Abstract
Hydrogel materials have been employed as biological scaffolds for tissue regeneration across a wide range of applications. Their versatility and biomimetic properties make them an optimal choice for treating the complex and delicate milieu of neural tissue damage. Aside from finely tailored hydrogel properties, which aim to mimic healthy physiological tissue, a minimally invasive delivery method is essential to prevent off-target and surgery-related complications. The specific class of injectable hydrogels termed self-assembling peptides (SAPs), provide an ideal combination of in situ polymerization combined with versatility for biofunctionlization, tunable physicochemical properties, and high cytocompatibility. This review identifies design criteria for neural scaffolds based upon key cellular interactions with the neural extracellular matrix (ECM), with emphasis on aspects that are reproducible in a biomaterial environment. Examples of the most recent SAPs and modification methods are presented, with a focus on biological, mechanical, and topographical cues. Furthermore, SAP electrical properties and methods to provide appropriate electrical and electrochemical cues are widely discussed, in light of the endogenous electrical activity of neural tissue as well as the clinical effectiveness of stimulation treatments. Recent applications of SAP materials in neural repair and electrical stimulation therapies are highlighted, identifying research gaps in the field of hydrogels for neural regeneration.
Keywords: self-assembling peptides, tissue engineering, neuroengineering, neuroregeneration, peptide synthesis, review, conductive biomaterials, scaffold, bioactive
1. Introduction
Neural tissue loss represents a complex clinical challenge, which translates to a heavy burden for society. As an indicator of impact, the economic loss has been estimated at $800 billion in the United States alone.1 Given the ever growing number of patients suffering from irreversible neural damage due to neurodegenerative diseases, traumatic brain injury, and spinal cord and peripheral nerve injury, a reliable strategy for neural repair and regeneration is a pressing healthcare necessity.2−5 The primary challenge in addressing neural tissue loss is its low regenerative capacity, which limits functional recovery after neural injury.2,6 Particularly, the injured central nervous system (CNS), which triggers an inhibitory response toward physiological regeneration, hinders functional recovery and promotes the formation of scar tissue.7,8 Although the peripheral nervous system (PNS) has more capacity for neuroregeneration, with recovery possible if the damage is relatively minor, larger injuries where nerve bundles must bridge lengths greater than 1 cm have limited solutions for functional recovery.9−12 In this context, neuroregeneration refers to a total or partial recovery of tissue functionality by neuronal regrowth or repair, including neurogenesis of the endogenous tissue, physiological repair mechanisms, and exogenous cell transplants.2 Considerable efforts have been made toward understanding the underlying mechanisms of neural repair, as well as the development of clinically relevant approaches to encourage neurogenesis, spanning drug development and delivery, tissue engineering, and electrical stimulation strategies.6,13−17 Critical to most tissue engineering approaches are biomaterials that act primarily as scaffolds for supporting cell delivery and growth but can also be used for drug delivery and provision of electrical stimuli.
The overarching aim of tissue engineering scaffolds is to use a material system to mimic the physicochemical properties of the natural tissue milieu.18,19 Biomimetic scaffolds, made from biologically inspired materials, provide environmental cues that target desired biological mechanisms.20,21,254 Such biomimetic cues can be used to control cell and tissue behavior, promoting neural tissue regeneration and repair. These elements can take the form of bioactive molecules and pharmaceuticals, as well as mechanical and topographical cues for physical support.6 These tissue scaffold materials need to be carefully designed and tailored to elicit the desired cellular responses and thus provide a therapeutic effect.
Hydrogel systems are the most commonly applied biomaterial for soft tissue engineering. Hydrogels are ideal for these applications because of their structural and mechanical similarity to the extracellular matrix components, their general cytocompatibility, and their capacity to provide biological cues.22,18,21,23−26 A variety of hydrogel materials have been investigated for neural applications, spanning from natural tissue components to entirely synthetic materials.26,27 Biologically sourced materials including acellularized tissue and extracellular matrix-derived macromolecules such as collagen, chitosan, and hyaluronic acid have been used extensively. They are advantageous because they are nontoxic, cytocompatible, simple to obtain, and have inherent bioactive cues, however biologically sourced materials carry a risk of immunogenicity and may be prone to batch-to-batch variability.28 Synthetic polymers present an alternative with significant benefits, including reproducibility and versatile tailoring through simple modifications of pendant groups. Common examples used in tissue engineering constructs include poly(ethylene glycol) (PEG), poly(vinyl alcohol) (PVA) and poly(ethylene oxide) (PEO).29−31 However, these purely synthetic hydrogels lack critical biological cues, limiting their biomimetic properties.28,32 As such, tuning of the physicochemical properties and biofunctionalization of these polymeric materials toward a more biomimetic material is often necessary. Biologically inspired proteins or polymers are a third class of material that provides a higher degree of control in contrast to biological polymers, but being based on natural amino acids (AAs) can be assembled to incorporate critical biological cues, such as adhesion sequences.33,34 These synthetic peptides can be cross-linked into tunable, nontoxic, and biofunctionalized hydrogels, making them a promising material choice for neuroregeneration applications.26,35−37
Cell scaffolds are intended to physically support the surrounding tissue during regeneration. Historically, the scaffold shape and size were defined preimplantation, leading to surgical invasiveness and long recovery periods.23,38,39 This was due to the need for material polymerization and implant definition prior to the surgery as a means of controlling the polymer structure and structural features.40,41 The more recent development of minimally invasive and in situ surgical approaches has fostered the development of injectable systems.42 These systems have found utility in neural repair, as they support localized treatment and minimize postsurgical complications, demonstrating versatility for translation to the clinic.6,20 Injectable materials permit the formation of a hydrogel in situ via the minimally invasive delivery of a hydrogel precursor to the desired location. Once injected, the hydrogel can be formed using a variety of physical or covalent cross-linking methods, including environmental stimuli such as temperature, pH and salt concentration.20,42−45 Both natural and synthetic polymers can be designed to be injectable, such as chitosan-based thermoresponsive hydrogels or injectable PEG polymers.46,47 The combination of hydrogel precursor and method of polymerization will determine the final molecular arrangement, allowing for finely controlled macromolecular conformations.48,49
The class of injectable materials termed self-assembling, offer a thermodynamic advantage by exploiting spontaneous physical interactions of the molecules in the environment, forming stable network microstructures.50 The design of self-assembling polymers requires a precise understanding of chemical structures and molecular interactions that impact on the assembly mechanisms from monomer or macromonomer into a hydrogel network.6,32,51 The addition of biofunctional groups must not chemically or structurally interfere with the self-assembling cross-linking mechanism of the polymer, and simultaneously the mechanical and structural properties need to be maintained within the physiological range.20 This complex design challenge requires versatile control over the polymer chemical and structural composition. Among all material types, peptide-based polymers offer the possibility to easily implement self-assembling mechanisms by mimicking natural aggregation processes, while maintaining the required physicochemical properties.52−54 The synthetic peptides that spontaneously assemble into ordered nanostructures under physiological conditions are named self-assembling peptides (SAPs).55−57 One of the major advantages of SAPs among other material types is their simple functionalization with adhesion molecules and their highly biocompatible components. SAP building blocks are effectively single AAs, which are an important component of the physiological environment.19,55 Besides the simple synthesis, functionalization and property modification, these materials allow for minimally invasive treatments, which are critical in neural injury or disease.55−58
This review examines the recent developments in SAP systems designed for neural applications, including methods to tailor SAP properties to optimize their performance as neural scaffolds which can guide neural repair. Key design criteria are identified from an overview of the physiological tissue properties, with the aim of replicating the main features of the neural environment within the biomaterial. Ways to control and tailor properties of SAP constructs, such as self-assembling mechanisms, mechanical properties, topography, and bioactivity are considered as biomimetic cues through the lens of cell–material interactions. Furthermore, the incorporation of conductive scaffolds and electrical stimulation within SAP constructs to promote neural regeneration is assessed. Finally, the latest SAP-based applications for neural regeneration are presented, to identify their advantages and limitations, highlighting the latest technological advances and unmet clinical needs.
2. Biomimetic Cues for Neural Repair
Cells need to sense specific biomimetic cues expected from the native ECM and healthy neural tissue to accomplish neural regeneration and repair. It is essential to consider these requirements for neural repair in the design of materials systems intended to address neural injury. Materials used for neural repair should therefore aim to mimic the neural environment with finely tuned physicochemical properties engineered to interact with the target cell types and tissue features.21,59 Understanding the specific injury environment that a biomaterial is intended to address is critical to the successful development of an injectable neural scaffold. The functionality and structure of physiological neural tissue relies on the synergy between a multitude of specialized cell types and a complex microbiological milieu. For instance, the CNS and PNS have different responses to injury and vary in their potential for regeneration.60−63
After a peripheral nerve injury, the distal segment of the axon undergoes an initial degeneration that inhibits growth in the initial stage, followed by the secretion of neurogenic signaling pathways by Schwann cells and the formation of growth cones for functional nerve regeneration.64 Conversely, the injury setting in the CNS triggers the reaction of microglia, astrocytes and oligodendrocytes, which inhibit regeneration and promote the creation of a glial scar.7 The two conditions present a different biochemical environment, characterized by specific ECM composition, signaling cues and cell types. Design of a biomaterial implant should consider all the relevant components and create a favorable environment for the proliferation, development and neurogenic behavior of target cell types. Drugs and bioactive molecules can also be incorporated within a material system for a multifunctional therapeutic approach.6,65,66 Biomaterials and in particular hydrogels may also be used as cell carriers in stem cell transplants to control cell fate and promote neuroregenerative processes.20,39,67 The material cues for this application should replicate the neural stem cell (NSC) niche, a biophysical microenvironment that regulates differentiation cues and cell fate.68 Cues toward neuronal lineage, as opposed to glial and epithelial, are preferred for an optimal integration with the endogenous nervous system.69,70
The design of biomaterials targeting neuroregeneration should account for the complex host–material interactions for specific injury environments, tailoring the cell interaction to the targeted tissue type, diseases environment and cell type.71,72 Specific design criteria for material parameters and composition should be defined by considering the key components of the native neural milieu and their effect on cell behavior. Among all material types, injectable materials require extremely precise tuning and characterization of the biomimetic features postassembly, because the in vivo polymerization does not allow for a preimplantation control of the material properties and self-assembling bioproducts. Fundamental material features such as mechanical properties, degradation mechanisms, biochemical composition, structural features, and conductivity should be investigated in light of both the physiological environment and the cell–material interactions to define effective injectable material properties and modifications.
2.1. Biological Cues
The primary requirement for neural repair is the presence of a biochemical environment that supports neural cell populations.18,27 Cell behavior can be directed toward neuroregeneration through the incorporation of bioactive cues within biomaterials.21,24−26,73,74 This material modification is exceptionally important in neural applications, given the low inherent low regenerative potential of this tissue type.75 The native ECM offers essential biochemical and structural cues to neural cells, which sense the environment through adhesion molecules, termed integrins.76−80 Integrins are specialized adhesion receptors that interact with peptide sequences present in the ECM and regulate cell–cell interactions.81 They interact with the cell cytoskeleton and influence gene expression, proliferation, and survival through bidirectional signaling with the biochemical environment.81−84 It follows that the presence of integrin-binding factors is a paramount design requirement in biomaterials. Specifically, this includes ensuring cell adhesion through the presence of naturally derived materials or the presence of biomimetic adhesion molecules.79−81,84
To inform the design of bioactive cues within hydrogels for neural repair, it is key to examine the native ECM components, which provide the necessary factors for healthy cell growth and differentiation. The brain ECM is a complex meshwork of multiple compounds. Aside from typical ECM components such as collagen, laminin, hyaluronic acid, and fibronectin, the brain ECM is extremely rich in glycosaminoglycans (GAGs), including chondroitin sulfate and hyaluronan.85,75,86,87 Chondroitin sulfate influences neural plasticity and cell behavior through sequences of sulfate groups on the GAG molecule backbone, conveying functional information through sulfation codes.75,88,89 In the case of the PNS, laminin, and collagen are fundamental ECM components for their role as Schwann cell regulators.90 It follows that ECM adhesion molecules are considered a powerful tool to direct cell behavior.79−81,84 In neural scaffolds laminin, collagen and hyaluronic acid are often selected as adhesion substrates in their natural or synthetic form.84,87 In particular, laminin-derived peptides in neural cultures are able to increase neural cell migration, proliferation and differentiation toward neuronal fate.84,91,92 Short bioactive sequences of AAs involved in the adhesion signaling, termed bioactive epitopes, are often exploited as adhesion cues in tissue engineering. The bioactive epitopes contained in laminin, collagen and fibronectin molecules, including RGD, IKVAV, and YIGSR, are the most widely used examples93 (the reader is referred to Koss and Unsworth;58 see Table 2 for a comprehensive review of adhesion molecules for neural regeneration). Moreover, GAGs such as chondroitin sulfate represent an effective element to introduce into a bioactive scaffold for the central role in the neural ECM.94−96 Adhesion molecules and their effect on neural cells should be carefully selected from the neural ECM components, tailoring the material composition toward the targeted regeneration application. Given the complexity of the natural biochemical milieu, replicating the biological cues in a material system is a design challenge. Often, hydrogel materials can be functionalized with a relatively small number bioactive molecules because of the low availability of chemical bonds that can be formed without affecting the self-assembling mechanism and molecular interactions.97,98 A trade-off between bioactivity and hydrogel stability and structure must be achieved.23,40
Table 2. Conductive Properties of the Neural Tissue.
| Brain (S/cm) | Spinal Cord (S/cm) | PNS (S/cm)a |
|---|---|---|
| 2, whole skull182 | 60, white matter, longitudinal183,184 | 9.1 inside nerve185 |
| 0.7, inner compact182 | ||
| 0.5, outer compact182 | 8.3, white matter, transverse183,184 | 15.9 epineurium186 |
| 47, gray matter182 | 23, gray matter183,184 | 57.1 endoneurium longitudinal186 |
| 8.3 endoneurium transverse186 |
Data for the PNS were derived from nerve resistivity values.
Aside from adhesion peptides, other bioactive molecules such as growth factors (GFs), cytokines, and signaling molecules are considered effective cues acting through regeneration-related molecular pathways in the CNS and PNS.75,89,99 GFs are a widespread class of proteins that can stimulate cell growth, differentiation, and wound healing.2,23,100,101 Cell-binding of GFs activate intracellular second messenger systems through cell surface membrane receptors that affect neural cell growth and differentiation.58,100,102,103 GFs are produced by healthy cell populations and can direct NSC differentiation toward specific cell types.74 Nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and tyrosine kinase (Trk) are important examples of a neurotrophic factors involved in neural development which enhance neuronal differentiation.100,102,103 Other methods of biochemical guidance include signaling molecules that drive gene cascades toward neural repair or differentiation.104,105 For instance, the delivery of a molecule dubbed TTK21 was recently proven to promote spinal cord regeneration and sprouting of sensory and motor axons through epigenetic reprogramming.104,105 In addition, the neural chemical signaling molecules neurotransmitters are known to influence neural plasticity and are involved in strengthening neural connections and glial cell stimulation.28,106,107 GFs and bioactive molecules can be incorporated in the material system to enhance neural regeneration or direct cell fate, and their effect can be tailored for regenerative or drug delivery applications.
2.2. Mechanical Properties
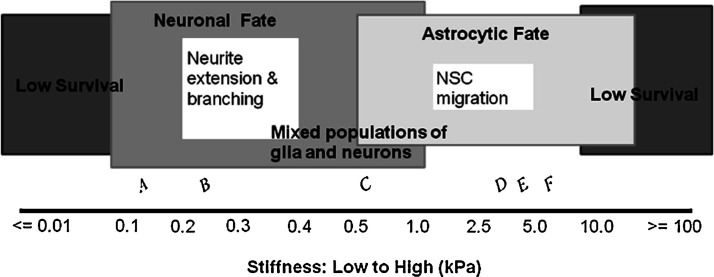
The mechanical properties of neural tissue vary depending on tissue type and location. In general, the brain has a low stiffness and presents viscoelastic properties, whereas nerves and the spinal cord show higher tensile strengths due to the alignment of the nerve fibers.108−110 More comprehensive properties of the brain, spinal cord, and PNS are presented in Table 1. The importance of biomimetic mechanical properties for neural cells is largely related to the mechanotransduction of key biological signals.6,21,24,111 Transmembrane proteins, primarily integrins, are intrinsically mechanosensitive and affect cell behavior and growth depending on substrate stiffness18,83 (Figure 1). Binding of these transmembrane proteins allows the mechanical signal to be converted into downstream chemical pathways, which are known to affect cell adhesion, morphology, and differentiation.93,112,113 For example, at stiffnesses comparable to physiological neural tissue (100–500 Pa), NSC differentiation can be directed toward a substantial neuronal subpopulation as opposed to astrocyte and oligodendrocyte populations.114−116 Koser et al.114 showed that axon length and degree of spreading varies with substrate stiffness. Softer substrates were shown to encourage more exploratory growth, better suited for synaptic formation, whereas stiffer substrates promoted faster, straighter, and more parallel growth of axons.117 A stiffness range above 200 kPa can lead to apoptotic activity and reduced viability of in vitro neural cultures.93 This phenomenon has also been observed in the clinical setting, where damage to the CNS causes the formation of scar tissue, or glial scar, which dramatically increases the stiffness of the tissue, leading to neural loss and cell death.7,8 Zhong et al.118 have performed a comprehensive review of mechano-sensing under 2D and 3D environments.
Table 1. Mechanical Properties of Brain, Spinal Cord, and PNS Tissue.
| Young’s modulus | compressive modulus | properties | |
|---|---|---|---|
| brain | 40 to 20 000 Pa, human108 | 3–6 kPa, rat116 | nonlinear viscoelastic behavior108 |
| 23.8 ± 10.5 kPa (50/s strain rate) | 3.4 kPa, embryonic rat forebrain122 | ||
| 38.5 ± 2.0 kPa (60/s strain rate), porcine121 | |||
| 3–10 kPa Young’s (elastic) modulus, human123 | |||
| spinal cord | 0.3–1.4 MPa, human109 | 8.1 ± 1.1 kPa, adult rat116 | |
| PNS | 1.2 MPa, mice lumbar nerve roots | ||
| 7 MPa, mice sciatic nerve110 |
Figure 1.
Effect of material stiffness on neural stem cell fate in vitro. A stiffness of around 1 kPa allows the presence of a mixed neural population, whereas excessively high or low values decrease cell survival. Reproduced with permission from ref (18). Copyright 2012 Elsevier.
When designing a biomaterial scaffold for neural repair, the mechanical properties should be based on physiological ranges, and design criteria should specifically target substrate stiffness to support neuron survival and direct cell behavior toward regenerative processes. Modifications of the elastic or compressive modulus can be implemented in material systems to match the target tissue features with relatively simple approaches that have been detailed in the literature.30,119,120 However, an engineering challenge can be identified in the design of injectable materials. Self-assembly mechanisms can be affected by variable physiological conditions and delivery methods, such as temperature, chemical composition of the target site, or injection speed.43 These features can cause difficulties in achieving precise mechanical properties to ensure physical support to the cells.42,47
Importantly, mechanical support provided to encapsulated cells changes dynamically with material degradation, which can be tuned to match natural tissue growth.39,124 Neural cells interact with their environment by degrading as well as producing ECM.18,30 Neural tissue physiological remodelling is a fundamental process in healthy tissue environments, involved in tissue turnover, synaptic plasticity and neural repair. Enzymes known as matrix metalloproteinases (MMPs) are responsible for ECM degradation and remodelling and promote tissue growth and differentiation.125 Neurons and glia secrete degradation MMPs and contribute to ECM remodelling in physiological conditions, brain injury, and other brain disorders such as cancer.126,85,127−129 Abnormal ECM dynamics, commonly present in injured or pathological tissue, may also cause imbalances in cell behaviors leading to immune and inflammatory response activation, which encompass the initial stage of spontaneous neural repair. For example, after spinal cord injury (SCI), the molecules released from damaged ECM can trigger and amplify the inflammatory response. The subsequent alterations of the ECM structural and chemical composition affect cell migration, communication, and survival toward a spontaneous regenerative response.85 These mechanisms affecting tissue remodelling can be replicated to provide both endogenous and exogenous cells with a substrate to degrade while proliferating and secreting new ECM.18,130 A balance between providing mechanical support and allowing space for tissue growth is a central requirement to achieve a physiological cell response to the biomaterial and avoiding adverse responses.125,131
The ideal scaffold provides initial mechanical and biochemical support to cells, and its degradation rate should match the ECM formation such that it allows for the regeneration and growth of the new tissue.18,21 A trade-off between controlled degradation and biocompatibility should be considered.18,36 A high degradation rate can lead to the accumulation of chemical degradation products, which in turn can encourage glial scarring and immune/foreign body response.18,128,132,133 Thus, the material composition and degradable chemical bonds should be engineered to match the natural tissue degradation rate of 2–6 weeks.134 Degradation is typically due to hydrolytic or enzymatic degradation.135,136 Functional groups such as MMP cleavable peptide linkages can be inserted into a biomaterial to match the degradation with local cell proliferation and metabolic activity.137 It is important to note that the degradation rate in vitro and in vivo can vary considerably because of the changes in environmental conditions.18,138
2.3. Architecture and Topography
The micro- and macroscale structures of neural tissue are linked to their physiological function.139 In the PNS, aligned nerve fibers are organized in fascicles depending on function, displaying a hierarchical architecture,140.141 The nerve sheath, composed of myelin and connective tissue, surrounds and insulates nerve fibers.141 The spinal cord has a similar aligned architecture, showing ascending and descending neurons organized in bundles, around 8–60 μm in size123.142 The brain structure is more homogeneous, with the white matter composed of aligned myelinated nerve fibers and the gray matter consisting of cell bodies and unmyelinated axons, with highly anisotropic structures.139 The brain ECM includes perineuronal nets (PNNs), which show lattice-like chondroitin sulfate structures around subpopulations of neurons. They act as growth and migration inhibitors to maintain the tissue structure.75 Replicating these physiological structures can be advantageous for a scaffold’s efficacy, given that the tissue architecture can directly affect cell behavior and function.139 Indeed, aside from sensing the substrate’s stiffness, surface and adhesion receptors can also respond to the architecture and topography of the environment.63
The spatial arrangement of micro- and nanoscale material features can influence cell adhesion, spreading, alignment, and morphology which in turn can alter cell behavior and gene expression.93,143−148 It is important to note that historically the majority of in vitro cell studies have been performed in 2D cultures.149,150 However, the native neural milieu and its physicochemical features are 3D. This implies a significant difference in the way cells are affected by environmental cues. The spatial distribution of the cues is more homogeneous and this affects cell attachment and shape toward a more biomimetic model.151−153 As a result, 3D spatial features of a construct can influence the neural cell response, and in vitro 3D cultures created by encapsulating cells within a biomaterial are a preferable method for replicating the neural environment.149 3D architectural cues can be introduced into the material system as topographical cues to neural cells. Topographical cues include every spatial feature and physical modification of the microenvironment, spanning from fibrous structures to roughness of the surface.80,154,155 Curtis et al.156 have reviewed how cells sense physical features of the environment at the nano- and microscale such as physical patterning, roughness, pits, grooves, and fiber alignment. Surface patterning and roughness affect cell attachment and migration,157,158 while chemical patterning modifies cell morphology.159 Aligned topography is found to be among the most effective in neural tissue regeneration, due to their polarized morphology, which mimics physiological patterns in neural tissue.28,32,63,93,143,160−163 Human NSCs are shown to differentiate toward the neuronal lineage when exposed to aligned microscale patterns, and neurite outgrowth can be enhanced by contact guidance.93,145,164−166 For example, dorsal root ganglia cells increase the maximum length of their neurites by 82% when exposed to core–sheath nanofibers.167 Baranes et al.168 showed that nanotopographies altered gene expression profiles of primary neurons isolated from medicinal leaches, upregulating axon-guidance signaling pathways, synaptogenesis and synaptic regulation, resembling the behavior of interconnected neurons. Human embryonic stem cells (hESCs) can be differentiated into a neuronal lineage by exposing them to an aligned ridge pattern, without the need for other differentiation-inducing agents.143 Similarly, human induced pluripotent stem cells (hiPSCs) can be differentiated into neuronal lineages when exposed to aligned microgrooves.169 This property can be exploited as a powerful method to control and tune the development of a neural progenitor cell population, and guide its growth at the same time.170,171 This cellular response is highly desirable for neural regeneration, and methods to create a material that elicits this cellular response in clinical applications are of utmost interest.10,172
Micro- and nanoscale structures can also influence local homeostasis by affecting the accessibility of soluble nutrients, ions and molecules, as well as tissue vascularization.173 Specifically, the porosity and pore size of the material should be tuned to allow for molecular diffusion while providing a stable structure for cell growth and proliferation.173,174 In neural applications, the pore interconnectivity is essential for neurite growth, with a desirable porosity of 90% and a suitable pore size pore size ranging from 10 to 100 μm.123,62,173,175−177
2.4. Conductive Properties
Neural cell behavior and growth can be substantially impacted by electrical cues, which are a widespread strategy for neuroregeneration treatments such as nerve repair.178 Endogenous electric fields are known to be present in neural development and would healing.179,180 Spontaneous activity in the CNS plays a role in the assembly of developing neural circuits, and axon regrowth is promoted by the electrical potential physiologically generated in the wound environment.179 Endogenous electrical signals consist of polarized ion transport within the biological tissue, which influences cell membrane potential and electrophysiological state.180,181 The conductive properties of different types of neural tissue are presented in Table 2.
