Abstract
The current pandemic responsible for the crippling of the health care system is caused by the novel SARS‐CoV‐2 in 2019 and leading to coronavirus disease 2019 (COVID‐19). The virus enters into humans by attachment of its Spike protein (S) to the ACE receptor present on the lung epithelial cell surface followed by cleavage of S protein by the cellular transmembrane serine protease (TMPRSS2). After entry, the SARS‐CoV‐2 RNA genome is released into the cytosol, where it highjacks host replication machinery for viral replication, assemblage, as well as the release of new viral particles. The major drug targets that have been identified for SARS‐CoV‐2 through host‐virus interaction studies include 3CLpro, PLpro, RNA‐dependent RNA polymerase, and S proteins. Several reports of natural compounds along with synthetic products have displayed promising results and some of them are Tripterygium wilfordii, Pudilan Xiaoyan Oral Liquid, Saponin derivates, Artemisia annua, Glycyrrhiza glabra L., Jinhua Qinggan granules, Xuebijing, and Propolis. This review attempts to disclose the natural products identified as anti‐SARS‐CoV‐2 based on in silico prediction and the effect of a variety of phytochemicals either alone and/or in combination with conventional treatments along with their possible molecular mechanisms involved for both prevention and treatment of the SARS‐CoV‐2 disease.
Keywords: coronaviruses, COVID‐19, MERS‐CoV, natural compounds, phytochemicals, prevention, RNA‐Virus, SARS‐CoV, SARS‐CoV‐2, therapy
1. INTRODUCTION
Coronaviruses belong to the family Coronaviridiae that consists of enveloped, single‐stranded, and positive‐strand RNA viruses with a helical nucleocapsid. They have been associated with acute and chronic respiratory, gastric, and neurologic conditions in humans and in vivo (Weiss & Navas‐Martin, 2005). Among the various coronaviruses isolated to date, the ones that infect humans include HCoV‐229E, HCoV‐OC43, HCoV‐NL63, HCoV‐HKU1, Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV, β‐coronaviruses), MERS‐CoV, and SARS‐CoV‐2 (Adhikari et al., 2021). SARS‐CoV was the responsible agent of a highly transmissible and often deadly (11% mortality) respiratory disease SARS, which resulted in an epidemic especially in the Asia region between 2002 and 2003. Another epidemic was reported in the year 2012 from Saudi Arabia, with a possible human transmission from camels of Middle East respiratory syndrome coronavirus (MERS‐CoV; 34% mortality). In comparison to the previously known human coronaviruses, SARS and MERS viruses exhibited an increase in virulence that was accompanied by severe pneumonia and the death of infected people (Guarner, 2020). Recently, a newly identified coronavirus that had emerged in December 2019 in Wuhan, China, causing coronavirus disease‐2019 (COVID‐19), has become a serious global threat to humankind by severely affecting not only the health of the people but also the economy of the nations across the globe. The pandemic has imposed a threat to all the countries in a similar fashion that is caused by SARS‐CoV2, earlier named 2019‐nCoV (Adhikari et al., 2021; Weiss & Navas‐Martin, 2005). As of January 12, 2020, over 88 million reported cases of SARS‐CoV‐2 with 1.9 million deaths worldwide have been confirmed by World Health Organization (WHO) official statistics (Bai et al., 2021). Although it comes from the same lineage, which has been responsible for SARS, however, this virus shows only 79% similarity with SARS‐CoV and 50% with MERS‐CoV (Lu et al., 2020; Rabaan et al., 2020). SARS‐CoV‐2 is believed to be transmitted to humans by zoonotic transfer, from insectivorous bats (Rhinolophus affinis) or pangolins (Manis javanica), and interestingly, more than 96% of the genome sequencing of SARS‐CoV‐2 was identical to the one of the bat CoV RaTG13 (Pagano, 2021; Shereen, Khan, Kazmi, Bashir, & Siddique, 2020; Zhou et al., 2020). SARS‐CoV‐2 is an enveloped, non‐segmented, single strands positive‐sense RNA virus (Huang et al., 2020) with crown‐like spikes on the viral outer membrane (Watanabe, Allen, Wrapp, McLellan, & Crispin, 2020). It has a diameter of 65 to 125 nm with a length of 29.9 kb (Shereen et al., 2020). The viral genome is transcribed into 16 non‐structural proteins (nsPs) and four main structural proteins (sPs). The structural proteins have been identified as spike glycoprotein (S), small envelope glycoprotein(E), membrane glycoprotein(M), and nucleocapsid protein (N) (Y. Chen, Liu, & Guo, 2020; Shereen et al., 2020; Walls et al., 2020) that is transcribed by the sub‐genomic RNA of the virus. The S glycoprotein is a transmembrane protein with a molecular weight of about 150 kDa localized in the virus outlying membrane. S protein constitutes a homo‐trimers in the viral surface and allows the binding of the SARS‐CoV‐2 envelope to the host cells by attaching with angiotensin‐converting enzyme 2 (ACE2) that is expressed in various cells types, including respiratory cells, myocardial cells, and kidney cells (Lan et al., 2020; Watanabe et al., 2020). Hence, patients who are infected with SARS‐CoV‐2 might develop respiratory syndromes such as pneumonia (Gibson, Qin, & Puah, 2020), which may lead to Acute Respiratory Distress Syndrome (ARDS). Apart from that, patients might also develop cardiac, hepatic, and digestive disorders. S protein is fragmented by the virus‐host cell furin‐like protease into two subunits known as S1 and S2. S1 would determine the host‐virus range and cellular tropism with the receptor‐binding domain make‐up while S2 is the fusion intermediate with the host cells (Rabaan et al., 2020; Walls et al., 2020). The N protein constitutes the nucleocapsid of the virus and is capable of binding to the viral RNA through its C‐terminal as well as N‐terminal domains. Due to the binding of the protein with the viral RNA, it is implicated in processes related to the viral genome, that is, the viral replication cycle, and the cellular response of host cells to viral infections (Schoeman & Fielding, 2019). Furthermore, N protein is highly phosphorylated and it has been proposed that phosphorylation triggers a structural change in the protein, which increases its affinity for the viral RNA (Chen, Liu, & Guo, 2020). In addition, it has been shown to interact with nsP3 and M protein suggesting its role in viral replication as well as packaging. M protein of the coronaviruses is the key structural protein of the viral envelope. Its interaction with nucleocapsid is crucial for the coronavirus assembly (Neuman et al., 2011; Sturman, Holmes, & Behnke, 1980). The self‐interaction of M proteins helps in the maintenance of the viral scaffold. Lastly, the E protein, which is the smallest protein in the SARS‐CoV‐2 structure has a central function in viral morphogenesis and assembly (Bianchi et al., 2020). Viroporins affects the viral entry into the host cell besides its production and maturation (Bianchi et al., 2020).
SARS‐CoV‐2 infection is triggered by the recognition and binding of the virus particle to the human ACE2 after which fusion of the virus and its host cellular membrane occurs (Chakraborty & Bhattacharjya, 2020; Wu et al., 2020). ACE 2 is a type 1 membrane protein localized in the lung, kidney, intestine, and heart. This protein is crucial for the maturation of angiotensin (a hormone that regulates vasoconstriction and blood pressure). The fusion between the viral envelope and host cell surface is mediated by the viral transmembrane fusion protein. Depending on the availability of proteases, coronavirus has two routes for entry and membrane fusion. First is the early pathway, which is adopted by the virus if the plasma protease like TMPRSS2 is available, and the virus fuses with the host membrane releasing the virus in the cytoplasm (Hoffmann et al., 2020; Simmons, Zmora, Gierer, Heurich, & Pohlmann, 2013). Further, Spike protein S also undergoes a conformational change and is primed by TMPRSS2 for membrane fusion (Figure 1) (Hoffmann et al., 2020; Simmons et al., 2013). If the membrane proteases are absent, then the coronavirus will be embodied by clathrin or non‐clathrin mediated endocytosis and this is referred to as the late pathway. In the latter pathway, activation of cathepsin L influences the viral‐host membrane fusion at the endosomal membrane. It has been demonstrated that protease availability and cell type govern the virus entry not only in SARS‐CoV‐2 but also in SARS‐CoV and MERS‐CoV. Once in the host cell, SARS‐CoV2 releases mRNA and takes over the host replication machinery to facilitate viral replication. SARS‐CoV2 genome, like other coronaviruses, is approximately 30 kb long positive single‐stranded, non‐segmented RNA molecule with 5′ cap and 3′ poly‐A tails. The viral genome during translation undergoes −1 ribosomal frameshift creating ORF1a and ORF1b. These two ORFs are translated into two polypeptides namely pp1a and pp1b. These two polypeptides are further processed by viral proteases to form 16 nonstructural proteins (nsPs) of which the first 11 nsPs are coded by ORF1a while ORF1b encodes nsP12‐16. nsPs have been shown to have multiple enzymatic functions. For example, papain‐like proteases (PLpro) and serine‐type Mpro (chymotrypsin‐like protease) also called 3CLpro protease, are encoded by nonstructural proteins (nsPs) nsp3 and nsp 5, respectively. This indicates that nsPs play a major role in many molecular processes during host cell invasion, and therefore, have been used for molecular detection of coronaviruses (Anand, Ziebuhr, Wadhwani, Mesters, & Hilgenfeld, 2003; T. Tang, Bidon, Jaimes, Whittaker, & Daniel, 2020). The next step in the viral replication involves the translation of the sub‐genomic RNA into four structural proteins that have been discussed earlier and a variable number of accessory proteins namely 3a, 3b, p6, 7a, 7b, 8b, 9b, and ORF14. The structural proteins undergo post‐translational modifications in the endoplasmic reticulum and are then shifted to the endoplasmic reticulum‐Golgi intermediate compartment (ERGIC). For viral assembly, once the genome has been replicated, it will interact with the N protein to form a nucleocapsid and relocate into the ERGIC. In this cellular organelle, the nucleocapsid will interact with other structural components to shape vesicles that will eventually be transported out of the cell via exocytosis (Masters, 2006).
FIGURE 1.
