Abstract
Transplantation of solid organs from donors with active SARS-CoV-2 infection has been advised against due to the possibility of disease transmission to the recipient. However, with the exception of lungs, conclusive data for productive infection of transplantable organs do not exist. While such data are awaited, the organ shortage continues to claim thousands of lives each year. In this setting, we put forth a strategy to transplant otherwise healthy extrapulmonary organs from SARS-CoV-2-infected donors. We transplanted 10 kidneys from five deceased donors with new detection of SARS-CoV-2 RNA during donor evaluation in early 2021. Kidney donor profile index ranged from 3% to 56%. All organs had been turned down by multiple other centers. Without clear signs or symptoms, the veracity of timing of SARS-CoV-2 infection could not be confirmed. With 8–16 weeks of follow-up, outcomes for all 10 patients and allografts have been excellent. All have been free of signs or symptoms of donor-derived SARS-CoV-2 infection. Our findings raise important questions about the nature of SARS-CoV-2 RNA detection in potential organ donors and suggest underutilization of exceptionally good extrapulmonary organs with low risk for disease transmission.
KEYWORDS: clinical decision-making, clinical research/practice, donors and donation: deceased, infection and infectious agents—viral, infectious disease, kidney transplantation/nephrology
Abbreviations: ACE2, angiotensin-converting enzyme 2; COVID-19, coronavirus disease 2019; KDPI, kidney donor profile index; PCR, polymerase chain reaction; RNA, ribonucleic acid; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TMPRSS2, transmembrane serine protease 2
1. INTRODUCTION
Since March 2020, over 33 million SARS-CoV-2 infections with more than a half million deaths have occurred in the United States.1 Despite uncertainties and pandemic-related disruptions, most transplant centers and organ procurement organizations have continued to provide these lifesaving therapies supported by infection prevention practices.2 , 3 Nationwide, only a modest decrease in deceased donor transplantation occurred in 2020 compared to 2019.4 However, the organ shortage persists with thousands of deaths on the transplant waitlist yearly, including over 4000 deaths while awaiting a kidney transplant in 2020.5
Those dying from severe COVID-19 are not considered for organ donation. Given the prevalence of infection, however, it was expected that those dying from other causes might also have active SARS-CoV-2 infection. By April 2020, SARS-CoV-2 screening was required for all potential organ donors to avoid the possibility of virus transmission to recipients through transplanted organs and to the medical teams during organ procurement. Those with detectable SARS-CoV-2 RNA have generally not been considered for donation unless there is evidence for successfully resolved infection.
While SARS-CoV-2 infection in the airway of potential donors is prohibitive of lung donation, transplantation of the extrapulmonary organs has been more controversial. An editorial by Kates, et al. published in July 2020 put forth a well-considered argument for use of such organs.6 Early in the pandemic, most organ procurement organizations considered SARS-CoV-2-infected donors to be ineligible for organ donation. Recent guidance from the Organ Procurement Transplant Network Disease Transmission Advisory Committee suggests balancing the risk for SARS-CoV-2 transmission possible effects on allograft quality and risk to procurement teams with the recipient’s risk for mortality and other complications on the waitlist.7 Still, many centers do not consider allografts from such donors. Without convincing evidence to date for productive (or transmissible) infection of most transplantable extrapulmonary organs, we put forth a strategy at our center for transplanting such organs from selected donors with SARS-CoV-2 RNA detection on screening. While not restricted to kidneys, our strategy resulted in the following 10 kidney transplants from five deceased donors.