Signaling pathways influencing the cell cycle, ion channel expression, and other gene cascades leading to proliferation, migration, and differentiation are activated by electrical activity.181,187,188 In the context of neuroregeneration, neuronal guidance through biomimetic electrical signals is a powerful tool to repair nerve and spinal cord injuries.189−192 The electrophysiological state of the stem cell niche is known to promote differentiation toward neural lineage and increased neural proliferation.189,190 The use of electrical cues in tissue engineering is extensive and spontaneous electrical potentials are a central element for neural development, thus the conductivity and electrochemical properties of scaffold materials used for neural repair are worthy of consideration.152,191,193−195 An ideal material will support the endogenous or exogenous electric field propagation to favor neural regeneration.193,194 In the context of SAPs, it is essential to ensure the compatibility of the self-assembling physiochemical mechanism with the propagation of electrical signals.191,196 Alternatively, electroactive scaffolds can be developed to actively promote electrical stimulation or exposure of cells to electric fields.189
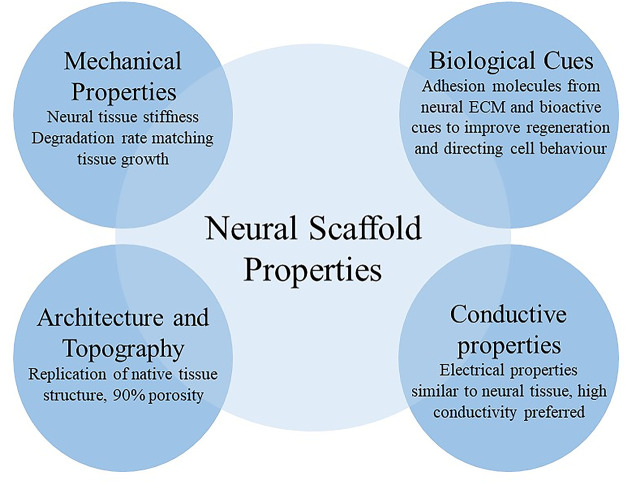
The reviewed design criteria cover an extensive range of material properties and relative cell–material interactions involved in neuroregeneration mechanisms (Figure 2). Bioactive cues ensure cytocompatibility and direct cell behavior, whereas mechanical properties ensure cell adhesion and proliferation through mechanotransduction. The scaffold topography can guide cell migration and differentiation. Lastly, conductive properties of the scaffolds allow the compatibility of hydrogels with stimulation treatments as well as supporting spontaneous electrical activity. A close investigation of the native neural environment is crucial and largely encouraged for defining material design criteria as well as fostering novel bioinspired hydrogel systems, toward a multifunctionalized highly effective self-assembling material.
Figure 2.
Design criteria for a neural scaffold can be divided into four categories: biological cues, mechanical properties, architecture and topography, and conductive properties.
3. Self-Assembly Biomaterials for Neural Repair
Scaffolds for tissue engineering neural repair should minimize invasiveness and provide topographical, structural, biomechanical and biochemical support for neural regeneration. Various attempts have been made using synthetic or biological materials; however, all these material modifications need to be considered with regards to their potential impact on the physicochemical properties of the biomaterial construct.60 Facilitating topographical cues via injectable materials is challenging as it typically requires in situ formation of structural elements. Self-assembling materials enable the formation of various topographies upon injection in vivo due to their responsiveness to local environments. Therefore, careful design of the material can lead to control over physiochemical properties in order to achieve a scaffold that meets the criteria for neural regeneration.197 Self-assembly is governed by supramolecular chemistry as it relies on non-covalent forces between molecules. It is therefore important to understand the forces that govern the self-assembly process in order to tune the assembled structures and their properties for a specific application. Methods have been developed to tune the topographical, mechanical, bioactive and conductive properties of self-assembling materials. The application of these methods to SAPs can be tailored to create a biomimetic and effective material support.
Noncovalent interactions between molecules are the driving force for the spontaneous formation of organized structures, a process called self-assembly that occurs readily in nature at various length scales. A variety of molecular driving forces can be used to create self-assembly systems.198 These intermolecular forces are dominated by hydrogen bonds, electrostatic interactions, hydrophobic interaction, and π–π interactions. Therefore, external stimulations to trigger self-assembly include the effect of pH, temperature, ionic charge and concentration as well as various other triggers such as enzymes and phototriggers. Different intramolecular driving forces and external stimulations can guide the self-assembly of polymer systems. These interactions have been extensively reviewed and are summarized in Table 3.199−202
Table 3. Driving Forces of Self-Assembly Adapted from Ref (205).
| internal Interaction | strength (kJ/mol) | properties |
|---|---|---|
| electrostatic | 50–300 | electric force between charged bodies also known as Coulomb force; it can either be attractive between opposite charges or repulsive between like charges;201 short range interaction, nonselective |
| coordination binding | 50–200 | short ranged, directional |
| hydrogen bonding | 5–120 | interaction between hydrogen atoms and electronegative atoms; long ranged, selective, directional |
| π–π stacking | 0–50 | attractive noncovalent interaction between stacked aromatic rings; short ranged, directional |
| hydrophobic | depends on solvent type | hydrophobic segments are shielded from the aqueous solution by aggregating inside the self-assembled structure; this results from the van der Waals forces between hydrocarbon molecules and the hydrogen bonding between water molecules; affected by ionic strength.206 |
| van der Waals | <5 | attractive force, short ranged, nondirectional, nonselective |
| covalent | 350 | short ranged, irreversible |
It is important to consider these known driving forces when tailoring the topographical, mechanical and electrical properties of SAPs for neural regeneration. Strong interactions such as ionic forces and coordination bonds require consideration in the design of a system that will self-assemble in the conditions found within the nervous system. Weaker interactions such as van der Waals electrostatic and hydrophobic interactions, H-bonding, and π–π stacking have strong influences on the self-assembled morphology, mechanical properties and bioactivity of SAPs. A balance between these forces can create molecules that will self-assemble into fibers in aqueous conditions but form a hydrogel when strong ions are introduced, thus enabling control over their gelation and subsequent material properties.203,204
To date, various types of self-assembling molecules in physiological environments have been explored ranging from synthetic small molecules, proteins, peptides, nucleic acids and hybrids as detailed in Table 4.
Table 4. Materials Used for Molecular Self-Assembly.
| hydrogelators | typical dominant forces driving self-assembly | features of the material | examples of neural cell response |
|---|---|---|---|
| DNA and nucleic acids220 | base pairing–hydrogen bonding | can be tailored to incorporate specific molecular recognition and exhibit excellent biocompatibility; mostly researched for cancer applications221,222 | DNA nanotubes covalently functionalized with RGDS epitopes; neural stem cells cultured on bioactive DNA nanotube substrates showed enhanced differentiation into neurons215 |
| proteins and short peptides198 | arrangement of hydrophobic and hydrophilic segments dictate secondary structure; hydrogen bonding, van der Waals electrostatic and hydrophobic interactions, H-bonding, and π–π stacking have all been used as gelator forces | can be protein functionalized with self-assembling short peptide sequences or short peptide sequences; low cost, biocompatible | RADA-16I showed axonal infiltration and strong integration with host tissue when injected after a spinal cord contusion lesion,223 and when seeded with HCMECs/D3 cells they promoted vascularization and augmented the host axon infiltration224 |
| hybrid biomolecules225 | electrostatic interactions between the charged AAs of the hydrophilic head, hydrogen bonding in the β-sheet forming regions as well as hydrophobic tail aggregation are dominant | typically consists of peptides segments functionalized with aromatic or alkyl groups; can also be DNA functionalized onto synthetic polymer chains self-assembly properties | Fmoc-FF: mulitpotent pericytes cultured for a week on the surface of Fmoc-FF/S coassembly showed neural differentiation on 1 kPa gel substrate226 |
| π–π stacking may occur if the synthetic component contains aromatic groups or the amino AAs contain aromatic groups in their side chains; can also rely on base pairing in ssDNA | peptide amphiphile: IKVAV functionalized self-assembling peptide amphiphile induced neural trans-differentiation in human bone marrow mesenchymal stem cells227 | ||
| synthetic228 | hydrophobicity, ionization and conformational change | block copolymers or designer small molecules that mimic self-assembly mechanisms found in nature | thermoresponsive PEG–PLAL loaded with BDNF and NGF led to neuronal differentiation of tonsil derived mesenchymal stem cells210 |
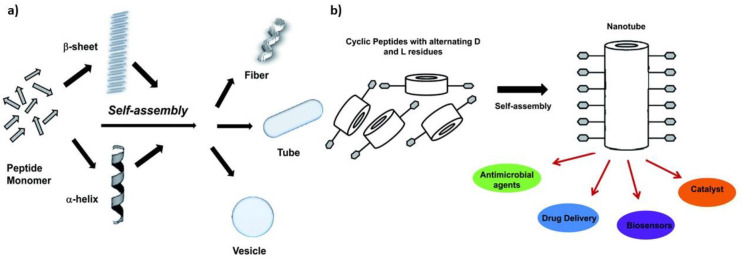
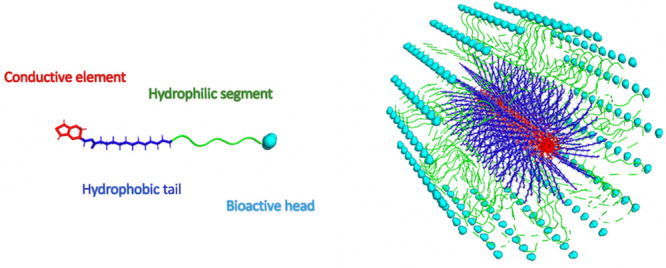
The chemical structure of these molecules allows control over size, shape, charge, and surface properties while maintaining low cytotoxicity.207−209 Most of these self-assembling molecules are in part driven by the interplay of hydrophobic and hydrophilic forces. For example, synthetic block copolymers comprised from alternating hydrophobic poly(l-alanine) and hydrophilic poly(ethylene glycol) segments form a self-assembling gel in aqueous conditions and this has been shown to support neuronal differentiation when loaded with growth factor releasing microspheres.210 Similarly, the RADA16-I is a peptide consisting of 16 AAs with alternating hydrophobic and hydrophilic residues. This drives its self-assembly in aqueous environments into a stable β-sheet structure.19 Alternately, Watson–Crick base pairing in DNA can be utilized to form self-assembling nanotubes of DNA segments, which can be functionalized with peptide sequences that promote neural differentiation.211 A common example of hybrid biomolecules are peptide amphiphiles (PAs), which consist of a hydrophilic peptide head, often followed by a β-sheet forming sequence, which is then capped with a hydrophobic segment. This leads to hydrophobic collapse in aqueous conditions.200 The hydrophobic tail can consist of alkyl chains, aromatic molecules such as Fmoc or other functional molecules.212,213 Nucleic acids and peptide or peptide amphiphiles are an ideal material because of their inherent low immunogenicity and versatile biofunctionality.214−216,98,57 PAs can easily be synthesized on both small scales for experimental study and large scale for application in the clinic.217 Peptides can also be functionalized with synthetic molecules in order to create amphiphilic molecules that self-assemble into a variety of different morphologies including fibers which promote the differentiation and elongation of neural stem cells, serving as a topographical guide for their growth.218,219 These self-assembling systems can be utilized to make materials across multiple length and spatial scales.207 Some of the most common morphologies are linear, trigonal, and cyclical structures, which then self-assemble to form various secondary and tertiary structures as illustrated by Figure 3.
Figure 3.
Possible self-assembled structures secondary and tertiary structures of (a) linear peptides and (b) cyclical peptides Adapted with permission from ref (212). Copyright 2010 Royal Society of Chemistry.
3.1. Topographical Material Modifications
The nanotopography of self-assembled structures can be modulated by varying the molecular structure or the environment in which the self-assembly occurs. More specifically, techniques such as changing the molecular design, electrostatic capping, pH, self-assembly molecule concentration or solvents have all been used to control the formation of micelles, β-sheets, α-helix, nanobelts, and membranes.229−232Figure 4 illustrates various structures formed under different conditions. For example, Ghosh et al.233 developed a PA that would transition from molecules dispersed in solution to micelles or nanofibers based on pH. A reduction in pH of 0.8 transformed micelles into nanofibers.233 This pH and concentration responsiveness is illustrated in Figure 5a. and can be used to design an injectable construct for neural repair which self-assembles when exposed to physiological pH.
Figure 4.
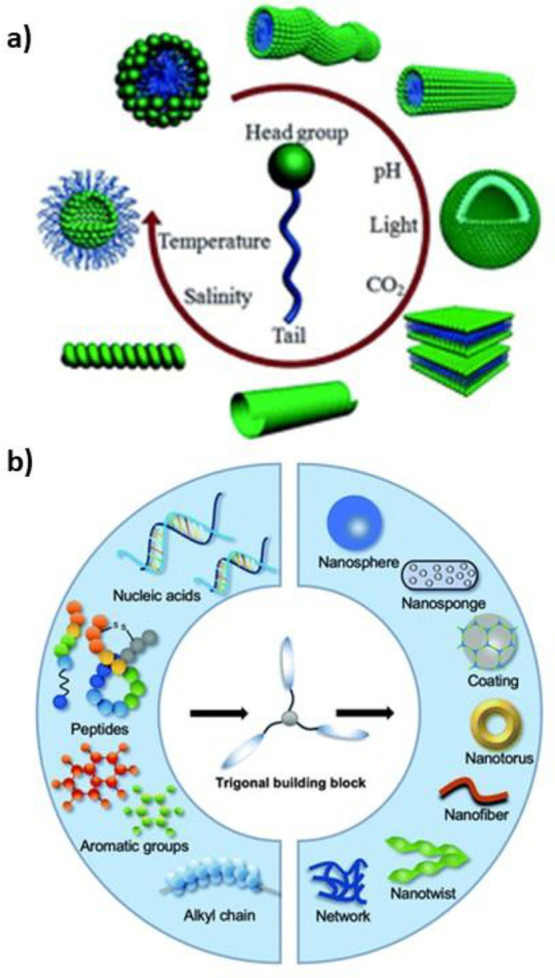
Schematic illustrations of self-assembled structures formed from various building blocks. (a) Amphiphilic building blocks adopting different morphologies. Reprinted with permission from ref (240). Copyright 2014 Royal Society of Chemistry. (b) Trigonal building blocks yielding different structures and morphologies. Reprinted with permission from ref (209). Copyright 2013 Royal Society of Chemistry.
Figure 5.
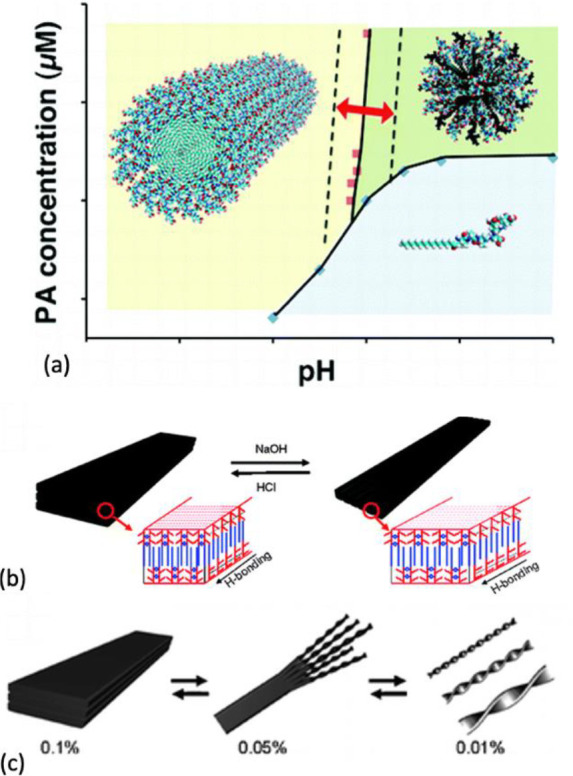
Effect of pH and concentration on self-assembly. (a) pH-dependent micellar, fibrillar, or dispersed topography. Reprinted with permission from ref (233). Copyright 2012 American Chemical Society. (b) Schematic illustration of pH change leading to the formation of nanobelts and varying concentration leading to a change in morphology from plaques to nanoribbons; (c) schematic illustration of morphology changes due to change in concentration. Reprinted with permission from ref (235). Copyright 2009 American Chemical Society.
The morphology of a self-assembly structure can also be fine-tuned by pH as shown by Cui et al.234 By varying the pH it was shown that a flat amphiphilic peptide nanobelt could be transformed into a grooved nanobelt with parallel nanochannels.228 Interestingly, a concentration-dependent modulation of morphologies was also demonstrated.228 Different structures including a split nanobelt with bristle morphology and twisted nanoribbons were achieved by reducing the concentration of PA molecules in the aqueous solution.235
Self-assembling fibers can be hierarchically organized in supramolecular crystals which can be aligned using various methods such as acoustic fields, pressure, magnetic fields,236 ultrasonication, electric fields, or external force fields.214,237,238 For example, Zhang et al.239 used shear force from the injection of an aqueous PA into an ionic solution to form a noodle-like hydrogel of aligned peptide nanofibers. This aligned PA was later functionalized with IKVAV and RGDS bioactive epitopes and shown to promote aligned neurite outgrowth in P19 mouse neurons.218 It also resulted in the formation of synapses and spontaneous electrical network formation after 2 weeks in culture with hippocampal neurons.218
Co-assembly is the incorporation of two or more distinct building blocks that self-assemble to form a structure, similar to the coassembly of proteins in nature. The combination of distinct components allows for the development of novel functional properties, and the tuning of supramolecular morphology and bioactivity as well as the physicochemical properties of the hydrogel. Various methods exist to obtain coassembly harnessing aromatic interactions, enzymatic action, electrostatic interaction, chemical stimuli, or electromechanical stimuli.241 Co-assembly and AA modification can also change dimensions and sizes of fibrous aggregates, fostering the formation of 1D or 3D networks.242,243 These techniques can be harnessed to create nanotopographies that can promote neural regeneration. Co-assembly can also be used to incorporate bioactive epitopes into the fibers in order to control cell fate.244
3.2. Mechanical Material Modifications
Self-assembling structures have tunable mechanical properties. By varying sequence charge, branching,245 concentration,246,247 coassembly, cross-linking,248 and solvent/ions interactions the mechanical properties can be tailored.212,249−251,213,252 The mechanical properties of SAPs that have been achieved using these methods can be found in Table 5. For example, Clarke et al.249 showed that by modifying peptide concentration and sequence charge of an oligopeptide the elastic modulus of the resulting hydrogel can be varied across 2 orders of magnitude from 2–200 kPa. Shear thinning and self-healing properties were also demonstrated through reassembly, which are of interest for in situ placement.249 Shantanu et al.111 explored the effect of varying gel stiffness on hippocampal cells. By varying the strength of the β-sheet interactions PAs with stiffness of 22.9 and 7.3 kPa were designed.111 Hippocampal neurons were subsequently cultured on peptide coated surfaces and it was found that the stiffness of the substrate greatly affected astrocyte density and neuronal maturation.111 Stiffer substrates led to an astrocyte density 10 times higher than softer substrates, while neuronal density was 30% lower on stiffer substrates compared to soft self-assembled fibers.111 This demonstrated that varying stiffness allows for control over the differentiation of neural cells.111 Furthermore, the effect of stiffness on neuron maturation, classified by morphological criteria, was apparent after only 20 h in culture.111 Interestingly, softer peptide amphiphile scaffolds showed faster maturation of neurons, which was not dependent on the presence of KDI or RGDS epitopes.111
Table 5. Stiffness of Various Self-Assembled Hydrogels in Cell Culture Conditions.
| method used to modulate stiffness | material | range of storage modulus (stiffness) obtained (kPa) | ref |
|---|---|---|---|
| concentration | RADA I | 0.046–0.735 | (246) |
| RADA II | |||
| concentration and sequence | 2–15 mg/mL | 0.5–3 | (247) |
| KFE-8 | |||
| KFE RGD | |||
| KFE RDG | |||
| pentapeptide | 2–200 | (253) | |
| co-assembly and concentration | SA5N | 10–200 | (255) |
| SA21 | |||
| Fmoc peptides | 2–30 | (256) | |
| sequence modifications | peptide amphiphile | 7–23 | (111) |
| branched (LDLK)3 peptides | 0.002–0.008 | (257) | |
| cross-linking | self-assembled peptides cross-linked with genipin | 1.5–120 | (248) |
Scaffold degradation allows cells to remodel the ECM, thus improving migration and viability.118 Degradation of self-assembling materials can be tuned by varying the molecular structure. For example, the incorporation of sequences that can be cleaved by MMPs has led to the degradation of β-sheet fibrillar materials.125,36,258 However, the expression of MMPs is hard to control in vivo. An alternative that has been investigated is the incorporation of ester bonds into self-assembled gels, rendering the degradation dependent on pH and water accessibility, a more predictable in vivo process. Collier et al.259 showed that by incorporating glycolic acid (Glc) within the peptide segment of an Fmoc-F-RGD SAP resulted in a linear degradation profile over 60 days. Placement of the Glc segment was critical, as substituting the glycine in the RGD sequence resulted in greatly reduced bioactivity of the adhesion epitope.259 Placement of the Glc segment next to intact RGD sequences permitted hydrolytic degradation without compromising the bioactivity of the RGD sequence.259 The stiffness of the degradable gel was around 1.5 kPa.259 When coassembled with and Fmoc-diphenylaniline peptide which has a stiffness of 30 kPa, a range of stiffnesses was obtained depending on the ratio up to a stiffness of 13 kPa for a 20:1 ratio of Fmoc-FF to Fmoc-F-Glc-RGD.259 Rho et al.260 showed that secondary hydrophobic interaction near the core of cyclical peptides can stabilize the peptide bonds without compromising on solubility in aqueous conditions.
