M. C., and P. J. White. Unpublished data.
- 27.Ji J, Clegg N J, Petersen K R, Jackson A L, Laird C D, Loeb L A. In vitro expansion of GGC:GCC repeats: identification of the preferred strand of expansion. Nucleic Acids Res. 1996;24:2835–2840. doi: 10.1093/nar/24.14.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson R E, Kovvali G K, Prakash L, Prakash S. Requirement of the yeast RTH1 5′=3′ exonuclease for the stability of simple repetitive DNA. Science. 1995;269:238–240. doi: 10.1126/science.7618086. [DOI] [PubMed] [Google Scholar]
- 29.Kang S, Ohshima K, Shimizu M, Amirhaeri S, Wells R D. Pausing of DNA synthesis in vitro at specific loci in CTG and CGG triplet repeats from human hereditary disease genes. J Biol Chem. 1995;270:27014–27021. doi: 10.1074/jbc.270.45.27014. [DOI] [PubMed] [Google Scholar]
- 30.Kolodner R. Biochemistry and genetics of eukaryotic mismatch repair. Genes Dev. 1996;10:1433–1442. doi: 10.1101/gad.10.12.1433. [DOI] [PubMed] [Google Scholar]
- 31.Klungland A, Lindahl T. Second pathway for completion of human DNA base-excision-repair: reconstitution with purified proteins and requirement for DNase IV (FEN1) EMBO J. 1997;16:3341–3348. doi: 10.1093/emboj/16.11.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kokoska R J, Stefanovic L, Tran H P, Resnick M A, Gordenin D A, Petes T D. Destabilisation of yeast micro- and mini-satellite DNA sequences by mutations affecting a nuclease involved in Okazaki fragment processing (rad27) and DNA polymerase δ (pol3-t) Mol Cell Biol. 1998;18:2779–2788. doi: 10.1128/mcb.18.5.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunkel T A, Resnick M A, Gordenin D A. Mutator specificity and disease: looking over the FENce. Cell. 1997;88:155–158. doi: 10.1016/s0092-8674(00)81832-2. [DOI] [PubMed] [Google Scholar]
- 34.Kunst C, Warren S. Cryptic and polar variation of the fragile X repeat could result in predisposing normal alleles. Cell. 1994;77:853–861. doi: 10.1016/0092-8674(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 35.Lavedan C N, Garrett L, Nussbaum R L. Trinucleotide repeats (CGG)22TGG (CGG)43TGG(CGG)21 from the fragile X gene remain stable in transgenic mice. Hum Genet. 1997;100:407–414. doi: 10.1007/s004390050525. [DOI] [PubMed] [Google Scholar]
- 36.Lieber M R. The FEN-1 family of structure specific nucleases in eukaryotic DNA replication, recombination and repair. Bioessays. 1997;19:233–240. doi: 10.1002/bies.950190309. [DOI] [PubMed] [Google Scholar]
- 37.Malter H E, Iber J C, Willemsen R, de Graaff E, Tarleton J C, Leisti J, Warren S T, Oostra B A. Characterization of the full fragile X syndrome mutation in fetal gametes. Nat Genet. 1997;15:165–169. doi: 10.1038/ng0297-165. [DOI] [PubMed] [Google Scholar]
- 38.Mangiarini L, Sathasivam K, Mahal A, Mott R, Seller M, Bates G P. Instability of highly expanded CAG repeats in mice transgenic for the Huntington’s disease mutation. Nat Genet. 1997;15:197–200. doi: 10.1038/ng0297-197. [DOI] [PubMed] [Google Scholar]
- 39.Maurer D J, O’Callaghan B L, Livingston D M. Orientation dependence of trinucleotide CAG repeat instability in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:6617–6622. doi: 10.1128/mcb.16.12.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMurray C T. Mechanisms of DNA expansion. Chromosoma. 1995;104:2–13. doi: 10.1007/BF00352220. [DOI] [PubMed] [Google Scholar]
- 41.Mieczkowski P A, Fikus M U, Ciesla Z. Characterisation of a novel DNA damage inducible gene of Saccharomyces cerevisiae, DIN7, which is a structural homolog of the RAD2 and RAD27 DNA repair genes. Mol Gen Genet. 1997;253:655–665. doi: 10.1007/s004380050369. [DOI] [PubMed] [Google Scholar]
- 42.Mitas M. Trinucleotide repeats associated with human disease. Nucleic Acids Res. 1997;25:2245–2253. doi: 10.1093/nar/25.12.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monckton D G, Coolbaugh M I, Ashizawa K T, Siciliano M J, Caskey C T. Hypermutable myotonic dystrophy CTG repeats in transgenic mice. Nat Genet. 1997;15:193–196. doi: 10.1038/ng0297-193. [DOI] [PubMed] [Google Scholar]
- 44.Moutou C, Vincent M C, Biancalana V, Mandel J L. Transition from premutation to full mutation in fragile X syndrome is likely to be prezygotic. Hum Mol Genet. 1997;6:971–979. doi: 10.1093/hmg/6.7.971. [DOI] [PubMed] [Google Scholar]
- 44a.Newlon, C. Personal communication.