2. METHODS AND RESULTS
2.1. Donor and recipient selection criteria
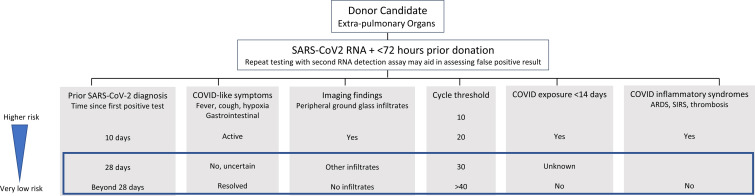
Our strategy included considering all SARS-CoV-2-positive deceased donors with otherwise acceptable donor characteristics and healthy appearing kidneys with good organ function. Lower airway testing was requested and considered reassuring when negative. We aimed to avoid donors with severe COVID-19 or with COVID-related inflammatory syndromes ( Figure 1). A cycle threshold as a surrogate for viral load was requested but not required. The strategy was approved by our transplant center leadership.
FIGURE 1.
Factors to consider when accepting extrapulmonary organs from SARS-CoV-2 RNA-positive organ donors to minimize the theoretical and unknown absolute risk for SARS-CoV-2 transmission to recipients and organ procurement teams and to minimize the potential subclinical effect on end organ function from SARS-CoV-2 infection and inflammatory syndromes. All such information may not be readily available or consistently reliable. The boxed region would be considered exceedingly low risk for most recipients by our program [Color figure can be viewed at wileyonlinelibrary.com]
There were no recipient selection criteria other than willingness to accept such an organ. All candidates were explained the risks and benefits of accepting a kidney from a SARS-CoV-2 RNA-positive donor and all provided clinical consent. None of our own center candidates declined an offer. Recipients received standard immunosuppression, including induction with rabbit antithymocyte globulin, prograf, mycophenolate mofetil, and prednisone, and posttransplant care. All were monitored closely for signs of COVID-like illness with plans for chest imaging and SARS-CoV-2 testing when present. Since circulated virus from the kidney would likely infect the lower airway prior to detection in the upper airway, we did not consider upper airway testing to be a helpful screening tool. COVID-specific infection prevention precautions (N95, eye protection in addition to universal surgical precautions) were recommended during organ recovery but not during organ implantation. Recipients were followed as for all our early organ transplant recipients with caregivers using routine surgical masking, eye protection, gloves, and hand hygiene. No added precautions were recommended by Infection Prevention at our institution. Approval from our Institutional Review Board was obtained for preparation and submission of this report.
2.2. Donor characteristics
Since February 2021, seven deceased donors from three different organ procurement organizations were considered by our program. Two donors were not accepted due to bilateral ground glass pulmonary infiltrates and death from cerebrovascular accidents. Five were accepted for kidney donation. The donor details are summarized in Table 1. All donors were tested positive for SARS-CoV-2 RNA by nasopharyngeal swab (NPS) polymerase chain reaction (PCR) by a variety of testing platforms after hospital admission and within 3 days of donation. All but donor 3 had additional tests during the index hospitalization that were negative, including three from lower airway specimens within 2 days of donation. None had record of recent COVID-19 or prior positive SARS-CoV-2 testing. However, donor 1 had a detectable SARS-CoV-2 IgG and a reported exposure to infected relatives the month before. None had reported COVID-like symptoms or signs prior to or during admission. None had infiltrates on chest X-ray or computed tomography scan suggestive of severe COVID-19. Donor 3 tested positive for hepatitis C RNA and had a terminal creatinine level higher than 1.5 mg/dl. A biopsy specimen showed no pathological change. The kidney donor profile index (KDPI) was extremely favorable for three donors. Organ offers were turned down by other transplant centers prior to allocation to our candidates. One donor was administered the SARS-CoV-2 monoclonal antibody on the day of donation. Two donors had other organs allocated elsewhere.
TABLE 1.