3.3. Incorporating Biomolecular Components
A wide range of bioactive cues have been incorporated within biomaterials intended for neural repair. SAPs offer the possibility of multifunctionalizing the material system, by simultaneously incorporating bioactive molecules in the peptide sequence and within the scaffold structure. The versatility of their biofunctionalization is a major advantage in the field of neural scaffold materials.152,153 An overview of recently explored bioactive cues incorporated in SAP materials is presented in Table 6. Adhesion molecules consist of bioactive epitopes derived from large molecules found in the neural ECM and they interact with the cells through integrins.138 These molecules are necessary for cell survival, migration, and differentiation and cell behavior can be influenced by modifying the scaffold’s adhesion cues.261 Decellularized ECM materials or purified single ECM components can be engineered as injectable natural scaffolds to preserve the physiological chemical environment.42,65,138,262 Hyaluronan, methylcellulose, chitosan, and fibrin among other materials can be used to design in situ forming biomaterials for neural repair and drug delivery.42,65,263,264 However, such materials can present batch-to-batch variability, and tuning their composition or material properties can be challenging.39,42 Synthetic SAPs offer the possibility of multiple functionalizations with targeted molecules and epitopes in predefined concentrations.130 Thus, bioactive ECM components can be included in self-assembling material design maintaining constant biochemical and physical conditions.58,265
Table 6. Bioactive Molecules for Neural Engineering Incorporated in SAPs.
| bioactive molecule | SAP system | inclusion method | physical and biological action | ref |
|---|---|---|---|---|
| Adhesion Molecules | ||||
| IKVAV | RADA16-IKVAV | addition at one extremity of the peptide sequence by covalent bond | enhanced survival of encapsulated NSCs and glial scar reduction | (134,268) |
| improvement of neuroregeneration after 6 weeks in traumatic brain injury murine models | ||||
| RADA16-IKVAV/-RGD | addition at one extremity of the peptide sequence by covalent bond and SAP combination | increased spinal cord embryonic primary cell viability and increased neural differentiation compared to 2D substrates and nonfunctionalized peptide (RADA16-I) | (296,297) | |
| enhanced neural differentiation in primary embryonic rat NSCs in vitro | ||||
| promoted nerve functional regrowth in vivo | ||||
| PA-IKVAV | addition at one extremity of the peptide sequence by covalent bond | increase in neural stem cell neurogenesis and neuronal differentiation in vitro | (267,275,166) | |
| improvement in Alzheimer’s symptoms and neurogenesis in vivo | ||||
| YIGSR | RADA16-GG-YIGSR | addition at one extremity of the peptide sequence by covalent bond | increase in neuronal differentiation, restoration of memory/learning function in Alzheimer’s mice models, rescued synaptic function, decrease in pro-inflammatory factors | (234) |
| RGD | RADA16-I | addition at one extremity of the peptide sequence by covalent bond | promoted primary murine NSC proliferation and differentiation with mechanical and rheological properties comparable with neural tissue | (272) |
| RADA4 | addition at one extremity of the peptide sequence by covalent bond | supported proliferation and differentiation of primary mouse NSCs compared to Matrigel control | (298) | |
| Growth Factors | ||||
| BDNF and GDNF | Fmoc-DDIKVAV | SAP functonalied with chitosan molecule, cross-linking between chitosan polysaccharide amine group and BDNF sulfhydryl group | increase GF lifespan by over 40 times | (101) |
| structural and biochemical peptide properties maintained | ||||
| βFGF | RADA16-DGE | electrostatic interaction with negatively charged peptide terminus | clinically viable drug release profiles, increased neural stem cell proliferation | (283) |
| GDNF | Fmoc-DIKVAV | addition of IKVAV at one extremity of the peptide sequence by covalent bond | sustained released of GDNF from 1 to 172 h | (282) |
| GDNF physical entrapment by gelation | implants of cell-loaded material system in Parkinson’s disease murine models promotes graft cell survival, reinnervation of the host tissue, and overall endogenous tissue repair | |||
| bone marrow homing peptide 1 and 2 (BMHP1, BMHP2) motifs | RAD16-I | GF motives directly extended from the peptide sequence by covalent bond | enhanced NSC survival and differentiation promoted differentiation toward neural and glial fate in vitro | (286) |
| amelioration of locomotor recovery in rats | ||||
| Drugs and Proteins | ||||
| lipophilic drugs (pindolol, quinine, and timolol maleate) | RADA16-II | physical entrapment | clinically viable release profile of lipophilic drugs was obtained, while maintaining the peptide nanostructure | (299) |
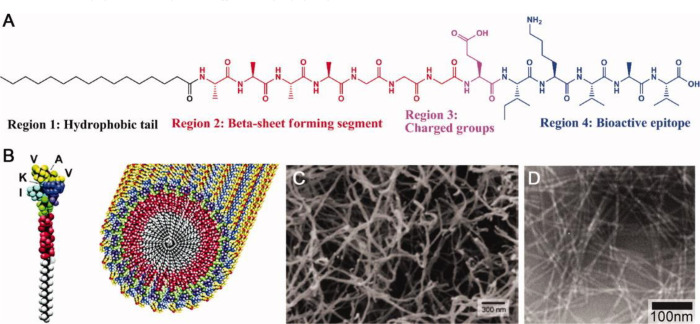
Adhesion epitopes can be introduced in the peptide sequence, and therefore they are often designed to be as short as possible so as not to interfere with the nanostructure and self-assembly mechanism.58 Neural bioactive peptide motifs tested for use in biomaterials are usually derived from the amino-acidic sequence of the neural cell adhesion molecule (NCAM), fibrin, laminin, and fibronectin.34,58 Aside from the universal adhesion molecule RGD laminin-derived epitope, IKVAV can be considered the most popular example in neural engineering for its role in neural stem cell differentiation and glial scar reduction, especially when combined with SAPs such as RADA-IKVAV and PA sequences.97,266−268 Epitope peptides can be synthesized directly at any site of the SAP backbone sequence or chemically ligated as a postsynthesis modification, in a linear or branched fashion.265,269−272 Solid phase peptide synthesis is one of the most common techniques, chosen for the relatively simple method and versatility.270,273−275 Investigation on the effect of different bioactive epitopes, epitope density and exposure are possible because of the highly controllable chemical structure of SAPs and precise material purification methods.97,275 Silva et al.275 synthesized a peptide-amphiphile material that assembles into nanofibers at physiological pH and functionalized it by chemically binding the IKVAV epitope at one extremity of the sequence (Figure 6).276 The PA-IKVAV showed optimal NSC survival compared to 2D laminin controls in vitro.275 The IKVAV epitope density was then modified by mixing the material with different concentrations of the same SAP sequence functionalized with a nonphysiological sequence instead of IKVAV.275 The results showed that neuronal differentiation increased with IKVAV epitope density as opposed to astrocytic development.275 The same material was shown by Yang et al.267 to improve cognitive impairments and increase hippocampal neurogenesis when implanted in Alzheimer’s transgenic mice. Tysseling-Mattiace et al.267,277 also reported the reduction glial scar formation, the regeneration of sensory fibers and significant behavioral improvements in an in vivo murine model of spinal cord injury. Cui et al.234 presented similar results with the SAP RADA16 functionalized with the motif YIGSR, a laminin-derived epitope that also promotes neural differentiation and proliferation. These results demonstrate the effectiveness and versatility of SAPs in disease-targeted neuroregeneration.
Figure 6.
SAP PA-IKVAV. (A) Molecular structure composed of four functional regions dedicated to different functions, highlighting the versatility and multifunctionality of SAP systems. (B) Molecular graphics of the PA-IKVAV molecules, also assembled into a nanofiber. (C, D) Scanning electron microscopy and transmission electron microscopy (respectively) of self-assembled PA-IKVAV nanofibers. Reproduced with permission from refs (276) and (273). Copyright 2004 The American Association for the Advancement of Science and 2010 John Wiley and Sons.
Combining multiple functionalizations within the same SAP can be used to target different pathways and achieve synergistic effects. For example, Galler et al.278 synthesized a multidomain SAP containing both the degradable MMP-2 motif and the adhesion peptide RGD in different peptide locations, observing enhanced cell viability, spreading, and migration. The epitope distribution and topography can also be controlled through chemical interactions with specific AAs,34,279 thereby affecting the cell overall behavior. Sur et al.279 functionalized PA nanofibers by binding RGD epitopes on specific glycine sites, which was shown to affect cell spreading on the scaffold nanostructure.
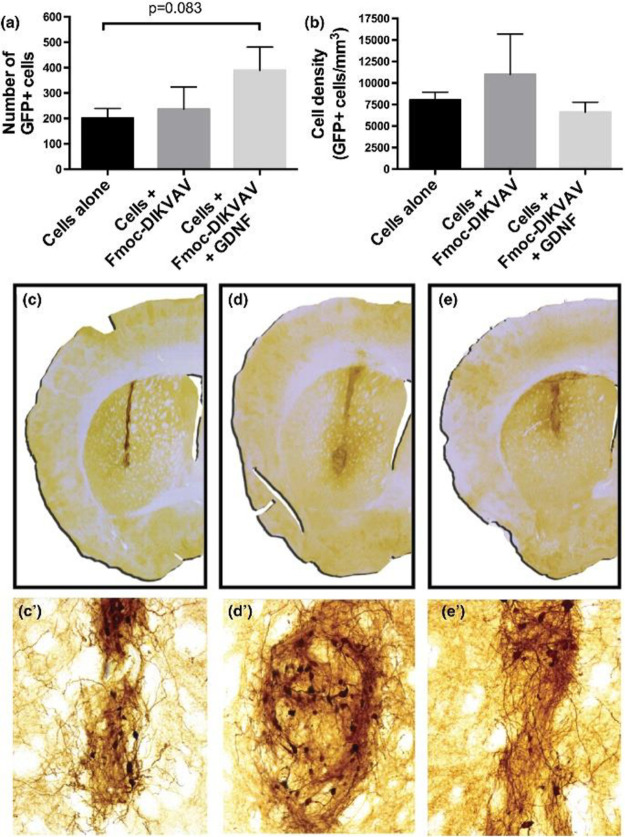
In addition to adhesion molecules, other bioactive elements such as GFs and neurotrophins can affect both cell behavior and cell fate.130,280 GFs are a powerful and widespread tool for regeneration applications, however their administration route and method must be finely controlled because of the short half-life, relatively large size, slow tissue penetration, and the potential toxic effects at high levels when delivered systemically.280 SAPs are considered an optimal GF delivery method because they offer protection from degradation, controlled spatial and temporal release and local administration.280−282 GF molecules can be inserted directly into the SAP sequence as seen for adhesion molecules,281,283 or they can be chemically conjugated with the hydrogel. Other methods of incorporation include the use of GF-specific binding sequences or biotin–streptavidin–biotin bonds.43,281,284,285 Gelain et al.286 extended the peptide sequence of RAD16-I by directly adding bone marrow homing peptide 1 and 2 (BMHP1, BMHP2) motifs, achieving an increase in primary NSC proliferation and neural differentiation, whereas maintaining a stable and precise GF delivery and concentration. GFs can also be encapsulated into the polymer network through physical bonds which break upon hydrogel degradation.43,282,283 Finally, Gelain et al.283 achieved clinically viable GF release profiles incorporating negatively charged AA sequences to the SAP RADA16-I. The positively charged basic-fibroblast cytokine (βFGF) electrostatically interacted with the SAP terminus, allowing for a gradual release, which increased NSC proliferation.283 GFs can also be combined with adhesion epitopes using different incorporation methods, to enable effective delivery to the tissues. Rodriguez et al.282 synthesized the SAP Fmoc-DIKVAV as a single peptide chain and subsequently incorporated glial cell line derived neurotrophic factor (GDNF) by physically entrapping the molecule within the hydrogel upon gelation. This allowed for a dual effect on NSC differentiation and proliferation by the IKVAV epitope and NGF, which improved the regeneration effect of a NSC transplant in Parkinson’s disease mice models (Figure 7)282
Figure 7.
In vivo effect of a SAP biofunctionalized with the adhesive molecule IKVAV and the growth factor GDNF in a Parkinson’s disease murine model. (a, b) The effect of the functionalized hydrogel is more pronounced than the cell implanted alone, as shown by the GFP+ cell density 10 weeks post-transplantation. The transplant has different outcomes in vivo, where (c) the cell line alone showed a lower graft survival than (d) the cells with the SAP N-fluorenylmethyloxycarbonyl (Fmoc)-DIKVAV and (e) the SAP combined with the GDNF growth factor. Reproduced with permission from ref (282). Copyright 2017 John Wiley and Sons.
Self-assembling drug-loaded microparticles287,288 and genetically modified cells for GF production are other delivery approaches.289 RAD16-I was employed to create cell-encapsulating microgel beads, which were able to support cell proliferation and diffusion of nutrients.130 Indeed, other signaling proteins and drugs such as neurotransmitters, gene vectors, and signaling molecules can be encapsulated into the SAP structure for self-assembling, resulting in delivery profiles similar to GF delivery.43,56,283,290−293 The MAX8 β-hairpin SAP designed by Branco et al.290 exploits the positive charge of the hydrogel network to bind and release negatively charged molecules with different isoelectric points. Koutsopoulos et al.291 investigated the delivery properties of RADA16 with different proteins physically encapsulated during the self-assembling process. The findings reveal the structural stability of the SAP when employed as a drug delivery system, and the size-dependent protein release.291 Importantly, the molecular structure, size, charge, and biological effects need to be investigated case by case to reach an appropriate release and administration route.290,291
SAP material systems can also be used to design stimuli-controlled drug delivery systems.43 Different physiological stimuli can modify the material interaction with the encapsulated bioactive molecule and trigger its release.43 For example, the material degradation of a Fmoc-based SAP can be tuned with the temporal release of GF motifs, resulting in optimal drug release profiles, as shown by Bruggeman et al.101 Drugs and molecules can also be linked to the material with enzymatically cleavable bonds,125,278,294 or chemical links subject to change in pH, temperature, and magnetic fields.43,56,135,295 This feature introduces significant advantages for delivery approaches that require spatially or temporally targeted delivery methods.
4. Considerations for Electrical Stimulation
Electrical stimulation is a powerful tool for neural repair. The therapeutic effect of stimulation is supported by a range of treatments targeting diverse injury settings and applications.14,300 It is therefore important to consider how electrical stimulation can be incorporated in self-assembling hydrogel systems in order to achieve neural regeneration. Examples of widespread clinically implemented electrical stimulation methods are deep brain stimulation (DBS) for brain diseases and functional electrical stimulation (FES) for spinal cord injuries.14,301 However, although these devices are designed to replace lost function, without scaffold support, there is minimal capacity for neural tissue regeneration. In fact, the implantation and presence of a rigid device can result in further neural cell loss. The employment of scaffold materials within bioelectronics applications has been gaining attention over the past decade, including the use of soft polymeric electronics for implants, neural interface coating materials, and drug delivery systems.302,303 These technologies have revealed both the potential for organic conductors applied in electrical stimulation and the need for scaffold materials that are compatible with electrical stimulation.30,304,305 Coupling bionic devices with tissue engineered scaffolds is an emergent area where conductive SAPs may find application. However, it is essential to ensure the compatibility of the self-assembling mechanism, which is often driven by electrostatic interactions with the propagation of electrical signals.191,196
Electroactive scaffolds have been developed to actively promote electrical stimulation116,306−310 and ionically porous materials have been used to ensure that cells are effectively exposed to electric fields.189 Incorporation of conductive materials into self-assembling scaffolds has been investigated as a method of providing cell scaffolds with conductive elements. Carbon based nanomaterials such as nanotubes (CNT) and graphene have been explored to confer electroactivity to scaffolds. Although they demonstrate good conductivity and polymer composites have been designed with appropriate mechanical properties, the regulatory pathway for new materials and in particular carbon nanomaterials has hindered their clinical translation.311,312 Conductive polymers (CPs)313 have also emerged as a potential solution due to their high charge injection capacity and ionic conductance.304,313−316 CPs are characterized by alternating single and double bonds along the backbone, termed π-conjugation, which cause a delocalization of electrons. Some of the most commonly used CPs for in vivo studies are poly(3,4-ethylenedioxythiophene) (PEDOT), polypyrrole (PPy), and polyaniline (PANI).60 The primary disadvantage of CPs are their poor mechanical stability and limited conformational control.305,317,318 The addition of bioactive cues to CPs to improve cell attachment and proliferation can also have a significant impact on the polymer properties, preventing the possibility of a multifunctional biomimetic scaffold from a bulk CP.319
To improve the scaffold electrical properties while maintaining cytocompatibility and mechanical tuneability, researchers can introduce conducting elements such as CPs or CNTs to softer and more tunable materials.320 Conductive hydrogels (CHs) have been developed pursuing this concept and applied to flexible bioelectronic applications.314,321,322 The coupling of these conductive materials to self-assembling hydrogels has also been investigated.33,306,323−325 Relevant examples of natural and synthetic self-assembling materials compatible with electrical stimulation or that possess intrinsic conductive properties can be found in Table 7. Peptides inspired by bacterial pili have shown some extremely high conductivities as reviewed by Hochbaum et al.326 However, this conductivity has been shown to be highly dependent on the secondary and tertiary structures,327 making it difficult to tailor these systems for neural repair applications.
Table 7. Conductive Self-Assembling Hydrogels and Polymers.
| material | electrical properties | degradation and toxicity | application | ref |
|---|---|---|---|---|
| tetra(aniline)-based cationic amphiphile self-assembled into a nanowire thin film | acid-doped emeraldine salt of aniline was spin-coated into a thin film and dried under a vacuum; conductivity of 2.7 ± 0.3 mS cm–1 was calculated based on four-point probe resistance measurements | self-assembled in aqueous solution but biocompatibility still needs to be studied | designed for application in sensors and device; gel formation was not investigated | (358) |
| amphiphilic peptide-functionalized with an alkyl spacer and tetra(aniline) | tetra(aniline) fibers were doped with HCl and dried under a vacuum; conductivity was measured via a two-point probe to be 6.97 × 10–6 S/cm | co-assembled into a porous nanofiber network with a diameter of 10 nm; PC12 study showed good biocompatibility | PC12 study showed improved neurite outgrowth and more advanced differentiation after 6 days in vitro; demonstrated the potential as an electroactive scaffold for neural culture in vitro | (357) |
| tetra(aniline) terpolymer that forms aggregates with a TANI core and PEG corona | drop-cast aqueous samples had a conductivity of 2.1 × 10–4 when measured via a four-point probe | coculture with chondrocytes showed good biocompatibility and the gels showed a 90% decrease in viscosity over 100 min in PBS at 37 °C; in vivo systemic injection showed strong electroactive intrinsic antioxidant behavior | showed potential for treatment of oxidative stress in diabetic rats yielding normalized ROS levels and enzymatic antioxidants | |
| amphiphilic peptide functionalized with an alkyl spacer and BTBT | displayed extended π-delocalization within the hydrophobic core resulting in a conductivity of 6.0 × 10–6 S cm–1 without doping | self-assembled into nanofibers of 11–13 nm in aqueous media but no cell studies have been made | bioelectronics and possibly tissue engineering | (359) |
| single-walled carbon nanotubes in collagen and Matrigel hydrogel | bulk conductivity of 1723 ×10–3 S/m | improved neurite outgrowth dorsal root ganglia primary cells from P2 neonatal rats under 8 h DC stimulation | elastic modulus of 37–50 Pa | (306) |
| bundled carbon nanotubes entrapped in β-Vhex nanofibers | conductivity of 0.02 S/cm and impedance of 0.2 MΩ as measured by filling a nonconductive microtube with gel and placing 2 electrodes on each side | biostable with little degradation when injected into the brain cortex; no difference in microglial activation relative to the control | soft neural interface to improve neural signal recordings | (360) |
| PEDOT polymer confined within peptide amphiphile nanostructures | finite window of conductivity: maximum on the forward sweep at 5.52 × 10–5 S cm–1 at 0.12 V and global maximum of 6.57 × 10–5 S cm–1 on the reverse sweep at −0.01 V | (33) | ||
| chitosan/gelatin porous scaffolds assembled with conductive poly(3,4-ethylenedioxythiophene) nanoparticles | 5.82 × 10–5 to 6.22 × 10–1 hydrated, acellularized, 6.45 × 10–5 to 6.81 × 10–1 with cells | 30–70% biodegradation in 8 weeks, PC12 cell viability maintained throughout the study | in vitro study of PC12 cell viability, adhesion and proliferation, morphology and epigenetic investigation | (361) |
| Fmoc-FF-PANI hydrogel | from 10 to 2 to 10–1 S/cm; high cell viability of cardiomyocytes grown on the composite hydrogel demonstrates its noncytotoxicity | degradation of ∼62% was obtained after 20 days | living dynamic range pressure sensing and electroconductive interface for electrogenic cardiac cells | (362) |
| conductive collagen/poly pyrrole-b-polycaprolactone hydrogel | 1–5 mS/cm | PC12 cell viability did not differ from collagen after 48 h of incubation | bioprinting for neural tissue constructs | (363) |
| bacterial derived α-helix peptide self-assembly into nanofibers | shows ohmic charge conduction in aqueous states and conductance AFM measured a conductivity of 1.12 ± 0.77 S cm–1 for individual nanofibers; conductance of 10–6 S cm–1 for a 0.3 wt % gel measured with EIS; interestingly, conductivity decreases with increasing peptide concentration | potential for bioelectronics applications | (327) | |
| peptide thiophene hybrids | 1wv% peptide hybrid gels combined with EDOT–OH and pTSA; conductivities range from 3 × 10–3 to 1.5 × 10–2 S cm–1 | storage modulus ranging from 27 to 100 kPa, no cell studies | possible application in tissue engineering | (364) |
| polydiacetylene conjugated peptide consisting of a polymerized polydiacetylene core flanked by peptides | self-assembled hydrogel of aligned nanofibers with polymerized polydiacetylene at the core | (365) | ||
| diphenylalanine peptide nanowires | 2D culture of primary neocortical neurons exhibit enhanced viability, neurotransmitter release, and lower fraction of non- oxidative glucose metabolism | 2D cell culture | (366) | |
| T4P-like peptide decorated with gold nanoparticle | 445–427 S cm–1 | cardiac cell cultures on nanofiber film for 5 days; the film supported the assembly of single cells into synchronized cardiac patches | microelectronics, sensors, integrated in electroresponsive tissues | (367) |
4.1. Conductive SAPs
Self-assembly of small molecules enables a bottom-up control of material properties. Aromatic compounds are mostly incorporated into hydrogels for biomedical applications because they enhance the formation and stability of hydrogels in self-assembling systems.328 For example, Fmoc functionalization of SAPs have been shown to aid self-assembly by enhancing π–π stacking.329 In peptide-based hydrogels, aromatic compounds are used as gelators, helping form hydrogels that are mechanically stable and biocompatible for various applications such as drug carriers or antifouling/antibacterial gels.330 Aromatic compounds are used to cap the N-terminus in solid phase peptide synthesis, and can therefore easily be integrated into the material synthesis.331,328 The incorporation of aromatic compounds and oligomers in self-assembling molecules has been explored to make conductive materials for a wide variety of applications such as electronics, optics, optoelectronics, photovoltaics, magnetic and piezoelectric devices, sensors, drug releasing hydrogels, and catalysts.328,330,332−339
The field of nanoarchitectonics has studied the hierarchical organization of self-assembling molecules into functional layers, sensors, bioactive components, and artificial living systems. Conductive layers have therefore been developed by incorporating aromatic molecules and small linker molecules to various self-assembling molecules.340 Supramolecular electronics have studied the assembly of π-conjugates into electronic nanowires.341 Many of these self-assemblies occur in organic solvents, which inherently limits their application to neural regeneration where a key design criterion is in situ formation via injectable preproducts. Furthermore, the stability and degradation of electroactive scaffolds in physiological environments is not well understood. Despite these current limitations, the approaches taken in these associated fields that employ self-assembly techniques demonstrate the tuneability and feasibility of developing 3D conductive self-assembling networks for neural repair.342,343 For example, highly conductive BTBT amphiphiles are commonly formed in organic solvents, but recently, it has been demonstrated that self-assembly of these molecules can be achieved in aqueous conditions, in a first step toward a tissue engineering application.344 Interest in these π-conjugated peptides for biomaterial applications will continue to grow because of the tuneability and nontoxic nature of self-assembling electroactive molecules.345,346 Understanding the various methods that have been used to tailor the electronic properties of π-conjugated oligomer self-assembling systems is crucial to determining their compatibility for neural tissue engineering.