- 45.Oberlé I, Rousseau F, Heitz D, Kretz C, Devys D, Hanauer A, Boue J, Bertheas M, Mandel J. Instability of a 550bp DNA fragment and abnormal methylation in fragile X syndrome. Science. 1991;252:1097–1102. doi: 10.1126/science.252.5009.1097. [DOI] [PubMed] [Google Scholar]
- 46.Pearson C E, Ewel A, Acharya S, Fishel R A, Sinden R R. Human MSH2 binds to trinucleotide repeat DNA structures associated with neurodegenerative disease. Hum Mol Genet. 1997;6:1117–1123. doi: 10.1093/hmg/6.7.1117. [DOI] [PubMed] [Google Scholar]
- 47.Pierreti M, Zhang F, Fu Y, Warren S, Oostra B, Caskey C, Nelson D. Absence of expression of the FMR1 gene in fragile X patients. Cell. 1991;66:817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- 48.Reagan M S, Pittenger C, Siede W, Friedberg E C. Characterization of a mutant strain of Saccharomyces cerevisiae with a deletion of the RAD27 gene, a structural homolog of the RAD2 nucleotide excision repair gene. J Bacteriol. 1995;177:364–371. doi: 10.1128/jb.177.2.364-371.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reyniers E, Vits L, De Boulle K, Van Roy B, Van Velzen D, de Graaff E, Verkerk A J, Jorens H Z, Darby J K, Oostra B, et al. The full mutation in the FMR-1 gene of male fragile X patients is absent in their sperm. Nat Genet. 1993;4:143–146. doi: 10.1038/ng0693-143. [DOI] [PubMed] [Google Scholar]
- 50.Richards R I, Sutherland G S. Simple repeat DNA is not replicated simply. Nat Genet. 1994;6:114–116. doi: 10.1038/ng0294-114. [DOI] [PubMed] [Google Scholar]
- 51.Richards R I, Sutherland G S. Dynamic mutations: possible mechanisms and significance in human disease. Trends Biochem Sci. 1997;22:432–435. doi: 10.1016/s0968-0004(97)01108-0. [DOI] [PubMed] [Google Scholar]
- 52.Samadashwily G M, Raca R, Mirkin S M. Trinucleotide repeats affect DNA replication in vivo. Nat Genet. 1997;17:298–304. doi: 10.1038/ng1197-298. [DOI] [PubMed] [Google Scholar]
- 53.Schweitzer J K, Livingston D M. Destabilisation of CAG trinucleotide repeat tracts by mismatch repair mutations in yeast. Hum Mol Genet. 1997;6:349–355. doi: 10.1093/hmg/6.3.349. [DOI] [PubMed] [Google Scholar]
- 54.Schweitzer J K, Livingston D M. Expansions of CAG repeat tracts are frequent in a yeast mutant defective in Okazaki fragment maturation. Hum Mol Genet. 1998;7:69–74. doi: 10.1093/hmg/7.1.69. [DOI] [PubMed] [Google Scholar]
- 55.Shimizu M, Gellibolian R, Oostra B A, Wells R D. Cloning, characterisation and properties of CGG triplet repeats from the FMR-1 gene. J Mol Biol. 1996;258:614–626. doi: 10.1006/jmbi.1996.0273. [DOI] [PubMed] [Google Scholar]
- 56.Snow K, Tester D, Kruckeberg K, Schaid D, Thibodeau S. Sequence analysis of the fragile X trinucleotide repeat: implications for the origin of the fragile X mutation. Hum Mol Genet. 1994;3:1543–1551. doi: 10.1093/hmg/3.9.1543. [DOI] [PubMed] [Google Scholar]
- 57.Sommers C H, Miller E J, Dujon B, Prakash S, Prakash L. Conditional lethality of null mutations in RTH1 that encodes the yeast counterpart of a mammalian 5′- to 3′-exonuclease required for lagging strand DNA synthesis in reconstituted systems. J Biol Chem. 1995;270:4193–4196. doi: 10.1074/jbc.270.9.4193. [DOI] [PubMed] [Google Scholar]
- 58.Strand M, Prolla T A, Liskay R M, Petes T D. Destabilisation of tracts of simple repetitive DNA in yeast by mutations affecting DNA mismatch repair. Nature. 1993;365:274–276. doi: 10.1038/365274a0. [DOI] [PubMed] [Google Scholar]