Characteristics of donors with SARS-CoV-2 detection
| Donor 1 | Donor 2 | Donor 3 | Donor 4 | Donor 5 | |
|---|---|---|---|---|---|
| Donor age, years | 17 | 28 | 31 | 6 | 23 |
| Cause of death | Asphyxia | Cardiac arrest | Drug overdose HCV NAT+ | Drowning | Asphyxia |
| Hospital LOS, days | 8 | 22 | 4 | 3 | 7 |
| Brain death | Yes | Yes | No, DCD | Yes | Yes |
| Prior COVID-19, Recent exposure | No, Exposure <45d | No | No | No | No |
| SARS-CoV-2 testing, Days from test to donation | |||||
| Nasopharyngeal RNAa | Positive (–3, –3) Negative (–9) |
Positive (0, 0) Negative (–22, –15, –3) |
Positive (–3, –1) | Positive (–3) Negative (–1) |
Positive (–1, 0) Negative (–7, –1) |
| Cycle threshold, Ct | NA | Ct 40, 38 | NA | NA | Ct 31, 41 |
| Nasopharyngeal Ag | — | Negative (–22, –15, –7) | — | — | — |
| Lower airwayb | — | Tracheal aspirate Negative (–1) |
BAL Negative (–2) |
— | BAL Negative (–1) |
| SARS-CoV-2 Ab | IgG+ | — | — | — | — |
| Chest imaging | CXR (–2) Right basilar infiltrate CT (–9) Minimal ground glass, left upper contusion |
CXR (–7) Opacification left lung, patchy airspace opacities | CT (–1) Right pneumothorax, Right lower lobe collapse |
CXR (–1) Perihilar airspace opacities in batwing distribution |
CT (–1) Dense lower airway consolidations, right upper focus ground glass opacity |
| COVID therapies | No | No | No | No | REGEN-CoV mAB |
| Terminal Cr, mg/dl | 1.5 | 1.0 | 5.7 | 0.48 | 0.6 |
| KDPI, % | 3 | 13 | 56 | 56 | 4 |
| Other organs transplanted | Liver | Heart Liver |
None | None | None |
Abbreviations: Ab, antibody; Ag, antigen; BAL, bronchoalveolar lavage; Cr, creatinine; CT, computed tomography; CXR, chest X-ray plain film; DCD, donor after cardiac death; HCV NAT, hepatitis C nucleic acid amplification test; KDPI, kidney donor profile index; LOS, length of stay.
CompuNet qualitative SARS-CoV-2 RT PCR, Aptima SARS-CoV-2 RNA, Cepheid SARS-CoV-2 RT PCR, XpertXpress SARS-CoV-2 Flu RSV, “Expedited COVID-19 by PCR.”
Simplexa SARS-CoV-2 PCR, “SARS-CoV-2 by PCR.”
2.3. Recipient characteristics and outcomes
Ten kidneys were accepted for 10 adult recipients. Recipients consented to receive an organ from a “COVID-positive” donor with a low (but not zero) risk for disease transmission and unknown subclinical effects of SARS-CoV-2 infection on organ function. Recipient details are summarized in Table 2. Transplantation for one recipient was preemptive and the remainder were on dialysis. Median waitlist time was 1251 days with two recipients having short wait times. One recipient had a mild COVID-19 illness with positive SARS-CoV-2 RNA testing 3 months before transplantation with full recovery. This same recipient tested positive for SARS-CoV-2 RNA on the day of transplant considered to be RNA shedding. All other recipients had negative screening tests and none had COVID-like symptoms on admission for transplantation. There were no surgical complications. One recipient experienced postoperative atrial fibrillation and two were readmitted, one with gastroparesis and one with ileus related to underlying comorbidities. One patient experienced delayed graft function requiring dialysis. At follow-up (56–112 days), all kidneys were functioning well. All posttransplant SARS-CoV-2 tests have been negative. Since our protocol did not include routine NPS SARS-CoV-2 testing, most of these tests were sent for other reasons during hospitalization and follow-up. One patient with pulmonary fibrosis due to preexisting interstitial lung disease developed worsened respiratory symptoms posttransplant and tested negative for SARS-CoV-2 RNA by NPS. Otherwise none had signs or symptoms typical of acute COVID-19. No procurement team transmissions occurred.