To develop a biomimetic scaffold understanding how the incorporation of π-conjugated systems affects topographical, mechanical, and conductive properties is key. Varying the molecular structure has been shown to tune the secondary structure and morphology of aromatic self-assembled molecules.347−349 Peptide π-conjugates are often composed of an AA region, a CP region, and sometimes a hydrophobic alkyl tail region. Modifying the various components of this molecular structure can affect several material properties. Lehrman et al.350 showed that by varying the AA side chain of peptide thiophene structures allows for control over the resulting nanostructure. By substituting AA of varying size and hydrophobicity, it was demonstrated that π–π stacking and hydrogen bonding both contribute to self-assembly but are also competitive forces.350 It was suggested that the control of nanostructures arises from the optimization of the balance between π–π stacking, intermolecular hydrogen bonding, and attractive van de Waals forces.350 Changing this balance results in different morphologies including flat structures, spiral sheets, or nanotubes.350 Panda et al.351 also showed that by varying the alkyl spacer length between the peptide and the aromatic component of the self-assembling structure the chirality of a triblock β-sheet fiber could be tuned. All-atom molecular simulations were subsequently used to design peptide sequences to control peptide chirality and electron delocalization properties.352 Peptide chirality affects the conformation and morphology of the resulting structure and is therefore of great interest for bioactivity.353 The core oligomer length was also shown to influence the phase behavior and morphology of self-assembled structures. Different oligomer lengths can lead to the formation of high-aspect-ratio fiber networks or disordered aggregates.354 Alternatively, varying the solvent has been shown to change the self-assembled morphology.355,356 Doping of the conjugated structures has also been shown to alter the morphology of self-assembled molecules. Mushtaq et al.335 showed that PEGylated tetra (aniline) self-assembled into spherical nanostructures. It was shown that these nanostructures can be doped using HCl, which increases their size.335 These structures were found to be electroactive through cyclic voltammetry and UV–vis spectroscopy investigations.335 These polymers were shown to have excellent cytocompatibility when injected into the liver of rats.336
Finally, it has been shown that biological benefits can be achieved from these electrically modified SAPs. Guler et al.357 incorporated tetra(aniline) into an SAP nanofiber and demonstrated that neurite outgrowth and differentiation of PC12 cells was enhanced 6 days after induction with NGF. It was demonstrated that this conductive SAP upregulated the phosphorylation level of the ERK1/2 pathway relative to the nonfunctionalized peptide in an investigation of the upstream pathways of NGF induced neural-like differentiation.357 Although these studies show the significant promise of SAPs, many do not characterize the SAP electrical properties in physiological conditions.
The hierarchical organization of self-assembling π-conjugated fibers is of interest for neural tissue engineering since combining their conductive properties with topographical cues could lead to synergistic effects on neural cells. Aligned fibers are of interest for both spinal cord and peripheral nerve regeneration as they mimic the topography found in vivo. Wall et al.368 demonstrated the hierarchical organization of self-assembling π-conjugated fibers into aligned macroscopic domains. Aligned macroscopic domains of optoelectronic structures were fabricated using a shear-flow assembly as shown in Figure 8.368 As discussed above, topographical cues from self-assembled aligned fibrous networks have been shown to guide neurite outgrowth, which is particularly applicable to mimicking spinal cord architecture for regeneration after SCI. Wu et al.369 studied the effect of combining topographical cues with the photoconductive polymer poly(3-hexylthiophene) (P3HT) on neurite outgrowth. The growth and differentiation of PC12 cells was assessed on a homogeneous P3HT film, self-assembled P3HT nanofibers, and electrospun P3HT/poly(e-caprolactone) (PCL) microfibers with and without light irradiation.369 This enabled an understanding of the effect of electrical stimulation due to the photoconductive polymer in conjunction with different topographies.369 The electrical stimulation provided by the LED irradiation was shown to consistently promote increased neurite outgrowth on all topographies and the self-assembled nanofibers promoted the longest neurite outgrowth.369
Figure 8.
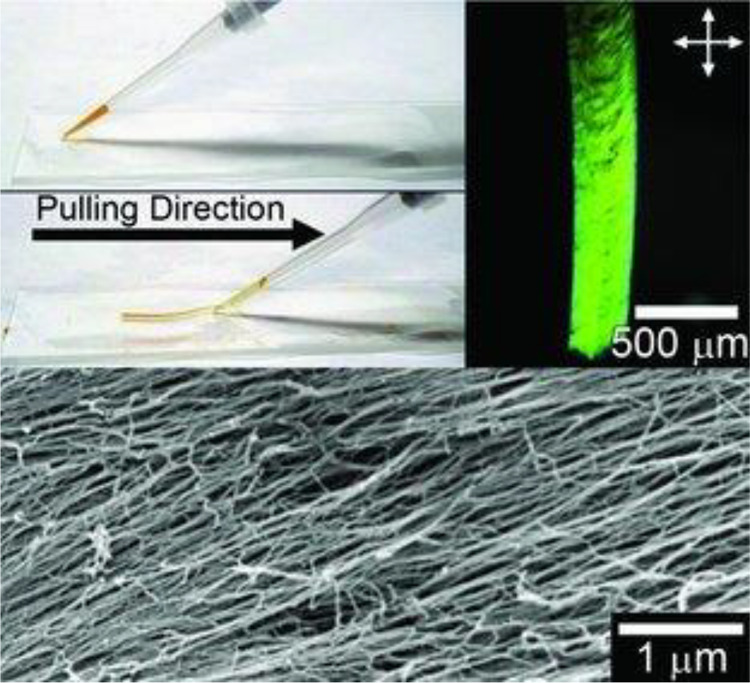
Alignment of π-conjugated peptide hydrogel using shear flow assembly. Reproduced with permission from ref (368). Copyright Advanced Materials 2011.
It is important to understand how these modifications to molecular structure will affect the conductivity of the self-assembled π-conjugates. Ardoña et al.370 studied the influence of varying peptide sequence on mechanical and electrical properties and found that varying the length of AA side chains varied the topography, mechanical, and electrical properties of the formed gels. It was demonstrated that the storage modulus could be increased from 3 to 20 kPa, with sheet resistance increasing from 5 to 17 kΩ sq–1 and secondary structures varying from α- to β-helix. Varying AA sequences changed the local conformation and stacking of π-conjugates.370 Interestingly, the steric effects of the larger aliphatic tails or aromatic groups were found to vary intermolecular electronic coupling within the nanostructures as well as stacking, which was correlated to a reduced sheet resistance.370 This demonstrates the tuneability of self-assembled π-conjugated peptides for neural tissue engineering. Thurston et al.371 investigated the effect on the electronic properties further through molecular modeling and density functional theory calculations to show that smaller AAs favor linear stacking within the peptide dimer, and this improves the delocalization of electrons. This confirmed that varying the AA sequence changes intermolecular forces and charge transport properties and is therefore an important tool to consider when designing a tissue engineering scaffold for neural regeneration.
Several methods have been explored to enhance the electron transport of self-assembled fibers. Nanofibers have been used as a template to guide the self-assembly of conductive polymers, with additional CP added to the self-assembly dispersion before gelation and oxidation.362 The conductive polymer segments can also be covalently cross-linked along the fiber axis to increase conductivity by adding free conductive polymer to the solution. Blatz et al.372 explored this idea by functionalizing a peptide with EDOT–OH. The EDOT-modified peptide was polymerized with a 1:3 molar ratio of additional EDOT–OH in organic solvent as shown in Figure 9b.372 Conductivity in the order of 10 × 10–4 S cm–1 were reported, but it was hypothesized that doping the structure could greatly increase its conductivity.372 Murphy et al.364 synthesized a library of 12 tetrapeptides and functionalized them with EDOT–OH, subsequently polymerizing them with a 1:1 molar ratio and EDOT–OH in aqueous solutions and doped the gel with pTSA.364 This yielded gels with conductivities in the order of 1 × 10–2 S cm–1.364 Conductivity of the conjugated systems can also be improved by incorporating dopants within the molecular structure of self-assembling systems.373 Yang et al.374 created a 3D nanostructured CH for application as a pseudocapacitor. Ionic and electronic conductive properties of PPy and PANI functionalized with different molecules that act as both gelators and dopants were investigated.374 These molecules self-assemble into conductive fibers as shown in Figure 9a and can readily form a hydrogel with morphologies dependent on trypan blue (TB) concentrations.374 Conductivities as high as 3.3 S/cm were obtained for the PPy-TB molecule shown in Figure 9c.374 This hydrogel was designed for energy storage applications, and it is biocompatibility has not been explored but trypan blue is known to be toxic.375 However, this is a good demonstration of the use of dopants to increase conductivity in self-assembled π-conjugated systems as other dopants are readily used in the synthesis of CPs for neural tissue engineering.316 Another strategy to increase the conductivity is the coassembly of different self-assembling molecules.376,241 The coassembly of electron donors and acceptors has been shown to increase conductivity. For example, to create a 1D nanowire that self-assembles in aqueous media, Khalily et al.377 coassembled n-type and p-type short peptide-chromophore conjugates. The coassembly showed increased conductivity relative to either the n-type or p-type fibers alone. The n/p-co-assembled nanowires are approximately 2400 times more conductive that the n-type wires, and are 10 times more conductive than the p-type alone.377
Figure 9.

Methods for increasing conductivity of π-conjugated self-assembling systems. (a) SEM of self-doping PPy-TB and (c) Molecular structure of PPy-TB. Reprinted with permission from ref (374). Copyright 2019 American Chemical Society. (b) Schematic of EDOT–OH polymerization along the fiber axis. Reprinted with permission from ref (372). Copyright 2013 American Chemical Society.
Understanding the relationship between molecular structure and π-stacking is important in the development of conductive self-assembled systems containing π-conjugates. It is therefore interesting to note changes in π-stacking of chromophore self-assemblies. Varying the AA sequence of a PA has also been shown to tune chromophore packing and their resulting photophysics. Tovar et al.378 showed that variations of the AA side chain residues at various locations along the AA side chains led to changes in π-stacking. π-stacking has been shown to have profound effects on the conductivity of self-assembled structure metal–organic frameworks,379,380 we can therefore infer that conductive peptide packing will be affected by the AA sequence leading to changes in conductivity. This is further reinforced by Lee et al.’s381 recent report of very high conductivity in a self-assembly system composed of a π-chromophore core flanked by an alkyl spacer and pentapeptide on both sides. Unexpected mineralization of KCl along the glutamic AA in HCl vapor deposition was obtained following KOH treatment, which is thought to have led to proton doping along with very strong packing and stability.381 This method yielded conductivities as high as 1800 S cm–1 when incorporating alkyl spacers between the peptides and π-conjugation.381 Obtaining such conductivity values demonstrates the potential for the application of molecular self-assembly to form conductive networks.
4.2. Switchable SAPs
The uses of electrical stimulation can be targeted not only to neural tissue but also toward specific SAP material features. The possibility of finely tuning the stimulation parameters has promoted the concept of electrically responsive materials.198−200 This option confers precise temporal control over material properties and polymerization, fostering the development of cutting-edge applications such as stimuli-responsive drug delivery.200,206 This technology provides clear advantages in the field of neural repair, where the sensitive and complex environment requires temporally and spatially precise interventions. Material features such as bioactive cues, wettability, and protein absorption, as well as assembly and disassembly could be controlled by electrical stimulation,201−204.205 For instance, bioactive biotin molecules can be reversibly exposed depending on the surface potential,200 and the presence of an electric voltage can maintain drug-loaded nanoparticles in an assembled configuration to control drug delivery.206 It is also known that the natural hydrogel chitosan reversibly self-assembles via electrodeposition of films which are physically cross-linked.207,208 Similarly, the redox reaction triggered by electrical stimulation of polydopamine (PDA) coatings have been shown to promote cell spreading, proliferation, and differentiation on a titanium electrode.209 This class of materials termed “switchable” can be used to implement highly controllable electrically mediated therapies, allowing for temporal and spatial control of bioactive, topographical, electrochemical, and structural cues to neural cells. Although the properties mentioned are representative of the wide range of possibilities offered by switchable materials, today they only serve as a proof of concept toward an innovative vision for SAP materials.
5. Applications
In vivo applications of SAP hydrogels have shown great promise for neural regeneration. Multiple studies have shown that SAPs elicit minimal inflammation and scar formation while promoting vasculature formation, axonal regrowth, and synaptic formation.19,216 In the PNS, SAPs have been investigated for treatment of crush and resections of sciatic nerves,11 cavernous nerves,382383 and facial nerves.219 Recently, Richter et al.219 compared SAP performance to an autograft (using the resected nerve segment) for the regeneration of the facial nerve after a 7.5 mm resection. A hollow collagen tube was filled with an aligned PA and neural regeneration was assessed by electrophysiological stimulation and recording across the nerve resection site.219 It was found that the PA showed similar performance to the autograft which is an impressive outcome because autografts are considered the gold standard in treatment.219 This PA did not present any bioactive epitopes, but presents evidence that the regenerative potential of SAPs could match autografts and lead to improvements in functional recovery. This is further supported by a 10 mm sciatic nerve resection study led by Yang et al.384 Functional recovery similar to that of an autograft was demonstrated by using a SAP functionalized with both IKVAV and RGI, which mimics both laminin and BDNF.384
In the CNS treatment of spinal cord injury with SAPs has shown reduced inflammation, cavitation, and scar formation. The promotion of axonal growth and guidance, vascularization, and functional recovery has been observed in animal models.26,216,224,382,385−388 In one of the most recent applications to spinal cord regeneration the properties of self-assembling systems were leveraged in order to create a synergistic scaffold. Xiao et al.389 have combined topography with bioactivity and drug release. RADA16 SAP containing FGL (neural cell adhesion molecule) was tailored to release Taxol for spinal cord injury in a rat model.389 Taxol has been shown useful in vitro but has difficulty crossing the BBB and therefore requires localized drug delivery. Following a T9 contusion, the SAPs were injected into the lesion site and rats were evaluated up to 8 weeks after injury. The rats injected with a Taxol-loaded scaffold showed the most functional recovery with a ranking of 15 on the BBB scale, cell infiltration, and neurite extension across the lesion, as well as reduced glial activation and inflammation, and reduced cavity formation.389 This is an example of the advantage of tailoring SAPs to control multiple properties critical to regeneration of the nervous system.
SAPs are also an emerging option to accomplish brain regeneration in the context of traumatic brain injury and neurodegenerative diseases.58,390 Cell-loaded, drug-loaded, or bioactive injectable SAPs are a strategy for restoring brain tissue and function.58 For example, a SAP functionalized with the laminin epitope IKVAV was found to promote the proliferation and differentiation of endogenous NSCs and to improve the learning and memory impairment in an Alzheimer’s disease mice model.267
Although there is a plethora of studies on self-assembly systems for neural regeneration both in vivo and in vitro, no complete functional recovery has been observed to date in long peripheral nerve gap injuries or severe central nervous system injuries. Complementary electrical stimulation approaches such as DBS are among the leading treatments for late stage neurodegeneration14 and bridging the gap between regeneration therapies and electrical stimulation devices is becoming a necessity.391 Smart conductive materials are emerging for bioelectronics applications where the versatility of SAPs could be harnessed for functional electro-neural interface therapies promoting neural regeneration. Chromophore and electroactive peptides have been studied for in vivo drug delivery and tracing and imaging of tumors and have demonstrated good biocompatibility and stability.178,343,392,393 However, more work needs to be done on the application of electroactive self-assembling scaffolds for neural regeneration. Material development of biocompatible and electrically tunable SAPs needs to be undertaken with specific attention to biocompatibility of the components and self-assembly mechanism for injection in vivo. Additionally, the stability and degradation of electroactive scaffolds in physiological environments need to be understood to characterize its effect on stiffness and electroactivity of the scaffold. The properties of these novel materials can then be tailored to suit the material properties of neural tissue enabling the understanding of the degree of synergistic effects possible through utilizing multiple biomolecules, topographies, and coassemblies. Ultimately the translation of materials developed in the lab not only to in vivo models that are known to have regenerative capacity but to the clinic where the systemic patient disease or injury state can impact cell–material interactions needs to be investigated.
6. Conclusion
Self-assembling materials provide a significant opportunity for developing next-generation neural regeneration scaffolds. There are challenges in designing injectable biomimetic SAPs, but because of their bottom-up design, these self-assembled structures are highly tailorable. Varying molecular structure and intramolecular interactions, as well as ionic concentrations, can ensure that bioactivity, mechanical, and topographical properties can meet neural tissue engineering regeneration criteria. SAPs offer significant improvement by filling the heterogeneous injury cavities, promoting cell survival, migration, and differentiation as well as axonal growth into through the lesion site. However, in CNS lesions and extensive PNS damage, SAPs do not completely restore function to the injured tissue.
Future developments of SAPs must consider the design of materials that provide a combination of biomimetic cues to promote the regeneration of neural tissue through provision of multiple physical and biochemical cues. Historically, SAPs and indeed other hydrogel materials for neural regeneration have not been tailored through combining all of these biomimetic cues but rather focused on a single or two desired properties. More complex combinatorial systems that better replicate the natural neural milieu may address the current limitations faced by tissue engineering approaches to neural regeneration. Specifically, it is thought that electrical properties will be critical to supporting these electroactive tissues. Various methods exist to incorporate electrical activity into self-assembling systems for the development of nanoelectronics; however, few studies have assessed their application to neural regeneration. Some studies have investigated the application of electroactive or chromophore functionalized self-assembling systems for responsive in vivo drug delivery which suggests the potential biocompatibility of electrically functionalized SAPs. However, key limitations in the current research include minimal characterization of electroactive properties and a lack of understanding of how these components impact degradation and subsequent long-term electroactivity of the scaffold.
Glossary
Abbreviations
- SAP
self-assembling peptide
- ECM
extracellular matrix
- CNS
central nervous system
- PNS
peripheral nervous system
- NSC
neural stem cells
- GF
growth factor
- NGF
nerve growth factor
- BDNF
brain-derived neurotrophic factor
- Trk
tyrosine kinase
- GAG
glycosamminoglycans
- MMP
matrix metalloproteinases
- SCI
spinal cord injury
- Glc
glycolic acid
- NCAM
neural cell adhesion molecule
- PA
peptide amphiphile
- βFGF
basic-fibroblast cytokine
- GDNF
glial cell line derived neurotrophic factor
- DBS
deep brain stimulation
- FES
functional electrical stimulation
- CNT
carbon nanotubes
- TB
trypan blue
- PDA
polydopamine
Author Contributions
† S.P. and G.E.K. contributed equally to this work. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors acknowledge funding support of the EPSRC Healthcare Technology Challenge Award (EP/R004498/1), the EPSCR CDT in Neurotechnology grant (EP/L016737/1) and the ERC Consolidator Grant in Living Bionics (771985–1).
The authors declare no competing financial interest.
