Abstract
Intestinal ischemia reperfusion (I/R) injury is a tissue and organ injury that frequently occurs during surgery and significantly contributes to the pathological processes of severe infection, injury, shock, cardiopulmonary insufficiency and other diseases. However, the mechanism of intestinal I/R injury remains to be elucidated. A mouse model of intestinal I/R injury was successfully established and the model mice were treated with remote ischemic post-conditioning (RIPOC) and/or an ERK inhibitor (CC-90003), respectively. Histopathological changes of the intestinal mucosa were determined by hematoxylin and eosin staining. In addition, the levels of high-mobility group box 1 (HMGB1) and receptor for advanced glycation end products (RAGE) expression were confirmed by reverse transcription-quantitative polymerase chain reaction, western blotting and immunohistochemistry assays. The levels of antioxidants, oxidative stress markers (8-OHdG) and interleukin 1 family members were evaluated by ELISA assays and the levels of NF-κB pathway proteins were analyzed by western blotting. The data demonstrated that RIPOC could attenuate the histopathological features of intestinal mucosa in the intestinal I/R-injury mouse models via the ERK pathway. It was also revealed that HMGB1 and RAGE expression in the mouse models could be markedly reduced by RIPOC (P<0.05) and that these reductions were associated with inhibition of the ERK pathway. Furthermore, it was demonstrated that RIPOC produced significant antioxidant and anti-inflammatory effects following an intestinal I/R injury and that these effects were mediated via the ERK pathway (P<0.05). In addition, RIPOC was demonstrated to suppress the NF-κB (p65)/NLR family pyrin domain containing 3 (NLRP3) inflammatory pathways in the intestinal I/R injury mouse models via the ERK pathway. The findings of the present study demonstrated that RIPOC helped to protect mice with an intestinal I/R injury by downregulating the ERK pathway.
Keywords: ischemia-reperfusion, extracellular signal-regulated kinase, inflammation, antioxidants
Introduction
Intestinal ischemia reperfusion (I/R) injury is one of the commonest tissue and organ injuries that occur during surgeries, such as abdominal aortic aneurysm surgery, cardiopulmonary bypass surgery and intestinal transplantation surgery (1). Intestinal I/R injury can cause pathophysiological changes of the intestinal mucosa (2) and not only causes the destruction of intestinal tissue, but also results in secondary damage to distant organs and tissues, thus inducing multi-organ dysfunction syndrome and systemic inflammatory response syndrome (3). As a result of these changes, the small intestine is often called ‘the origin of multiple organ failure after trauma’ (4,5). Previous studies have revealed the pathological mechanisms of intestinal I/R injury, which include microvascular damage to ischemic organs, the release of inflammatory cytokines, ATP depletion, oxygen free radical injury, leukocyte adhesion, calcium overload and apoptosis (2,6). I/R injury evokes reactive oxygen species (ROS) production and results in DNA damage, cell apoptosis and tubule destruction (7). In previous studies, cell and animal experiments have investigated the pathogenesis of intestinal I/R injuries (8,9). However, due to their complicated mechanisms, the underlying pathological factors involved in intestinal I/R injuries remain to be elucidated. Therefore, additional studies on the mechanism of intestinal I/R injuries can provide a theoretical basis for their clinical treatment.
Remote ischemic pre-conditioning (RIPOC) is a new type of organ protection method that allows the short-term ischemia and reperfusion of one organ to protect another distal organ (10,11). The first study of RIPOC found that preconditioning of the anterior descending artery of a dog heart with 4 cycles of 5 min ischemia followed by 5 min of reperfusion helped to protect against distant myocardial I/R injury (12). A subsequent study demonstrated that I/R preconditioning of tissues and organs in the farther regions could also help to protect organs (13). Those studies notably enhanced the operability of RIPOC and the possibility of its clinical use. However, due to the unpredictability of clinical ischemic events, such as anesthesia methods, anesthetic drugs, complications and individual differences, the current clinical applications of RIPOC remain limited (14,15). RIPOC can be implemented following the occurrence of ischemic events with good controllability and its use and applications are being extensively studied (16,17). However, the mechanism by which RIPOC protects against intestinal I/R injury has not yet been fully elucidated.
High-mobility group box 1 (HMGB1) is a highly conserved DNA-binding protein that is passively released by dead cells or actively secreted into the extracellular environment by inflammatory cells under conditions of cellular stress to regulate innate and adaptive immunity (18). The known HMGB1 receptors include receptor for advanced glycation end products (RAGE), Toll-like receptor (TLR) 2 and TLR4 (19). Studies have verified that RAGE is the earliest identified HMGB1 receptor that belongs to the immunoglobulin superfamily (20,21). The binding of RAGE to its ligands can result in the activation of multiple protein kinases including MAPKs, RaC/cell division control protein 42 homolog and Janus kinase/STATs and thereby activate the NF-κB signaling pathway (22). In addition, the biological function of HMGB1 also depends on the involvement of RAGE (20). However, it is unclear whether the RAGE/HMGB pathway is involved in the protective effect of RIPOC against intestinal I/R injury.
The present study established a mouse model of intestinal I/R injury and observed the influence of RIPOC on histopathological features, antioxidant capacity and inflammation in the injured mice. In addition, the regulatory effects of RIPOC on the RAGE/HMGB pathway in the intestinal I/R injury mouse models was also confirmed. The results of the present study suggested RIPOC as an approach for protecting tissues against I/R injury by attenuating oxidative stress and preventing inflammation.