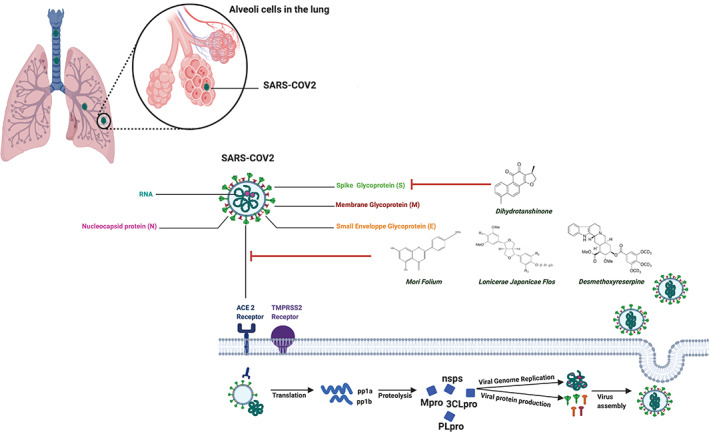
Inhibition of SARS‐CoV2 cell entry that depends on ACE2 and TMPRSS2. RNA, Ribonucleic acid; ACE2, Angiotensin‐converting enzyme 2; TMPRSS2, Type II transmembrane serine protease; PLpro, papain‐like proteases; Mpro (3CLpro), chymotrypsin‐like protease; pp1a, polyproteins 1a; pp1b, polyproteins 1b; nsps, nonstructural proteins; PLpro, papain‐like proteases; Mpro (3CLpro), chymotrypsin‐like protease
To date, the successful approach that has been found to have concrete ameliorating effects on SARS‐CoV‐2 infection is plasma therapy. The substantial drug targets of SARS‐CoV‐2 that have been identified include 3CLpro (Mpro), PLpro, RNA‐dependent RNA polymerase, and S proteins (Faheem Kumar et al., 2020; Prajapat et al., 2020). Irrespective of the impact of the pandemic on healthcare systems, alternate forms of therapy, that is, traditional medicine or their synthetic derivatives have shown some promising results in restricting SARS‐CoV‐2 viral infection, the most famous being hydroxychloroquine and chloroquine phosphate (Cortegiani, Ingoglia, Ippolito, Giarratano, & Einav, 2020). Furthermore, numerous other molecules derived from various medicinal plants have been reported to exhibit significant SARS‐CoV‐2 activities through targeting SARS‐CoV‐2 activity by meddling with every step of the interaction of the virus with its host cell (Brendler et al., 2020). These products have been identified either in silico as targeting MPro aster pentapeptide A, ligustrazine, salvianolic acid B, Zingiberis Rhizoma Recens, Asteris Radix et Rhizoma, Notoginseng Radix et Rhizoma, Chuanxiong Rhizoma, Salviae Miltiorrhizae Radix et Rhizoma, Zingiberis Rhizoma, Dianthi Herba, Rhei Radix et Rhizoma, Cistanches Herba, gingerketophenol, ginkgol alcohol, ferulic acid, etc., and Codonopsis Radix, Notopterygii Rhizoma et Radix, Zingiberis Rhizoma Recens, Ginkgo Semen, Chuanxiong Rhizoma, Trichosanthis Fructus, Paeoniae Radix Alba, Psoraleae Fructus, Sophorae Flavescentis Radix, Notoginseng Radix et Rhizoma, Angelicae Sinensis Radix (Ma et al., 2020; Yang et al., 2020), Betulinic acid, Coumaroyltyramine, Cryptotanshinone, Desmethoxyreserpine, Dihomo‐γ‐linolenic acid, Kaempferol, Lignan, N‐cis‐feruloyltyramine, Quercetin, Sugiol, Tanshinone IIa (D. H. Zhang, Wu, Zhang, Deng, & Peng, 2020), hillyraeoidin‐E (PR), pseudoheptafuhalol‐C (PHF) and a triterpenoidal saponin (GPA) (Khalifa et al., 2020), coumarin analogs psoralen, bergapten, imperatorin, heraclenin, heraclenol, saxalin, oxepeucedanin, angelicin, toddacoumaquinone, aesculetin (Chidambaram, Ali, Alarifi, Radhakrishnan, & Akbar, 2020); phlorotannins (Gentile et al., 2020; Li et al., 2017); S‐protein Castanospermine and Karuquinone B (Al‐Sehemi et al., 2020), Lonicerae Japonicae Flow [(Niu et al., 2020), hesperidin, emodin, chrysin (Basu, Sarkar, & Maulik, 2020), Quercetin (Derosa, Maffioli, D'Angelo, & Di Pierro, 2021); PLproCoumaroyltyramine, Cryptotanshinone, Kaempferol, Moupinamide, N‐cis‐feruloyltyramine, Quercetin, Tanshinone IIa (Gentile et al., 2020); tested in in vitro such as Saponin derivates, inhibition of the virus adsorption, entrance, and replication (Cheng, Ng, Chiang, & Lin, 2006); Pudilan Xiaoyan Oral Liquid, suppressed viral replication (Deng et al., 2020); Curcuma longa, S protein/virus entry inhibition (Brendler et al., 2020; T. Y. Chen et al., 2013; Zahedipour et al., 2020); Propolis, inhibiting TMPRSS2 expression and ACE2 anchorage (Berretta, Silveira, Condor Capcha, & De Jong, 2020; Hori, Zamboni, Carrao, Goldman, & Berretta, 2013; Machado et al., 2012; Pineros et al., 2020); Artemisia annua, inhibits pulmonary fibrosis (Haq et al., 2020); Tripterygium wilfordii, suppression of TMPRSS2, inhibiting viral entry through the reduction of the spike protein cleavage in ACE2 receptor‐mediated viral penetration (Habtemariam et al., 2020; Hoffmann et al., 2020; Ren et al., 2017; Shi et al., 2018); Pudilan Xiaoyan Oral Liquid (Deng et al., 2020); and clinically including Glycyrrhiza glabra L.(Cinatl et al., 2003); Jinhua Qinggan granules (H. Chen, Song, Gao, Zhao, & Ma, 2020); Xuebijing (Wen, Zhou, Jiang, & Huang, 2020). This review is intended to deliver cumulative pieces of information regarding the natural compounds or their derivatives that have shown potential preventive as well as therapeutic effects on SARS‐CoV‐2 either in silico, in vitro, in vivo, and or clinically.
2. IN SILICO IDENTIFIED PHYTOCHEMICALS AGAINST SARS‐COV‐2
During the first SARS‐CoV pandemic, patients with SARS who were treated with Traditional Chinese Medicines (TCM) had a shortened hospitalization time, limited side effects, and recovery from initial symptoms of the virus (Boozari & Hosseinzadeh, 2021; Pagano, 2021). Interestingly, genomic and biochemical interactions along with pathogenesis and in silico structural studies of SARS‐CoV‐2 disclosed that it is closely linked to the SARS coronavirus, implying that TCM used for SARS might possess useful curative benefits during the current pandemic. Hence, the development of bioinformatics tools is beneficial to predict the effectiveness of natural products and TCM in SARS‐CoV‐2 treatment (figure 2).
FIGURE 2.
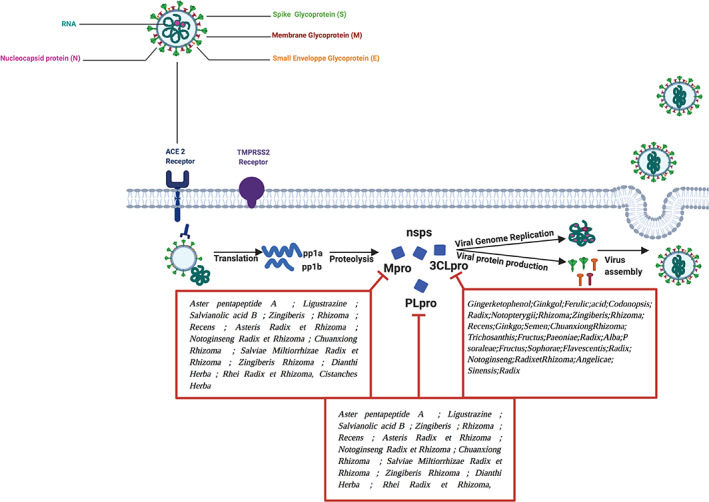
Inhibition of SARS‐CoV2 viral genome replication and viral protein production. RNA, ribonucleic acid, ACE2, Angiotensin‐converting enzyme 2; TMPRSS2, Type II transmembrane serine protease; PLpro, papain‐like proteases, Mpro (3CLpro), chymotrypsin‐like protease; pp1a, polyproteins 1a; pp1b, polyproteins 1b; nsps, nonstructural proteins; PLpro, papain‐like proteases; Mpro (3CLpro), chymotrypsin‐like protease
2.1. TCM targeting SARS‐CoV‐2/pulmonary fibrosis
Using molecular docking technology and the crystal structures of both Mpro and PLP, virtual screening of TCMD 2009 database (On‐Line Database of TCM) was conducted by Ma et al. The products scored in the top 100 were chosen as candidates then classified by the number of hit molecules. According to the Mpro inhibitors screening, 12,322 potential active compounds were selected, and the most promising included aster pentapeptide A, ligustrazine, salvianolic acid B, etc., and Zingiberis Rhizoma Recens, Asteris Radix et Rhizoma, Notoginseng Radix et Rhizoma, Chuanxiong Rhizoma, Salviae Miltiorrhizae Radix et Rhizoma, Zingiberis Rhizoma, Dianthi Herba, Rhei Radix et Rhizoma, Cistanches Herba (Ma et al., 2020). Several potential active components were obtained by PLP inhibitor screening, along with ACE2 inhibitors, the most active photo components, including gingerketophenol, ginkgol alcohol, ferulic acid, etc., and Codonopsis Radix, Notopterygii Rhizoma et Radix, Zingiberis Rhizoma Recens, Ginkgo Semen, Chuanxiong Rhizoma, Trichosanthis Fructus, Paeoniae Radix Alba, Psoraleae Fructus, Sophorae Flavescentis Radix, Notoginseng Radix et Rhizoma, Angelicae Sinensis Radix (figure 2) (Ma et al., 2020).