References
- Gooch C. L.; Pracht E.; Borenstein A. R. The burden of neurological disease in the United States: A summary report and call to action. Ann. Neurol. 2017, 81 (4), 479–484. 10.1002/ana.24897. [DOI] [PubMed] [Google Scholar]
- Enciu A. M.; Nicolescu M. I.; Manole C. G.; Mureşanu D. F.; Popescu L. M.; Popescu B. O. Neuroregeneration in neurodegenerative disorders. BMC Neurol. 2011, 11, 75. 10.1186/1471-2377-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane C. A.; Hardy J.; Schott J. M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25 (1), 59–70. 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- Kim S. U.; de Vellis J. Stem cell-based cell therapy in neurological diseases: A review. J. Neurosci. Res. 2009, 87 (10), 2183–2200. 10.1002/jnr.22054. [DOI] [PubMed] [Google Scholar]
- Lee R. C.; Lee M. H.; Wu C. C.; Couto e Silva A.; Possoit H.; Hsieh T.-H.; Minagar A.; Lin H. Cerebral ischemia and neuroregeneration. Neural Regener. Res. 2018, 13, 373–385. 10.4103/1673-5374.228711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam R. Y.; Fuehrmann T.; Mitrousis N.; Shoichet M. S. Regenerative therapies for central nervous system diseases: A biomaterials approach. Neuropsychopharmacology 2014, 39 (1), 169–188. 10.1038/npp.2013.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter K. A.; Buck A. C.; Self W. K.; Capadona J. R. Stab injury and device implantation within the brain results in inversely multiphasic neuroinflammatory and neurodegenerative responses. J. Neural Eng. 2012, 9 (4), 046020. 10.1088/1741-2560/9/4/046020. [DOI] [PubMed] [Google Scholar]
- Wellman S. M.; Kozai T. D. Y. Understanding the Inflammatory Tissue Reaction to Brain Implants to Improve Neurochemical Sensing Performance. ACS Chem. Neurosci. 2017, 8 (12), 2578–2582. 10.1021/acschemneuro.7b00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosshauer B.; Dreesmann L.; Schaller H.-E.; Sinis N. Synthetic Nerve Guide Implants in Humans: A Comprehensive Survey. Neurosurgery 2006, 59 (4), 740–748. 10.1227/01.NEU.0000235197.36789.42. [DOI] [PubMed] [Google Scholar]
- Yucel D.; Kose G. T.; Hasirci V. Polyester based nerve guidance conduit design. Biomaterials 2010, 31 (7), 1596–1603. 10.1016/j.biomaterials.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Li A.; et al. A bioengineered peripheral nerve construct using aligned peptide amphiphile nanofibers. Biomaterials 2014, 35 (31), 8780–8790. 10.1016/j.biomaterials.2014.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayavenkataraman S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020, 106, 54–69. 10.1016/j.actbio.2020.02.003. [DOI] [PubMed] [Google Scholar]
- Manthey A. L.; et al. Using Electrical Stimulation to Enhance the Efficacy of Cell Transplantation Therapies for Neurodegenerative Retinal Diseases: Concepts, Challenges, and Future Perspectives. Cell Transplant. 2017, 26 (6), 949–965. 10.3727/096368917X694877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D. M.; Koppes A. N.; Hardy J. G.; Schmidt C. E. Electrical Stimuli in the Central Nervous System Microenvironment. Annu. Rev. Biomed. Eng. 2014, 16 (1), 397–430. 10.1146/annurev-bioeng-121813-120655. [DOI] [PubMed] [Google Scholar]
- Kam N.; Jan E.; Kotov N. A. Electrical stimulation of neural stem cells mediated by humanized carbon nanotube composite made with extracellular matrix protein. Nano Lett. 2009, 9 (1), 273–278. 10.1021/nl802859a. [DOI] [PubMed] [Google Scholar]
- Feng Z.; Zhao G.; Yu L. Neural Stem Cells and Alzheimer’s Disease: Challenges and Hope. Am. J. Alzheimer’s Dis. Other Dementias 2009, 24 (1), 52–57. 10.1177/1533317508327587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner F. B.; et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 2018, 563 (7729), 65–93. 10.1038/s41586-018-0649-2. [DOI] [PubMed] [Google Scholar]
- Aurand E. R.; Lampe K. J.; Bjugstad K. B. Defining and designing polymers and hydrogels for neural tissue engineering. Neurosci. Res. 2012, 72 (3), 199–213. 10.1016/j.neures.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J.; So K.-F.; Wu W., Self-Assembling Peptides Mediate Neural Regeneration.. In Neural Regeneration; So K.-F., Xu X.-M., Eds.; Academic Press: Oxford, U.K., 2015; Chapter 14, pp 229–236. [Google Scholar]
- Orive G.; Anitua E.; Pedraz J. L.; Emerich D. F. Biomaterials for promoting brain protection, repair and regeneration. Nat. Rev. Neurosci. 2009, 10 (9), 682–692. 10.1038/nrn2685. [DOI] [PubMed] [Google Scholar]
- Horner P. J.; Gage F. H. Regenerating the damaged central nervous system. Nature 2000, 407 (6807), 963–970. 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- Yu L.; Ding J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008, 37 (8), 1473–1481. 10.1039/b713009k. [DOI] [PubMed] [Google Scholar]
- Hoffman A. S. Hydrogels for biomedical applications. Adv. Drug Delivery Rev. 2012, 64, 18–23. 10.1016/j.addr.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Chan B. P.; Leong K. W. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur. Spine J. 2008, 17 (S4), 467–479. 10.1007/s00586-008-0745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutolf M. P.; Raeber G. P.; Zisch A. H.; Tirelli N.; Hubbell J. A. Cell-Responsive Synthetic Hydrogels. Adv. Mater. 2003, 15 (11), 888–892. 10.1002/adma.200304621. [DOI] [Google Scholar]
- Subramanian A.; Krishnan U. M.; Sethuraman S. Development of biomaterial scaffold for nerve tissue engineering: Biomaterial mediated neural regeneration. J. Biomed. Sci. 2009, 16, 108. 10.1186/1423-0127-16-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feksa L. R.; Troian E. A.; Muller C. D.; Viegas F.; Machado A. B.; Rech V. C.. Hydrogels for biomedical applications. In Nanostructures for the Engineering of Cells, Tissues and Organs: From Design to Applications; Elsevier, 2018; Chapter 11, p 403. 10.1016/B978-0-12-813665-2.00011-9 [DOI] [Google Scholar]
- Gumera C.; Rauck B.; Wang Y. Materials for central nervous system regeneration: Bioactive cues. J. Mater. Chem. 2011, 21 (20), 7033–7051. 10.1039/c0jm04335d. [DOI] [Google Scholar]
- Heo D. N.; et al. Multifunctional hydrogel coatings on the surface of neural cuff electrode for improving electrode-nerve tissue interfaces. Acta Biomater. 2016, 39, 25–33. 10.1016/j.actbio.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Aregueta-Robles U. A.; Martens P. J.; Poole-Warren L. A.; Green R. A. Tailoring 3D hydrogel systems for neuronal encapsulation in living electrodes. J. Polym. Sci., Part B: Polym. Phys. 2018, 56, 273. 10.1002/polb.24558. [DOI] [Google Scholar]
- Liyanage W.; Ardoña H. A. M.; Mao H. Q.; Tovar J. D. Cross-Linking Approaches to Tuning the Mechanical Properties of Peptide π-Electron Hydrogels. Bioconjugate Chem. 2017, 28 (3), 751–759. 10.1021/acs.bioconjchem.6b00593. [DOI] [PubMed] [Google Scholar]
- Sensharma P.; Madhumathi G.; Jayant R. D.; Jaiswal A. K. Biomaterials and cells for neural tissue engineering: Current choices. Mater. Sci. Eng., C 2017, 77, 1302–1315. 10.1016/j.msec.2017.03.264. [DOI] [PubMed] [Google Scholar]
- Tovar J. D.; Rabatic B. M.; Stupp S. I. Conducting polymers confined within bioactive peptide amphiphile nanostructures. Small 2007, 3 (12), 2024–2028. 10.1002/smll.200600645. [DOI] [PubMed] [Google Scholar]
- Webber M. J.; Tongers J.; Renault M. A.; Roncalli J. G.; Losordo D. W.; Stupp S. I. Development of bioactive peptide amphiphiles for therapeutic cell delivery. Acta Biomater. 2010, 6 (1), 3–11. 10.1016/j.actbio.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong A.; Aguilar M. I.; Del Borgo M. P.; Sobey C. G.; Broughton B. R. S.; Forsythe J. S. Self-assembling injectable peptide hydrogels for emerging treatment of ischemic stroke. J. Mater. Chem. B 2019, 7 (25), 3927–3943. 10.1039/C9TB00257J. [DOI] [Google Scholar]
- Giano M. C.; Pochan D. J.; Schneider J. P. Controlled biodegradation of Self-assembling β-hairpin Peptide hydrogels by proteolysis with matrix metalloproteinase-13. Biomaterials 2011, 32 (27), 6471–6477. 10.1016/j.biomaterials.2011.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C.; et al. Bioactive Self-Assembling Peptide Hydrogels Functionalized with Brain-Derived Neurotrophic Factor and Nerve Growth Factor Mimicking Peptides Synergistically Promote Peripheral Nerve Regeneration. ACS Biomater. Sci. Eng. 2018, 4 (8), 2994–3005. 10.1021/acsbiomaterials.8b00536. [DOI] [PubMed] [Google Scholar]
- Hou Q.; De Bank P. A.; Shakesheff K. M. Injectable scaffolds for tissue regeneration. J. Mater. Chem. B 2004, (13), 1915–1923. 10.1039/B401791A. [DOI] [Google Scholar]
- Hsieh F. Y.; Tseng T. C.; Hsu S. H. Self-healing hydrogel for tissue repair in the central nervous system. Neural Regener. Res. 2015, 10 (12), 1922–1923. 10.4103/1673-5374.169624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y.; Nan D.; Jin H.; Qu X. Recent advances of injectable hydrogels for drug delivery and tissue engineering applications. Polym. Test. 2020, 81, 106283. 10.1016/j.polymertesting.2019.106283. [DOI] [Google Scholar]
- Fan D. Y.; Tian Y.; Liu Z. J. Injectable Hydrogels for Localized Cancer Therapy. Front. Chem. 2019, 7, 675. 10.3389/fchem.2019.00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakulska M. M.; Ballios B. G.; Shoichet M. S. Injectable hydrogels for central nervous system therapy. Biomedical Materials 2012, 7 (2), 024101. 10.1088/1748-6041/7/2/024101. [DOI] [PubMed] [Google Scholar]
- Habibi N.; Kamaly N.; Memic A.; Shafiee H. Self-assembled peptide-based nanostructures: Smart nanomaterials toward targeted drug delivery. Nano Today 2016, 11 (1), 41–60. 10.1016/j.nantod.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzelczak M.; Liz-Marzán L. M.; Klajn R. Stimuli-responsive self-assembly of nanoparticles. Chem. Soc. Rev. 2019, 48 (5), 1342–1361. 10.1039/C8CS00787J. [DOI] [PubMed] [Google Scholar]
- Hoban D. B.; Newland B.; Moloney T. C.; Howard L.; Pandit A.; Dowd E. The reduction in immunogenicity of neurotrophin overexpressing stem cells after intra-striatal transplantation by encapsulation inaninsitu gelling collagen hydrogel. Biomaterials 2013, 34 (37), 9420–9429. 10.1016/j.biomaterials.2013.08.073. [DOI] [PubMed] [Google Scholar]
- Akimoto A. M. Design of Tetra-arm PEG-crosslinked Thermoresponsive Hydrogel for 3D Cell Culture. Anal. Sci. 2016, 32 (11), 1203–1205. 10.2116/analsci.32.1203. [DOI] [PubMed] [Google Scholar]
- Ruel-Gariépy E.; Leroux J. C. In situ-forming hydrogels - Review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58 (2), 409–426. 10.1016/j.ejpb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Hu W.; Wang Z.; Xiao Y.; Zhang S.; Wang J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7 (3), 843–855. 10.1039/C8BM01246F. [DOI] [PubMed] [Google Scholar]
- Ayub N. F.; Hashim S.; Jamaluddin J.; Adrus N. New UV LED curing approach for polyacrylamide and poly(: N -isopropylacrylamide) hydrogels. New J. Chem. 2017, 41 (13), 5613–5619. 10.1039/C7NJ00176B. [DOI] [Google Scholar]
- Li J.; Xing R.; Bai S.; Yan X. Recent advances of self-assembling peptide-based hydrogels for biomedical applications. Soft Matter 2019, 15 (8), 1704–1715. 10.1039/C8SM02573H. [DOI] [PubMed] [Google Scholar]
- Qin X. H.; Wang X.; Rottmar M.; Nelson B. J.; Maniura-Weber K. Near-Infrared Light-Sensitive Polyvinyl Alcohol Hydrogel Photoresist for Spatiotemporal Control of Cell-Instructive 3D Microenvironments. Adv. Mater. 2018, 30 (10), 1–7. 10.1002/adma.201705564. [DOI] [PubMed] [Google Scholar]
- Holmes T. C. Novel peptide-based biomaterial scaffolds for tissue engineering. Trends Biotechnol. 2002, 20 (1), 16–21. 10.1016/S0167-7799(01)01840-6. [DOI] [PubMed] [Google Scholar]
- Van Tomme S. R.; Storm G.; Hennink W. E. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2008, 355 (1–2), 1–18. 10.1016/j.ijpharm.2008.01.057. [DOI] [PubMed] [Google Scholar]
- Loo Y.; Goktas M.; Tekinay A. B.; Guler M. O.; Hauser C. A. E.; Mitraki A. Self-Assembled Proteins and Peptides as Scaffolds for Tissue Regeneration,. Adv. Healthcare Mater. 2015, 4 (16), 2557–2586. 10.1002/adhm.201500402. [DOI] [PubMed] [Google Scholar]
- Tatman P. D.; Muhonen E. G.; Wickers S. T.; Gee A. O.; Kim E. S.; Kim D. H. Self-assembling peptides for stem cell and tissue engineering. Biomater. Sci. 2016, 4 (4), 543–554. 10.1039/C5BM00550G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French K. M.; Somasuntharam I.; Davis M. E. Self-assembling peptide-based delivery of therapeutics for myocardial infarction. Adv. Drug Delivery Rev. 2016, 96, 40–53. 10.1016/j.addr.2015.04.023. [DOI] [PubMed] [Google Scholar]
- Levin A.; Hakala T. A.; Schnaider L.; Bernardes G. J. L.; Gazit E.; Knowles T. P. J. Biomimetic peptide self-assembly for functional materials. Nat. Rev. Chem. 2020, 4, 615. 10.1038/s41570-020-0215-y. [DOI] [Google Scholar]
- Koss K. M.; Unsworth L. D. Neural tissue engineering: Bioresponsive nanoscaffolds using engineered self-assembling peptides. Acta Biomater. 2016, 44, 2–15. 10.1016/j.actbio.2016.08.026. [DOI] [PubMed] [Google Scholar]
- Mouw J. K.; Ou G.; Weaver V. M. Extracellular matrix assembly: A multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15 (12), 771–785. 10.1038/nrm3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni R.; Ali A.; Shavandi A.; Clarkson A. N. Current and novel polymeric biomaterials for neural tissue engineering. J. Biomed. Sci. 2018, 25 (1), 1–21. 10.1186/s12929-018-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino F. M.; Ganat Y.; Zhang Y.; Zheng W. Stem cells in neurodevelopment and plasticity. Neuropsychopharmacology 2001, 25 (6), 805–815. 10.1016/S0893-133X(01)00349-9. [DOI] [PubMed] [Google Scholar]
- Entekhabi E.; Haghbin Nazarpak M.; Moztarzadeh F.; Sadeghi A. Design and manufacture of neural tissue engineering scaffolds using hyaluronic acid and polycaprolactone nanofibers with controlled porosity. Mater. Sci. Eng., C 2016, 69, 380–387. 10.1016/j.msec.2016.06.078. [DOI] [PubMed] [Google Scholar]
- Hoffman-Kim D.; Mitchel J. A.; Bellamkonda R. V. Topography, Cell Response, and Nerve Regeneration. Annu. Rev. Biomed. Eng. 2010, 12, 203–231. 10.1146/annurev-bioeng-070909-105351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland S. S. The anatomy and physiology of nerve injury. Muscle Nerve 1990, 13 (9), 771–784. 10.1002/mus.880130903. [DOI] [PubMed] [Google Scholar]
- Macaya D.; Spector M. Injectable hydrogel materials for spinal cord regeneration: a review. Biomed. Mater. 2012, 7 (1), 012001. 10.1088/1748-6041/7/1/012001. [DOI] [PubMed] [Google Scholar]
- Hadavi D.; Poot A. A. Biomaterials for the treatment of alzheimer’s disease. Front. Bioeng. Biotechnol. 2016, 4, 49. 10.3389/fbioe.2016.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshayedi P.; Nih L. R.; Llorente I. L.; Berg A. R.; Cinkornpumin J.; Lowry W. E.; Segura T.; Carmichael S. T. Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials 2016, 105, 145. 10.1016/j.biomaterials.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbán N.; Guillemot F.. Neurogenesis in the embryonic and adult brain: Same regulators, different roles. Front. Cell. Neurosci. 2014, 8, 10.3389/fncel.2014.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H.-F.; Lee Y.-S.; Tang T. K.; Cheng J.-Y. Pulsed DC Electric Field-Induced Differentiation of Cortical Neural Precursor Cells. PLoS One 2016, 11 (6), e0158133 10.1371/journal.pone.0158133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian J.; Zheng J.; Li S.; Luo L.; Ding F. Sequential Differentiation of Embryonic Stem Cells into Neural Epithelial-Like Stem Cells and Oligodendrocyte Progenitor Cells. PLoS One 2016, 11 (5), e0155227 10.1371/journal.pone.0155227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadian H.; Maleki H.; Fathollahi A.; Salehi M.; Gholizadeh S.; Derakhshankhah H.; Allahyari Z.; Jaymand M. Naturally occurring biological macromolecules-based hydrogels: Potential biomaterials for peripheral nerve regeneration. Int. J. Biol. Macromol. 2020, 154, 795–817. 10.1016/j.ijbiomac.2020.03.155. [DOI] [PubMed] [Google Scholar]
- Yao S.; Liu X.; Wang X.; Merolli A.; Chen X.; Cui F. Directing neural stem cell fate with biomaterial parameters for injured brain regeneration. Prog. Nat. Sci. 2013, 23 (2), 103–112. 10.1016/j.pnsc.2013.02.009. [DOI] [Google Scholar]
- Dvir T.; Timko B. P.; Kohane D. S.; Langer R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2011, 6 (1), 13–22. 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira A. I.; Duckworth J. K.; Hermanson O. Getting the right stuff: Controlling neural stem cell state and fate in vivo and in vitro with biomaterials. Cell Res. 2007, 17 (1), 56–61. 10.1038/sj.cr.7310141. [DOI] [PubMed] [Google Scholar]
- Miyata S.; Kitagawa H. Formation and remodeling of the brain extracellular matrix in neural plasticity: Roles of chondroitin sulfate and hyaluronan. Biochim. Biophys. Acta, Gen. Subj. 2017, 1861 (10), 2420–2434. 10.1016/j.bbagen.2017.06.010. [DOI] [PubMed] [Google Scholar]
- Li Y. Neural differentiation from pluripotent stem cells: The role of natural and synthetic extracellular matrix. World J. Stem Cells 2014, 6 (1), 11. 10.4252/wjsc.v6.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay E. D.Cell Biology of Extracellular Matrix; Springer, 1988. 10.1007/978-1-4613-0881-2 [DOI] [Google Scholar]
- Ruoslahti E; Pierschbacher M. New perspectives in cell adhesion: RGD and integrins. Science (Washington, DC, U. S.) 1987, 238 (4826), 491–497. 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Bačáková L.; Filová E.; Rypáček F.; Švorčík V.; Starý V. Cell Adhesion on Artificial Materials for Tissue Engineering. Physiol. Res. 2004, 53, 35–45. [PubMed] [Google Scholar]
- Cavalcanti-Adam E. A.; Volberg T.; Micoulet A.; Kessler H.; Geiger B.; Spatz J. P. Cell Spreading and Focal Adhesion Dynamics Are Regulated by Spacing of Integrin Ligands. Biophys. J. 2007, 92 (8), 2964–2974. 10.1529/biophysj.106.089730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barczyk M.; Carracedo S.; Gullberg D. Integrins. Cell Tissue Res. 2010, 339 (1), 269–280. 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B. D.; Grashoff C.; Schwartz M. A. Dynamic molecular processes mediate cellular mechanotransduction. Nature 2011, 475 (7356), 316–323. 10.1038/nature10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumi A.; Orr A. W.; Tzima E.; Schwartz M. A. Integrins in Mechanotransduction. J. Biol. Chem. 2004, 279 (13), 12001–12004. 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- Gardiner N. J. Integrins and the extracellular matrix: Key mediators of development and regeneration of the sensory nervous system. Dev. Neurobiol. 2011, 71 (11), 1054–1072. 10.1002/dneu.20950. [DOI] [PubMed] [Google Scholar]
- Gaudet A. D.; Popovich P. G. Extracellular matrix regulation of inflammation in the healthy and injured spinal cord. Exp. Neurol. 2014, 258, 24–34. 10.1016/j.expneurol.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasung L.; et al. Exploring early human brain development with structural and physiological neuroimaging. NeuroImage 2019, 187, 226–254. 10.1016/j.neuroimage.2018.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long K. R.; et al. Extracellular Matrix Components HAPLN1, Lumican, and Collagen I Cause Hyaluronic Acid-Dependent Folding of the Developing Human Neocortex. Neuron 2018, 99 (4), 702–719. 10.1016/j.neuron.2018.07.013. [DOI] [PubMed] [Google Scholar]
- Gama C. I.; et al. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat. Chem. Biol. 2006, 2 (9), 467–473. 10.1038/nchembio810. [DOI] [PubMed] [Google Scholar]
- Mizumoto S.; Yamada S.; Sugahara K. Molecular interactions between chondroitin-dermatan sulfate and growth factors/receptors/matrix proteins. Curr. Opin. Struct. Biol. 2015, 34, 35–42. 10.1016/j.sbi.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Chernousov M. A.; Yu W. M.; Chen Z. L.; Carey D. J.; Strickland S. Regulation of schwann cell function by the extracellular matrix. Glia 2008, 56 (14), 1498–1507. 10.1002/glia.20740. [DOI] [PubMed] [Google Scholar]
- Hall P. E.; Lathia J. D.; Miller N. G. A.; Caldwell M. A.; Ffrench-Constant C. Integrins Are Markers of Human Neural Stem Cells. Stem Cells 2006, 24 (9), 2078–2084. 10.1634/stemcells.2005-0595. [DOI] [PubMed] [Google Scholar]
- Flanagan L. A.; Rebaza L. M.; Derzic S.; Schwartz P. H.; Monuki E. S. Regulation of human neural precursor cells by laminin and integrins. J. Neurosci. Res. 2006, 83 (5), 845–856. 10.1002/jnr.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stukel J. M.; Willits R. K. Mechanotransduction of Neural Cells Through Cell-Substrate Interactions. Tissue Eng., Part B 2016, 22 (3), 173–182. 10.1089/ten.teb.2015.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. F.; Hsu W. C.; Chiu W. T.; Wang J. Y. Functional improvement and neurogenesis after collagen-GAG matrix implantation into surgical brain trauma. Biomaterials 2012, 33 (7), 2067–2075. 10.1016/j.biomaterials.2011.11.040. [DOI] [PubMed] [Google Scholar]
- Wang M.; Liu X.; Lyu Z.; Gu H.; Li D.; Chen H. Glycosaminoglycans (GAGs) and GAG mimetics regulate the behavior of stem cell differentiation. Colloids Surf., B 2017, 150, 175–182. 10.1016/j.colsurfb.2016.11.022. [DOI] [PubMed] [Google Scholar]
- Wang M.; et al. A new avenue to the synthesis of GAG-mimicking polymers highly promoting neural differentiation of embryonic stem cells. Chem. Commun. 2015, 51 (84), 15434–15437. 10.1039/C5CC06944K. [DOI] [PubMed] [Google Scholar]
- Horgan C. C.; et al. Characterisation of minimalist co-assembled fluorenylmethyloxycarbonyl self-assembling peptide systems for presentation of multiple bioactive peptides. Acta Biomater. 2016, 38, 11–22. 10.1016/j.actbio.2016.04.038. [DOI] [PubMed] [Google Scholar]
- Yadav N.; Chauhan M. K.; Chauhan V. S. Short to ultrashort peptide-based hydrogels as a platform for biomedical applications. Biomater. Sci. 2020, 8 (1), 84–100. 10.1039/C9BM01304K. [DOI] [PubMed] [Google Scholar]
- Sart S.; Agathos S. N.; Li Y. Engineering stem cell fate with biochemical and biomechanical properties of microcarriers. Biotechnol. Prog. 2013, 29 (6), 1354–1366. 10.1002/btpr.1825. [DOI] [PubMed] [Google Scholar]
- Nilbratt M.; Porras O.; Marutle A.; Hovatta O.; Nordberg A. Neurotrophic factors promote cholinergic differentiation in human embryonic stem cell-derived neurons. J. Cell. Mol. Med. 2010, 14 (6b), 1476–1484. 10.1111/j.1582-4934.2009.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggeman K. F.; Rodriguez A. L.; Parish C. L.; Williams R. J.; Nisbet D. R. Temporally controlled release of multiple growth factors from a self-assembling peptide hydrogel. Nanotechnology 2016, 27 (38), 385102. 10.1088/0957-4484/27/38/385102. [DOI] [PubMed] [Google Scholar]
- Tuszynski M. H.; et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat. Med. 2005, 11 (5), 551–555. 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- Sofroniew M. V; Howe C. L; Mobley W. C Nerve Growth Factor Signaling, Neuroprotection, and Neural Repair. Annu. Rev. Neurosci. 2001, 24 (1), 1217–1281. 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- Hutson T. H.; Di Giovanni S. The translational landscape in spinal cord injury: focus on neuroplasticity and regeneration. Nat. Rev. Neurol. 2019, 15 (12), 732–745. 10.1038/s41582-019-0280-3. [DOI] [PubMed] [Google Scholar]
- Hutson T. H.; et al. Cbp-dependent histone acetylation mediates axon regeneration induced by environmental enrichment in rodent spinal cord injury models. Sci. Transl. Med. 2019, 11 (487), 2064. 10.1126/scitranslmed.aaw2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L.; Rigo J.-M.; Rocher V.; Belachew S.; Malgrange B.; Rogister B.; Leprince P.; Moonen G. Neurotransmitters as early signals for central nervous system development. Cell Tissue Res. 2001, 305 (2), 187–202. 10.1007/s004410000343. [DOI] [PubMed] [Google Scholar]
- Borta A.; Höglinger G. U. Dopamine and adult neurogenesis. J. Neurochem. 2007, 100 (3), 587–595. 10.1111/j.1471-4159.2006.04241.x. [DOI] [PubMed] [Google Scholar]
- Franze K.; Janmey P. A.; Guck J. Mechanics in neuronal development and repair. Annu. Rev. Biomed. Eng. 2013, 15, 227–251. 10.1146/annurev-bioeng-071811-150045. [DOI] [PubMed] [Google Scholar]
- Clarke E. C., Spinal Cord Mechanical Properties, in Studies in Mechanobiology, Tissue Engineering and Biomaterials, Vol. 3, Springer, 2011; pp 25–40. [Google Scholar]
- Nicholson K. J.; Winkelstein B. A.. Nerve and Nerve Root Biomechanics. In Neural Tissue Biomechanics; Studies in Mechanobiology, Tissue Engineering and Biomaterials; Springer, 2011; Vol. 3, pp 203–229. [Google Scholar]
- Sur S.; Newcomb C. J.; Webber M. J.; Stupp S. I. Tuning supramolecular mechanics to guide neuron development. Biomaterials 2013, 34 (20), 4749–4757. 10.1016/j.biomaterials.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A. J.; Sen S.; Sweeney H. L.; Discher D. E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677. 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Flanagan L. A.; Ju Y.-E.; Marg B.; Osterfield M.; Janmey P. A. Neurite branching on deformable substrates. NeuroReport 2002, 13 (18), 2411–2415. 10.1097/00001756-200212200-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J.; Bao M.; Bruekers S. M. C.; Huck W. T. S. Collagen Gels with Different Fibrillar Microarchitectures Elicit Different Cellular Responses. ACS Appl. Mater. Interfaces 2017, 9 (23), 19630–19637. 10.1021/acsami.7b03883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha K.; et al. Substrate modulus directs neural stem cell behavior. Biophys. J. 2008, 95 (9), 4426–4438. 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidlits S. K.; et al. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials 2010, 31 (14), 3930–3940. 10.1016/j.biomaterials.2010.01.125. [DOI] [PubMed] [Google Scholar]