Materials and methods
Animals
A total of 40 male specific pathogen-free (SPF) C57 BL/6 mice (8 weeks old; weight range, 20–30 g) were acclimated for a week prior to being used for experiments. The mice were housed in a room with a 12-h light/dark cycle, 40–60% humidity and a controlled temperature of 18–23°C; food and water were available ad libitum. All animal experiments were performed in compliance with ethical standards of Shandong Provincial Hospital Affiliated to Shandong University. The present study was approved by the Ethics committee of Shandong Provincial Hospital affiliated to Shandong University (approval no. 2019-330).
Establishment of an intestinal I/R injury model
Mice were anesthetized by intraperitoneal injection of 60 mg/kg pentobarbital sodium before surgery to avoid pain. Next, the superior mesenteric artery (SMA) was exposed and the root of the superior mesenteric artery was blocked using a non-traumatic vascular clip, resulting in complete intestinal ischemia for 1 h, followed by reperfusion of the clip for 1 h. Heart rate, blood pressure and body temperature were monitored during experiments to evaluated the stress response. Mice in the sham group were also anesthetized, however, the SMA was isolated but not blocked. All the mice were euthanized with pentobarbital sodium (i.p., 60 mg/kg) and then decapitated when they reached a humane end of life (gentle heartbeat, even breathing, stable body temperature). Mice which did not breath for over 3 min and without heartbeat were identified as having succumbed. Samples of colorectal tissue were collected and fixed in 4% paraformaldehyde for further examination by hematoxylin and eosin (H&E) staining and immunohistochemistry. Other tissue samples were quick-frozen in liquid nitrogen and stored at −80°C. Serum samples were collected and stored at −80°C. No animal succumbed during the experiment before sacrifice. The whole experiments lasted for ≤1.5 h.
Animal groups
The mice were randomly assigned to four separate groups: i) the Sham group (only laparotomy was performed and the SMA was separated without clamping); ii) the I/R group (treated as aforementioned; iii) I/R+RIPOC group (following ischemia for 45 min, three cycles of 30 sec artery perfusion/30 sec artery blocking were performed and the remaining steps were the same as for the I/R group) and 10 mice per group; and iv) the I/R+RIPOC+CC-90003 group. CC9003 was purchased from MedChemExpress (cat. no. HY-112570).
H&E staining
The separated intestinal tubes were fixed with 4% paraformaldehyde (EMD Millipore) for 12 h at 4°C, dehydrated by gradient alcohol (70% alcohol for 1 h, 85% alcohol for 1 h, 95% alcohol for 1 h, 100% alcohol for 30 min, 100% alcohol for 1 h, 100% alcohol for 30 min). Then the samples were embedded in paraffin and cut into 4-µm sections. After being roasted at 38°C for 6 h, the sections were subjected to a series of operations including dewaxing with xylene, hydration with a gradient alcohol series, hematoxylin staining at room temperature for 8 min, differentiation in 1% alcohol and hydrochloric acid for 3 sec, bluing in Scott blue buffer for 10 min, and eosin for 10 sec. After rehydrating and clearing with a gradient alcohol series, the sections were observed under a light microscope (CKX41, Olympus Corporation) at ×200 and ×400 magnification with 5 fields. The histological assessment was performed according to Chiu's score (23). Briefly, it was as follows: 0, normal; 1, development of Gruenhagen's space, along with capillary congestion; 2, increase in epithelial space with moderate lifting of epithelial layer; 3, markedly lifted epithelial; 4, denuded villi with lamina propria and dilated capillaries; 5, digestion and disintegration of lamina propria and hemorrhage and ulceration.
Immunohistochemistry (IHC) assays
As described in previous studies (24,25), the 4-µm sections were dewaxed in xylene and then rehydrated with a gradient alcohol series (100-70%); following which, they were subjected to antigen retrieval with 0.01 M citrate buffer (cat. no. AR0024; Wuhan Boster Biological Technology, Ltd.) and incubation with 3% H2O2 at room temperature for 10 min. Next, the sections were blocked with 10% bovine serum albumin (BSA; Sigma-Aldrich; Merck KGaA; cat. no. A2153), incubated with primary antibodies against HMGB1 (1:100; cat. no. ab77302; Abcam) and RAGE (1:100; cat. no. ab3611; Abcam) overnight at 4°C, and then incubated with an HRP-labeled secondary antibody (1:50, Abcam; cat. no. ab205719) at room temperature for 2 h. Subsequently, the sections were hatched by exposure to 50 µl of peroxidase-labeled polymer (cat. no. K4003; Dako; Agilent Technologies, Inc.) and 100 µl of substrate-chromogen (cat. no. K3464; Dako; Agilent Technologies, Inc.) for 2 min. The results were examined under a light microscope (Olympus Corporation) at ×200 magnification with 6 fields.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total tissue RNAs were extracted from the separated tissues, which were then homogenized on ice using TRIzol® reagent (Thermo Fisher Scientific, Inc.). The RNA concentration was detected with a GeneQuant 1300 spectrophotometer (Cytiva) according to the manufacturer's protocols. A 1-µl sample of total RNA was reverse-transcribed into cDNA in a 20 µl system using an All-in-One First-Strand cDNA Synthesis kit (GeneCopoeia, Inc.) based on the manufacturer's protocols. Then RT-qPCR was performed using a SYBR-Green qPCR kit (cat. no. F-416L; Finnzymes; Thermo Fisher Scientific, Inc.) on a CFX96 Real-time PCR Detection System C1000 (Bio-Rad Laboratories, Inc.). The thermocycling conditions were as follows: 95°C for 2 min; followed by 40 cycles of 95°C for 10 sec, 60°C for 34 sec and 72°C for 33 sec. The primers used for RT-qPCR were synthesized by Takara Biotechnology Co., Ltd. and the gene expression was quantitated by the 2−ΔΔCq method (26) from 3 repeated experiments. The primer sequences were as following: GAPDH forward, 5′-CCTCGTCTCATAGACAAGATGGT-3′ and reverse, 5′-GGGTAGAGTCATACTGGAACATG-3′; HMGB1 forward, 5′-TGTTCTGAGTACCGCCCAAA-3′ and reverse, 5′-CTTGGCGGCCTTCTTTTCAT-3′; and RAGE forward, 5′-TCACAGAAACCGGTGATGAAG-3′ and reverse, 5′-CTCGAGTCTGGGTTGTCGTT-3′.