Pulmonary fibrosis that has been identified as one of the primary SARS‐CoV‐2 sequelae, leads to a deterioration of the lungs along with dyspnea. Furthermore, it has a life‐threatening impact on patients leading to poor prognosis and high mortality, and poor prognosis. Vascular endothelial growth factor receptor (VEGFR) and fibroblast growth factor receptor (FGFR) regulate the molecular pathway of pulmonary fibrosis and Chinese herbal preparations have shown a strong therapeutic effect on pulmonary fibrosis. The therapeutics modulation of pulmonary fibrosis by TCM was explored by screening the candidates for VEGFR and FGFR inhibition. The docking analysis of VEGFR and FGFR was set up to obtain the potential active ingredients, which were filtered by the docking score. The candidates for treating pulmonary fibrosis mostly belonged to the lung and spleen. Therefore, Qiyin Prescription and Buzhong Yiqi Decoction were shown to include the largest number of top‐ranked potential herbs (Bian, Ma, Ren, Zhang, & Qiao, 2020). Interestingly, Guo F et al have developed a prediction platform TCMATCOV to examine the effectiveness of the anti‐SARS‐CoV‐2 pneumonia syndrome of TCM. This SARS‐CoV‐2 network model was constructed by protein–protein interactions of differentially expressed genes in mouse pneumonia caused by SARS‐CoV‐2. TCMATCOV has used a quantitative evaluation algorithm of disease‐targeted organs after a multi‐target drug attack to predict potential drug effects. The results have predicted 106 TCM as potential drug candidates anti‐SARS‐CoV‐2. The results were compatible with the proportion of the classic anti‐SARS‐CoV‐2 prescriptions used by clinicians, which strongly supports that TCMATCOV has a good prediction ability to bring to light the effective TCMs. The outcome of this study has shown that the top‐scored flavors of TCM are mainly spicy and bitter, while the main meridian systems organs targeted by these TCMs are lung, heart, spleen, liver, and stomach meridian. Hence, the TCMATCOV platform possesses the potential for the discovery of possible effective TCM remedies for SARS‐CoV‐2 (Guo et al., 2020).
2.2. Mpro
Amid well‐described coronaviruses, Mpro has been described as a top drug target. Indeed, the numerous cleavage sites fragmented by Mpro (11 sites), usually Leu‐Gln↓(Ser, Ala, Gly) on the polyprotein replicase 1ab. Hence, inhibiting this enzyme would ease the suppression of viral replication. Furthermore, as no stated human proteases with such a cleavage particularity have been described, it is believed that the inhibitors against this enzyme are more likely to be specific and have lower toxic effects. The three‐dimensional X‐ray crystal structure of this catalyst has recently been solved by Zhang et al., which gives opportunities for structure‐based drug design toward the Mpro targets (Gurung, Ali, Lee, Farah, & Al‐Anazi, 2020; Zhang et al., 2020). In that regard, the group has executed a rational screen to identify TCM products that inhibit Mpro principally by entering the region between domain 2 and 3, knowing that this region is crucial for Mpro to form a dimer and include Betulinic acid, Coumaroyltyramine, Cryptotanshinone, Desmethoxyreserpine, Dihomo‐ϒ‐linolenic acid, Kaempferol, Lignan, N‐cis‐feruloyltyramine, Quercetin, Sugiol, Tanshinone IIa (Zhang, Wu, et al., 2020). Another study led by Padhi et al. have utilized in silico evaluation based on the binding affinity and molecular docking to screen 2,300 FDA‐approved drugs and a large number of small molecules of natural origin, and identified putaminoxin D and jasmonic acid as being largely favorable in inhibiting SARS‐CoV‐2 Mpro (Padhi et al., 2021). Whereas the analysis of phytochemicals databases by Gurung et al. led to the identification of three lead molecules Bonducellpin D, 5,7‐dimethoxyflavanone‐4′‐O‐β‐d‐glucopyranoside, and Caesalmin B (Gurung et al., 2020). Likewise, post‐screening, the most potent natural compounds that target Mpro residue identified by Khalifa et al. were two tannins, phillyraeoidin‐E (PR), pseudoheptafuhalol‐C (PHF), and a triterpenoidal saponin (GPA) (Khalifa et al., 2020), while another in silico study led by Chidambaram et al. has selected top coumarin analogs Psoralen, bergapten, imperatorin, heraclenin, heraclenol, saxalin, oxepeucedanin, angelicin, toddacoumaquinone, and aesculetin (Figure 2) were the utmost suggested compounds originated in therapeutic plants that might act as significant inhibitors of Mpro (Chidambaram et al., 2020). Using the same approach on Marine Natural Product Library, 17 potential SARS‐CoV‐2 Mpro inhibitors have been found. The most promising inhibitors of the SARS‐CoV‐2 Mpro have been identified as being part of a class of molecules called phlorotannins, oligomers of phloroglucinol (1,3,5 trihydroxybenzene) that are isolated from Sargassum spinuligerum brown alga (Gentile et al., 2020; Li et al., 2017).
2.3. S protein
Investigation of 31,000 natural compounds by Al‐Sehemi et al. for their ability to bind to S‐glycoprotein has distinguished two top compounds Castanospermine and Karuquinone B that were selected based on their binding affinity to ACE2 and pharmacokinetic data. Castanospermine having a higher binding affinity and hence SARS‐CoV‐2 inhibition through S‐protein (Al‐Sehemi et al., 2020). The same observations have been made on Lonicerae Japonicae Flow (Niu et al., 2020). Using the same principle, Basu et al. have identified hesperidin, emodin, and chrysin phytochemicals as S protein/ACE2 binding inhibitors. Interestingly, these compounds have been reported to have shown comparable spike protein inhibiting efficacy as that of known inhibitors such as chloroquine and hydroxychloroquine (Basu et al., 2020). Quercetin a flavonol that belongs to flavonoid compounds is derived from quercetum (oak forest) known for its antiinflammatory and antiviral properties, is an FDA‐approved compound as part of antioxidant and anti‐allergy medicines (Fischer, Speth, Fleig‐Eberenz, & Neuhaus, 1997). Coronil, an herbal formulation of Tinospora cordifolia, Withania somnifera, and Ocimum sanctum was reported to suppress the SARS‐CoV‐2 S‐protein dependent entry of the virus in A549 cells by inhibiting the interaction of spike protein with ACE‐2 (Figure 2). Coronil was reported to regulate the levels of IL‐6, IL‐1β, and TNF‐α in A549 cells (Balkrishna, Haldar, Singh, Roy, & Varshney, 2021). In silico modeling of small molecule candidates of known drugs and natural compounds was performed to identify S Protein/ACE2 binding inhibitors. The studies have shown that quercetin was one of the most potent compounds (Abian et al., 2020; Derosa et al., 2021).
2.4. PLpro
Zhang et al. Have highlighted that among the natural compounds they have screened, 13 were found to target PLpro mainly through binding in the locus across the thumb and palm domain. This could disrupt with substrate penetrating PLpro active sites, located underneath the two domains and include Coumaroyltyramine, Cryptotanshinone, Kaempferol, Moupinamide, N‐cis‐feruloyltyramine, Quercetin, Tanshinone IIa (Zhang, Wu, et al., 2020). The results of these studies might be used as a base reference to elaborate efficient treatments to target the SARS‐CoV‐2 outbreak. Eventually in vitro and in vivo studies need to be carried out to validate their potential as drug candidate molecules.
3. ANTI‐SARS‐COV‐2 COMPOUNDS FROM NATURAL SOURCES
3.1. Curcuma longa
Curcumin (diferuloylmethane) is a biphenolic phytochemical found in turmeric, which is well characterized for being an immunomodulator with various pharmacological actions, against various chronic conditions, including inflammation, viral infections, and cancers (Sandur et al., 2007). Curcumin has poor bioavailability in its raw form; therefore, derivatives of the original form have been developed (Shanmugam et al., 2015). It has been widely shown that curcumin has powerful anti‐viral effects. These effects of curcumin have been shown in the treatment of multiple viral diseases, including those due to vesicular stomatitis virus, parainfluenza virus type 3, vesicular stomatitis virus, flock house virus, herpes simplex virus, human papillomavirus, and respiratory syncytial virus (Moghadamtousi et al., 2014). Interestingly, a world map of the distribution of SARS‐CoV‐2 shows that countries in Southeast Asia, which have the most important production along with being the first consumers of curcumin worldwide, have shown a very low number of deaths attributed to SARS‐CoV‐2 infections (Taiwan having had efficient rules along with adequate health facilities has been excluded from this cited study) in comparison with western European countries that have far better health systems available (Rocha & de Assis, 2020).
3.2. Artemisia annua L.
Pulmonary fibrosis is seen in SARS‐CoV‐2 infection with advanced gravity (Ojo, Balogun, Williams, & Ojo, 2020). Numerous findings suggest that pulmonary diseases are correlated with oxidative stress, therefore, the consumption of antioxidant compounds from natural products is effective in lung fibrosis (Day, 2008). Artemisia annua L. is used in both the Asian and African continent in a form of tea or press juice for the treatment of malaria and associated fever, chills, and its well‐known active compound Artemisinin (ARS), is now used all over the world as an antimalarial drug but also to target hepatitis B virus, bovine viral diarrhea virus and Epstein–Barr virus, and Herpes Simplex virus type 1 (for which the methanolic extracts of A. annua had the most effective antiviral effect) (Efferth, 2017) Interestingly, during the SARS epidemic that occurred in 2002, Artemisia annua L. has shown significant activity. Annua extract is known for its antioxidant activity that is presumably due to its high phenolic content (Haq et al., 2020).
3.3. Tripterygium wilfordii
Celastrol (tripterine), a phytochemical extracted from the roots of Tripterygium wilfordii (Thunder god vine) has been widely investigated, for its antiinflammatory as well as anticancer potential, which is believed to be mainly modulated through its ability to inhibit the production of cytokines and chemokines that could conduct to the suppression of leukocyte migration into the inflamed joints, leading to antiinflammatory activity. Celastrol has also shown promising results both in vitro and in vivo against SARS‐CoV‐2 (Khalili, Karimi, Moradi, & Shirzad, 2018; Ren et al., 2017; Shi et al., 2018; M. Zhang et al., 2019).
