Recent media reports from Israel,1 France,2 and the United States military3 have raised the possibility of a link between COVID‐19 mRNA vaccines and myopericarditis in young adults. We present a case series of eight young adults seen in emergency departments (EDs) of a U.S. health care system who developed myopericarditis shortly after their second dose of a COVID‐19 mRNA vaccine.
We queried our local institutional health care system database4 for all diagnoses of myopericarditis (ICD‐10 codes I30.XX, I51.4, and I40.9) in patients aged 16 to 25 years old who presented to an ED between January 1 and May 20, 2021. These dates were selected to reflect the vaccination schedule for COVID‐19 mRNA vaccines for young adults within the state of Connecticut (noting that all persons aged 16 years and older became eligible for vaccination from April 1, 2021). Our health care system is a mix of suburban community (n = 6), urban community (n = 2), and urban academic (n = 1) EDs in the northeastern United States. All cases were reviewed for temporal relationship to a second dose of a COVID‐19 mRNA vaccine. Demographics, presenting characteristics, troponin levels, electrocardiogram, echocardiogram, and cardiac MRI were subsequently manually abstracted and analyzed. This study was approved by our local institutional review board (IRB# 2000027975).
During the study period, 18,671 patients aged 16 to 25 years received two doses of the Pfizer‐BioNTech BNT162b2 (Pfizer) vaccine, 5,999 received two doses of the Moderna mRNA‐1273 vaccine, and three received a combination of the two. We identified eight patients 16 to 24 years of age who developed myopericarditis within 8 days of their second mRNA vaccine—all of whom presented between the dates of May 4 and 17, 2021 (Table 1). All patients were male, six identified as White, and all received two doses of the Pfizer vaccine. On review of medical records, none had a prior diagnosis of COVID‐19 or any relevant past medical history. All eight patients presented with chest pain as the chief complaint, and seven presented within 3 days of the second vaccine dose. None were symptomatic prior to their second vaccine dose or had other reported adverse events. Vitals signs ranges on arrival were heart rate 57 to 109, systolic blood pressure 106 to 138, temperature 36.5 to 37.9 C, respiratory rate 12 to 21, and oxygen saturation 97% to 100% on room air.
TABLE 1.
Characteristics of eight patients presenting with myopericarditis after second COVID‐19 mRNA vaccine
| Patient | Age (years) | Gender | Vaccine | Chief complaint | Days after second vaccine | Hospital LOS (days) | Initial ECG findings | Initial troponin (Trop I unless specified, ref range = 0.00–0.08 ng/mL) | Echo findings | Cardiac MRI findings |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 18 | M | Pfizer | Chest pain | 1 | 3 | STE II, III, aVF, V3‐6 | 1.09a | Normal | Generalized edema, hyperemia, and fibrosis |
| 2 | 21 | M | Pfizer | Chest pain | 1 | 2 | STE I, II, III, aVF, V4‐6 | 10.70 | Normal | LVEF 53%. Thickened pericardium with delayed enhancement. Edematous inferolateral epicardium |
| 3 | 17 | M | Pfizer | Chest pain | 7 | 4 | STE I, V3‐6 | 10.94 | Normal | Diffuse fibrosis. Delayed enhancement of anterolateral LV |
| 4 | 23 | M | Pfizer | Chest pain | 1 | 1 | STE V1‐3, STD III, aVF | 11.44 | Normal | N/A |
| 5 | 24 | M | Pfizer | Chest pain | 3 | 1 | Normal | 0.54 | Mild basal inferoseptal hypokinesis. Normal LVEF. | N/A |
| 6 | 19 | M | Pfizer | Chest pain | 1 | 2 | STE I, II, III, aVF, V4‐6 | 24.50 | Normal | Normal |
| 7 | 23 | M | Pfizer | Chest pain | 2 | 2 | STE II, V3‐5 | 14.20 | Normal | N/A |
| 8 | 16 | M | Pfizer | Chest pain | 2 | 2 | STE II, III, aVF, V4‐6 | 4.03 | Normal | Diffuse edema. Delayed sub‐epicardial enhancement and hypokinesis of inferolateral LV |
Abbreviations: LOS, length of stay; LV, left ventricle; LVEF, left ventricular ejection fraction; STE, ST elevation.
Troponin T (ULN < 0.01 ng/ml).
All patients were found to have troponin elevations (either I or T), and seven of eight had electrocardiogram (ECG) changes (Figure 1). Seven patients received an echocardiogram, one of whom demonstrated hypokinesis with normal ejection fraction and the remainder being normal. Five patients underwent cardiac MRI, with common findings of edema, fibrosis, and delayed enhancement meeting established diagnostic criteria for myocarditis.5 Two patients underwent CTA chest, both demonstrating only nonspecific unilateral axillary lymphadenopathy. For the five patients in whom it was measured, all had detectable levels of SARS‐CoV‐2 spike receptor binding domain antibody (range = 158 to greater than the assay upper limit [2,500] u/mL) thought to be reflective of vaccination status, and none had reactive SARS‐CoV‐2 nucleocapsid antibody testing, suggesting that none had had prior COVID‐19 infection.6 All eight patients were admitted: four to the pediatric ICU (consistent with local protocols for MIS‐C–like illnesses) and four to the hospital floor. During admission, none of the patients required oxygen supplementation or vasopressor support. Four patients received steroids, four received intravenous immunoglobulin (all of whom were at our urban academic center), three received aspirin, and five received nonsteroidal anti‐inflammatory drugs (NSAIDs). One patient underwent a cardiac catheterization, without evidence of coronary artery disease. The range of hospital length of stay was 1 to 4 days, after which all were discharged home. Seven had documented discharge exercise precautions. Discharge medications included steroid taper (n = 4), aspirin (n = 4), NSAIDs (n = 3), and colchicine (n = 1). None of the eight patients had alternative explanations for their myopericarditis identified during hospitalization.