TABLE 2.
Recipients of kidneys from SARS-CoV-2 RNA-positive donors: characteristics and outcomes
| Recipient 1 | Recipient 2 | Recipient 3 | Recipient 4 | Recipient 5 | Recipient 6 | Recipient 7 | Recipient 8 | Recipient 9 | Recipient 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Donor | 1 | 1 | 2 | 2 | 3 | 3 | 4 | 4 | 5 | 5 |
| Age | 35 | 29 | 44 | 38 | 66 | 55 | 40 | 27 | 24 | 41 |
| Cause ESRD | C1Q nephropathy | Lupus nephropathy | IgA nephropathy | IgA nephropathy | Diabetic nephropathy | Diabetic nephropathy | Sjogren’s syndrome | Diffuse GN | Diffuse GN | Septic shock |
| Waitlist time, days | 1346 | 1051 | 1356 | 1314 | 499 | 332 | 1089 | 1793 | 2366 | 1188 |
| Dialysis type | None | PD | HD | HD | HD | HD | HD | HD | HD | HD |
| Prior COVID-19 | No | No | Yes 3 mos prior |
No | No | No | No | No | No | No |
| COVID vaccination | No | No | No | No | Dose 1 (–14d) | Dose 1, 2 (–32, –8) | Dose 1, 2 (–32, –4) | Dose 1 (–11) | Dose 1 (–14) | No |
| Induction therapy | rATG | rATG | rATG | rATG | rATG | rATG | rATG | rATG | rATG | rATG |
| Maintenance IS | CNI, MMF, CS | CNI, MMF, CS | CNI, MMF, CS | CNI, MMF, CS | CNI, MMF, CS | CNI, MMF, CS | CNI, MMF, CS | CNI, MMF, CS | CNI, MMF, CS | CNI, MMF, CS |
| Complications | ||||||||||
| Early | None | None | None | None | None | Delayed graft function | Atrial fibrillation | None | None | None |
| Readmission | No | Yes, d4 Gastroparesis |
No | No | No | No | No | No | No | Yes Ileus |
| Creatinine (md/dl) | ||||||||||
| Baseline | 4.7 | 12 | 7.9 | 7.0 | 6.5 | 9.1 | 11 | 11.4 | 9.4 | 7.7 |
| 2 weeks | 1.9 | 1 | 1.9 | 2.7 | 4.8 | 2.5 | 2.4 | 1.9 | 1.4 | 1.9 |
| 8 weeks | 1.7 | 0.9 | 1.9 | 1.9 | 1.3 | 1.2 | 1.8 | 1.7 | 0.9 | 1.3 |
| 12 weeks | 1.6 | 1.1 | 1.9 | 1.6 | 1.4 | 1.1 | 2.1 | 1.6 | NA | NA |
| SARS-CoV-2 testing (NPS) | Negative BL, d2 |
Negative BL, d2, d4 |
Positive BL Negative d28 |
Negative BL, d12 |
Negative BL, d3 |
Negative BL |
Negative BL, d32 |
Negative BL, d7 |
Negative BL, d3 |
Negative BL,d3, d7, d21 |
| COVID-19 symptoms | None | None | None | None | None | None | None (worsened cough, known ILD) | None | None | None |
Abbreviations: BL, baseline; CNI, calcineurin inhibitor; CS, corticosteroids; ESRD, end-stage renal disease; GN, glomerulonephritis; HD, hemodialysis; ILD, interstitial lung disease; MMF, mycophenolate mofetil; NA, not applicable; NPS, nasopharyngeal swab; PD, peritoneal dialysis; rATG, rabbit antithymocyte globulin.
3. DISCUSSION
It is ordinary practice to weigh the risks of accepting an organ and the risk of dying on the waitlist, a practice made more complex by the SARS-CoV-2 pandemic. Overall, by considering these SARS-CoV-2-positive donors, we were able to transplant organs of excellent quality to our patients with no clinical evidence for virus transmission or allograft injury and no reported transmission events to organ procurement teams.