3.4. Saponin derivatives
Glycyrrhiza species (licorice) have displayed antiviral effects against numerous viral infections (Brendler et al., 2020; Fiore et al., 2008). Cinatl et al. have tested ribavirin, 6‐azauridine, pyrazofurin, mycophenolic acid, and glycyrrhizin, which are saponin derivatives discovered in licorice roots of Glycyrrhiza glabra L., Fabaceae toward two clinical samples of SARS‐CoV (one from Frankfurt patients and the second from Hong Kong). They have been shown to successfully inhibit the adsorption, entrance, and replication of SARS‐CoV‐2 in its host cells (Cinatl et al., 2003). Saikosaponin B2 has also been proved to have a strong activity as an inhibitor of virus‐host cell binding and entrance modulating the early stage of SARS‐CoV‐2 replication (Cheng et al., 2006).
3.5. Pudilan Xiaoyan Oral Liquid
Pudilan Xiaoyan Oral Liquid (PDL) is a TCM concoction that contains Indigowoad Root (Isatis Indigotica), Bunge Corydalis (Corydalis Bungeana), Mongolian Dandelion (Taraxacum Mongolicum), Scutellaria Amoena (Scutellaria Baicalensis). PDL has shown an important suppressive effect against bacterial and viral infections and is known for its wide therapeutic use in mumps, pharyngitis, children's acute tonsillitis, acute bronchitis, among other respiratory diseases. PDL has been shown to effectively suppress viral replication of the SARS‐CoV‐2 (Deng et al., 2020).
3.6. Xuanfei Baidu Tang (XFBD)
Xuanfei Baidu Tang (XFBD) is a herbal compound that has been described in the Chinese pharmacopeia, 326 of the 1,224 putative XFBD targets were combined with the disease targets of SARS‐CoV‐2, among which 109 targets were enhanced in the disease pathways of viral infection and lung injury (Wang et al., 2020).
3.7. Lonicerae Japonicae Flos and Morus alba L.
Lonicerae Japonicae Flos (Jinyinhua), flowers or flower buds of Lonicera japonica Thunberg, is a widely used TCM. Various basic and clinical studies have demonstrated that JYH has strong effects on infectious diseases (Li, Li, Fu, Song, & Fu, 2019). Morus alba L., from the Moraceae family, is a short‐lived mulberry tree. The species originates from northern China and is largely farmed around the world. The leaves, bark, branches, and fruits of Morus alba L. have been widely applied for medicinal uses due to their various therapeutic effects, including the antimicrobial and antioxidant ones. Lonicerae Japonicae Flos combined with Morus alba L. when taken with modern medicine, symptomatic supportive treatment for SARS‐CoV‐2 has shown significantly better effects than symptomatic supportive therapy alone (Huang et al., 2020).
3.8. Jinhua Qinggan granules
Jinhua Qinggan granule (JHQGG) is a Chinese multi‐herbal formula that has been first developed as a medicine for H1N1 influenza and has been highly suggested for suspicious clinical cases of SARS‐CoV‐2, indeed JHQGG has been shown to shorten the timing of viral nucleic acid detectability and a quick viral clearance rate, in addition to speed the pneumonia recovery time of the treated SARS‐CoV‐2 patients, all that without adverse reactions (Chen, Song, et al., 2020).
3.9. Xuebijing
Xuebijing (made of Flos Carthami, Radix Paeoniae Rubra, Rhizoma Chuanxiong, Radix Salviae Miltiorrhizae et Radix Angelicae Sinensis), a Chinese medicine extract infusion formula, approved by the China FDA, is demonstrated to contain active compounds that lead to cytokine reduction, anti‐coagulation, and neutralization of released bacterial cytotoxins. Outcomes of clinical studies have shown a tremendous improvement in patients demonstrated by increased survival, and reduction in hospital admissions, along with an enhanced clinical picture after Xuebijing infusion (Editorial Board of Chinese Critical Care, 2019; Song et al., 2019).
4. QUERCETIN
A study led by Derosa et al. has shown that Quercetin, a flavonol that belongs to flavonoid compounds is derived from quercetum (oak forest) known for its antiinflammatory and antiviral properties could suppress SARS‐CoV‐2 cell entry, replication, and inhibit 3CLpro and PLpro in vitro (Abian et al., 2020; Derosa et al., 2021; Gentile et al., 2020). Hence, Quercetin is currently undergoing several clinical trials [NCT04578158, NCT04377789, NCT04468139, and NCT04622865] as anti‐SARS‐CoV‐2 candidate (Abian et al., 2020; Islam et al., 2020).
5. MOLECULAR MECHANISMS OF NATURAL COMPOUNDS AS SARS‐COV‐2 AGENTS
5.1. In vitro studies
5.1.1. Curcuma longa
The multi‐targeting anti‐viruses power of curcumin arises from its faculty to interfere with numerous molecular pathways hence initiating cellular signaling pathways involved in pulmonary disease, metabolic diseases, liver diseases, and inflammation (Brendler et al., 2020). Former studies have proved that curcumin interoperates directly with about thirty proteins, among which DNA polymerase, thioredoxin reductase, focal adhesion kinase, protein kinase (PK), tubulin, and lipoxygenase (LOX). Furthermore, curcumin regulates key intercellular signaling cascades, which are crucial for an efficient virus replication such as attenuation of NF‐κB and PI3K/Akt signaling. It also impacts cellular post‐transcriptional and post‐translational variations, hence limiting the viral multiplication by interfering with key steps in their replication cycle, among which genome replication, and viral attachment (Pourhajibagher et al., 2021). In addition, it has been demonstrated that curcumin treatment can modulate the structure of the S protein in viruses, and hence block the viruses' entry into the cells (T. Y. Chen et al., 2013). Using molecular docking, curcumin was shown to bind to RBD‐S, PD‐ACE2, and SARS‐CoV‐2 protease (Soni et al., 2020; Zahedipour et al., 2020).
5.1.2. Pudilan Xiaoyan Oral Liquid
In Vero E6 cells, PDL with an EC50 = 1.078 mg/ml has been observed to effectively suppress the viral duplication of SARS‐CoV‐2, suggesting that virus infection was vigorously blocked by PDL (Deng et al., 2020).
5.1.3. Saponin derivatives
In human fetal lung fibroblasts, Saikosaponins A, B2, C, and D were tested against SARS‐CoV‐2. No cytotoxicity has been detected with saikosaponins A, B2, C, and D up to a concentration of 25 μmol/L, where, identically, all the compounds have shown a strong SARS‐CoV‐2 inhibition. The highest efficiency has been observed with saikosaponin B2 leading to 100 ± 0.2% of inhibition at 25 μmol/L. EC50 values for saikosaponins A, B2, C, and D were defined to be 8.6 ± 0.3 (SI = 26.6), 1.7, 19.9, and 13.2, respectively. Additional investigations have been led and have confirmed that saikosaponin B2 had the strongest activity as a virus‐host cell attachment and penetration inhibition (Cheng et al., 2006).
5.2. Propolis
Propolis, a resinous material produced by honey bees from plant exudates, has long been used in traditional herbal medicine and is widely consumed as a health aid and immune system booster. There is considerable evidence that propolis can reduce and alleviate the symptoms of inflammatory diseases by affecting various metabolic cycles. Recently, several studies have shown that propolis extract and some of its components act against several important targets of SARS‐CoV‐2, such as reducing TMPRSS2 expression, and reducing ACE2 anchorage; this is in addition to immuno‐modulation of monocytes/macrophages (reducing the production of and eliminating IL‐1 beta and IL‐6), reduction of the transcription factors NF‐KB and JAK2/STAT3 and blocking PAK1, which determine inflammatory activities and fibrosis caused by SARS‐CoV‐2 (Berretta et al., 2020; Hori et al., 2013; Machado et al., 2012; Pineros et al., 2020).
5.3. In vivo studies
5.3.1. Artemisia annua
Pulmonary fibrosis, found in SARS‐CoV‐2 infected people is proved to be mediated by Interleukin‐1 (IL‐I) induction that occurs after virus binding to TLR. IL‐I is a pleiotropic cytokine, highly inflammatory, the mediator of fever, and fibrosis. HSP47 is associated with collagen accumulation along with TGF‐β1 and its downstream molecules Smad3 and α‐SMA; which are up‐regulated in pulmonary fibrosis. In rats, Artemisia annua has been shown to inhibit all the cited molecules and their corresponding pathways, which inhibits pulmonary fibrosis and there may be used as a possible treatment for SARS‐CoV 2 (Haq et al., 2020).
5.3.2. Tripterygium wilfordii
TMPRSS2 is located in epithelial cells of the lung (Kim, Heinlein, Hackman, & Nelson, 2006) and upper airways. Airway protease like Epitheliasin is directly implicated in the initiation of viral infections with Influenza and coronaviruses (Bugge, Antalis, & Wu, 2009). In the TMPRSS2 knockout murine model with MERS‐CoV and SARS‐CoV 2 infection, the inflammatory reaction was shown to be activated through the NF‐kB pathway by its regulation by TMPRSS2 implying that the NF‐κB pathway and TMPRSS2 are interlinked (Iwata‐Yoshikawa et al., 2019). Furthermore, the suppression of TMPRSS2 might have a double effect on SARS‐CoV 2, by inhibiting viral entry through the reduction of the spike protein cleavage in ACE2 receptor‐mediated viral penetration (Hoffmann et al., 2020) and that of suppressing the NF‐κB pathway, which conducts to a lower proinflammatory response with a weaker severity of lung pathology on SARS and MERS‐CoV, with celastrol potentially interfering in both mechanisms. Furthermore, celastrol antiinflammatory potential, through suppression of NF‐κB signaling, in rats that have undergone lipopolysaccharide (LPS)‐induced ARDS, an improvement in the inflammation‐mediated injury along with the expression levels of pro‐inflammatory cytokines (TNF‐α, IL‐1, IL‐6, and IL‐8) and NF‐κB has been shown (Habtemariam et al., 2020). Moreover, celastrol has shown promising results in reducing chronic obstructive pulmonary disease in mice via antiinflammatory mechanisms, including the reduction of the levels of the inflammatory cytokine such as IL‐8, TNF‐α, and monocyte chemoattractant protein‐1 while increasing the antioxidant defenses that includes superoxide dismutase and catalase (Shi et al., 2018). In patients suffering from asthma or with enhanced airway hyper‐responsiveness, a positive outcome for orally administered celastrol both in the disease level and Th17 proinflammatory cells suppression was observed in vivo (Zeng, Lin, Zheng, Zhang, & Zhang, 2018) whereas, under ventilator‐induced lung injury in mice, celastrol was shown to ameliorate the outcome (Ren et al., 2017).