Western blot analysis
The intestinal tube tissues in each group were homogenized on ice and their total proteins were extracted using RIPA lysate buffer (cat. no. AR0105; Wuhan Boster Biological Technology, Ltd.). The total protein concentration in each sample was determined using a BCA Protein Assay kit (cat. no. 233225; Thermo Fisher Scientific, Inc.). A 20-µg aliquot of total protein from each sample was separated by 10% SDS-PAGE (cat. no. NP0322BOX; Thermo Fisher Scientific, Inc.) and the protein bands were transferred onto PVDF membranes (PerkinElmer, Inc.), which were subsequently blocked with 5% powdered skimmed milk at room temperature for 2 h. Following blocking, the membranes were incubated with primary antibodies at 4°C overnight, and then incubated with HRP-conjugated secondary antibodies (1:2,000; cat. nos. ab205719 or ab6721; Abcam) at room temperature for 1 h. Next, ECL reagent (Pierce; Thermo Fisher Scientific, Inc.) was used to visualize the immunostained proteins and the relative amounts of protein were determined using a ChemiDoc Imaging System (Bio-Rad Laboratories, Inc.). The primary antibodies used were: HMGB1 (1:1,000; cat. no. ab77302), RAGE (1:1,000; cat. no. ab3611), p-ERK1/2 (1:1,000; cat. no. ab214362), ERK1/2 (1:1,000; cat. no. ab17942), p-p65 (1:1,000; cat. no. ab86299), p65 (1:1,000; cat. no. ab32536), p-IKBa (1:1,000; cat. no. ab24783), IKBa (1:1,000; cat. no. ab7217), NLRP3 (1:1,000; cat. no. ab214185) and GAPDH (1:2,000; cat. no. ab9485; all from Abcam). GAPDH was used as an internal reference. The gray value in each group was determined using ImageJ software (version 1.51d; National institutes of Health).
Flow cytometric analysis for ROS
The level of ROS was analyzed using blue fluorescent dye dihydroethidium (DHE, Invitrogen; Thermo Fisher Scientific, Inc.) as described in a previous study (23). The prepared samples were incubated with 2.5 mmol/l DHE solution for 25 min at 37°C. Following washing with PBS three times, the fluorescence intensity in each group was determined by flow cytometry (Beckman CytoFLEX; Beckman Coulter, Inc.).
ELISA assays
A total of 5 ml blood was collected from the vein and heparin using anticoagulation. Following centrifugation (3,000 × g for 10 min at 4°C), the blood serum was collected and stored at −20°C until use. The concentrations of IL-18 (cat. no. E-EL-M0730c), IL-33 (cat. no. E-EL-M2642c), malondialdehyde (MDA; cat. no. E-EL-0060c) and thioredoxin (Trx; cat. no. E-EL-M1134c; all from Elabscience, Inc.), superoxide dismutase (SOD; cat. no. CK-E20348; Yuanye Bio-Technology, Co. Ltd.), glutathione peroxidase (GPx; cat. no. 703102, Cayman Chemical Company) and 8-OHdG (cat. no. E-EL-0028c; Elabscience, Inc.) in blood serum were determined using the corresponding ELISA kits according to instructions provided by the manufacturer. The experimental data were obtained by measuring the absorbance at 450 nm with a microplate reader (Thermo Fisher Scientific, Inc.).
Statistical analysis
All data were analyzed using SPSS version 19.0 (IBM Corp.) and results are presented as the mean value ± standard deviation of data obtained from at least 3 independent experiments. One-way ANOVA with post hoc Tukey's test was used to analyze the experimental data. P<0.05 was considered to indicate a statistically significant difference.
Results
RIPOC ameliorates the histopathological features of intestinal mucosa in mice with intestinal I/R injury via the ERK pathway
To investigate the effects of I/R, mice were used in the present study, as described by Gubernatorova et al (27). H&E staining was performed to determine whether RIPOC could protect the intestinal tissue of mice following intestinal I/R injury. As demonstrated in Fig. 1, the intestinal mucosa in the sham group had a normal appearance, whereas in the I/R group, the small intestine contained villi that exhibited obvious edema. Furthermore, the mucosa and villi in the I/R group were disorganized; most of the villous mucosa were deciduous and the glands were damaged. Following treatment with RIPOC, these histopathological features in the I/R group demonstrated improvement. Furthermore, it was found that CC-90003 treatment could further ameliorate the histopathological features (Fig. 1B).
Figure 1.
RIPOC ameliorates the histopathological features of intestinal mucosa in mice with intestinal I/R injury via the ERK pathway. I/R injury mouse models were treated with RIPOC and/or CC-90003 and histopathological changes in intestinal mucosa were evaluated by (A) hematoxylin and eosin staining in the sham group and treated model group and (B) evaluated according to Chiu's score. **P<0.01 vs. Sham; #P<0.05 vs. I/R; $P<0.05 vs. the I/R+RIPOC group. RIPOC, remote ischemic post-conditioning; I/R, ischemia reperfusion.