The transmission of SARS-CoV-2 via organ transplantation is to be avoided and careful donor screening is essential. Recent reports of SARS-CoV-2 transmissions from donor lungs in the setting of negative NPS testing have led to more stringent criteria for screening lung donors by lower airway sampling.8, 9, 10 For other organs, the theoretical risk for transmission must be weighed against the risk of dying from complications from end-stage organ disease.
Concern for productive SARS-CoV-2 infection of extrapulmonary organs is based on several observations. While transfusion-associated infection has not been identified, very low levels of viremia during the course of airway infection could theoretically result in infection of other organs.11 Both angiotensin-converting enzyme 2 receptors (ACE2), the portal of entry for SARS-CoV-2, and the priming cofactor transmembrane serine protease 2 (TMPRSS2) are distributed throughout the body but do not alone predict pathologic organ infection.12 SARS-CoV-2 RNA has been detected in multiple organs and body fluids (including blood, stool, and urine) during COVID-19, but is not proof of infective virus.13 , 14 Citing over 100 publications, Gaussen, et al. recently delineated the still inconclusive data for productive infection of extrapulmonary organs and tissues.15 The lack of correlation between RNA detection and histopathologic evidence suggesting active infection challenged the reports of electron micrograph images of SARS-CoV-2 virions, and highly inconsistent detection of viable virus from pathologic tissue and body fluids raises significant doubts. So while there is biologic plausibility and circumstantial evidence for infection, it is unclear how close our science is to confirming the presence of viable virus in extrapulmonary organs.
Similar concerns for the potential for donor-derived transmission were levied during the 2003 SARS-CoV and the 2009 H1N1 influenza A outbreaks but the scope and duration of these outbreaks were limited.16 , 17 Nucleic acid from RNA respiratory viruses can be detected in extrapulmonary organs during infection, and influenza A has been shown to infect other organs during fatal infection.18 However, evidence for donor-derived transmission of any RNA respiratory viruses from extrapulmonary organs is absent.19
There are few published case reports of extrapulmonary organ transplants from donors with SARS-CoV-2 infection with none resulting in definitive organ-derived COVID-19.20, 21, 22 Two were living donor liver transplants performed prior to routine testing of donors and recipients. One donor was mildly symptomatic at donation. A donor recipient pair (a mother urgently donating to her infant) both developed COVID-19 symptoms within 4 days of transplantation. While donor-derived infection could not be excluded, it is more likely that donor and recipient had acquired infection from a common source prior to transplantation. The third case was a deceased donor with SARS-CoV-2 detected from bronchoalveolar lavage fluid. The liver was transplanted to a recipient with SARS-CoV-2 infection 1 month prior to transplantation with evidence of resolving infection.22 Additional cases have emerged of inadvertent use of organs from donors with SARS-CoV-2 infection with clinically meaningful viral transmission events only to three recipients of lungs and none of the 12 recipients of extrapulmonary organs.7
Our donor data highlight several important points pertinent to assessing donor risk for SARS-CoV-2 transmission. Most importantly, none of these donors were clearly actively infected. None had overt clinical manifestations of COVID-19, though assessing such signs and symptoms is challenging during terminal illness for other causes and when patient histories may be incomplete. Assessing infiltrates as more or less “COVID-appearing” is worthwhile given the common occurrence of various infiltrates in organ donors but may not be reliable. When uncertain, negative results of SARS-CoV-2 RNA from lower airway samples provide some reassurance.