5.3.3. Pudilan Xiaoyan Oral Liquid
In transgenic mice, SARS‐CoV‐2‐infected hACE2, dispensed by intragastric route with PDL (4 ml/kg) from 1 hour post virus inoculation, then once daily for 5 days, has been shown to reduce the viral RNA copies in the lungs in SARS‐CoV‐2‐infected hACE2 mice with PDL treatment in comparison with the model control group. In SARS‐CoV‐2‐infected hACE2 mice, lung tissues at 5 dpi had restrained pneumonia with interstitial hyperplasia. Furthermore, the alveolar interstitium was thickened with inflammatory cells, inflammatory cells and infiltration around blood vessels were observed. In comparison, the lung tissues from PDL‐treated mice displayed mild interstitial pneumonia along with limited amounts of inflammatory cells infiltrated, even though the alveolar interstitium expanded (Deng et al., 2020).
6. NATURAL COMPOUNDS FOR ORGANS PROTECTION
6.1. Liver and kidney injury
6.1.1. Curcumin
Post‐infection by SARS‐CoV‐2, an enhanced incidence of acute kidney injury is observed, which is most probably due to the presence of the virus, the inflammatory response, or a synergistic impact of both these factors on kidneys. It has been demonstrated that patients with acute renal injury have a higher mortality rate. ACE2 is strongly expressed in the kidneys (Mizuiri & Ohashi, 2015; Toapanta et al., 2021). A decrease in ACE2 and an enhancement in ACE expression might eventually lead to renal damage in diabetes (Mizuiri & Ohashi, 2015). Xu et al. have demonstrated that curcumin can lead to the over‐expression of ACE2. ACE2 mRNA leading to an improved renal blood flow with a potential anti‐fibrotic effect in kidneys in type 2 diabetic rat models. Curcumin potentially reduces renal fibrosis at priming and activation stages by suppressing the inflammation caused by reduced MCP‐1, NFκB, TNF‐α, IL‐1𝛽, COX‐2, and cav‐1 levels. Curcumin also increases the expression of antiinflammatory factors such as neural precursor cell expressed developmentally down‐regulated protein 4 (NEDD4), mannose‐6‐phosphate receptor binding protein 1(M6PRBP1), and heme oxygenase‐1 (HO‐1). Curcumin also targets MAPK/ERK, TGF‐α/smads, and PPAR‐𝛾 pathways in animal models of kidney disease (Sun et al., 2017). Thus, curcumin could likely be beneficial for the treatment of SARS‐CoV‐2 associated renal inflammation.
6.1.2. Tripterygium wilfordii
While using celastrol, organ protective effects have been noticed especially in renal injury under diabetes or drug‐induced nephrotoxicity (M. Tang et al., 2018).
6.2. Cardiovascular damage
ACE2 plays a key role in cardiovascular function as well as in the development of diabetes and hypertension (Turner, Hiscox, & Hooper, 2004). SARS‐CoV‐2 infectious process starts by the binding of the virus spike protein to ACE2 receptors, which are vastly expressed in different organs such as the heart, lungs, and kidney. As a result, SARS‐CoV‐2 leads to respiratory syndrome through its entrance to the alveolar epithelial cells. It has been determined that these syndromes are more prominent in subjects with cardiovascular disease, which might be due to their highest ACE2 display. Given that ACE2 operates as a receptor for SARS‐CoV‐2, the safety and potential effects of anti‐hypertension therapy with angiotensin‐receptor blockers or ACE inhibitors in SARS‐CoV‐2 clinical cases might be investigated (Mourad & Levy, 2020; Zheng, Ma, Zhang, & Xie, 2020). Nevertheless, the decrease of ACE2 activity is injurious to the heart, since it contributes to cardiac dysfunction, which is due to a higher stimulation of the AT1 receptor by angiotensin II (Gheblawi et al., 2020; Mourad & Levy, 2020; Zheng et al., 2020). In this context, it has been shown that curcumin considerably lowers mean arterial blood pressure and ameliorates cardiac fibrosis in vivo by up‐regulating angiotensin type II receptor, down‐regulated angiotensin II type I receptor, and increasing ACE2 in the myocardium without notable harmful side effects (Pang et al., 2015). In SARS‐CoV‐2 infected patients, cardiovascular symptoms appear due to the systemic inflammatory response initiated by the imbalance response of type 1 and type 2 T helper cells. In addition, it has been demonstrated that curcumin reduces inflammation and necrotic tissue in the myocardial ischemia–reperfusion model in the rat by suppressing the early growth response‐1 and inhibiting TNF‐α and IL‐6. Curcumin reduces myocardial ischemia–reperfusion injury by suppressing the c‐Jun‐N‐terminal kinase (JNK) and NF‐κB nuclear translocation phosphorylation (Mokhtari‐Zaer, Marefati, Atkin, Butler, & Sahebkar, 2018).
6.3. Clinical studies
6.3.1. Glycyrrhiza glabra L.
At the clinical center of Frankfurt University, Cinatl et al. have tested ribavirin, 6‐azauridine, pyrazofurin, mycophenolic acid, and glycyrrhizin, which are saponin derivatives in two samples of SARS‐CoV‐2 positive patients named FFM‐1 and FFM‐2 that have been isolated from the serums of the SARS‐CoV‐2 subjects. Glycyrrhizin, which has previously demonstrated its effectiveness in suppressing HIV‐1 and hepatitis C virus infections, has shown promising effects against SARS‐type coronavirus in Vero cells by effectively inhibiting the replicative process at its initial stage, but also the adsorption, and penetration of the virus in its host cells (SI of 67). Notably, it has also been highlighted that its effect was more limited when added during the virus adsorption (EC50 = 600 mg/L), whereas it had the highest inhibition when added after the virus adsorption (EC50 = 300 mg/L, 364.5 μM). Moreover, a dose of 1,000 mg/L glycyrrhizin treatment resulted in a lower viral antigen expression. The authors have also determined that the glycyrrhizin mechanism of action was likely the induction of nitric oxide synthase (Cinatl et al., 2003). Finally, a new clinical study led by Safa et al. to investigate Licorice root extract effect on clinical symptoms of SARS‐CoV‐2 is ongoing (Safa et al., 2020).
6.3.2. Jinhua Qinggan granules
Eighty cases of SARS‐CoV‐2 positive patients in Beijing YouAn Hospital, China, were analyzed. All the subjects have been treated with symptomatic along with supportive therapy. Forty‐four of these patients took JHQGGs (treatment group) within the first day of hospitalization, while the 36 other patients (control group) did not. The duration of viral nucleic acid detection and pneumonia absorption amelioration between the two groups was compared. The average time of viral nucleic acid detection was (7 ± 4) days in the Jinhua Qinggan administration group, in which 56.82% of the patients had negative nucleic acid tests within 7 days or less versus (10 ± 4) days for the control group with a significantly higher viral clearance rate for the Jinhua Qinggan group compared with the control one. Furthermore, the pneumonia recovery time indicated by chest CT was (8 ± 4) days in the Jinhua Qinggan group, versus (10 ± 5) days for the control (Chen, Song, et al., 2020).
6.3.3. Xuebijing
In Changsha hospital, china, sixty severe SARS‐CoV‐2 patients were admitted from January to March 2020 and had been randomly distributed into three groups of 20 cases each, the first group has been treated with a routine treatment (Treatment according to the national health commission protocol), the second one with Xuebijing 50 m that was injected two times a day for 7 days, and the last one with Xuebijing 100 ml also through injection twice a day for 7 days. The blood routine test, C‐reactive protein (CRP), erythrocyte sedimentation rate (ESR), acute physiology and chronic health evaluation II (APACHE II) score, SARS‐CoV‐2 nucleic acid test, and disease classification of the patients before treatment versus after the treatment protocol was observed. Post‐treatment, the white blood cell count (WBC) and lymphocyte count (LYM) of all the groups increased, whereas CRP and ESR decreased. In comparison with Xuebijing 50 ml group, the increase of WBC, and the decrease of CRP and ESR were more significant in Xuebijing 100 ml group. Interestingly, post‐Xuebijing treatment, the APACHE II score decreased more significantly in the Xuebijing treated groups in a dose‐dependent manner compared to the routine group. In addition, post‐treatment the SARS‐CoV‐2 nucleic acid test to some extent turned negative, with nine cases in the routine treatment group, eight cases in Xuebijing 50 ml group, and nine cases in Xuebijing 100 ml group, without significant difference. Overall, the patients with the best recovery and better scores were those treated with Xuebijing 100 ml (Wen et al., 2020).
7. CONCLUSION
Naturally occurring phytochemicals offer excellent resources for drug design and conception. Additional chemical modulations of these compounds, oriented by computer‐based docking simulations, might also strengthen their effectiveness and/or selectivity. Among the main promising products for the treatment of SARS‐CoV‐2 in humans that have been discussed in this review Curcuma longa, Artemisia annua L., Tripterygium wilfordii, Saponin derivates, PDL, XFBD, Lonicerae Japonicae Flos and Morus alba L., JHQGGs, and Xuebijing. Nevertheless, these compounds may have adverse or toxic effects at certain concentrations, and therefore, further investigations are necessary to define safe therapeutic doses for each compound before its clinical application. Preliminary studies could investigate the products that have formerly been approved for drug use or are generally considered to be safe by drug regulating agencies and administrations. Finally, it is hoped that the scientific community will continue to develop safe, and effective anti‐SARS‐CoV‐2 therapeutic agents from naturally derived compounds.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Merarchi, M. , Dudha, N. , Das, B. C. , & Garg, M. (2021). Natural products and phytochemicals as potential anti‐SARS‐CoV‐2 drugs. Phytotherapy Research, 35(10), 5384–5396. 10.1002/ptr.7151
[Correction added on 08 July 2021, after first online publication: The affiliation details for author Myriam Merarchi have been updated in this version].