- 59.Sutcliffe J S, Nelson D L, Zhang F, Pieretti M, Caskey C T, Saxe D, Warren S T. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet. 1992;1:397–400. doi: 10.1093/hmg/1.6.397. [DOI] [PubMed] [Google Scholar]
- 60.Tishkoff D X, Filosi N, Gaida G M, Kolodner R D. A novel mutation avoidance mechanism dependent upon S. cerevisiae RAD27 is distinct from DNA mismatch repair. Cell. 1997;88:253–263. doi: 10.1016/s0092-8674(00)81846-2. [DOI] [PubMed] [Google Scholar]
- 61.Tishkoff D X, Boerger A L, Betrand P, Filosi N, Gaida G M, Kane M F, Kolodner R D. Identification and characterisation of Saccharomyces cerevisiae EXO1, a gene encoding an exonuclease that interacts with MSH2. Proc Natl Acad Sci USA. 1997;94:7487–7492. doi: 10.1073/pnas.94.14.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Usdin K, Woodford K J. CGG repeats associated with DNA instability and chromosome fragility form structures that block DNA synthesis in vitro. Nucleic Acids Res. 1995;23:4202–4209. doi: 10.1093/nar/23.20.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verkerk A, Pieretti M, Sutcliffe J, Fu Y, Kuhl D, Pizzuti A, Reiner O, Richards S, Victoria M, Zhang F, Eussen B, van Ommen G, Blonden L, Riggins G, Chastain J, Kunst C, Galjaad H, Caskey C, Nelson D, Oostra B, Warren S. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 64.Wach A, Brachat A, Pohlmann R, Phillippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y H, Griffith J. Expanded CTG triplet blocks from the myotonic dystrophy gene create the strongest known natural nucleosome positioning elements. Genomics. 1995;25:570–573. doi: 10.1016/0888-7543(95)80061-p. [DOI] [PubMed] [Google Scholar]
- 66.Watt P M, Hickson I D, Borts R H, Louis E J. SGS1, a homologue of the Bloom’s and Werner’s syndrome genes, is required for maintenance of genome stability in Saccharomyces cerevisiae. Genetics. 1996;144:935–945. doi: 10.1093/genetics/144.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilson D M, Carney J P, Coleman M A, Adamson A W, Christensen M, Lamerdin J E. Hex1: a new human Rad2 nuclease family member with homology to yeast exonuclease 1. Nucleic Acids Res. 1998;26:3762–3768. doi: 10.1093/nar/26.16.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wohrle D, Hirst M, Davies K, Steinbach P. Genotype variation in fragile X fetal tissues. Hum Genet. 1992;89:114–116. doi: 10.1007/BF00207057. [DOI] [PubMed] [Google Scholar]
- 69.Wohrle D, Hennig I, Vogel W, Steinbach P. Mitotic stability of fragile X mutations in differentiated cells indicates early post-conceptional trinucleotide repeat expansion. Nat Genet. 1993;4:140–142. doi: 10.1038/ng0693-140. [DOI] [PubMed] [Google Scholar]
- 70.Wohrle D A, Salat U A, Glaser D A, Mucke J A, Meiselstosiek M A, Schindler D A, Vogel W A, Steinbach P. Unusual mutations in high functioning fragile X males: apparent instability of expanded un-methylated CGG repeats. J Med Genet. 1998;35:103–111. doi: 10.1136/jmg.35.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu S, Pritchard M, Kremer E, Lynch M, Nancarrow J, Baker E, Holman K, Mulley J, Warren S, Schlessinger D, Sutherland G, Richards R. Fragile X genotype characterised by an unstable region of DNA. Science. 1991;252:1179–1181. doi: 10.1126/science.252.5009.1179. [DOI] [PubMed] [Google Scholar]
- 72.Zhong N, Yang W, Dobkin C, Brown W. Fragile X gene instability: anchoring AGGs and linked microsatellites. Am J Hum Genet. 1995;57:351–361. [PMC free article] [PubMed] [Google Scholar]