RIPOC downregulates HMGB1 and RAGE expression in the intestinal I/R injury model mice via the ERK pathway
To further explore the possible molecular mechanism by which RIPOC protects against intestinal lesions induced by intestinal I/R injury, the changes in expression of I/R injury-related proteins were analyzed. As demonstrated in Fig. 2A, intestinal I/R injury significantly increased the levels of HMGB1 and RAGE expression, whereas RIPOC reduced these increases. It was also determined that CC-90003 could reduce the levels of HMGB1 and RAGE mediated by RIPOC in the intestinal I/R injury mouse models even further (P<0.05, P<0.01; Fig. 2A). In addition, western blot and IHC studies were performed to verify the influence of RIPOC or CC-90003 on HMGB1 and RAGE expression in the intestinal I/R injury mouse models and the results were similar to those obtained from the RT-qPCR assays (Fig. 2B and C).
Figure 2.
RIPOC downregulates HMGB1 and RAGE expression in the intestinal I/R injury mouse models via the ERK pathway. RIPOC and/or CC-90003 were used to treat the I/R injury mouse models. The levels of HMGB1 and RAGE were confirmed by (A) reverse transcription-quantitative polymerase chain and (B) western blot analysis, respectively. (C) The effects of RIPOC and CC-90003 on HMGB1 expression in the intestinal I/R mouse models were determined by immunohistochemistry. Magnification, ×400; Scale bar, 20 µm. **P<0.01 vs. the sham group; #P<0.05 vs. the I/R group; $P<0.05 vs. the I/R+RIPOC group. RIPOC, remote ischemic post-conditioning; HMGB1, high-mobility group box 1; RAGE, receptor for advanced glycation end products; I/R, ischemia reperfusion.
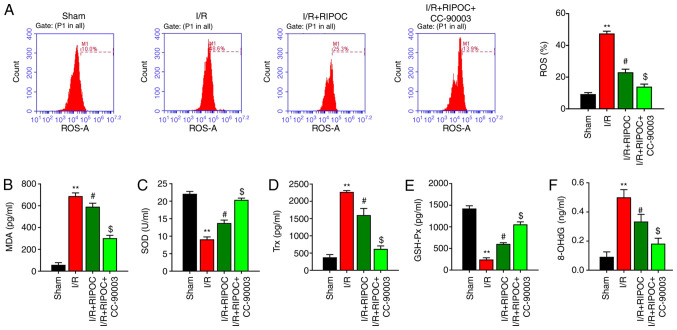
RIPOC decreases ROS, MDA and Trx levels and increases SOD and GSH-Px levels in an intestinal I/R injury mouse model via the ERK pathway
To confirm whether RIPOC exerted an antioxidant affect in the intestinal I/R injury mouse model, multiple oxidative stress indices were examined. A flow cytometric analysis revealed that the levels of ROS in the intestinal I/R injury mouse models were significantly higher compared with the sham mice and those increased levels could be reduced by RIPOC; in addition, the inhibitory effect of RIPOC on ROS production could be further accentuated by CC-90003 (P<0.05, P<0.01; Fig. 3A). In addition, MDA and Trx levels were higher and the SOD and GSH-Px levels were lower in the I/R model group compared with the sham group; and these changes could be attenuated by RIPOC. Concurrently, it was also revealed that CC-90003 could further accentuate the decreases in MDA and Trx levels as well as the increases in SOD and GSH-Px levels mediated by RIPOC in the intestinal I/R injury mouse model (P<0.05, P<0.01; Fig. 3B-E). 8-OHdG, a marker of oxidative stress, was enhanced in the intestinal I/R injury mouse model (P<0.01), while it was significantly decreased by RIPOC treatment compared with I/R mouse model (P<0.05) and further blocked by ERK inhibitor, CC-90003 (P<0.05; Fig. 3F).
Figure 3.
RIPOC decreases ROS, MDA and Trx levels and increases SOD and GSH-Px levels in intestinal I/R injury mouse models via the ERK pathway. (A) Following treatment of the I/R injury mouse models with RIPOC and/or CC-90003, the levels of ROS were identified using a flow cytometer equipped with a dihydroethidium fluorescent probe. The concentrations of (B) MDA, (C) SOD, (D) Trx, (E) GSH-Px and (F) 8-OHdG in each group were examined using the corresponding commercial ELISA kits. **P<0.01 vs. the sham group; #P<0.05 vs. the I/R group; $P<0.05 vs. the I/R+RIPOC group. RIPOC, remote ischemic post-conditioning; ROS, reactive oxygen species; MDA, malondialdehyde; Trx, thioredoxin; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; I/R, ischemia reperfusion.
RIPOC reduces IL-1β, IL-18 and IL-33 levels in mice with intestinal I/R injury via the ERK pathway
The present study next investigated the possible mechanism by which RIPOC affects the levels of interleukin-1 family cytokines (IL-1β, IL-18 and IL-33) in mice following intestinal I/R injury. As demonstrated in Fig. 4A-C, the levels of IL-1β, IL-18 and IL-33 in the I/R injury mice were significantly increased and RIPOC treatment efficiently reversed those increases. The data also demonstrated that CC-90003 could further enhance the reversal effect of RIPOC on the upregulation of IL-1β, IL-18 and IL-33 in mice following intestinal I/R injury.
Figure 4.
RIPOC reduces the levels of IL-1β, IL-18, IL-33 and NLRP3 in mice with intestinal I/R injury via the ERK pathway. Intestinal I/R injury mouse models were treated with RIPOC or/and CC-90003, respectively. The levels of (A) IL-1β, (B) IL-18 and (C) IL-33 were analyzed by ELISA. (D) Western blot assays were performed to evaluate p-ERK1/2, ERK1/2, p-p65, p65, p-IKBα, IKBα and NLRP3 expression. The relative levels of p-ERK1/2 and p-IKBα were defined as the ratio of phosphorylated protein to total protein. **P<0.01 vs. the sham group; #P<0.05 vs. the I/R group; $P<0.05 vs. the I/R+RIPOC group. RIPOC, remote ischemic post-conditioning; NLRP3, NLR family pyrin domain containing 3; I/R, ischemia reperfusion; p-, phosphorylated.