DATA AVAILABILITY STATEMENT
None.
REFERENCES
- Abian, O. , Ortega‐Alarcon, D. , Jimenez‐Alesanco, A. , Ceballos‐Laita, L. , Vega, S. , Reyburn, H. T. , … Velazquez‐Campoy, A. (2020). Structural stability of SARS‐CoV‐2 3CLpro and identification of quercetin as an inhibitor by experimental screening. International Journal of Biological Macromolecules, 164, 1693–1703. 10.1016/j.ijbiomac.2020.07.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari, B. , Marasini, B. P. , Rayamajhee, B. , Bhattarai, B. R. , Lamichhane, G. , Khadayat, K. , … Parajuli, N. (2021). Potential roles of medicinal plants for the treatment of viral diseases focusing on COVID‐19: A review. Phytotherapy Research, 35(3), 1298–1312. 10.1002/ptr.6893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Sehemi, A. G. , Olotu, F. A. , Dev, S. , Pannipara, M. , Soliman, M. E. , Carradori, S. , & Mathew, B. (2020). Natural products database screening for the discovery of naturally occurring SARS‐Cov‐2 spike glycoprotein blockers. ChemistrySelect, 5(42), 13309–13317. 10.1002/slct.202003349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand, K. , Ziebuhr, J. , Wadhwani, P. , Mesters, J. R. , & Hilgenfeld, R. (2003). Coronavirus main proteinase (3CLpro) structure: basis for design of anti‐SARS drugs. Science, 300(5626), 1763–1767. 10.1126/science.1085658 [DOI] [PubMed] [Google Scholar]
- Bai, L. , Zhao, Y. , Dong, J. , Liang, S. , Guo, M. , Liu, X. , … Xu, K. (2021). Coinfection with influenza A virus enhances SARS‐CoV‐2 infectivity. Cell Research, 31(4), 395–403. 10.1038/s41422-021-00473-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkrishna, A. , Haldar, S. , Singh, H. , Roy, P. , & Varshney, A. (2021). Coronil, a tri‐herbal formulation, attenuates spike‐protein‐mediated SARS‐CoV‐2 viral entry into human alveolar epithelial cells and pro‐inflammatory cytokines production by inhibiting spike protein‐ACE‐2 interaction. Journal of Inflammation Research, 14, 869–884. 10.2147/JIR.S298242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu, A. , Sarkar, A. , & Maulik, U. (2020). Molecular docking study of potential phytochemicals and their effects on the complex of SARS‐CoV2 spike protein and human ACE2. Scientific Reports, 10(1), 17699. 10.1038/s41598-020-74715-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta, A. A. , Silveira, M. A. D. , Condor Capcha, J. M. , & De Jong, D. (2020). Propolis and its potential against SARS‐CoV‐2 infection mechanisms and COVID‐19 disease: Running title: Propolis against SARS‐CoV‐2 infection and COVID‐19. Biomedicine & Pharmacotherapy, 131, 110622. 10.1016/j.biopha.2020.110622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian, Y. Q. , Ma, J. , Ren, Y. , Zhang, Y. L. , & Qiao, Y. J. (2020). Discovery of intervention effect of Chinese herbal formulas on COVID‐19 pulmonary fibrosis treated by VEGFR and FGFR inhibitors. Zhongguo Zhong Yao Za Zhi, 45(7), 1481–1487. 10.19540/j.cnki.cjcmm.20200315.401 [DOI] [PubMed] [Google Scholar]
- Bianchi, M. , Benvenuto, D. , Giovanetti, M. , Angeletti, S. , Ciccozzi, M. , & Pascarella, S. (2020). Sars‐CoV‐2 envelope and membrane proteins: Structural differences linked to virus characteristics? BioMed Research International, 2020, 4389089. 10.1155/2020/4389089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boozari, M. , & Hosseinzadeh, H. (2021). Natural products for COVID‐19 prevention and treatment regarding to previous coronavirus infections and novel studies. Phytotherapy Research, 35(2), 864–876. 10.1002/ptr.6873 [DOI] [PubMed] [Google Scholar]
- Brendler, T. , Al‐Harrasi, A. , Bauer, R. , Gafner, S. , Hardy, M. L. , Heinrich, M. , … Williamson, E. M. (2020). Botanical drugs and supplements affecting the immune response in the time of COVID‐19: Implications for research and clinical practice. Phytotherapy Research, 1–19. 10.1002/ptr.7008 [DOI] [PubMed] [Google Scholar]
- Bugge, T. H. , Antalis, T. M. , & Wu, Q. (2009). Type II transmembrane serine proteases. The Journal of Biological Chemistry, 284(35), 23177–23181. 10.1074/jbc.R109.021006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty, H. , & Bhattacharjya, S. (2020). Mechanistic insights of host cell fusion of SARS‐CoV‐1 and SARS‐CoV‐2 from atomic resolution structure and membrane dynamics. Biophysical Chemistry, 265, 106438. 10.1016/j.bpc.2020.106438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. , Song, Y. P. , Gao, K. , Zhao, L. T. , & Ma, L. (2020). Efficacy and safety of Jinhua Qinggan granules for coronavirus disease 2019 (COVID‐19): A protocol of a systematic review and meta‐analysis. Medicine (Baltimore), 99(24), e20612. 10.1097/MD.0000000000020612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, T. Y. , Chen, D. Y. , Wen, H. W. , Ou, J. L. , Chiou, S. S. , Chen, J. M. , … Hsu, W. L. (2013). Inhibition of enveloped viruses infectivity by curcumin. PLoS One, 8(5), e62482. 10.1371/journal.pone.0062482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Liu, Q. , & Guo, D. (2020). Emerging coronaviruses: Genome structure, replication, and pathogenesis. Journal of Medical Virology, 92(4), 418–423. 10.1002/jmv.25681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, P. W. , Ng, L. T. , Chiang, L. C. , & Lin, C. C. (2006). Antiviral effects of saikosaponins on human coronavirus 229E in vitro. Clinical and Experimental Pharmacology & Physiology, 33(7), 612–616. 10.1111/j.1440-1681.2006.04415.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidambaram, S. K. , Ali, D. , Alarifi, S. , Radhakrishnan, S. , & Akbar, I. (2020). In silico molecular docking: Evaluation of coumarin based derivatives against SARS‐CoV‐2. Journal of Infection and Public Health, 13(11), 1671–1677. 10.1016/j.jiph.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinatl, J. , Morgenstern, B. , Bauer, G. , Chandra, P. , Rabenau, H. , & Doerr, H. W. (2003). Glycyrrhizin, an active component of liquorice roots, and replication of SARS‐associated coronavirus. Lancet, 361(9374), 2045–2046. 10.1016/s0140-6736(03)13615-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortegiani, A. , Ingoglia, G. , Ippolito, M. , Giarratano, A. , & Einav, S. (2020). A systematic review on the efficacy and safety of chloroquine for the treatment of COVID‐19. Journal of Critical Care, 57, 279–283. 10.1016/j.jcrc.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day, B. J. (2008). Antioxidants as potential therapeutics for lung fibrosis. Antioxidants & Redox Signaling, 10(2), 355–370. 10.1089/ars.2007.1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, W. , Xu, Y. , Kong, Q. , Xue, J. , Yu, P. , Liu, J. , … Bao, L. (2020). Therapeutic efficacy of Pudilan Xiaoyan Oral Liquid (PDL) for COVID‐19 in vitro and in vivo. Signal Transduction and Targeted Therapy, 5(1), 66. 10.1038/s41392-020-0176-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derosa, G. , Maffioli, P. , D'Angelo, A. , & Di Pierro, F. (2021). A role for quercetin in coronavirus disease 2019 (COVID‐19). Phytotherapy Research, 35(3), 1230–1236. 10.1002/ptr.6887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Editorial Board Of Chinese Critical Care, M . (2019). Xuebijing injection versus placebo for critically ill patients with severe community‐acquired pneumonia: a randomized controlled trial: research results and clinical value. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue, 31(10), 1199–1203. 10.3760/cma.j.issn.2095-4352.2019.10.004 [DOI] [PubMed] [Google Scholar]
- Efferth, T. (2017). From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Seminars in Cancer Biology, 46, 65–83. 10.1016/j.semcancer.2017.02.009 [DOI] [PubMed] [Google Scholar]
- Faheem Kumar, B. K. , Sekhar, K. , Kunjiappan, S. , Jamalis, J. , Balana‐Fouce, R. , … Sankaranarayanan, M. (2020). Druggable targets of SARS‐CoV‐2 and treatment opportunities for COVID‐19. Bioorganic Chemistry, 104, 104269. 10.1016/j.bioorg.2020.104269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore, C. , Eisenhut, M. , Krausse, R. , Ragazzi, E. , Pellati, D. , Armanini, D. , & Bielenberg, J. (2008). Antiviral effects of Glycyrrhiza species. Phytotherapy Research, 22(2), 141–148. 10.1002/ptr.2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, C. , Speth, V. , Fleig‐Eberenz, S. , & Neuhaus, G. (1997). Induction of zygotic polyembryos in wheat: Influence of Auxin polar transport. Plant Cell, 9(10), 1767–1780. 10.1105/tpc.9.10.1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile, D. , Patamia, V. , Scala, A. , Sciortino, M. T. , Piperno, A. , & Rescifina, A. (2020). Putative inhibitors of SARS‐CoV‐2 main protease from a library of marine natural products: A virtual screening and molecular modeling study. Marine Drugs, 18(4), 225. 10.3390/md18040225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheblawi, M. , Wang, K. , Viveiros, A. , Nguyen, Q. , Zhong, J. C. , Turner, A. J. , … Oudit, G. Y. (2020). Angiotensin‐converting enzyme 2: SARS‐CoV‐2 receptor and regulator of the renin‐angiotensin system: Celebrating the 20th anniversary of the discovery of ACE2. Circulation Research, 126(10), 1456–1474. 10.1161/CIRCRESAHA.120.317015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, P. G. , Qin, L. , & Puah, S. H. (2020). COVID‐19 acute respiratory distress syndrome (ARDS): Clinical features and differences from typical pre‐COVID‐19 ARDS. The Medical Journal of Australia, 213(2), 54–56 e51. 10.5694/mja2.50674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarner, J. (2020). Three emerging coronaviruses in two decades: The story of SARS, MERS, and now COVID‐19. American Journal of Clinical Pathology, 153(4), 420–421. 10.1093/ajcp/aqaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, F. F. , Zhang, Y. Q. , Tang, S. H. , Tang, X. , Xu, H. , Liu, Z. Y. , … Yang, H. J. (2020). TCMATCOV—A bioinformatics platform to predict efficacy of TCM against COVID‐19. Zhongguo Zhong Yao Za Zhi, 45(10), 2257–2264. 10.19540/j.cnki.cjcmm.20200312.401 [DOI] [PubMed] [Google Scholar]