- Moeendarbary E.; Weber I. P.; Sheridan G. K.; Koser D. E.; Soleman S.; Haenzi B.; Bradbury E. J.; Fawcett J.; Franze K.; et al. The soft mechanical signature of glial scars in the central nervous system. Nat. Commun. 2017, 8, 14787. 10.1038/ncomms14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J.; Yang Y.; Liao L.; Zhang C. Matrix stiffness-regulated cellular functions under different dimensionalities. Biomater. Sci. 2020, 8 (10), 2734–2755. 10.1039/C9BM01809C. [DOI] [PubMed] [Google Scholar]
- Vedadghavami A.; Minooei F.; Mohammadi M. H.; Khetani S.; Rezaei Kolahchi A.; Mashayekhan S.; Sanati-Nezhad A. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 2017, 62, 42–63. 10.1016/j.actbio.2017.07.028. [DOI] [PubMed] [Google Scholar]
- Lossada F.; Hoenders D.; Guo J.; Jiao D.; Walther A. Self-Assembled Bioinspired Nanocomposites. Acc. Chem. Res. 2020, 53, 2622. 10.1021/acs.accounts.0c00448. [DOI] [PubMed] [Google Scholar]
- Rashid B.; Destrade M.; Gilchrist M. D. Mechanical characterization of brain tissue in compression at dynamic strain rates. J. Mech. Behav. Biomed. Mater. 2012, 10, 23–38. 10.1016/j.jmbbm.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Lampe K.; Mooney R.; B K. B.-J. Effect of macromer weight percent on neural cell growth in 2D and 3D nondegradable PEG hydrogel culture. J. Biomed. Mater. Res. A 2010, 94, 1162. 10.1002/jbm.a.32787. [DOI] [PubMed] [Google Scholar]
- Niemczyk B.; Sajkiewicz P.; Kolbuk D. Injectable hydrogels as novel materials for central nervous system regeneration. J. Neural Eng. 2018, 15 (5), 051002. 10.1088/1741-2552/aacbab. [DOI] [PubMed] [Google Scholar]
- Sakai S.; Hirose K.; Taguchi K.; Ogushi Y.; Kawakami K. An injectable, in situ enzymatically gellable, gelatin derivative for drug delivery and tissue engineering. Biomaterials 2009, 30 (20), 3371–3377. 10.1016/j.biomaterials.2009.03.030. [DOI] [PubMed] [Google Scholar]
- Chau Y.; et al. Incorporation of a matrix metalloproteinase-sensitive substrate into self-assembling peptides - A model for biofunctional scaffolds. Biomaterials 2008, 29 (11), 1713–1719. 10.1016/j.biomaterials.2007.11.046. [DOI] [PubMed] [Google Scholar]
- Song I.; Dityatev A. Crosstalk between glia, extracellular matrix and neurons. Brain Res. Bull. 2018, 136, 101–108. 10.1016/j.brainresbull.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Rusanescu G.; Mao J. Peripheral nerve injury induces adult brain neurogenesis and remodelling. J. Cell. Mol. Med. 2017, 21 (2), 299–314. 10.1111/jcmm.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A.; et al. Mechanical strength vs. degradation of a biologically-derived surgical mesh over time in a rodent full thickness abdominal wall defect. Biomaterials 2016, 108, 81–90. 10.1016/j.biomaterials.2016.08.053. [DOI] [PubMed] [Google Scholar]
- Lu P.; Takai K.; Weaver V. M.; Werb Z. Extracellular Matrix degradation and remodeling in development and disease. Cold Spring Harbor Perspect. Biol. 2011, 3 (12), a005058 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu E. C.; Zhang S.; Hauser C. A. E. Self-Assembling Peptides as Cell-Interactive Scaffolds. Adv. Funct. Mater. 2012, 22 (3), 456–468. 10.1002/adfm.201101905. [DOI] [Google Scholar]
- Lutolf M. P.; et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc. Natl. Acad. Sci. U. S. A. 2003, 100 (9), 5413–8. 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane T. J.; Londono R.; Turner N. J.; Badylak S. F. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 2012, 33 (6), 1771–1781. 10.1016/j.biomaterials.2011.10.054. [DOI] [PubMed] [Google Scholar]
- Bjugstad K. B.; Lampe K.; Kern D. S.; Mahoney M. Biocompatibility of poly (ethylene glycol)-based hydrogels in the brain: An analysis of the glial response across space and time. J. Biomed. Mater. Res., Part A 2010, 95, 79. 10.1002/jbm.a.32809. [DOI] [PubMed] [Google Scholar]
- Cheng T.-Y.; Chen M.-H.; Chang W.-H.; Huang M.-Y.; Wang T.-W. Neural stem cells encapsulated in a functionalized self-assembling peptide hydrogel for brain tissue engineering. Biomaterials 2013, 34 (8), 2005–2016. 10.1016/j.biomaterials.2012.11.043. [DOI] [PubMed] [Google Scholar]
- Mura S.; Nicolas J.; Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12 (11), 991–1003. 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- Matson J. B.; Stupp S. I. Drug release from hydrazone-containing peptide amphiphiles. Chem. Commun. 2011, 47 (28), 7962–7964. 10.1039/c1cc12570b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha A. K.; et al. Matrix metalloproteinase-13 mediated degradation of hyaluronic acid-based matrices orchestrates stem cell engraftment through vascular integration. Biomaterials 2016, 89, 136–147. 10.1016/j.biomaterials.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey G. S.; Dziki J. L.; Badylak S. F. Extracellular matrix-based materials for regenerative medicine. Nature Reviews Materials 2018, 3 (7), 159–173. 10.1038/s41578-018-0023-x. [DOI] [Google Scholar]
- Budday S.; et al. Mechanical characterization of human brain tissue. Acta Biomater. 2017, 48, 319–340. 10.1016/j.actbio.2016.10.036. [DOI] [PubMed] [Google Scholar]
- Myers R. R. Anatomy and microanatomy of peripheral nerve. Neurosurgery Clinics of North America 1991, 2 (1), 1–20. 10.1016/S1042-3680(18)30753-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perez F.; Udina E.; Navarro X.. Extracellular matrix components in peripheral nerve regeneration. In Tissue Engineering of the Peripheral Nerve: Stem Cells and Regeneration Promoting Factors; International Review of Neurobiology; Elsevier: 2013; Vol. 108, Chapter 10, pp 257–275. 10.1016/B978-0-12-410499-0.00010-1 [DOI] [PubMed] [Google Scholar]
- Brown A. G.Organization in the Spinal Cord: The Anatomy and Physiology of Identified Neurones; Springer, 1981. [Google Scholar]
- Lee M. R.; et al. Direct differentiation of human embryonic stem cells into selective neurons on nanoscale ridge/groove pattern arrays,. Biomaterials 2010, 31 (15), 4360–4366. 10.1016/j.biomaterials.2010.02.012. [DOI] [PubMed] [Google Scholar]
- McBeath R.; Pirone D. M.; Nelson C. M.; Bhadriraju K.; Chen C. S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment,. Dev. Cell 2004, 6 (4), 483–495. 10.1016/S1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- Yang K.; et al. Multiscale, hierarchically patterned topography for directing human neural stem cells into functional neurons,. ACS Nano 2014, 8 (8), 7809–7822. 10.1021/nn501182f. [DOI] [PubMed] [Google Scholar]
- Tonazzini I.; Meucci S.; Faraci P.; Beltram F.; Cecchini M. Neuronal differentiation on anisotropic substrates and the influence ofnanotopographical noise on neurite contact guidance,. Biomaterials 2013, 34 (25), 6027–6036. 10.1016/j.biomaterials.2013.04.039. [DOI] [PubMed] [Google Scholar]
- Panseri S. Electrospun micro- and nanofiber tubes for functional nervous regeneration in sciatic nerve transections. BMC Biotechnol. 2008, 8, 39. 10.1186/1472-6750-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain D.; Mattiassi S.; Goh E.; Yim E. Extracellular matrix and biomimetic engineering microenvironment for neuronal differentiation,. Neural Regener. Res. 2020, 15 (4), 573–585. 10.4103/1673-5374.266907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampaloni F.; Reynaud E. G.; Stelzer E. H. K. The third dimension bridges the gap between cell culture and live tissue,. Nat. Rev. Mol. Cell Biol. 2007, 8 (10), 839–845. 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- Pasca S. P. The rise of three-dimensional human brain cultures. Nature 2018, 553 (7689), 437–445. 10.1038/nature25032. [DOI] [PubMed] [Google Scholar]
- Aregueta-Robles U. A.; Lim K. S.; Martens P. J.; Lovell N. H.; Poole-Warren L. A.; Green R.. Producing 3D neuronal networks in hydrogels for living bionic device interfaces. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS; IEEE: Piscataway, NJ, 2015. [DOI] [PubMed]
- Bertucci C.; Koppes R.; Dumont C.; Koppes A. Neural responses to electrical stimulation in 2D and 3D in vitro environments. Brain Res. Bull. 2019, 152, 265–284. 10.1016/j.brainresbull.2019.07.016. [DOI] [PubMed] [Google Scholar]
- Bissell M. J.; Hall H. G.; Parry G. How does the extracellular matrix direct gene expression?. J. Theor. Biol. 1982, 99 (1), 31–68. 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Haeger A.; Wolf K.; Zegers M. M.; Friedl P. Collective cell migration: guidance principles and hierarchies. Trends Cell Biol. 2015, 25 (9), 556–566. 10.1016/j.tcb.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Stevenson P. M.; Donald A. M. Identification of Three Regimes of Behavior for Cell Attachment on Topographically Patterned Substrates. Langmuir 2009, 25 (1), 367–376. 10.1021/la802859v. [DOI] [PubMed] [Google Scholar]
- Curtis A.; Wilkinson C. Topographical control of cells. Biomaterials 1997, 18, 1573. 10.1016/S0142-9612(97)00144-0. [DOI] [PubMed] [Google Scholar]
- Beduer A.; Vieu C.; Arnauduc F.; Sol J.-C.; Loubinoux I.; Vaysse L. Engineering of adult human neural stem cells differentiation through surface micropatterning. Biomaterials 2012, 33, 504. 10.1016/j.biomaterials.2011.09.073. [DOI] [PubMed] [Google Scholar]
- Martin J. Y.; et al. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J. Biomed. Mater. Res. 1995, 29 (3), 389–401. 10.1002/jbm.820290314. [DOI] [PubMed] [Google Scholar]
- Li F.; Li B.; Wang Q.-M.; Wang J. H.-C. Cell shape regulates collagen type I expression in human tendon fibroblasts. Cell Motil. Cytoskeleton 2008, 65 (4), 332–341. 10.1002/cm.20263. [DOI] [PubMed] [Google Scholar]
- López-Fagundo C.; Mitchel J. A.; Ramchal T. D.; Dingle Y. T. L.; Hoffman-Kim D. Navigating neurites utilize cellular topography of Schwann cell somas and processes for optimal guidance. Acta Biomater. 2013, 9 (7), 7158–7168. 10.1016/j.actbio.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D. Y.; Krebsbach P. H.; Hollister S. J. Brain cortex regeneration affected by scaffold architectures: Laboratory investigation. J. Neurosurg. 2008, 109 (4), 715–722. 10.3171/JNS/2008/109/10/0715. [DOI] [PubMed] [Google Scholar]
- Cooper A.; Bhattarai N.; Zhang M. Fabrication and cellular compatibility of aligned chitosan-PCL fibers for nerve tissue regeneration. Carbohydr. Polym. 2011, 85 (1), 149–156. 10.1016/j.carbpol.2011.02.008. [DOI] [Google Scholar]
- Mashinchian O.; et al. Regulation of stem cell fate by nanomaterial substrates. Nanomedicine 2015, 10 (5), 829–847. 10.2217/nnm.14.225. [DOI] [PubMed] [Google Scholar]
- Franze K. The mechanical control of nervous system development. Development 2013, 140 (15), 3069–3077. 10.1242/dev.079145. [DOI] [PubMed] [Google Scholar]
- Hulsman M.; et al. Analysis of high-throughput screening reveals the effect of surface topographies on cellular morphology. Acta Biomater. 2015, 15, 29–38. 10.1016/j.actbio.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Bugnicourt G.; Brocard J.; Nicolas A.; Villard C. Nanoscale Surface Topography Reshapes Neuronal Growth in Culture. Langmuir 2014, 30, 4441. 10.1021/la5001683. [DOI] [PubMed] [Google Scholar]
- Xie J.; et al. Conductive Core-Sheath Nanofibers and Their Potential Application in Neural Tissue Engineering. Adv. Funct. Mater. 2009, 19 (14), 2312–2318. 10.1002/adfm.200801904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranes K.; et al. Comparing Transcriptome Profiles of Neurons Interfacing Adjacent Cells and Nanopatterned Substrates Reveals Fundamental Neuronal Interactions. Nano Lett. 2019, 19 (3), 1451–1459. 10.1021/acs.nanolett.8b03879. [DOI] [PubMed] [Google Scholar]
- Pan F.; Zhang M.; Wu G.; Lai Y.; Greber B.; Scholer H. R.; Chi L. Topographic effect on human induced pluripotent stem cells differentiation towards neuronal lineage. Biomaterials 2013, 34, 8131. 10.1016/j.biomaterials.2013.07.025. [DOI] [PubMed] [Google Scholar]
- Weightman A.; Jenkins S.; Pickard M.; Chari D.; Yang Y. Alignment of multiple glial cell populations in 3D nanofiber scaffolds: Toward the development of multicellular implantable scaffolds for repair of neural injur,. Nanomedicine 2014, 10 (2), 291–295. 10.1016/j.nano.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Recknor J. B.; Sakaguchi D. S.; Mallapragada S. K. Directed growth and selective differentiation of neural progenitor cells on micropatterned polymer substrates. Biomaterials 2006, 27 (22), 4098–4108. 10.1016/j.biomaterials.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Meco E.; Lampe K. J. Microscale architecture in biomaterial scaffolds for spatial control of neural cell behavior. Front. Mater. 2018, 5, 1. 10.3389/fmats.2018.00002. [DOI] [Google Scholar]
- Hollister S. J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4 (7), 518–524. 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- Keskar V.; Marion N. W.; Mao J. J.; Gemeinhart R. A. In Vitro Evaluation of Macroporous Hydrogels to Facilitate Stem Cell Infiltration, Growth, and Mineralization. Tissue Eng., Part A 2009, 15 (7), 1695–1707. 10.1089/ten.tea.2008.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L. H.; Lai J. H.; Yu S.; Yang F. Dynamic tissue engineering scaffolds with stimuli-responsive macroporosity formation,. Biomaterials 2013, 34 (17), 4251–4258. 10.1016/j.biomaterials.2013.02.051. [DOI] [PubMed] [Google Scholar]
- Mahoney M. J.; Chen R. R.; Tan J.; Mark Saltzman W. The influence of microchannels on neurite growth and architecture. Biomaterials 2005, 26 (7), 771–778. 10.1016/j.biomaterials.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Feng X.; Lu X.; Huang D.; Xing J.; Feng G.; Jin G.; Yi X.; Li L.; Lu Y.; Nie D.; Chen X.; Zhang L.; Gu Z.; Zhang X. 3D Porous Chitosan Scaffolds Suit Survival and Neural Differentiation of Dental Pulp Stem Cells. Cell. Mol. Neurobiol. 2014, 34, 859. 10.1007/s10571-014-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M.; Selberg J.; Rolandi M. Endogenous Bioelectrics in Development, Cancer, and Regeneration: Drugs and Bioelectronic Devices as Electroceuticals for Regenerative Medicine. iScience 2019, 22, 519–533. 10.1016/j.isci.2019.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaig C. D.; Song B.; Rajnicek A. M. Electrical dimensions in cell science,. J. Cell Sci. 2009, 122 (23), 4267–4276. 10.1242/jcs.023564. [DOI] [PubMed] [Google Scholar]
- Kirkby L. A.; Sack G. S.; Firl A.; Feller M. B. A role for correlated spontaneous activity in the assembly of neural circuits. Neuron 2013, 80 (5), 1129–1144. 10.1016/j.neuron.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston D. J.; McLaughlin K. A.; Levin M. Bioelectric controls of cell proliferation: Ion channels, membrane voltage and the cell cycle. Cell Cycle 2009, 8 (21), 3527–3536. 10.4161/cc.8.21.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann H.; Pisano G.; Beltrachini L. Variation in Reported Human Head Tissue Electrical Conductivity Values. Brain Topogr. 2019, 32 (5), 825–858. 10.1007/s10548-019-00710-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latikka J.; Kuurne T.; Eskola H. Conductivity of living intracranial tissues. Phys. Med. Biol. 2001, 46 (6), 1611–1616. 10.1088/0031-9155/46/6/302. [DOI] [PubMed] [Google Scholar]
- Howell B.; Lad S. P.; Grill W. M.; Glorioso J. C. Evaluation of intradural stimulation efficiency and selectivity in a computational model of spinal cord stimulation. PLoS One 2014, 9 (12), e0114938. 10.1371/journal.pone.0114938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayami T.; Iramina K.; Chen X. Effect of external tissue resistivity on threshold level of myelinated nerve fiber. Electron. Commun. Japan 2010, 93 (2), 50–56. 10.1002/ecj.10065. [DOI] [Google Scholar]
- Pelot N. A.; Behrend C. E.; Grill W. M. On the parameters used in finite element modeling of compound peripheral nerves. J. Neural Eng. 2019, 16 (1), 016007. 10.1088/1741-2552/aaeb0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.; Li Y.; Chen J.; Zhou H.; Tan S.. Electrical stimulation elicits neural stem cells activation: New perspectives in CNS repair. Front. Human Neurosci. 2015, 9, 10.3389/fnhum.2015.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X.; Arocena M.; Penninger J.; Gage F. H.; Zhao M.; Song B. PI3K mediated electrotaxis of embryonic and adult neural progenitor cells in the presence of growth factors. Exp. Neurol. 2011, 227 (1), 210–217. 10.1016/j.expneurol.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G.; Li K. The electrically conductive scaffold as the skeleton of stem cell niche in regenerative medicine. Mater. Sci. Eng., C 2014, 45, 671–681. 10.1016/j.msec.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Maziarz A. How electromagnetic fields can influence adult stem cells: Positive and negative impacts. Stem Cell Res. Ther. 2016, 7 (1), 54. 10.1186/s13287-016-0312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrivikraman G.; Boda S. K.; Basu B. Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: A tissue engineering perspective. Biomaterials 2018, 150, 60–86. 10.1016/j.biomaterials.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Zhu R.; Sun Z.; Li C.; Ramakrishna S.; Chiu K.; He L. Electrical stimulation affects neural stem cell fate and function in vitro. Exp. Neurol. 2019, 319, 112963. 10.1016/j.expneurol.2019.112963. [DOI] [PubMed] [Google Scholar]
- Markx G. H. The use of electric fields in tissue engineering: A review. Organogenesis 2008, 4 (1), 11–17. 10.4161/org.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M.; Stevenson C. G. Regulation of cell behavior and tissue patterning by bioelectrical signals: Challenges and opportunities for biomedical engineering. Annu. Rev. Biomed. Eng. 2012, 14 (1), 295–323. 10.1146/annurev-bioeng-071811-150114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariza C. A.; et al. The Influence of Electric Fields on Hippocampal Neural Progenitor Cells. Stem Cell Rev. Reports 2010, 6 (4), 585–600. 10.1007/s12015-010-9171-0. [DOI] [PubMed] [Google Scholar]
- Yuk H.; Lu B.; Zhao X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019, 48 (6), 1642–1667. 10.1039/C8CS00595H. [DOI] [PubMed] [Google Scholar]
- Papadimitriou L.; Manganas P.; Ranella A.; Stratakis E. Biofabrication for neural tissue engineering applications. Mater. Today Bio 2020, 6, 100043. 10.1016/j.mtbio.2020.100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Gong C.; Yuan X.; Wei G. Controlling the self-assembly of biomolecules into functional nanomaterials through internal interactions and external stimulations: A review. Nanomaterials 2019, 9 (2), 285. 10.3390/nano9020285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop K. J. M.; Wilmer C. E.; Soh S.; Grzybowski B. A. Nanoscale forces and their uses in self-assembly. Small 2009, 5 (14), 1600–1630. 10.1002/smll.200900358. [DOI] [PubMed] [Google Scholar]
- Hendricks M. P.; Sato K.; Palmer L. C.; Stupp S. I. Supramolecular Assembly of Peptide Amphiphiles. Acc. Chem. Res. 2017, 50, 2440–2448. 10.1021/acs.accounts.7b00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekiz M. S.; Cinar G.; Khalily M. A.; Guler M. O Self-assembled peptide nanostructures for functional materials. Nanotechnology 2016, 27 (40), 402002. 10.1088/0957-4484/27/40/402002. [DOI] [PubMed] [Google Scholar]
- Dhotel A.; Chen Z.; Delbreilh L.; Youssef B.; Saiter J. M.; Tan L. Molecular motions in functional self-assembled nanostructures. Int. J. Mol. Sci. 2013, 14 (2), 2303–2333. 10.3390/ijms14022303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.; et al. A self-assembly pathway to aligned monodomain gels. Nat. Mater. 2010, 9 (7), 594–601. 10.1038/nmat2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z.; et al. Co-assembly of Peptide Amphiphiles and Lipids into Supramolecular Nanostructures Driven by Anion-π Interactions. J. Am. Chem. Soc. 2017, 139 (23), 7823–7830. 10.1021/jacs.7b02058. [DOI] [PubMed] [Google Scholar]
- Leite D.; Barbu E.; Pilkington G.; Lalatsa A. Peptide Self-Assemblies for Drug Delivery. Curr. Top. Med. Chem. 2015, 15 (22), 2277–2289. 10.2174/1568026615666150605120456. [DOI] [PubMed] [Google Scholar]
- Bogunia M.; Makowski M. Influence of Ionic Strength on Hydrophobic Interactions in Water: Dependence on Solute Size and Shape. J. Phys. Chem. B 2020, 124, 10326. 10.1021/acs.jpcb.0c06399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwari F.; Shoji Y.; Fukushima T. Supramolecular scaffolds enabling the controlled assembly of functional molecular units. Chem. Sci. 2018, 9 (8), 2028–2041. 10.1039/C7SC04340F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.; Jeong S. M.; Park J. W. Electrical switching between vesicles and micelles via redox-responsive self-assembly of amphiphilic rod-coils. J. Am. Chem. Soc. 2011, 133 (14), 5206–5209. 10.1021/ja200297j. [DOI] [PubMed] [Google Scholar]
- Long K.; Liu Y.; Li Y.; Wang W. Self-assembly of trigonal building blocks into nanostructures: molecular design and biomedical applications. J. Mater. Chem. B 2020, 8 (31), 6739–6752. 10.1039/D0TB01128B. [DOI] [PubMed] [Google Scholar]
- Patel M.; Moon H. J.; Jung B. K.; Jeong B. Microsphere-Incorporated Hybrid Thermogel for Neuronal Differentiation of Tonsil Derived Mesenchymal Stem Cells. Adv. Healthcare Mater. 2015, 4 (10), 1565–1574. 10.1002/adhm.201500224. [DOI] [PubMed] [Google Scholar]
- Stephanopoulos N.; et al. Bioactive DNA-Peptide Nanotubes Enhance the Differentiation of Neural Stem Cells Into Neurons. Nano Lett. 2015, 15 (1), 603–609. 10.1021/nl504079q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R.; et al. Tuning the mechanical and morphological properties of self-assembled peptide hydrogels via control over the gelation mechanism through regulation of ionic strength and the rate of pH change. RSC Adv. 2015, 5 (1), 301–307. 10.1039/C4RA13266A. [DOI] [Google Scholar]
- Stephanopoulos N.; Ortony J. H.; Stupp S. I. Self-assembly for the synthesis of functional biomaterials. Acta Mater. 2013, 61 (3), 912–930. 10.1016/j.actamat.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C.; Ji W.; Xing R.; Li J.; Gazit E.; Yan X. Hierarchically oriented organization in supramolecular peptide crystals. Nat. Rev. Chem. 2019, 3 (10), 567–588. 10.1038/s41570-019-0129-8. [DOI] [Google Scholar]
- Stephanopoulos N.; et al. Bioactive DNA-peptide nanotubes enhance the differentiation of neural stem cells into neurons. Nano Lett. 2015, 15 (1), 603–609. 10.1021/nl504079q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B.; Yuan X.; Jiang D. Molecular self-assembly guides the fabrication of peptide nanofiber scaffolds for nerve repair. RSC Adv. 2014, 4 (45), 23610–23621. 10.1039/C4RA01826E. [DOI] [Google Scholar]
- Nagarkar R. P.; Schneider J. P. Synthesis and primary characterization of self-assembled peptide-based hydrogels. Methods Mol. Biol. 2008, 474, 61–77. 10.1007/978-1-59745-480-3_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns E. J.; et al. Aligned neurite outgrowth and directed cell migration in self-assembled monodomain gels. Biomaterials 2014, 35 (1), 185–195. 10.1016/j.biomaterials.2013.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J. J.; McClendon M. T.; Stephanopoulos N.; Álvarez Z.; Stupp S. I.; Richter C.-P. Electrophysiological assessment of a peptide amphiphile nanofiber nerve graft for facial nerve repair. J. Tissue Eng. Regener. Med. 2018, 12 (6), 1389–1401. 10.1002/term.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Zhu Y.; Liu H.; Wang L. Tailoring DNA Self-assembly to Build Hydrogels. Top. Curr. Chem. 2020, 378 (2), 32. 10.1007/s41061-020-0295-7. [DOI] [PubMed] [Google Scholar]
- Lattuada E.; et al. DNA-GEL, Novel Nanomaterial for Biomedical Applications and Delivery of Bioactive Molecules. Front. Pharmacol. 2020, 11, 1–13. 10.3389/fphar.2020.01345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa M.; et al. Injectable, self-gelling, biodegradable, and immunomodulatory DNA hydrogel for antigen delivery. J. Controlled Release 2014, 180 (1), 25–32. 10.1016/j.jconrel.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Guo J.; et al. Reknitting the injured spinal cord by self-assembling peptide nanofiber scaffold. Nanomedicine 2007, 3 (4), 311–321. 10.1016/j.nano.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Tran K. A.; Partyka P. P.; Jin Y.; Bouyer J.; Fischer I.; Galie P. A. Vascularization of self-assembled peptide scaffolds for spinal cord injury repair. Acta Biomater. 2020, 104, 76–84. 10.1016/j.actbio.2019.12.033. [DOI] [PubMed] [Google Scholar]
- Panda J. J.; Chauhan V. S. Short peptide based self-assembled nanostructures: Implications in drug delivery and tissue engineering. Polym. Chem. 2014, 5 (15), 4418–4436. 10.1039/C4PY00173G. [DOI] [Google Scholar]
- Alakpa E. V.; et al. Tunable Supramolecular Hydrogels for Selection of Lineage-Guiding Metabolites in Stem Cell Cultures. Chem. 2016, 1 (2), 298–319. 10.1016/j.chempr.2016.07.001. [DOI] [Google Scholar]
- Ji W.; Álvarez Z.; Edelbrock A. N.; Sato K.; Stupp S. I. Bioactive Nanofibers Induce Neural Transdifferentiation of Human Bone Marrow Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2018, 10 (48), 41046–41055. 10.1021/acsami.8b13653. [DOI] [PubMed] [Google Scholar]
- Hoang Thi T. T.; Sinh L. H.; Huynh D. P.; Nguyen D. H.; Huynh C. Self-Assemblable Polymer Smart-Blocks for Temperature-Induced Injectable Hydrogel in Biomedical Applications. Front. Chem. 2020, 8, 1–23. 10.3389/fchem.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapter 14 Rules for the Manufacture, Testing and Certification of Materials. Rules and Regulations for the Classification of Naval Ships; Lloyd’s Register, 2000; pp 1–35.