RIPOC attenuates p-ERK1/2, p-p65 and NLRP3 expression and enhances p-IKBα expression in mice following intestinal I/R injury via the ERK pathway
To further determine the mechanism by which RIPOC protects against intestinal I/R injury, the intestinal I/R injury mouse model were treated with RIPOC and/or CC-90003. A western blotting analysis demonstrated that compared with the sham group, the levels of p-ERK1/2, p-p65, p-IKBα and NLRP3 were significantly increased in intestinal I/R injury group, while RIPOC could significantly attenuate the increases in p-ERK1/2, p-p65, p-IKBα and NLRP3 expressions in intestinal I/R injury mice. In addition, CC-90003 could further inhibit the increases in p-ERK1/2, p-p65, p-IKBα and NLRP3 expressions mediated by RIPOC following intestinal I/R injury (Fig. 4D).
Discussion
The small intestine is crucial for maintaining normal physiological activities of the human body and is responsible for the uptake, digestion and absorption of nutrients (28). The small intestine is also the largest reservoir of bacteria and endotoxins and a vital neuroendocrine and immune organ (29). In pathological conditions, the small intestine performs functions that influence all systems of the body (29). Previous studies have demonstrated that intestinal I/R injury is a universal pathophysiological process that occurs in patients suffering from shock, severe trauma and undergoing resuscitation, all of which cause a series of pathophysiological changes to occur in intestinal tissues (30,31). Intestinal I/R injury can also cause secondary damage to distant organs, in addition to intestinal tissue damage (32). However, the relevant mechanisms and treatment strategies of Intestinal I/R injury are still not entirely clear. The present study established a mouse model of intestinal I/R injury and verified that intestinal I/R injury could seriously damage the structure of intestinal mucosa.
RIPOC, as a new non-pharmaceutical intervention, can alleviate long-term I/R-injured target organs via transient ischemic preconditioning of limbs and other distant organs (33). Studies have demonstrated that RIPOC has a significant protective effect when used in treatment of ischemic stroke (34,35), myocardial ischemia reperfusion injury (36,37), or acute ST-elevation myocardial infarction (38). For example, RIPOC improves the transplantation of mesenchymal stem cells in reperfused myocardium (17). Furthermore, limb RIPOC was demonstrated to relieve cerebral I/R injury via AMPK-dependent autophagy (16), attenuate I/R injury in rat skin flaps by reducing oxidative stress (39) and help to protect against cerebral I/R injury in a rat model (40). The present study verified that RIPOC could improve the histopathological features of intestinal mucosa in mice following intestinal I/R injury.
Previous studies have verified that the intestinal mucosal barrier damage caused by I/R injury is mainly attributable to low perfusion pressure, oxygen-free radical injuries and the action of cytokines (41,42). While free radicals are constantly produced, there are also certain enzymes that decompose them in an organism (43). The main endogenous antioxidant enzymes include SOD, CAT and GSH-Px (44). Cytokines including TNF-α, IL-6, IL-1β, IL-18 and IL-33 serve important roles in I/R injuries and greatly contribute to the occurrence of diseases caused by I/R injuries (45,46). I/R injury in the liver significantly promotes ROS expression and the activation of NLRP3 inflammasomes. In contrast, a ROS antagonist such as NAC alleviates hepatic injury by suppressing the activation of NLRP3 (47). The present study detected relevant indicators and found that intestinal I/R injury could markedly reduce the antioxidant capacity of intestinal tissue and promote an inflammatory response, while RIPOC could enhance the antioxidant capacity of intestinal tissue and reduce an inflammatory response.
ERK, as one of the most characteristic members of the MAPK family, regulates a range of cellular properties and activities, including cell metabolism, cell viability, inflammation, cell necrosis and apoptosis (48). The present study demonstrated that the protective effect of RIPOC against intestinal I/R injury was regulated by the ERK pathway. Furthermore, the levels of HMGB1 and RAGE expression could be markedly downregulated by RIPOC in the intestinal I/R injury mouse models. The main HMGB1 receptors in cells are RAGE and TLRs, such as TLR2 and TLR4 (49). The activation of those receptors can subsequently activate various signaling pathways, such as the MAPK pathway and NF-κB signaling pathway and thus upregulate various inflammatory factors and promote an inflammatory cascade reaction (50). The present study further demonstrated that RIPOC could reduce the levels of p-ERK1/2, p-p65, p-IKBα and NLRP3 in mice with intestinal I/R injury by affecting the ERK pathway. Other studies have suggested that the HMGB1/NF-κB pathway participates in cerebral and myocardial I/R injuries (51–53). A previous study demonstrated that hepatic ischemia/reperfusion injury is mediated by the ERK/NF-κB pathway (54). Furthermore, Yang et al (51) suggested that quercetin attenuates hepatic I/R injury by mediating the ERK/NF-κB pathway (55). The present study demonstrated for the first time, to the best of the authors' knowledge, that HMGB1 and RAGE expression can be attenuated by an ERK inhibitor and thus NF-κB and NLRP3 expression was also suppressed. Therefore, it was hypothesized that RIPOC may protect against I/R injury by mediating the ERK/HMGB1/RAGE/NF-κB pathway.
In fact, RIPOC is a harmless approach providing a practical and potential tool to attenuate I/R injury (56). Although it has demonstrated a beneficial outcome to patients, it remains to be further explored. Therefore, further investigation is required to explore the effect of RIPOC at various doses on normal animals.