- Gurung, A. B. , Ali, M. A. , Lee, J. , Farah, M. A. , & Al‐Anazi, K. M. (2020). Unravelling lead antiviral phytochemicals for the inhibition of SARS‐CoV‐2 M(pro) enzyme through in silico approach. Life Sciences, 255, 117831. 10.1016/j.lfs.2020.117831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habtemariam, S. , Nabavi, S. F. , Berindan‐Neagoe, I. , Cismaru, C. A. , Izadi, M. , Sureda, A. , & Nabavi, S. M. (2020). Should we try the antiinflammatory natural product, celastrol, for COVID‐19? Phytotherapy Research, 34(6), 1189–1190. 10.1002/ptr.6711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq, F. U. , Roman, M. , Ahmad, K. , Rahman, S. U. , Shah, S. M. A. , Suleman, N. , … Ullah, W. (2020). Artemisia annua: Trials are needed for COVID‐19. Phytotherapy Research, 34(10), 2423–2424. 10.1002/ptr.6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , Schroeder, S. , Kruger, N. , Herrler, T. , Erichsen, S. , … Pohlmann, S. (2020). SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280 e278. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori, J. I. , Zamboni, D. S. , Carrao, D. B. , Goldman, G. H. , & Berretta, A. A. (2013). The inhibition of inflammasome by Brazilian Propolis (EPP‐AF). Evidence‐Based Complementary and Alternative Medicine, 2013, 418508. 10.1155/2013/418508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. , … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 395(10223), 497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J. , Tao, G. , Liu, J. , Cai, J. , Huang, Z. , & Chen, J. X. (2020). Current prevention of COVID‐19: Natural products and herbal medicine. Frontiers in Pharmacology, 11, 588508. 10.3389/fphar.2020.588508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, M. T. , Sarkar, C. , El‐Kersh, D. M. , Jamaddar, S. , Uddin, S. J. , Shilpi, J. A. , & Mubarak, M. S. (2020). Natural products and their derivatives against coronavirus: A review of the non‐clinical and pre‐clinical data. Phytotherapy Research, 34(10), 2471–2492. 10.1002/ptr.6700 [DOI] [PubMed] [Google Scholar]
- Iwata‐Yoshikawa, N. , Okamura, T. , Shimizu, Y. , Hasegawa, H. , Takeda, M. , & Nagata, N. (2019). TMPRSS2 contributes to virus spread and immunopathology in the airways of Murine models after coronavirus infection. Journal of Virology, 93(6), e01815–18. 10.1128/JVI.01815-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifa, S. A. M. , Yosri, N. , El‐Mallah, M. F. , Ghonaim, R. , Guo, Z. , Musharraf, S. G. , … El‐Seedi, H. R. (2020). Screening for natural and derived bio‐active compounds in preclinical and clinical studies: One of the frontlines of fighting the coronaviruses pandemic. Phytomedicine, 85, 153311. 10.1016/j.phymed.2020.153311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili, N. , Karimi, A. , Moradi, M. T. , & Shirzad, H. (2018). In vitro immunomodulatory activity of celastrol against influenza A virus infection. Immunopharmacology and Immunotoxicology, 40(3), 250–255. 10.1080/08923973.2018.1440591 [DOI] [PubMed] [Google Scholar]
- Kim, T. S. , Heinlein, C. , Hackman, R. C. , & Nelson, P. S. (2006). Phenotypic analysis of mice lacking the Tmprss2‐encoded protease. Molecular and Cellular Biology, 26(3), 965–975. 10.1128/MCB.26.3.965-975.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan, J. , Ge, J. , Yu, J. , Shan, S. , Zhou, H. , Fan, S. , … Wang, X. (2020). Structure of the SARS‐CoV‐2 spike receptor‐binding domain bound to the ACE2 receptor. Nature, 581(7807), 215–220. 10.1038/s41586-020-2180-5 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Fu, X. , Duan, D. , Liu, X. , Xu, J. , & Gao, X. (2017). Extraction and Identification of Phlorotannins from the Brown Alga, Sargassum fusiforme (Harvey) Setchell. Marine Drugs, 15(2), 45. 10.3390/md15020049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Li, W. , Fu, C. , Song, Y. , & Fu, Q. (2019). Lonicerae japonicae flos and Lonicerae flos: A systematic review of ethnopharmacology, phytochemistry and pharmacology. Phytochemistry Reviews, 19, 1–61. 10.1007/s11101-019-09655-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, R. , Zhao, X. , Li, J. , Niu, P. , Yang, B. , Wu, H. , … Tan, W. (2020). Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet, 395(10224), 565–574. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, J. , Huo, X. Q. , Chen, X. , Zhu, W. X. , Yao, M. C. , Qiao, Y. J. , & Zhang, Y. L. (2020). Study on screening potential traditional Chinese medicines against 2019‐nCoV based on Mpro and PLP. Zhongguo Zhong Yao Za Zhi, 45(6), 1219–1224. 10.19540/j.cnki.cjcmm.20200216.401 [DOI] [PubMed] [Google Scholar]
- Machado, J. L. , Assuncao, A. K. , da da Silva, M. C. , Dos Reis, A. S. , Costa, G. C. , Arruda Dde, S. , do Rocha B. A., Vaz, M. M. , Paes, A. M. , Guerra, R. N. , Berretta, A. A. , & Nascimento, F. R. (2012). Brazilian green propolis: Anti‐inflammatory property by an immunomodulatory activity. Evidence‐based Complementary and Alternative Medicine, 2012, 157652. 10.1155/2012/157652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters, P. S. (2006). The molecular biology of coronaviruses. Advances in Virus Research, 66, 193–292. 10.1016/S0065-3527(06)66005-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuiri, S. , & Ohashi, Y. (2015). ACE and ACE2 in kidney disease. World Journal of Nephrology, 4(1), 74–82. 10.5527/wjn.v4.i1.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadamtousi, S. Z. , Kadir, H. A. , Hassandarvish, P. , Tajik, H. , Abubakar, S. , & Zandi, K. (2014). A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Research International, 2014, 186864. 10.1155/2014/186864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhtari‐Zaer, A. , Marefati, N. , Atkin, S. L. , Butler, A. E. , & Sahebkar, A. (2018). The protective role of curcumin in myocardial ischemia‐reperfusion injury. Journal of Cellular Physiology, 234(1), 214–222. 10.1002/jcp.26848 [DOI] [PubMed] [Google Scholar]
- Mourad, J. J. , & Levy, B. I. (2020). Interaction between RAAS inhibitors and ACE2 in the context of COVID‐19. Nature Reviews. Cardiology, 17(5), 313. 10.1038/s41569-020-0368-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman, B. W. , Kiss, G. , Kunding, A. H. , Bhella, D. , Baksh, M. F. , Connelly, S. , … Buchmeier, M. J. (2011). A structural analysis of M protein in coronavirus assembly and morphology. Journal of Structural Biology, 174(1), 11–22. 10.1016/j.jsb.2010.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu, M. , Wang, R. L. , Wang, Z. X. , Zhang, P. , Bai, Z. F. , Jing, J. , … Xiao, X. H. (2020). Rapid establishment of traditional Chinese medicine prevention and treatment of 2019‐nCoV based on clinical experience and molecular docking. Zhongguo Zhong Yao Za Zhi, 45(6), 1213–1218. doi:10.19540/j.cnki.cjcmm.20200206.501 [DOI] [PubMed] [Google Scholar]
- Ojo, A. S. , Balogun, S. A. , Williams, O. T. , & Ojo, O. S. (2020). Pulmonary fibrosis in COVID‐19 survivors: Predictive factors and risk reduction strategies. Pulm Med, 2020, 6175964. 10.1155/2020/6175964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padhi, S. , Masi, M. , Chourasia, R. , Rajashekar, Y. , Rai, A. K. , & Evidente, A. (2021). ADMET profile and virtual screening of plant and microbial natural metabolites as SARS‐CoV‐2 S1 glycoprotein receptor binding domain and main protease inhibitors. European Journal of Pharmacology, 890, 173648. 10.1016/j.ejphar.2020.173648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano, E. (2021). The pharmacological potential of plant compounds and preparations in COVID‐19: A PTR virtual issue. Phytotherapy Research, 35(4), 1683–1685. 10.1002/ptr.6961 [DOI] [PubMed] [Google Scholar]
- Pang, X. F. , Zhang, L. H. , Bai, F. , Wang, N. P. , Garner, R. E. , McKallip, R. J. , & Zhao, Z. Q. (2015). Attenuation of myocardial fibrosis with curcumin is mediated by modulating expression of angiotensin II AT1/AT2 receptors and ACE2 in rats. Drug Design, Development and Therapy, 9, 6043–6054. 10.2147/DDDT.S95333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineros, A. R. , de Lima, M. H. F. , Rodrigues, T. , Gembre, A. F. , Bertolini, T. B. , Fonseca, M. D. , … Bonato, V. L. D. (2020). Green propolis increases myeloid suppressor cells and CD4(+)Foxp3(+) cells and reduces Th2 inflammation in the lungs after allergen exposure. Journal of Ethnopharmacology, 252, 112496. 10.1016/j.jep.2019.112496 [DOI] [PubMed] [Google Scholar]