Importantly, positive SARS-CoV-2 RNA results without signs or symptoms of COVID-19 may reflect extremely disparate situations. First, the tests could be falsely positive. Concerns for false positive tests have been raised for SARS-CoV-2 antigen tests and for few RNA detection methods.23 However, none of the testing platforms used for these donors have been implicated in false positive testing errors. More importantly, for all but one of our donors, a second test performed on a different testing platform was also positive making a false positive test highly unlikely. The SARS-CoV-2-positive test results could also indicate disparate phases in the course of infection, including true asymptomatic infection when viral loads are high or resolved infection when there is no productive infection. The detection of SARS-CoV-2 RNA has been detected in upper and lower airway samples for months following resolved infection.24 , 25 Inconsistent SARS-CoV-2 RNA detection as was seen for some of our donors is more likely to occur during this period when RNA is present at the lower limits of detection of the assay. Multiple cases have now been reported using organs from SARS-CoV-2 RNA-positive donors with prior documented and resolved infection.26 The cycle threshold, an inverse correlate with the viral load, may help determine the likelihood of productive infection (either extremely early or late in infection) but is not available from many SARS-CoV-2 RNA testing platforms and was not available in time to influence clinical decisions for most of these donors. SARS-CoV-2 serology may also aid in assessing acute or resolved infection but the commercial tests, authorized under emergency use, have not been well validated for clinical use.27
In this setting, one might also consider risk mitigation strategies. For example, accepting these organs only for those recipients with some immunologic protection as a result of prior infection or vaccination. While advised, our data do not indicate that vaccination or documentation of antibody detection be required to accept such organs. Use of remdesivir, monoclonal antibodies, or convalescent plasma in the perioperative period might be attractive but may also be unnecessary and may carry their own risks to the recipient. It is unclear whether the administration of monoclonal antibody to one of our donors on the day of donation was of any benefit.
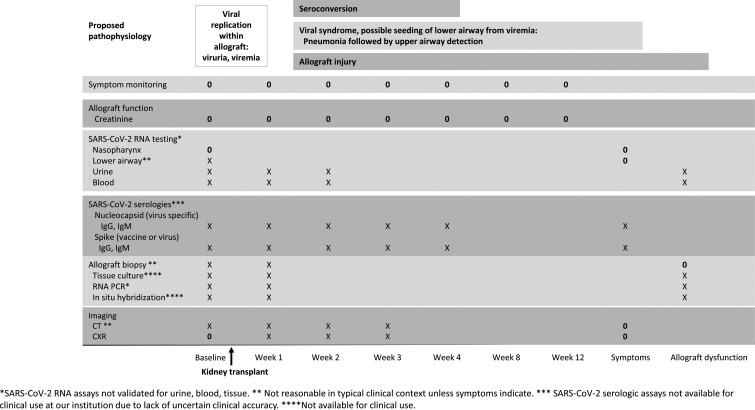
Short of the clinical outcomes that we monitored, it is not clear how to monitor recipients for transmitted infection. Assays to detect SARS-CoV-2 RNA or culture to detect viable virus in blood, urine, or kidney tissue may best identify early infection from the allograft but are not available to most clinicians. Nasopharyngeal detection may only occur after significant lower airway involvement in this setting. Thus, screening at this site may reflect new community-acquired acquisition. Assessing for serologic conversion after transplant might be of interest but results could be compromised by immune suppression, blood products, or vaccine-related immunity. We have outlined an ideal methodology for monitoring such patients for transmitted infection in Figure 2. However, this is not currently clinically practicable for reasons listed in the figure. Given current testing limitations and limited evidence, our approach and recommendations are to monitor our recipients for clinical signs and symptoms of COVID-like illness and initiate appropriate testing at that time.
FIGURE 2.