The conclusion of the present study is that RIPOC exerted strong antioxidant and anti-inflammatory effects in mice following intestinal I/R injury and it produced those effects via an ERK-mediated HMGB1/RAGE/NF-κB pathway. The present study suggested RIPOC as a potential method for treating intestinal I/R injury induced by serious infection and traumatic shock. However, the specific mechanism of the therapeutical effect has not been fully investigated and further studies are required.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Authors' contributions
LM and JW designed the experiments. LM, JW and JL performed the experiments. LM, JW, NZ and MC performed the experiments and collected experimental data. LM, JW, JL and XZ analyzed the data. XZ verified the accuracy of the data analysis. XZ and JL provided the resource supports. LM and JW drafted the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All animal experiments were performed in compliance with ethical standards of Shandong Provincial Hospital Affiliated to Shandong University. The present study was approved by the Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong University (approval no. 2019-330).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Gonzalez LM, Moeser AJ, Blikslager AT. Animal models of ischemia-reperfusion-induced intestinal injury: Progress and promise for translational research. Am J Physiol Gastrointest Liver Physiol. 2015;308:G63–G75. doi: 10.1152/ajpgi.00112.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu Q, Ren H, Ren J, Liu Q, Wu J, Wu X, Li G, Wang G, Gu G, Guo K, et al. Released mitochondrial DNA following intestinal ischemia reperfusion induces the inflammatory response and gut barrier dysfunction. Sci Rep. 2018;8:7350. doi: 10.1038/s41598-018-25387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M, Verhaegh R, Tsagakis K, Brencher L, Zwanziger D, Jakob HG, Groot H, Dohle DS. Impact of acute intestinal ischemia and reperfusion injury on hemodynamics and remote organs in a rat model. Thorac Cardiovasc Surg. 2018;66:99–108. doi: 10.1055/s-0037-1603935. [DOI] [PubMed] [Google Scholar]
- 4.Ferrada P, Wolfe L, Duchesne J, Fraga GP, Benjamin E, Alvarez A, Campbell A, Wybourn C, Garcia A, Morales C, et al. Management of duodenal trauma: A retrospective review from the Panamerican Trauma Society. J Trauma Acute Care Surg. 2019;86:392–396. doi: 10.1097/TA.0000000000002157. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Kong P, Chen C, Tang J, Jin X, Yan J, Wang Y. Targeting IL-17A improves the dysmotility of the small intestine and alleviates the injury of the interstitial cells of cajal during sepsis. Oxid Med Cell Longev. 2019;2019:1475729. doi: 10.1155/2019/1475729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mester A, Magyar Z, Sogor V, Tanczos B, Stark Y, Cherniavsky K, Bidiga L, Peto K, Nemeth N. Intestinal ischemia-reperfusion leads to early systemic micro-rheological and multiorgan microcirculatory alterations in the rat. Clin Hemorheol Microcirc. 2018;68:35–44. doi: 10.3233/CH-170278. [DOI] [PubMed] [Google Scholar]
- 7.Ameli M, Hashemi MS, Moghimian M, Shokoohi M. Protective effect of tadalafil and verapamil on testicular function and oxidative stress after torsion/detorsion in adult male rat. Andrologia. 2018;50:e13068. doi: 10.1111/and.13068. [DOI] [PubMed] [Google Scholar]
- 8.Jia Z, Lian W, Shi H, Cao C, Han S, Wang K, Li M, Zhang X. Ischemic post-conditioning protects against intestinal ischemia/reperfusion injury via the HIF-1α/miR-21 axis. Sci Rep. 2017;7:16190. doi: 10.1038/s41598-017-16366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheu EG, Wakatsuki K, Oakes S, Carroll MC, Moore FD., Jr Prevention of intestinal ischemia-reperfusion injury in humanized mice. Surgery. 2016;160:436–442. doi: 10.1016/j.surg.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giannopoulos G, Vrachatis DA, Panagopoulou V, Vavuranakis M, Cleman MW, Deftereos S. Remote ischemic conditioning and renal protection. J Cardiovasc Pharmacol Ther. 2017;22:321–329. doi: 10.1177/1074248417702480. [DOI] [PubMed] [Google Scholar]
- 11.Heusch G. Remote ischemic conditioning in cardiovascular surgery. J Cardiovasc Pharmacol Ther. 2017;22:297–301. doi: 10.1177/1074248416687874. [DOI] [PubMed] [Google Scholar]
- 12.Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–899. doi: 10.1161/01.CIR.87.3.893. [DOI] [PubMed] [Google Scholar]
- 13.Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K, Redington AN, MacAllister R. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881–2883. doi: 10.1161/01.CIR.0000043806.51912.9B. [DOI] [PubMed] [Google Scholar]
- 14.Anttila V, Haapanen H, Yannopoulos F, Herajärvi J, Anttila T, Juvonen T. Review of remote ischemic preconditioning: From laboratory studies to clinical trials. Scand Cardiovasc J. 2016;50:355–361. doi: 10.1080/14017431.2016.1233351. [DOI] [PubMed] [Google Scholar]
- 15.Gill R, Kuriakose R, Gertz ZM, Salloum FN, Xi L, Kukreja RC. Remote ischemic preconditioning for myocardial protection: Update on mechanisms and clinical relevance. Mol Cell Biochem. 2015;402:41–49. doi: 10.1007/s11010-014-2312-z. [DOI] [PubMed] [Google Scholar]