- Habila N.; Kulkarni K.; Lee T.-H.; Al-Garawi Z. S.; Serpell L. C.; Aguilar M.-I.; Del Borgo M. P.; et al. Transition of Nano-Architectures Through Self-Assembly of Lipidated β3-Tripeptide Foldamers. Front. Chem. 2020, 8, 217. 10.3389/fchem.2020.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zottig X.; Al-Halifa S.; Babych M.; Quittot N.; Archambault D.; Bourgault S. Guiding the Morphology of Amyloid Assemblies by Electrostatic Capping: from Polymorphic Twisted Fibrils to Uniform Nanorods. Small 2019, 15 (33), e1901806. 10.1002/smll.201901806. [DOI] [PubMed] [Google Scholar]
- Matsuurua K. Rational design of self-assembled proteins and peptides for nano- and micro-sized architectures. RSC Adv. 2014, 4 (6), 2942–2953. 10.1039/C3RA45944F. [DOI] [Google Scholar]
- Ghosh A.; Haverick M.; Stump K.; Yang X.; Tweedle M. F.; Goldberger J. E. Fine-Tuning the pH Trigger of Self-Assembly. J. Am. Chem. Soc. 2012, 134 (8), 3647–3650. 10.1021/ja211113n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G.-h.; Shao S.-j.; Yang J.-j.; Liu J.-r.; Guo H.-d. Designer Self-Assemble Peptides Maximize the Therapeutic Benefits of Neural Stem Cell Transplantation for Alzheimer’s Disease via Enhancing Neuron Differentiation and Paracrine Action. Mol. Neurobiol. 2016, 53 (2), 1108–1123. 10.1007/s12035-014-9069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H.; Muraoka T.; Cheetham A. G.; Stupp S. I. Self-Assembly of Giant Peptide Nanobelts. Nano Lett. 2009, 9 (3), 945–951. 10.1021/nl802813f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. C.; Cámara-Torres M.; Rahimi K.; Köhler J.; Möller M.; De Laporte L. Nerve Cells Decide to Orient inside an Injectable Hydrogel with Minimal Structural Guidance. Nano Lett. 2017, 17 (6), 3782–3791. 10.1021/acs.nanolett.7b01123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinde Z. O.; van der Heijden N. J.; Domigan L. J.; McGillivray D. J.; Williams D. E. Aligned assembly in a 2-D gel of a water-soluble peptide. Langmuir 2020, 36, 11292. 10.1021/acs.langmuir.0c01944. [DOI] [PubMed] [Google Scholar]
- Pappas C. G.; et al. Alignment of nanostructured tripeptide gels by directional ultrasonication. Chem. Commun. 2015, 51 (40), 8465–8468. 10.1039/C5CC02049B. [DOI] [PubMed] [Google Scholar]
- Zhang S.; et al. A self-assembly pathway to aligned monodomain gels-supp materials. Nat. Mater. 2010, 9 (7), 594–601. 10.1038/nmat2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S.; Song A.; Hao J. Self-assembled structures of amphiphiles regulated via implanting external stimuli. RSC Adv. 2014, 4 (79), 41864–41875. 10.1039/C4RA04849K. [DOI] [Google Scholar]
- Makam P.; Gazit E. Minimalistic peptide supramolecular co-assembly: Expanding the conformational space for nanotechnology,. Chem. Soc. Rev. 2018, 47 (10), 3406–3420. 10.1039/C7CS00827A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing P.; Li P.; Chen H.; Hao A.; Zhao Y. Understanding Pathway Complexity of Organic Micro/Nanofiber Growth in Hydrogen-Bonded Coassembly of Aromatic Amino Acids. ACS Nano 2017, 11 (4), 4206–4216. 10.1021/acsnano.7b01161. [DOI] [PubMed] [Google Scholar]
- Shen Z.; Wang T.; Liu M. H-bond and π-π stacking directed self-assembly of two-component supramolecular nanotubes: Tuning length, diameter and wall thickness. Chem. Commun. 2014, 50 (17), 2096–2099. 10.1039/c3cc48350a. [DOI] [PubMed] [Google Scholar]
- Berns E. J.; et al. A tenascin-C mimetic peptide amphiphile nanofiber gel promotes neurite outgrowth and cell migration of neurosphere-derived cells. Acta Biomater. 2016, 37, 50–58. 10.1016/j.actbio.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese R.; Fontana F.; Marchini A.; Gelain F. Branched peptides integrate into self-assembled nanostructures and enhance biomechanics of peptidic hydrogels. Acta Biomater. 2018, 66, 258–271. 10.1016/j.actbio.2017.11.026. [DOI] [PubMed] [Google Scholar]
- Sieminski A. L.; Was A. S.; Kim G.; Gong H.; Kamm R. D. The stiffness of three-dimensional ionic self-assembling peptide gels affects the extent of capillary-like network formation. Cell Biochem. Biophys. 2007, 49 (2), 73–83. 10.1007/s12013-007-0046-1. [DOI] [PubMed] [Google Scholar]
- Hogrebe N. J.; et al. Independent control of matrix adhesiveness and stiffness within a 3D self-assembling peptide hydrogel. Acta Biomater. 2018, 70, 110–119. 10.1016/j.actbio.2018.01.031. [DOI] [PubMed] [Google Scholar]
- Pugliese R.; Moretti L.; Maiuri M.; Romanazzi T.; Cerullo G.; Gelain F. Superior mechanical and optical properties of a heterogeneous library of cross-linked biomimetic self-assembling peptides. Mater. Des. 2020, 194, 108901. 10.1016/j.matdes.2020.108901. [DOI] [Google Scholar]
- Vandenberg M. A.; Sahoo J. K.; Zou L.; McCarthy W.; Webber M. J. Divergent Self-Assembly Pathways to Hierarchically Organized Networks of Isopeptide-Modified Discotics under Kinetic Control. ACS Nano 2020, 14 (5), 5491–5505. 10.1021/acsnano.9b09610. [DOI] [PubMed] [Google Scholar]
- Dang-I A. Y.; et al. Antimicrobial Activity with Enhanced Mechanical Properties in Phenylalanine-Based Chiral Coassembled Hydrogels: The Influence of Pyridine Hydrazide Derivatives. ACS Appl. Bio Mater. 2020, 3 (4), 2295–2304. 10.1021/acsabm.0c00075. [DOI] [PubMed] [Google Scholar]
- Vybornyi M.; Wijnands S.; Jeon B.-J.; Saleh O.; Meijer E. W.. A DNA-Small Molecule Conjugate Modulates the Complexity of Multicomponent Supramolecular Polymerization in Biorelevant Environments ChemRxviv, 2020, 10.26434/chemrxiv.12845930.v1. [DOI] [Google Scholar]
- Greenfield M. A.; Hoffman J. R.; Olvera de la Cruz M.; Stupp S. I. Tunable mechanics of peptide nanofiber gels. Langmuir 2010, 26 (5), 3641–3647. 10.1021/la9030969. [DOI] [PubMed] [Google Scholar]
- Clarke D. E.; Parmenter C. D. J.; Scherman O. A. Tunable Pentapeptide Self-Assembled β-Sheet Hydrogels,. Angew. Chem., Int. Ed. 2018, 57 (26), 7709–7713. 10.1002/anie.201801001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude S.; Ingham E.; Aggeli A. Biomimetic self-assembling peptides as scaffolds for soft tissue engineering. Nanomedicine 2013, 8 (5), 823–847. 10.2217/nnm.13.65. [DOI] [PubMed] [Google Scholar]
- Scelsi A.; et al. Tuning of hydrogel stiffness using a two-component peptide system for mammalian cell culture,. J. Biomed. Mater. Res., Part A 2019, 107 (3), 535–544. 10.1002/jbm.a.36568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckes K. M.; Baek K.; Suggs L. J. Design and Evaluation of Short Self-Assembling Depsipeptides as Bioactive and Biodegradable Hydrogels. ACS Omega 2018, 3 (2), 1635–1644. 10.1021/acsomega.7b01641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese R.; Fontana F.; Marchini A.; Gelain F. Branched peptides integrate into self-assembled nanostructures and enhance biomechanics of peptidic hydrogels. Acta Biomater. 2018, 66, 258–271. 10.1016/j.actbio.2017.11.026. [DOI] [PubMed] [Google Scholar]
- Chakroun R. W.; et al. Supramolecular Design of Unsymmetric Reverse Bolaamphiphiles for Cell-Sensitive Hydrogel Degradation and Drug Release. Angew. Chem., Int. Ed. 2020, 59 (11), 4434–4442. 10.1002/anie.201913087. [DOI] [PubMed] [Google Scholar]
- Tian Y. F.; Hudalla G. A.; Han H.; Collier J. H. Controllably degradable β-sheet nanofibers and gels from self-assembling depsipeptides. Biomater. Sci. 2013, 1 (10), 1037–1045. 10.1039/c3bm60161g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho J. Y. Dual self-assembly of supramolecular peptide nanotubes to provide stabilisation in water. Nat. Commun. 2019, 10 (1), 4708. 10.1038/s41467-019-12586-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Luca A. C.; Lacour S. P.; Raffoul W.; di Summa P. G. Extracellular matrix components in peripheral nerve repair: How to affect neural cellular response and nerve regeneration?. Neural Regen. Res. 2014, 9 (22), 1943–1948. 10.4103/1673-5374.145366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo P. M.; et al. Biologic scaffolds composed of central nervous system extracellular matrix,. Biomaterials 2012, 33 (13), 3539–3547. 10.1016/j.biomaterials.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A.; Ahmed S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. 10.1016/j.ijbiomac.2017.12.078. [DOI] [PubMed] [Google Scholar]
- Van Vlierberghe S.; Dubruel P.; Schacht E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12 (5), 1387–1408. 10.1021/bm200083n. [DOI] [PubMed] [Google Scholar]
- Zhou M.; et al. Self-assembled peptide-based hydrogels as scaffolds for anchorage-dependent cells. Biomaterials 2009, 30 (13), 2523–2530. 10.1016/j.biomaterials.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Tysseling-Mattiace V. M.; Sahni V.; Niece K. L.; Birch D.; Czeisler C.; Fehlings M. G.; Stupp S. I.; Kessler J. A. Self-Assembling Nanofibers Inhibit Glial Scar Formation and Promote Axon Elongation after Spinal Cord Injury. J. Neurosci. 2008, 28, 3814. 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.; et al. Self-assembling nanofibers improve cognitive impairment in a transgenic mice model of Alzheimer’s disease. Neurosci. Lett. 2013, 556, 63–68. 10.1016/j.neulet.2013.09.063. [DOI] [PubMed] [Google Scholar]
- Li Q.; Chau Y. Neural differentiation directed by self-assembling peptide scaffolds presenting laminin-derived epitopes. J. Biomed. Mater. Res. Part A 2010, 9999 (3), 688. 10.1002/jbm.a.32707. [DOI] [PubMed] [Google Scholar]
- Goktas M.; Cinar G.; Orujalipoor I.; Ide S.; Tekinay A. B.; Guler M. O. Self-Assembled Peptide Amphiphile Nanofibers and PEG Composite Hydrogels as Tunable ECM Mimetic Microenvironment. Biomacromolecules 2015, 16 (4), 1247–1258. 10.1021/acs.biomac.5b00041. [DOI] [PubMed] [Google Scholar]
- Hartgerink J. D.; Beniash E.; Stupp S. I. Peptide-amphiphile nanofibers: A versatile scaffold for the preparation of self-assembling materials. Proc. Natl. Acad. Sci. U. S. A. 2002, 99 (8), 5133–5138. 10.1073/pnas.072699999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington D. A.; Cheng E. Y.; Guler M. O.; Lee L. K.; Donovan J. L.; Claussen R. C.; Stupp S. I.; et al. Branched peptide-amphiphiles as self-assembling coatings for tissue engineering scaffolds. J. Biomed. Mater. Res., Part A 2006, 78 (1), 157–167. 10.1002/jbm.a.30718. [DOI] [PubMed] [Google Scholar]
- Cunha C.; Panseri S.; Villa O.; Silva D.; Gelain F. 3D culture of adult mouse neural stem cells within functionalized self-assembling peptide scaffolds. Int. J. Nanomed. 2011, 6, 943–955. 10.2147/IJN.S17292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrendt R.; White P.; Offer J. Advances in Fmoc solid-phase peptide synthesis. J. Pept. Sci. 2016, 22 (1), 4–27. 10.1002/psc.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders A. M.; Kale T. S.; Katz H. E.; Tovar J. D. Solid-Phase Synthesis of Self-Assembling Multivalent π-Conjugated Peptides. ACS Omega 2017, 2 (2), 409–419. 10.1021/acsomega.6b00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva G. A.; et al. Selective Differentiation of Neural Progenitor Cells by High-Epitope Density Nanofibers. Science (Washington, DC, U. S.) 2004, 303 (5662), 1352–1355. 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- Cui H.; Webber M. J.; Stupp S. I. Self-assembly of peptide amphiphiles: From molecules to nanostructures to biomaterials. Biopolymers 2010, 94 (1), 1–18. 10.1002/bip.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tysseling-Mattiace V. M.; Sahni V.; Niece K. L.; Birch D.; Czeisler C.; Fehlings M. G.; Stupp S. I.; Kessler J. A. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J. Neurosci. 2008, 28, 3814. 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galler K. M.; Aulisa L.; Regan K. R.; D’Souza R. N.; Hartgerink J. D. Self-assembling multidomain peptide hydrogels: Designed susceptibility to enzymatic cleavage allows enhanced cell migration and spreading. J. Am. Chem. Soc. 2010, 132 (9), 3217–3223. 10.1021/ja910481t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur S.; Tantakitti F.; Matson J. B.; Stupp S. I. Epitope topography controls bioactivity in supramolecular nanofibers. Biomater. Sci. 2015, 3 (3), 520–532. 10.1039/C4BM00326H. [DOI] [PubMed] [Google Scholar]
- Tayalia P.; Mooney D. J. Controlled growth factor delivery for tissue engineering. Adv. Mater. 2009, 21 (32–33), 3269–3285. 10.1002/adma.200900241. [DOI] [PubMed] [Google Scholar]
- Miller R. E.; Kopesky P. W.; Grodzinsky A. J. Growth factor delivery through self-assembling peptide scaffolds. Clin. Orthop. Relat. Res. 2011, 469 (10), 2716–2724. 10.1007/s11999-011-1891-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A. L.; et al. Using minimalist self-assembling peptides as hierarchical scaffolds to stabilise growth factors and promote stem cell integration in the injured brain. J. Tissue Eng. Regen. Med. 2018, 12 (3), e1571 10.1002/term.2582. [DOI] [PubMed] [Google Scholar]
- Gelain F.; Unsworth L. D.; Zhang S. Slow and sustained release of active cytokines from self-assembling peptide scaffolds. J. Controlled Release 2010, 145 (3), 231–239. 10.1016/j.jconrel.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Matson J. B.; Zha R. H.; Stupp S. I. Peptide self-assembly for crafting functional biological materials. Curr. Opin. Solid State Mater. Sci. 2011, 15 (6), 225–235. 10.1016/j.cossms.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R. N.; Shah N. A.; Del Rosario Lim M. M.; Hsieh C.; Nuber G.; Stupp S. I. Supramolecular design of self-assembling nanofibers for cartilage regeneration. Proc. Natl. Acad. Sci. U. S. A. 2010, 107 (8), 3293–3298. 10.1073/pnas.0906501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelain F.; Bottai D.; Vescovi A.; Zhang S. Designer Self-Assembling Peptide Nanofiber Scaffolds for Adult Mouse Neural Stem Cell 3-Dimensional Cultures. PLoS One 2006, 1 (1), e119 10.1371/journal.pone.0000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers V. F. M.; Lee R. T. Local delivery of proteins and the use of self-assembling peptides. Drug Discovery Today 2007, 12 (13–14), 561–568. 10.1016/j.drudis.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Elliott Donaghue I.; Tam R.; Sefton M. V.; Shoichet M. S. Cell and biomolecule delivery for tissue repair and regeneration in the central nervous system. J. Controlled Release 2014, 190, 219–227. 10.1016/j.jconrel.2014.05.040. [DOI] [PubMed] [Google Scholar]
- Gelse K.; Von der Mark K.; Aigner T.; Park J.; Schneider H. Articular cartilage repair by gene therapy using growth factor-producing mesenchymal cells. Arthritis Rheum. 2003, 48 (2), 430–441. 10.1002/art.10759. [DOI] [PubMed] [Google Scholar]
- Branco M. C.; Pochan D. J.; Wagner N. J.; Schneider J. P. The effect of protein structure on their controlled release from an injectable peptide hydrogel. Biomaterials 2010, 31 (36), 9527–9534. 10.1016/j.biomaterials.2010.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsopoulos S.; Unsworth L. D.; Nagai Y.; Zhang S. Controlled release of functional proteins through designer self-assembling peptide nanofiber hydrogel scaffold. Proc. Natl. Acad. Sci. U. S. A. 2009, 106 (12), 4623–4628. 10.1073/pnas.0807506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X.; et al. Development of self-assembling peptide nanovesicle with bilayers for enhanced EGFR-targeted drug and gene delivery. Biomaterials 2016, 82, 194–207. 10.1016/j.biomaterials.2015.12.015. [DOI] [PubMed] [Google Scholar]
- Goel R.; Gopal S.; Gupta A. Self-assembly of β-alanine homotetramer: Formation of nanovesicles for drug delivery. J. Mater. Chem. B 2015, 3 (28), 5849–5857. 10.1039/C5TB00652J. [DOI] [PubMed] [Google Scholar]
- Jun H. W.; Yuwono V.; Paramonov S. E.; Hartgerink J. D. Enzyme-mediated degradation of peptide-amphiphile nanofiber networks. Adv. Mater. 2005, 17 (21), 2612–2617. 10.1002/adma.200500855. [DOI] [Google Scholar]
- Wang H.; Lv L.; Xu G.; Yang C.; Sun J.; Yang Z. Molecular hydrogelators consist of Taxol and short peptides/amino acids. J. Mater. Chem. 2012, 22 (33), 16933–16938. 10.1039/c2jm32203j. [DOI] [Google Scholar]
- Sun Y.; et al. Functional Self-Assembling Peptide Nanofiber Hydrogels Designed for Nerve Degeneration. ACS Appl. Mater. Interfaces 2016, 8 (3), 2348–2359. 10.1021/acsami.5b11473. [DOI] [PubMed] [Google Scholar]
- Zhang N.; He L.; Wu W. Self-assembling peptide nanofibrous hydrogel as a promising strategy in nerve repair after traumatic injury in the nervous system. Neural Regener. Res. 2016, 11 (5), 717–718. 10.4103/1673-5374.182687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsopoulos S.; Zhang S. Long-term three-dimensional neural tissue cultures in functionalized self-Assembling peptide hydrogels, Matrigel and Collagen I. Acta Biomater. 2013, 9 (2), 5162–5169. 10.1016/j.actbio.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Briuglia M. L.; Urquhart A. J.; Lamprou D. A. Sustained and controlled release of lipophilic drugs from a self-assembling amphiphilic peptide hydrogel. Int. J. Pharm. 2014, 474 (1–2), 103–111. 10.1016/j.ijpharm.2014.08.025. [DOI] [PubMed] [Google Scholar]
- McIntyre C. C.; Grill W. M.; Sherman D. L.; Thakor N. V. Cellular Effects of Deep Brain Stimulation: Model-Based Analysis of Activation and Inhibition. J. Neurophysiol. 2004, 91 (4), 1457–1469. 10.1152/jn.00989.2003. [DOI] [PubMed] [Google Scholar]
- Jackson A.; Zimmermann J. B. Neural interfaces for the brain and spinal cord - Restoring motor function. Nat. Rev. Neurol. 2012, 8 (12), 690–699. 10.1038/nrneurol.2012.219. [DOI] [PubMed] [Google Scholar]
- Zhang A.; Lieber C. M. Nano-Bioelectronics. Chem. Rev. 2016, 116 (1), 215–257. 10.1021/acs.chemrev.5b00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivnay J.; Owens R. M.; Malliaras G. G. The rise of organic bioelectronics. Chem. Mater. 2014, 26 (1), 679–685. 10.1021/cm4022003. [DOI] [Google Scholar]
- Goding J.; Gilmour A.; Martens P.; Poole-Warren L.; Green R. Interpenetrating Conducting Hydrogel Materials for Neural Interfacing Electrodes. Adv. Healthcare Mater. 2017, 6 (9), 1601177. 10.1002/adhm.201601177. [DOI] [PubMed] [Google Scholar]
- Green R.; Abidian M. R. Conducting Polymers for Neural Prosthetic and Neural Interface Applications. Adv. Mater. (Weinheim, Ger.) 2015, 27, 7620. 10.1002/adma.201501810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppes A. N.; et al. Robust neurite extension following exogenous electrical stimulation within single walled carbon nanotube-composite hydrogels. Acta Biomater. 2016, 39, 34–43. 10.1016/j.actbio.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X.; Xiao Y.; Xiao H.; Harris G.; Wang T.; Che J. Topographic guidance based on microgrooved electroactive composite films for neural interface. Colloids Surf., B 2016, 145, 768–776. 10.1016/j.colsurfb.2016.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noshadi I.; et al. Engineering Biodegradable and Biocompatible Bio-ionic Liquid Conjugated Hydrogels with Tunable Conductivity and Mechanical Properties. Sci. Rep. 2017, 7 (1), 4345. 10.1038/s41598-017-04280-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H. T.; et al. Electric field stimulation through a biodegradable polypyrrole-co- polycaprolactone substrate enhances neural cell growth. J. Biomed. Mater. Res., Part A 2014, 102 (8), 2554–2564. 10.1002/jbm.a.34925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J. G.; et al. Into the groove: Instructive silk-polypyrrole films with topographical guidance cues direct DRG neurite outgrowth. J. Biomater. Sci., Polym. Ed. 2015, 26 (17), 1327–1342. 10.1080/09205063.2015.1090181. [DOI] [PubMed] [Google Scholar]
- Hwang J. Y.; Shin U. S.; Jang W. C.; Hyun J. K.; Wall I. B.; Kim H. W. Biofunctionalized carbon nanotubes in neural regeneration: A mini-review. Nanoscale 2013, 5 (2), 487–497. 10.1039/C2NR31581E. [DOI] [PubMed] [Google Scholar]