Proposed monitoring strategy for recipients of allografts from donors with SARS-CoV-2 infection given proposed pathophysiology. Ideal testing strategy and practical testing strategy included, based on the availability of reliable and reasonable clinical tools. “X” tests would require research capacity. “0” tests: Our monitoring strategy
We aimed to avoid donors with a possibility of subclinical organ injury that might occur in the setting of severe COVID-19 and did not accept kidneys from two donors with more extensive pulmonary infiltrates and new onset ischemic stroke. Kidney pathologies from autopsy series of those dying from severe disease include endothelial damage, collapsing glomerulopathy, obstructive erythrocyte aggregates of capillaries, and inflammation.28 , 29 Since kidney injury is most likely due to effects of the inflammatory response, it is unlikely in the early phases of infection but not known if it could be present in later phases without common clinical markers.30
In conclusion, we demonstrated successful short-term outcomes for kidney transplantation from selected donors with detectable SARS-CoV-2 RNA. Recipients experienced no clinical signs of donor-derived SARS-CoV-2 infection, and there were no transmission events to organ procurement teams. Longer term follow-up is warranted. That many of our donors were on the exceedingly low end of the risk spectrum for transmissible virus and that other centers turned down, these organs for highly ranked candidates suggest that there is underutilization of otherwise excellent quality organs. Scientific data that would support restricting or expanding the use of SARS-CoV-2-infected donor organs are eagerly awaited. In the meantime, we advocate for the cautious use of SARS-CoV-2 RNA-positive donors for transplantation of most extrapulmonary organs.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyarsky BJ, Massie AB, Love AD, et al. Early experiences with COVID-19 testing in transplantation. Transplant Direct. 2020;6(7):e572. doi: 10.1097/TXD.0000000000001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danziger-Isakov L, Blumberg EA, Manuel O, Sester M. Impact of COVID-19 in solid organ transplant recipients. Am J Transplant. 2021;21(3):925–937. doi: 10.1111/ajt.16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-19 and Solid Organ Transplantation. 2021. https://unos.org/covid/. Accessed April 18, 2021.
- 5.United Network for Organ Sharing Removal Reasons by Year. 2021. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/. Accessed April 18, 2021.
- 6.Kates OS, Fisher CE, Rakita RM, et al. Emerging evidence to support not always “just saying no” to SARS-CoV-2 positive donors. Am J Transplant. 2020;20(11):3261–3262. doi: 10.1111/ajt.16119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Organ Procurement Transplantation Network Ad Hoc Disease Transmission Advisory Committee Summary of Current Evidence and Information—Donor SARS-CoV-2 Testing and Organ Recovery from Donors and a History of COVID-19. 2021. https://optn.transplant.hrsa.gov/media/4424/sars-cov-2-summary-of-evidence.pdf. Accessed April 26, 2021.
- 8.International Society of Heart and Lung Transplantation COVID-19 Task Force Deceased donor and recipient selection for cardiothoracic transplantation during the COVID-19 pandemic. 2021. https://ishlt.org/ishlt/media/documents/COVID-19_GuidanceDocument_Deceased-donor-and-recipient-selection-for-cardiothoracic-transplantation.pdf. Accessed April 12, 2021.