- 16.Guo H, Zhao L, Wang B, Li X, Bai H, Liu H, Yue L, Guo W, Bian Z, Gao L, et al. Remote limb ischemic post-conditioning protects against cerebral ischemia-reperfusion injury by activating AMPK-dependent autophagy. Brain Res Bull. 2018;139:105–113. doi: 10.1016/j.brainresbull.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Q, Yu T, Huang K, Lu J, Zhang H, Hu S. Remote ischemic post-conditioning ameliorates the mesenchymal stem cells engraftment in reperfused myocardium. PLoS One. 2016;11:e0146074. doi: 10.1371/journal.pone.0146074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S, Zhang Y. HMGB1 in inflammation and cancer. J Hematol Oncol. 2020;13:116. doi: 10.1186/s13045-020-00950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang WS, Kim JJ, Lee MJ, Lee EK, Park SK. Ectodomain shedding of RAGE and TLR4 as a negative feedback regulation in high-mobility group box 1-activated aortic endothelial cells. Cell Physiol Biochem. 2018;51:1632–1644. doi: 10.1159/000495651. [DOI] [PubMed] [Google Scholar]
- 20.Bangert A, Andrassy M, Müller AM, Bockstahler M, Fischer A, Volz CH, Leib C, Göser S, Korkmaz-Icöz S, Zittrich S, et al. Critical role of RAGE and HMGB1 in inflammatory heart disease. Proc Natl Acad Sci USA. 2016;113:E155–E164. doi: 10.1073/pnas.1522288113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mou K, Liu W, Han D, Li P. HMGB1/RAGE axis promotes autophagy and protects keratinocytes from ultraviolet radiation-induced cell death. J Dermatol Sci. 2017;85:162–169. doi: 10.1016/j.jdermsci.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Qiu Y, Chen Y, Zeng T, Guo W, Zhou W, Yang X. High-mobility group box-B1 (HMGB1) mediates the hypoxia-induced mesenchymal transition of osteoblast cells via activating ERK/JNK signaling. Cell Biol Int. 2016;40:1152–1161. doi: 10.1002/cbin.10616. [DOI] [PubMed] [Google Scholar]
- 23.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 24.Qu H, Yin H, Yan S, Tao M, Xie Y, Chen W. Inhibitor of growth 4 suppresses colorectal cancer growth and invasion by inducing G1 arrest, inhibiting tumor angiogenesis and reversing epithelial-mesenchymal transition. Oncol Rep. 2016;35:2927–2935. doi: 10.3892/or.2016.4626. [DOI] [PubMed] [Google Scholar]
- 25.Ma X, Du T, Zhu D, Chen X, Lai Y, Wu W, Wang Q, Lin C, Li Z, Liu L, et al. High levels of glioma tumor suppressor candidate region gene 1 predicts a poor prognosis for prostate cancer. Oncol Lett. 2018;16:6749–6755. doi: 10.3892/ol.2018.9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Gubernatorova EO, Perez-Chanona E, Koroleva EP, Jobin C, Tumanov AV. Murine model of intestinal ischemia-reperfusion injury. J Vis Exp. 2016;111:53881. doi: 10.3791/53881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Zulfiqar M, Bluth MH, Bhalla A, Beydoun R. Molecular diagnostics in the neoplasms of small intestine and appendix: 2018 update. Clin Lab Med. 2018;38:343–355. doi: 10.1016/j.cll.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Volk N, Lacy B. Anatomy and physiology of the small bowel. Gastrointest Endosc Clin N Am. 2017;27:1–13. doi: 10.1016/j.giec.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Moriwaki T, Kawabata T, Goto S, Liu KX, Guo CY, Li TS. Nicaraven attenuates post-operative systemic inflammatory responses-induced tumor metastasis. Ann Surg Oncol. 2020;27:1068–1074. doi: 10.1245/s10434-019-08076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Ling Y, Cao Z, Shen J, Chen S, Liu W, Yuan B, Wen S. Targeting intestinal epithelial cell–programmed necrosis alleviates tissue injury after intestinal ischemia/reperfusion in rats. J Surg Res. 2018;225:108–117. doi: 10.1016/j.jss.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Hummitzsch L, Zitta K, Berndt R, Wong YL, Rusch R, Hess K, Wedel T, Gruenewald M, Cremer J, Steinfath M, et al. Remote ischemic preconditioning attenuates intestinal mucosal damage: Insight from a rat model of ischemia-reperfusion injury. J Transl Med. 2019;17:136. doi: 10.1186/s12967-019-1885-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng B, Guo QL, He ZJ, Ye Z, Yuan YJ, Wang N, Zhou J. Remote ischemic post-conditioning protects the brain from global cerebral ischemia/reperfusion injury by up-regulating endothelial nitric oxide synthase through the PI3K/Akt pathway. Brain Res. 2012;1445:92–102. doi: 10.1016/j.brainres.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 34.Zhao JJ, Xiao H, Zhao WB, Zhang XP, Xiang Y, Ye ZJ, Mo MM, Peng XT, Wei L. Remote ischemic post-conditioning for ischemic stroke: A systematic review and meta-analysis of randomized controlled trials. Chin Med J (Engl) 2018;131:956–965. doi: 10.4103/0366-6999.229892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang D, He XB, Wang Z, Li C, Gao BY, Wu JF, Bai YL. Remote limb ischemic post-conditioning promotes motor function recovery in a rat model of ischemic stroke via the up-regulation of endogenous tissue kallikrein. CNS Neurosci Ther. 2018;24:519–527. doi: 10.1111/cns.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, Wang J, Tu T, Iyan Z, Mungun D, Yang Z, Guo Y. Remote ischemic post-conditioning protects against myocardial ischemia-reperfusion injury by inhibition of the RAGE-HMGB1 pathway. BioMed Res Int. 2018;2018:4565630. doi: 10.1155/2018/4565630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang ZX, Li H, He JS, Chu HJ, Zhang XT, Yin L. Remote ischemic post-conditioning alleviates myocardial ischemia/reperfusion injury by up-regulating ALDH2. Eur Rev Med Pharmacol Sci. 2018;22:6475–6484. doi: 10.26355/eurrev_201810_16061. [DOI] [PubMed] [Google Scholar]