- Redondo-Gómez C.; Leandro-Mora R.; Blanch-Bermúdez D.; Espinoza-Araya C.; Hidalgo-Barrantes D.; Vega-Baudrit J. Recent Advances in Carbon Nanotubes for Nervous Tissue Regeneration. Adv. Polym. Technol. 2020, 2020, 1. 10.1155/2020/6861205. [DOI] [Google Scholar]
- Khan M. A.; Cantù E.; Tonello S.; Serpelloni M.; Lopomo N. F.; Sardini E. A review on biomaterials for 3D conductive scaffolds for stimulating and monitoring cellular activities. Appl. Sci. 2019, 9 (5), 961. 10.3390/app9050961. [DOI] [Google Scholar]
- Green R. A.; et al. Conductive Hydrogels: Mechanically Robust Hybrids for Use as Biomaterials. Macromol. Biosci. 2012, 12 (4), 494–501. 10.1002/mabi.201100490. [DOI] [PubMed] [Google Scholar]
- Balint R.; Cassidy N. J.; Cartmell S. H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10 (6), 2341–2353. 10.1016/j.actbio.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Goding J. A.; Gilmour A. D.; Aregueta-Robles U. A.; Hasan E. A.; Green R. A. Living Bioelectronics: Strategies for Developing an Effective Long-Term Implant with Functional Neural Connections. Adv. Funct. Mater. 2017, 1702969, 1702969. 10.1002/adfm.201702969. [DOI] [Google Scholar]
- Green R.; Lovell N.; Wallace G.; P.-W L. Conducting polymers for neural interfaces: challenges in developing an effective long-term implant. Biomaterials 2008, 29, 3393. 10.1016/j.biomaterials.2008.04.047. [DOI] [PubMed] [Google Scholar]
- Vallejo-Giraldo C.; Kelly A.; Biggs M. J. P. Biofunctionalisation of electrically conducting polymers. Drug Discovery Today 2014, 19 (1), 88–94. 10.1016/j.drudis.2013.07.022. [DOI] [PubMed] [Google Scholar]
- Green R. A.; Lovell N. H.; Wallace G. G.; Poole-Warren L. A. Conducting polymers for neural interfaces: Challenges in developing an effective long-term implant. Biomaterials 2008, 29, 3393. 10.1016/j.biomaterials.2008.04.047. [DOI] [PubMed] [Google Scholar]
- Green R. A.; Baek S.; Poole-Warren L. A.; Martens P. J. Conducting polymer-hydrogels for medical electrode applications. Sci. Technol. Adv. Mater. 2010, 11 (1), 014107. 10.1088/1468-6996/11/1/014107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Q.; Lei W.; Liu M. Conductive Hydrogels as Smart Materials for Flexible Electronic Devices. Chem. - Eur. J. 2018, 24 (64), 16930–16943. 10.1002/chem.201801302. [DOI] [PubMed] [Google Scholar]
- Mawad D.; Lauto A.; Wallace G. G.. Conductive Polymer Hydrogels; Springer: Cham, Switzerland, 2016; pp 19–44. [Google Scholar]
- He Q.; Cui Y.; Ai S.; Tian Y.; Li J. Self-assembly of composite nanotubes and their applications. Curr. Opin. Colloid Interface Sci. 2009, 14 (2), 115–125. 10.1016/j.cocis.2008.09.005. [DOI] [Google Scholar]
- Han T. H.; Lee W. J.; Lee D. H.; Kim J. E.; Choi E. Y.; Kim S. O. Peptide/graphene hybrid assembly into core/shell nanowires. Adv. Mater. 2010, 22 (18), 2060–2064. 10.1002/adma.200903221. [DOI] [PubMed] [Google Scholar]
- Deng Z.; Wang H.; Ma P. X.; Guo B. Self-healing conductive hydrogels: Preparation, properties and applications. Nanoscale 2020, 12 (3), 1224–1246. 10.1039/C9NR09283H. [DOI] [PubMed] [Google Scholar]
- Ing N. L.; El-Naggar M. Y.; Hochbaum A. I. Going the Distance: Long-Range Conductivity in Protein and Peptide Bioelectronic Materials. J. Phys. Chem. B 2018, 122 (46), 10403–10423. 10.1021/acs.jpcb.8b07431. [DOI] [PubMed] [Google Scholar]
- Ing N. L.; Spencer R. K.; Luong S. H.; Nguyen H. D.; Hochbaum A. I. Electronic Conductivity in Biomimetic α-Helical Peptide Nanofibers and Gels. ACS Nano 2018, 12 (3), 2652–2661. 10.1021/acsnano.7b08756. [DOI] [PubMed] [Google Scholar]
- Fleming S.; Ulijn R. V. Design of nanostructures based on aromatic peptide amphiphiles. Chem. Soc. Rev. 2014, 43 (23), 8150–8177. 10.1039/C4CS00247D. [DOI] [PubMed] [Google Scholar]
- Wakabayashi R.; Suehiro A.; Goto M.; Kamiya N. Designer aromatic peptide amphiphiles for self-assembly and enzymatic display of proteins with morphology control. Chem. Commun. 2019, 55 (5), 640–643. 10.1039/C8CC08163H. [DOI] [PubMed] [Google Scholar]
- Mondal S.; Das S.; Nandi A. K. A review on recent advances in polymer and peptide hydrogels. Soft Matter 2020, 16 (6), 1404–1454. 10.1039/C9SM02127B. [DOI] [PubMed] [Google Scholar]
- Tao K.; Levin A.; Adler-Abramovich L.; Gazit E. Fmoc-modified amino acids and short peptides: Simple bio-inspired building blocks for the fabrication of functional materials. Chem. Soc. Rev. 2016, 45 (14), 3935–3953. 10.1039/C5CS00889A. [DOI] [PubMed] [Google Scholar]
- Liang X.; Wang L.; Jeong K.; Lee M. Supramolecular conducting microfibers from amphiphilic tetrathiafulvalene-based organogelator. Chin. Chem. Lett. 2019, 30 (1), 123–126. 10.1016/j.cclet.2018.07.001. [DOI] [Google Scholar]
- Mijiddorj B.; Shirakata H.; Nakagawa T.; Ueda K.; Yokoyama Y.; Kawamura I. Stereochemical effects on the self-assembly of pyrenylalanine-phenylalanine dipeptide. Bull. Chem. Soc. Jpn. 2020, 93, 969. 10.1246/bcsj.20190376. [DOI] [Google Scholar]
- Dong Y.Cyclohexyl-substituted anthracene derivatives for high thermal stability organic semiconductors. Front. Chem. 2019, 7, 10.3389/fchem.2019.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushtaq I.; Akhter Z.; Shah F. U. Tunable Self-Assembled Nanostructures of Electroactive PEGylated Tetra(Aniline) Based ABA Triblock Structures in Aqueous Medium. Front. Chem. 2019, 7, 1–10. 10.3389/fchem.2019.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushtaq I.; et al. Engineering electroactive and biocompatible tetra(aniline)-based terpolymers with tunable intrinsic antioxidant properties in vivo. Mater. Sci. Eng., C 2020, 108, 110456. 10.1016/j.msec.2019.110456. [DOI] [PubMed] [Google Scholar]
- Faramarzi V.; et al. Light-triggered self-construction of supramolecular organic nanowires as metallic interconnects. Nat. Chem. 2012, 4 (6), 485–490. 10.1038/nchem.1332. [DOI] [PubMed] [Google Scholar]
- Panda S. S.; Katz H. E.; Tovar J. D. Solid-state electrical applications of protein and peptide based nanomaterials. Chem. Soc. Rev. 2018, 47 (10), 3640–3658. 10.1039/C7CS00817A. [DOI] [PubMed] [Google Scholar]
- Kumar R. J.; MacDonald J. M.; Singh T. B.; Waddington L. J.; Holmes A. B. Hierarchical self-assembly of semiconductor functionalized peptide α-helices and optoelectronic properties,. J. Am. Chem. Soc. 2011, 133 (22), 8564–8573. 10.1021/ja110858k. [DOI] [PubMed] [Google Scholar]
- Ariga K.; et al. Nanoarchitectonics beyond Self-Assembly: Challenges to Create Bio-Like Hierarchic Organization. Angew. Chem., Int. Ed. 2020, 59, 15424–15446. 10.1002/anie.202000802. [DOI] [PubMed] [Google Scholar]
- Schenning A. P. H. J.; Meijer E. W. Supramolecular electronics; nanowires from self-assembled π-conjugated systems. Chem. Commun. 2005, (26), 3245–3258. 10.1039/b501804h. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y. Programmed self-assembly of large π-conjugated molecules into electroactive one-dimensional nanostructures. Sci. Technol. Adv. Mater. 2012, 13 (3), 033001. 10.1088/1468-6996/13/3/033001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend E. J.; Alotaibi M.; Mills B. M.; Watanabe K.; Seddon A. M.; Faul C. F. J. Electroactive Amphiphiles for Addressable Supramolecular Nanostructures. ChemNanoMat 2018, 4 (8), 741–752. 10.1002/cnma.201800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalily M. A.; et al. The design and fabrication of supramolecular semiconductor nanowires formed by benzothienobenzothiophene (BTBT)-conjugated peptides. Nanoscale 2018, 10 (21), 9987–9995. 10.1039/C8NR01604F. [DOI] [PubMed] [Google Scholar]
- Tovar J. D. Supramolecular Construction of Optoelectronic Biomaterials. Acc. Chem. Res. 2013, 46 (7), 1527–1537. 10.1021/ar3002969. [DOI] [PubMed] [Google Scholar]
- Ardona H. A. M.; Tovar J. D. Peptide π-Electron Conjugates: Organic Electronics for Biology?. Bioconjugate Chem. 2015, 26 (12), 2290–2302. 10.1021/acs.bioconjchem.5b00497. [DOI] [PubMed] [Google Scholar]
- Desmarchelier A.; et al. Tuning the nature and stability of self-assemblies formed by ester benzene 1,3,5-tricarboxamides: The crucial role played by the substituents. Soft Matter 2016, 12 (37), 7824–7838. 10.1039/C6SM01601D. [DOI] [PubMed] [Google Scholar]
- Dibble J. P.; Troyano-Valls C.; Tovar J. D. A Tale of Three Hydrophobicities: Impact of Constitutional Isomerism on Nanostructure Evolution and Electronic Communication in π-Conjugated Peptides. Macromolecules 2020, 53, 7263. 10.1021/acs.macromol.0c01492. [DOI] [Google Scholar]
- Choi I.; Park I. S.; Ryu J. H.; Lee M. Control of peptide assembly through directional interactions. Chem. Commun. 2012, 48 (68), 8481–8483. 10.1039/c2cc31872e. [DOI] [PubMed] [Google Scholar]
- Lehrman J. A.; Cui H.; Tsai W. W.; Moyer T. J.; Stupp S. I. Supramolecular control of self-assembling terthiophene-peptide conjugates through the amino acid side chain. Chem. Commun. 2012, 48 (78), 9711–9713. 10.1039/c2cc34375d. [DOI] [PubMed] [Google Scholar]
- Panda S. S.; Shmilovich K.; Ferguson A. L.; Tovar J. D. Controlling Supramolecular Chirality in Peptide-π-Peptide Networks by Variation of the Alkyl Spacer Length. Langmuir 2019, 35 (43), 14060–14073. 10.1021/acs.langmuir.9b02683. [DOI] [PubMed] [Google Scholar]
- Panda S. S.; Shmilovich K.; Ferguson A. L.; Tovar J. D. Computationally Guided Tuning of Amino Acid Configuration Influences the Chiroptical Properties of Supramolecular Peptide-π-Peptide Nanostructures. Langmuir 2020, 36 (24), 6782–6792. 10.1021/acs.langmuir.0c00961. [DOI] [PubMed] [Google Scholar]
- Liu M.; Zhang L.; Wang T. Supramolecular chirality in self-Assembled systems. Chem. Rev. 2015, 115 (15), 7304–7397. 10.1021/cr500671p. [DOI] [PubMed] [Google Scholar]
- Jira E. R.; Shmilovich K.; Kale T. S.; Ferguson A.; Tovar J. D.; Schroeder C. M. Effect of Core Oligomer Length on the Phase Behavior and Assembly of π-Conjugated Peptides. ACS Appl. Mater. Interfaces 2020, 12 (18), 20722–20732. 10.1021/acsami.0c02095. [DOI] [PubMed] [Google Scholar]
- Wei D.; Ge L.; Wang Z.; Wang Y.; Guo R. Self-Assembled Dual Helical Nanofibers of Amphiphilic Perylene Diimides with Oligopeptide Substitution. Langmuir 2019, 35 (36), 11745–11754. 10.1021/acs.langmuir.9b01745. [DOI] [PubMed] [Google Scholar]
- Matmour R.; et al. Oligo(p-phenylenevinylene) peptide conjugates: Synthesis and self-assembly in solution and at the solid-liquid interface. J. Am. Chem. Soc. 2008, 130 (44), 14576–14583. 10.1021/ja803026j. [DOI] [PubMed] [Google Scholar]
- Arioz I.; et al. Biocompatible Electroactive Tetra(aniline)-Conjugated Peptide Nanofibers for Neural Differentiation. ACS Appl. Mater. Interfaces 2018, 10 (1), 308–317. 10.1021/acsami.7b16509. [DOI] [PubMed] [Google Scholar]
- Bell O. A.; et al. Self-Assembly of a Functional Oligo(Aniline)-Based Amphiphile into Helical Conductive Nanowires. J. Am. Chem. Soc. 2015, 137 (45), 14288–14294. 10.1021/jacs.5b06892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalily M. A.; et al. The design and fabrication of supramolecular semiconductor nanowires formed by benzothienobenzothiophene (BTBT)-conjugated peptides. Nanoscale 2018, 10 (21), 9987–9995. 10.1039/C8NR01604F. [DOI] [PubMed] [Google Scholar]
- Nam J.; et al. Supramolecular Peptide Hydrogel-Based Soft Neural Interface Augments Brain Signals through a Three-Dimensional Electrical Network. ACS Nano 2020, 14 (1), 664–675. 10.1021/acsnano.9b07396. [DOI] [PubMed] [Google Scholar]
- Wang S.; et al. Chitosan/gelatin porous scaffolds assembled with conductive poly(3,4-ethylenedioxythiophene) nanoparticles for neural tissue engineering. J. Mater. Chem. B 2017, 5 (24), 4774–4788. 10.1039/C7TB00608J. [DOI] [PubMed] [Google Scholar]
- Chakraborty P.; et al. A Self-Healing, All-Organic, Conducting, Composite Peptide Hydrogel as Pressure Sensor and Electrogenic Cell Soft Substrate,. ACS Nano 2019, 13 (1), 163–175. 10.1021/acsnano.8b05067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayavenkataraman S.; Vialli N.; Fuh J. Y. H.; Feng Lu W. Conductive collagen/polypyrrole-b-polycaprolactone hydrogel for bioprinting of neural tissue constructs. Int. J. Bioprinting 2019, 5, 31–43. 10.18063/ijb.v5i2.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James E. I.; Jenkins L. D.; Murphy A. R. Peptide-Thiophene Hybrids as Self-Assembling Conductive Hydrogels. Macromol. Mater. Eng. 2019, 304, 1900285. 10.1002/mame.201900285. [DOI] [Google Scholar]
- Diegelmann S. R.; Hartman N.; Markovic N.; Tovar J. D. Synthesis and alignment of discrete polydiacetylene-peptide nanostructures. J. Am. Chem. Soc. 2012, 134 (4), 2028–2031. 10.1021/ja211539j. [DOI] [PubMed] [Google Scholar]
- Walls A. B.; et al. Diphenylalanine Peptide Nanowires as a Substrate for Neural Cultures. Bionanoscience 2020, 10 (1), 224–234. 10.1007/s12668-019-00717-w. [DOI] [Google Scholar]
- Guterman T.; et al. Electrical Conductivity, Selective Adhesion, and Biocompatibility in Bacteria-Inspired Peptide-Metal Self-Supporting Nanocomposites. Adv. Mater. 2019, 31 (10), 1–9. 10.1002/adma.201807285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall B. D.; et al. Aligned Macroscopic Domains of Optoelectronic Nanostructures Prepared via Shear-Flow Assembly of Peptide Hydrogels. Adv. Mater. 2011, 23 (43), 5009–5014. 10.1002/adma.201102963. [DOI] [PubMed] [Google Scholar]
- Wu Y.; et al. Photoconductive Micro/Nanoscale Interfaces of a Semiconducting Polymer for Wireless Stimulation of Neuron-Like Cells. ACS Appl. Mater. Interfaces 2019, 11 (5), 4833–4841. 10.1021/acsami.8b19631. [DOI] [PubMed] [Google Scholar]
- Ardoña H. A. M.; Besar K.; Togninalli M.; Katz H. E.; Tovar J. D. Sequence-dependent mechanical, photophysical and electrical properties of pi-conjugated peptide hydrogelators. J. Mater. Chem. C 2015, 3 (25), 6505–6514. 10.1039/C5TC00100E. [DOI] [Google Scholar]
- Thurston B. A.; Shapera E. P.; Tovar J. D.; Schleife A.; Ferguson A. L. Revealing the Sequence-Structure-Electronic Property Relation of Self-Assembling π-Conjugated Oligopeptides by Molecular and Quantum Mechanical Modeling. Langmuir 2019, 35 (47), 15221–15231. 10.1021/acs.langmuir.9b02593. [DOI] [PubMed] [Google Scholar]
- Blatz T. J.; et al. Templating the 3D structure of conducting polymers with self-assembling peptides. J. Mater. Chem. B 2017, 5 (24), 4690–4696. 10.1039/C7TB00221A. [DOI] [PubMed] [Google Scholar]
- Nalluri S. K. M.; Shivarova N.; Kanibolotsky A. L.; Zelzer M.; Gupta S.; Frederix P. W. J. M.; Skabara P. J.; Gleskova H.; Ulijn R. V.; et al. Conducting nanofibers and organogels derived from the self-assembly of tetrathiafulvalene-appended dipeptides. Langmuir 2014, 30 (41), 12429–12437. 10.1021/la503459y. [DOI] [PubMed] [Google Scholar]
- Yang C.; et al. Tunable Three-Dimensional Nanostructured Conductive Polymer Hydrogels for Energy-Storage Applications. ACS Appl. Mater. Interfaces 2019, 11 (4), 4258–4267. 10.1021/acsami.8b19180. [DOI] [PubMed] [Google Scholar]
- van Dooren B. T. H. Biocompatibility of Trypan Blue with Human Corneal Cells. Arch. Ophthalmol. 2004, 122 (5), 736–742. 10.1001/archopht.122.5.736. [DOI] [PubMed] [Google Scholar]
- Rao K. V.; George S. J. Supramolecular alternate co-assembly through a non-covalent amphiphilic design: Conducting nanotubes with a mixed D-A structure. Chem. - Eur. J. 2012, 18 (45), 14286–14291. 10.1002/chem.201202168. [DOI] [PubMed] [Google Scholar]
- Khalily M. A.; et al. Fabrication of Supramolecular n/p-Nanowires via Coassembly of Oppositely Charged Peptide-Chromophore Systems in Aqueous Media. ACS Nano 2017, 11 (7), 6881–6892. 10.1021/acsnano.7b02025. [DOI] [PubMed] [Google Scholar]
- Wall B. D.; Zacca A. E.; Sanders A. M.; Wilson W. L.; Ferguson A. L.; Tovar J. D. Supramolecular polymorphism: Tunable electronic interactions within π-conjugated peptide nanostructures dictated by primary amino acid sequence. Langmuir 2014, 30 (20), 5946–5956. 10.1021/la500222y. [DOI] [PubMed] [Google Scholar]
- Miller L. L.; Mann K. R. π-Dimers and π-Stacks in Solution and in Conducting Polymers. Acc. Chem. Res. 1996, 29 (9), 417–423. 10.1021/ar9600446. [DOI] [Google Scholar]
- Xie L. S.; Alexandrov E. V.; Skorupskii G.; Proserpio D. M.; Dincǎ M. Diverse π-π Stacking motifs modulate electrical conductivity in tetrathiafulvalene-based metal-organic frameworks. Chem. Sci. 2019, 10 (37), 8558–8565. 10.1039/C9SC03348C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.; Panda S. S.; Tovar J. D.; Katz H. E. Unusually Conductive Organic-Inorganic Hybrid Nanostructures Derived from Bio-Inspired Mineralization of Peptide/Pi-Electron Assemblies. ACS Nano 2020, 14 (2), 1846–1855. 10.1021/acsnano.9b07911. [DOI] [PubMed] [Google Scholar]
- Angeloni N. L.; et al. Regeneration of the cavernous nerve by Sonic hedgehog using aligned peptide amphiphile nanofibers. Biomaterials 2011, 32 (4), 1091–1101. 10.1016/j.biomaterials.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S.; Bond C. W.; Harrington D. A.; Stupp S. I.; McVary K. T.; Podlasek C. A. Peptide amphiphile nanofiber hydrogel delivery of sonic hedgehog protein to the cavernous nerve to promote regeneration and prevent erectile dysfunction. Nanomedicine 2017, 13 (1), 95–101. 10.1016/j.nano.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.; et al. Self-assembling peptide hydrogels functionalized with LN- And BDNF- mimicking epitopes synergistically enhance peripheral nerve regeneration. Theranostics 2020, 10 (18), 8227–8249. 10.7150/thno.44276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki M.; et al. Synergistic effects of self-assembling peptide and neural stem/progenitor cells to promote tissue repair and forelimb functional recovery in cervical spinal cord injury. Biomaterials 2014, 35 (9), 2617–2629. 10.1016/j.biomaterials.2013.12.019. [DOI] [PubMed] [Google Scholar]
- Tysseling-Mattiace V. M.; et al. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J. Neurosci. 2008, 28 (14), 3814–3823. 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan A.; Rajadas J.; Seifalian A. M. Biochemical engineering nerve conduits using peptide amphiphiles. J. Controlled Release 2012, 163 (3), 342–352. 10.1016/j.jconrel.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Sever-Bahcekapili M.; et al. Neuroactive Peptide Nanofibers for Regeneration of Spinal Cord after Injury. Macromol. Biosci. 2020, 21, e2000234. 10.1002/mabi.202000234.. [DOI] [PubMed] [Google Scholar]
- Xiao Z.; Yao Y.; Wang Z.; Tian Q.; Wang J.; Gu L.; Li B.; Zheng Q.; Wu Y.; et al. Local Delivery of Taxol From FGL-Functionalized Self-Assembling Peptide Nanofiber Scaffold Promotes Recovery After Spinal Cord Injury. Front. Cell Dev. Biol. 2020, 8, 820. 10.3389/fcell.2020.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W. M.; et al. Hyaluronic acid-poly-D-lysine-based three-dimensional hydrogel for traumatic brain injury. Tissue Eng. 2005, 11 (3–4), 513–525. 10.1089/ten.2005.11.513. [DOI] [PubMed] [Google Scholar]
- Kotov N. A.; Winter J. O.; Clements I. P.; Jan E.; Timko B. P.; Campidelli S.; Pathak S.; Mazzatenta A.; Lieber C. M.; Prato M.; Bellamkonda R. V.; Silva G. A.; Kam N. W. S.; Patolsky F.; Ballerini L. Nanomaterials for neural interfaces. Adv. Mater. 2009, 21 (40), 3970–4004. 10.1002/adma.200801984. [DOI] [Google Scholar]
- Hsu C. H.; et al. Biomolding Technique to Fabricate the Hierarchical Topographical Scaffold of POMA to Enhance the Differentiation of Neural Stem Cells. ACS Biomater. Sci. Eng. 2017, 3 (8), 1527–1534. 10.1021/acsbiomaterials.7b00091. [DOI] [PubMed] [Google Scholar]
- He P. P.; Li X. D.; Wang L.; Wang H. Bispyrene-Based Self-Assembled Nanomaterials: In Vivo Self-Assembly, Transformation, and Biomedical Effects. Acc. Chem. Res. 2019, 52 (2), 367–378. 10.1021/acs.accounts.8b00398. [DOI] [PubMed] [Google Scholar]