- Pourhajibagher, M. , Azimi, M. , Haddadi‐Asl, V. , Ahmadi, H. , Gholamzad, M. , Ghorbanpour, S. , & Bahador, A. (2021). Robust antimicrobial photodynamic therapy with Curcumin‐Poly (Lactic‐co‐Glycolic Acid) nanoparticles against COVID‐19: A preliminary in vitro study in Vero cell line as a model. Photodiagnosis and Photodynamic Therapy, 34, 102286. 10.1016/j.pdpdt.2021.102286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajapat, M. , Sarma, P. , Shekhar, N. , Avti, P. , Sinha, S. , Kaur, H. , … Medhi, B. (2020). Drug targets for corona virus: A systematic review. Indian Journal of Pharmacology, 52(1), 56–65. 10.4103/ijp.IJP_115_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabaan, A. A. , Al‐Ahmed, S. H. , Haque, S. , Sah, R. , Tiwari, R. , Malik, Y. S. , … Rodriguez‐Morales, A. J. (2020). SARS‐CoV‐2, SARS‐CoV, and MERS‐COV: A comparative overview. Le Infezioni in Medicina, 28(2), 174–184. [PubMed] [Google Scholar]
- Ren, R. , Mao, Y. , Ruan, Z. , Wang, Y. , Zhang, Y. , Du, J. , & Yu, W. (2017). Celastrol attenuates ventilator induced lung injury in mouse through inhibition of MAPK pathway. International Journal of Clinical and Experimental Pathology, 10(9), 9302–9309. [PMC free article] [PubMed] [Google Scholar]
- Rocha, F. A. C. , & de Assis, M. R. (2020). Curcumin as a potential treatment for COVID‐19. Phytotherapy Research, 34(9), 2085–2087. 10.1002/ptr.6745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safa, O. , Hassani‐Azad, M. , Farashahinejad, M. , Davoodian, P. , Dadvand, H. , Hassanipour, S. , & Fathalipour, M. (2020). Effects of Licorice on clinical symptoms and laboratory signs in moderately ill patients with pneumonia from COVID‐19: A structured summary of a study protocol for a randomized controlled trial. Trials, 21(1), 790. 10.1186/s13063-020-04706-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandur, S. K. , Ichikawa, H. , Pandey, M. K. , Kunnumakkara, A. B. , Sung, B. , Sethi, G. , & Aggarwal, B. B. (2007). Role of pro‐oxidants and antioxidants in the anti‐inflammatory and apoptotic effects of curcumin (diferuloylmethane). Free Radical Biology & Medicine, 43(4), 568–580. 10.1016/j.freeradbiomed.2007.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeman, D. , & Fielding, B. C. (2019). Coronavirus envelope protein: current knowledge. Virology Journal, 16(1), 69. 10.1186/s12985-019-1182-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam, M. K. , Rane, G. , Kanchi, M. M. , Arfuso, F. , Chinnathambi, A. , Zayed, M. E. , … Sethi, G. (2015). The multifaceted role of curcumin in cancer prevention and treatment. Molecules, 20(2), 2728–2769. 10.3390/molecules20022728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shereen, M. A. , Khan, S. , Kazmi, A. , Bashir, N. , & Siddique, R. (2020). COVID‐19 infection: Origin, transmission, and characteristics of human coronaviruses. Journal of Advanced Research, 24, 91–98. 10.1016/j.jare.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, K. , Chen, X. , Xie, B. , Yang, S. S. , Liu, D. , Dai, G. , & Chen, Q. (2018). Celastrol alleviates chronic obstructive pulmonary disease by inhibiting cellular inflammation induced by cigarette smoke via the Ednrb/Kng1 signaling pathway. Frontiers in Pharmacology, 9, 1276. 10.3389/fphar.2018.01276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons, G. , Zmora, P. , Gierer, S. , Heurich, A. , & Pohlmann, S. (2013). Proteolytic activation of the SARS‐coronavirus spike protein: cutting enzymes at the cutting edge of antiviral research. Antiviral Research, 100(3), 605–614. 10.1016/j.antiviral.2013.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, Y. , Yao, C. , Yao, Y. , Han, H. , Zhao, X. , Yu, K. , … Bai, C. (2019). XueBiJing injection versus placebo for critically Ill patients with severe community‐acquired pneumonia: A randomized controlled Trial. Critical Care Medicine, 47(9), e735–e743. 10.1097/CCM.0000000000003842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni, V. K. , Mehta, A. , Ratre, Y. K. , Tiwari, A. K. , Amit, A. , Singh, R. P. , … Vishvakarma, N. K. (2020). Curcumin, a traditional spice component, can hold the promise against COVID‐19? European Journal of Pharmacology, 886, 173551. 10.1016/j.ejphar.2020.173551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman, L. S. , Holmes, K. V. , & Behnke, J. (1980). Isolation of coronavirus envelope glycoproteins and interaction with the viral nucleocapsid. Journal of Virology, 33(1), 449–462. 10.1128/JVI.33.1.449-462.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X. , Liu, Y. , Li, C. , Wang, X. , Zhu, R. , Liu, C. , … Li, Y. (2017). Recent Advances of curcumin in the prevention and treatment of renal fibrosis. BioMed Research International, 2017, 2418671. 10.1155/2017/2418671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, M. , Cao, X. , Zhang, K. , Li, Y. , Zheng, Q. Y. , Li, G. Q. , … Zhang, K. Q. (2018). Celastrol alleviates renal fibrosis by upregulating cannabinoid receptor 2 expression. Cell Death & Disease, 9(6), 601. 10.1038/s41419-018-0666-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, T. , Bidon, M. , Jaimes, J. A. , Whittaker, G. R. , & Daniel, S. (2020). Coronavirus membrane fusion mechanism offers a potential target for antiviral development. Antiviral Research, 178, 104792. 10.1016/j.antiviral.2020.104792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toapanta, N. , Torres, I. B. , Sellares, J. , Chamoun, B. , Seron, D. , & Moreso, F. (2021). Kidney transplantation and COVID‐19 renal and patient prognosis. Clinical Kidney Journal, 14(Suppl 1), i21–i29. 10.1093/ckj/sfab030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, A. J. , Hiscox, J. A. , & Hooper, N. M. (2004). ACE2: from vasopeptidase to SARS virus receptor. Trends in Pharmacological Sciences, 25(6), 291–294. 10.1016/j.tips.2004.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls, A. C. , Park, Y. J. , Tortorici, M. A. , Wall, A. , McGuire, A. T. , & Veesler, D. (2020). Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell, 181(2), 281–292 e286. 10.1016/j.cell.2020.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Li, X. , Zhang, J. H. , Xue, R. , Qian, J. Y. , Zhang, X. H. , … Zhang, B. L. (2020). Mechanism of Xuanfei Baidu Tang in treatment of COVID‐19 based on network pharmacology. Zhongguo Zhong Yao Za Zhi, 45(10), 2249–2256. 10.19540/j.cnki.cjcmm.20200325.401 [DOI] [PubMed] [Google Scholar]
- Watanabe, Y. , Allen, J. D. , Wrapp, D. , McLellan, J. S. , & Crispin, M. (2020). Site‐specific glycan analysis of the SARS‐CoV‐2 spike. Science, 369(6501), 330–333. 10.1126/science.abb9983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, S. R. , & Navas‐Martin, S. (2005). Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiology and Molecular Biology Reviews, 69(4), 635–664. 10.1128/MMBR.69.4.635-664.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, L. , Zhou, Z. , Jiang, D. , & Huang, K. (2020). Effect of Xuebijing injection on inflammatory markers and disease outcome of coronavirus disease 2019. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue, 32(4), 426–429. 10.3760/cma.j.cn121430-20200406-00386 [DOI] [PubMed] [Google Scholar]
- Wu, F. , Zhao, S. , Yu, B. , Chen, Y. M. , Wang, W. , Song, Z. G. , … Zhang, Y. Z. (2020). A new coronavirus associated with human respiratory disease in China. Nature, 579(7798), 265–269. 10.1038/s41586-020-2008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, F. , Zhang, Y. , Tariq, A. , Jiang, X. , Ahmed, Z. , Zhihao, Z. , … Bussmann, R. W. (2020). Food as medicine: A possible preventive measure against coronavirus disease (COVID‐19). Phytotherapy Research, 34(12), 3124–3136. 10.1002/ptr.6770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedipour, F. , Hosseini, S. A. , Sathyapalan, T. , Majeed, M. , Jamialahmadi, T. , Al‐Rasadi, K. , … Sahebkar, A. (2020). Potential effects of curcumin in the treatment of COVID‐19 infection. Phytotherapy Research, 34(11), 2911–2920. 10.1002/ptr.6738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, Z. , Lin, X. , Zheng, R. , Zhang, H. , & Zhang, W. (2018). Celastrol alleviates airway hyperresponsiveness and inhibits Th17 responses in obese asthmatic mice. Frontiers in Pharmacology, 9, 49. 10.3389/fphar.2018.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D. H. , Wu, K. L. , Zhang, X. , Deng, S. Q. , & Peng, B. (2020). In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. Journal of Integrative Medicine, 18(2), 152–158. 10.1016/j.joim.2020.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Lin, D. , Sun, X. , Curth, U. , Drosten, C. , Sauerhering, L. , … Hilgenfeld, R. (2020). Crystal structure of SARS‐CoV‐2 main protease provides a basis for design of improved alpha‐ketoamide inhibitors. Science, 368(6489), 409–412. 10.1126/science.abb3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M. , Chen, Y. , Yang, M. J. , Fan, X. R. , Xie, H. , Zhang, L. , … Yan, M. (2019). Celastrol attenuates renal injury in diabetic rats via MAPK/NF‐kappaB pathway. Phytotherapy Research, 33(4), 1191–1198. 10.1002/ptr.6314 [DOI] [PubMed] [Google Scholar]
- Zheng, Y. Y. , Ma, Y. T. , Zhang, J. Y. , & Xie, X. (2020). COVID‐19 and the cardiovascular system. Nature Reviews. Cardiology, 17(5), 259–260. 10.1038/s41569-020-0360-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, P. , Yang, X. L. , Wang, X. G. , Hu, B. , Zhang, L. , Zhang, W. , … Shi, Z. L. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579(7798), 270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
None.