- 38.Ghaffari S, Pourafkari L, Manzouri S, Nader ND. Effect of remote ischemic post-conditioning during thrombolysis in STEMI. Herz. 2018;43:161–168. doi: 10.1007/s00059-017-4550-9. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Xu H, Wang T, He J, Wei J, Wang T, Dong J. Remote limb ischemic post-conditioning attenuates ischemia-reperfusion injury in rat skin flapby limiting oxidative stress. Acta Cir Bras. 2016;31:15–21. doi: 10.1590/S0102-865020160010000003. [DOI] [PubMed] [Google Scholar]
- 40.Ramagiri S, Taliyan R. Protective effect of remote limb post-conditioning via upregulation of heme oxygenase-1/BDNF pathway in rat model of cerebral ischemic reperfusion injury. Brain Res. 2017;1669:44–54. doi: 10.1016/j.brainres.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 41.Ren G, Yuan X, Zhao X, Hao Q, Cao J, Wang Y, Gao Q, Dou J, Zeng Q. Characterization and evolution of intestine injury at the anhepatic phase in portal hypertensive rats. Exp Ther Med. 2018;16:4765–4771. doi: 10.3892/etm.2018.6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang Q. Research progress on intestinal barrier dysfunction and treatment of severe acute pancreatitis. Adv Emerg Med. 2017;6:1–6. [Google Scholar]
- 43.Mason RP. Imaging free radicals in organelles, cells, tissue, and in vivo with immuno-spin trapping. Redox Biol. 2016;8:422–429. doi: 10.1016/j.redox.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goc Z, Szaroma W, Kapusta E, Dziubek K. Protective effects of melatonin on the activity of SOD, CAT, GSH-Px and GSH content in organs of mice after administration of SNP. Chin J Physiol. 2017;60:1–10. doi: 10.4077/CJP.2017.BAF435. [DOI] [PubMed] [Google Scholar]
- 45.Huo Y, Liu S-X, Li L, Feng J, Li H-Y, Song G-Y. Circulating vaspin and IL-6 concentrations in second trimester pregnancy with gestational diabetes. Clin Exp Obstet Gynecol. 2019;46:211–215. [Google Scholar]
- 46.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 47.Shi C, Wang Q, Rao Z, Shi Y, Wei S, Wang H, Lu X, Wang P, Lu L, Zhou H, et al. Diabetes induces hepatocyte pyroptosis by promoting oxidative stress-mediated NLRP3 inflammasome activation during liver ischaemia and reperfusion injury. Ann Transl Med. 2020;8:739. doi: 10.21037/atm-20-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35:600–604. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- 49.Tirone M, Tran NL, Ceriotti C, Gorzanelli A, Canepari M, Bottinelli R, Raucci A, Di Maggio S, Santiago C, Mellado M, et al. High-mobility group box 1 orchestrates tissue regeneration via CXCR4. J Exp Med. 2018;215:303–318. doi: 10.1084/jem.20160217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bianchi ME, Crippa MP, Manfredi AA, Mezzapelle R, Rovere Querini P, Venereau E. High-mobility group box 1 protein orchestrates responses to tissue damage via inflammation, innate and adaptive immunity, and tissue repair. Immunol Rev. 2017;280:74–82. doi: 10.1111/imr.12601. [DOI] [PubMed] [Google Scholar]
- 51.Yang M, Ruidi A, Minghang L, Tian X, Lu X, Dong Z. β-caryophyllene mitigates cerebral ischemia reperfusion injury in mice by inhibiting HMGB1/TLR4/NF-κB pathway. Chin J Immunol. 2017;33:1009–1013. [Google Scholar]
- 52.Dong LY, Chen F, Xu M, Yao LP, Zhang YJ, Zhuang Y. Quercetin attenuates myocardial ischemia-reperfusion injury via downregulation of the HMGB1-TLR4-NF-κB signaling pathway. Am J Transl Res. 2018;10:1273–1283. [PMC free article] [PubMed] [Google Scholar]
- 53.Xie W, Zhu T, Dong X, Nan F, Meng X, Zhou P, Sun G, Sun X. HMGB1-triggered inflammation inhibition of notoginseng leaf triterpenes against cerebral ischemia and reperfusion injury via MAPK and NF-κB signaling pathways. Biomolecules. 2019;9:512. doi: 10.3390/biom9100512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu Q, Wu L, Liu T, Li S, Feng J, Mao Y, Fan X, Guo C, Wu J. Protective effects of levo-tetrahydropalmatine on hepatic ischemia/reperfusion injury are mediated by inhibition of the ERK/NF-κB pathway. Int Immunopharmacol. 2019;70:435–445. doi: 10.1016/j.intimp.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 55.Wu L, Zhang Q, Dai W, Li S, Feng J, Li J, Liu T, Xu S, Wang W, Lu X, et al. Quercetin pretreatment attenuates hepatic ischemia reperfusion-induced apoptosis and autophagy by inhibiting ERK/NF-κB Pathway. Gastroenterol Res Pract. 2017;9724217:2017. doi: 10.1155/2017/9724217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nielsen MB, Krogstrup NV, Oltean M, Nieuwenhuijs-Moeke GJ, Dor FJMF, Birn H, Jespersen B. Remote ischaemic conditioning and early changes in plasma creatinine as markers of one year kidney graft function-A follow-up of the CONTEXT study. PLoS One. 2019;14:e0226882. doi: 10.1371/journal.pone.0226882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.