- 9.Kaul DR, Valesano AL, Petrie JG, et al. Donor to recipient transmission of SARS-CoV-2 by lung transplantation despite negative donor upper respiratory tract testing. Am J Transplant. 2021. [DOI] [PMC free article] [PubMed]
- 10.Kumar D, Humar A, Keshavjee S, Cypel M. A call to routinely test lower respiratory tract samples for SARS-CoV-2 in lung donors. Am J Transplant. 2021;21:2623–2624. doi: 10.1111/ajt.16576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cappy P, Candotti D, Sauvage V, et al. No evidence of SARS-CoV-2 transfusion transmission despite RNA detection in blood donors showing symptoms after donation. Blood. 2020;136(16):1888–1891. doi: 10.1182/blood.2020008230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong M, Zhang J, Ma X, et al. ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed Pharmacother. 2020;131:110678. doi: 10.1016/j.biopha.2020.110678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Remmelink M, De Mendonça R, D’Haene N, et al. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care. 2020;24(1):495. doi: 10.1186/s13054-020-03218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaussen A, Hornby L, Rockl G, et al. Evidence of SARS-CoV-2 infection in cells, tissues and organs and the risk of transmission through transplantation. Transplantation. 2021;105:1405–1422. doi: 10.1097/TP.0000000000003744. [DOI] [PubMed] [Google Scholar]
- 16.Kumar D, Morris MI, Kotton CN, et al. Guidance on novel influenza A/H1N1 in solid organ transplant recipients. Am J Transplant. 2010;10(1):18–25. doi: 10.1111/j.1600-6143.2009.02960.x. [DOI] [PubMed] [Google Scholar]
- 17.Kumar D, Tellier R, Draker R, Levy G, Humar A. Severe acute respiratory syndrome (SARS) in a liver transplant recipient and guidelines for donor SARS screening. Am J Transplant. 2003;3(8):977–981. doi: 10.1034/j.1600-6143.2003.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frankova V, Jirasek A, Tumova B. Type A influenza: postmortem virus isolations from different organs in human lethal cases. Arch Virol. 1977;53(3):265–268. doi: 10.1007/BF01314671. [DOI] [PubMed] [Google Scholar]
- 19.Kaul DR, Vece G, Blumberg E, et al. Ten years of donor-derived disease: a report of the disease transmission advisory committee. Am J Transplant. 2021;21(2):689–702. doi: 10.1111/ajt.16178. [DOI] [PubMed] [Google Scholar]
- 20.Heinz N, Griesemer A, Kinney J, et al. A case of an Infant with SARS-CoV-2 hepatitis early after liver transplantation. Pediatr Transplant. 2020;24(8):e13778. doi: 10.1111/petr.13778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong HL, Kim SH, Choi DL, Kwon HH. A case of coronavirus disease 2019-infected liver transplant donor. Am J Transplant. 2020;20(10):2938–2941. doi: 10.1111/ajt.15997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manzia TM, Gazia C, Lenci I, et al. Liver transplantation performed in a SARS-CoV-2 positive hospitalized recipient using a SARS-CoV-2 infected donor. Am J Transplant. 2021;21:2600–2604. doi: 10.1111/ajt.16548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin L, Carlquist J, Sinclair W, Hall T, Lopansri BK, Bennett ST. Experience with false-positive test results on the TaqPath real-time reverse transcription-polymerase chain reaction coronavirus disease 2019 (COVID-19) testing platform. Arch Pathol Lab Med. 2021;145(3):259–261. doi: 10.5858/arpa.2020-0612-LE. [DOI] [PubMed] [Google Scholar]
- 24.Lang C, Jaksch P, Hoda MA, et al. Lung transplantation for COVID-19-associated acute respiratory distress syndrome in a PCR-positive patient. Lancet Respir Med. 2020;8(10):1057–1060. doi: 10.1016/S2213-2600(20)30361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li N, Wang X, Lv T. Prolonged SARS-CoV-2 RNA shedding: not a rare phenomenon. J Med Virol. 2020;92(11):2286–2287. doi: 10.1002/jmv.25952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neidlinger NA, Smith JA, D’Alessandro AM, et al. Organ recovery from deceased donors with prior COVID-19: a case series. Transpl Infect Dis. 2021;23(2):e13503. doi: 10.1111/tid.13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deeks JJ, Dinnes J, Takwoingi Y, et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev. 2020;6:CD013652. doi: 10.1002/14651858.CD013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossi GM, Delsante M, Pilato FP, et al. Kidney biopsy findings in a critically ill COVID-19 patient with dialysis-dependent acute kidney injury: a case against “SARS-CoV-2 nephropathy”. Kidney Int Rep. 2020;5(7):1100–1105. doi: 10.1016/j.ekir.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan KW, Hung IF, Tsang OT, et al. Mass screening is associated with low rates of acute kidney injury among COVID-19 patients in Hong Kong. Am J Nephrol. 2021;52(2):161–172. doi: 10.1159/000514234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.