FIGURE 1.
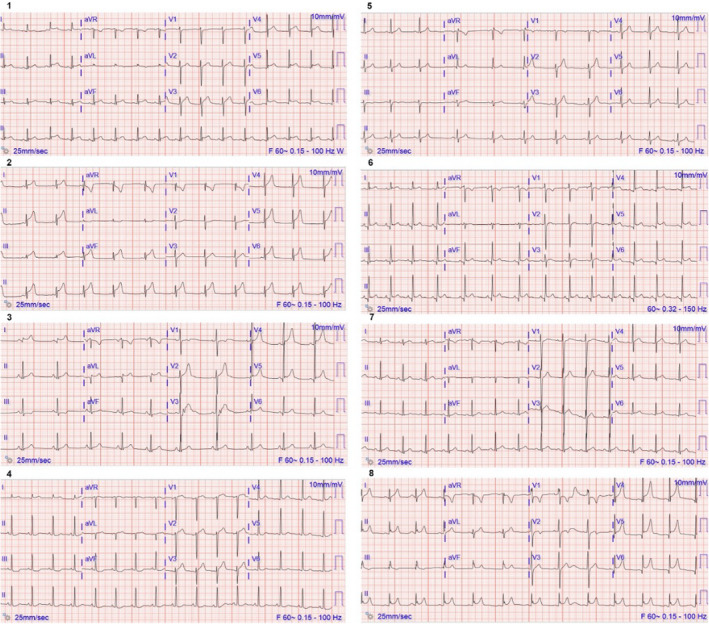
Presenting ED 10‐second 12‐lead ECGs from the eight patients in the study cohort, labeled numerically as per Table 1
Electronic health record review revealed two other ED cases of pericarditis within the study period and demographic population. Neither was temporally related to COVID‐19 vaccination. One case occurred in a patient with prior COVID‐19 infection who was discharged from the presenting ED, and the other was secondary to a known underlying autoimmune disorder.
This single‐center case series describes eight cases of myopericarditis in otherwise healthy young adults seen in the ED within a 2‐week window, with onset of symptoms within 7 days of administration of a second dose of a COVID‐19 mRNA vaccine. All patients experienced improvement in symptoms and were discharged home after a brief hospitalization. The timing of these cases coincided broadly with the expanded eligibility for COVID‐19 vaccination to all individuals aged 16 years and older in the state of Connecticut, which began on April 1, 2021. Comprehensive screening of all ED visits from January 1, 2021, to May 20, 2021, revealed no other unexplained cases of myopericarditis in our study population.
The small study sample size from a single health care system precludes meaningful analyses regarding associations between vaccination status, vaccine manufacturer, age, gender, or race and development of myopericarditis. These eight cases comprise 0.03% of the 24,673 individuals aged 16 to 25 years who have received two doses of a COVID‐19 mRNA vaccine in our health care system. Public health officials have announced ongoing monitoring of potential links between COVID‐19 vaccination and myopericarditis and further data are needed to establish the nature of any possible causal association.
CONFLICT OF INTEREST
The authors have no potential conflicts to disclose.
AUTHOR CONTRIBUTIONS
All authors contributed to the conception and design of the manuscript. R. Andrew Taylor supervised the creation of the study. Alex Fleming‐Nouri, Adrian D. Haimovich, David Yang, Wade L. Schulz, and Andreas Coppi performed the data collection. Alex Fleming‐Nouri, Adrian D. Haimovich, and David Yang drafted the manuscript, and all authors contributed to its revision. R. Andrew Taylor takes responsibility for the paper as a whole.
Supervising Editor: John Burton
REFERENCES
- 1.Lee BY. Are Rare Cases Of Myocarditis Linked To Pfizer, Moderna Covid‐19 Vaccines? Forbes Magazine. 2021. https://www.forbes.com/sites/brucelee/2021/04/27/are‐rare‐cases‐of‐myocarditis‐linked‐to‐pfizer‐moderna‐covid‐19‐vaccines/ (Accessed May 20, 2021).
- 2.France Investigates New Possible Side‐effect of Pfizer Coronavirus Vaccine. 2021. https://www.brusselstimes.com/news/167442/france‐investigates‐new‐possible‐side‐effect‐pfizer‐biontech‐coronavirus‐vaccine‐ansm‐myocarditis‐heart‐israel/ (Accessed May 20, 2021).
- 3.Kime P. Pentagon Tracking 14 Cases of Heart Inflammation in Troops after COVID‐19 Shots. 2021. https://www.military.com/daily‐news/2021/04/26/pentagon‐tracking‐14‐cases‐of‐heart‐inflammation‐troops‐after‐covid‐19‐shots.html (Accessed May 20, 2021).
- 4.Schulz WL, Durant TJ, Torre CJ Jr, Hsiao AL, Krumholz HM. Agile health care analytics: enabling real‐time disease surveillance with a computational health platform. J Med Internet Res 2020;22(5):e18707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedrich MG, Sechtem U, Schulz‐Menger J, et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. 2009;53(17):1475‐1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El‐Khoury JM, Schulz WL, Durant TJ. Longitudinal assessment of SARS‐CoV‐2 anti‐nucleocapsid and anti‐spike‐1‐RBD antibody testing following PCR‐detected SARS‐CoV‐2 infection. J Appl Lab Med. 2021. 10.1093/jalm/jfab030 [DOI] [PMC free article] [PubMed] [Google Scholar]


