Abstract
Cerebral toxoplasmosis and cerebral malaria are two important neurological diseases caused by protozoan parasites. In this review, we discuss recent findings regarding the innate immune responses of microglia and astrocytes to Toxoplasma and Plasmodium infection. In both infections, these tissue-resident glial cells perform a sentinel function mediated by alarmin crosstalk that licenses adaptive type 1 immunity in the central nervous system. Divergent protective or pathogenic effects of type 1 activation of these astrocytes and microglia are revealed depending on the inherent lytic potential of the protozoan parasite.
Keywords: Cerebral malaria, cerebral toxoplasmosis, microglia, astrocytes, alarmin, neuroinflammation
Introduction
Neurological diseases associated with neuroinflammation have an autoimmune, neurodegenerative, or infectious etiology1. Although central nervous system (CNS) infections caused by viral and bacterial pathogens have been better studied, neurotropic protozoan parasites also cause two major diseases, namely cerebral toxoplasmosis (CT) and cerebral malaria (CM). CT arises as a result of invasion of the CNS cells by Toxoplasma gondii parasites that exist in both lytic (tachyzoites) and latent (bradyzoites) forms. In immunocompromised patients, life-threatening necrotic encephalitis stems from a failure to control the lytic stage of the parasite. In immunocompetent individuals, T. gondii establishes a chronic infection that has been linked to several neuropsychiatric disorders such as anxiety, suicidal behaviors, and schizophrenia2–4. Unlike in CT, there is no threat of parasite replication in neural cells in CM, and neuropathological symptoms occur acutely. It is thought that occlusion of cerebral microvasculature by Plasmodium-infected red blood cells (iRBCs) causes increased blood–brain barrier (BBB) permeability, hemorrhage, and induction of cerebral hypoxia5. In a mouse model of experimental CM (ECM), inflammation caused by sequestered inflammatory leukocytes, including monocytes and CD8+ T cells, also contributed to the development of secondary neurological symptoms6,7. CM often appears as a severe and lethal neurological complication, but even after recovery, long-term neurological deficits such as cognitive and behavioral disorders persist in children with complicated malaria8.
Here, we discuss the most recent findings regarding the immune responses of two major CNS glial cells—microglia and astrocytes—during Toxoplasma and Plasmodium infection. In both CT and CM, microglia and astrocytes mount a shared T helper 1 (TH1) program of pro-inflammatory and anti-inflammatory cytokine cascades that have divergent protective and pathogenic effects largely dictated by the inherent lytic threat posed by the pathogen to the tissue integrity of the CNS. The mechanistic studies discussed in this short review largely use established mouse models to CT and CM that recapitulate key pathogenetic and clinical features of their respective human diseases.
Microglia responses in cerebral toxoplasmosis and cerebral malaria
Microglia are CNS-resident immune cells originating from yolk sac myeloid progenitors with multifaceted functions during physiological and pathological conditions9. Microglia constantly monitor their microenvironment and respond to pathogenic infections exhibiting reactive phenotypes and immune responses10. Moreover, engagement of microglia and astrocytes in the neurovascular unit allows the initiation of glial effector functions in response to BBB injury11. Several lines of evidence demonstrated microglia activation during Toxoplasma and Plasmodium infection. In vitro studies showed that upon exposure to T. gondii tachyzoites and Plasmodium-iRBCs, microglia underwent morphological changes and upregulated the expression of inflammatory markers12,13. An important question is whether the microglial phenotype is modulated through direct effects of the parasites or exposure to inflammatory mediators. Bhandage et al. revealed that T. gondii–infected microglia displayed a hypermotile phenotype dependent on the presence of live parasites in the cells14. Microglial hypermigratory behavior is mediated by autocrine GABAergic signaling in T. gondii–infected cells14. However, these data were derived from in vitro studies, and the behavior of infected microglia in vivo needs to be investigated.
In contrast to T. gondii infection, Plasmodium-iRBCs do not directly interact with microglia. Instead, hemodynamic alterations resulting from adherence of inflammatory leukocytes to postcapillary venule endothelium lead to leakage of plasma into the postcapillary space15. Exposure of microglia to inflammatory milieu in the postcapillary space may lead to their activation15. In addition, oxygen deprivation may provide additional cues for microglial activation16. Collectively, microglial activation can stem from direct interaction with the parasites or changes in the microenvironment surrounding the tissue harboring parasitic lesions.
A hallmark of microglial activation is their acquisition of a pro-inflammatory phenotype. The interleukin 1 (IL-1) family is one of the important groups of pro-inflammatory cytokines upregulated in reactive microglia during several neurodegenerative diseases17,18. The role of microglial IL-1 expression has been investigated in CT and CM. A recent study from the Harris lab indicates that IL-1α signaling is critical for the interplay between CNS cells during CT19. Using RNA sequencing, transgenic mice, and ex vivo cytokine assays, the authors showed that microglia was the major source of IL-1α in T. gondii–infected brain19. Moreover, IL-1α plays a critical role in the recruitment of immune cells and consequently restriction of parasite growth. The protective effect of microglial IL-1α is mediated by upregulating expression of adhesion molecules on endothelial cells19.
Though clearly protective in CT, microglial IL-1 has potential adverse effects on neuronal function in ECM. Reverchon et al. showed that induction of microglial IL-1β during ECM was associated with impairment of memory and learning20,21. Interestingly, this microglial IL-1 response appears to be driven by IL-33 produced by astrocytes and oligodendrocytes20,21. IL-1 itself enhanced IL-33 production by oligodendrocytes, suggesting a potent synergistic crosstalk between microglia and oligodendrocytes mediated by alarmins that may augment the neuroinflammatory responses and neurological symptoms during ECM. Although these results suggest that the reduction of endogenous IL-33 may be a therapeutic target for ECM and alleviates neuronal damage, other studies show that exogenous administration of IL-33 in the early stages of ECM has protective outcomes. IL-33 treatment with or without coadministration of antimalaria drugs results in a reduction of cerebral lesions and amelioration of neuropathological symptoms22,23. The protective effects of exogenous IL-33 were associated with TH2 cell polarization, regulatory T-cell response and decreased inflammasome activation in microglia, and most likely a reduction in destructive TH1 response22,23.
Tumor necrosis factor alpha (TNF-α) is another major pro-inflammatory cytokine contributing to the development of CT and CM pathogenesis. In CT, TNF-α signaling has a protective role as it is crucial for microglial production of nitric oxide and restriction of parasite growth24,25. Conversely in ECM, it appears that TNF-α signaling is detrimental as it mediates intercellular adhesion molecule 1 (ICAM-1) upregulation in brain endothelial cells and leukocyte sequestration26. In vitro studies showed that TNF-α was upregulated in microglia during T. gondii infection and Plasmodium-derived extracellular vesicle/iRBC stimulation13,27,28. In addition, microglial TNF-α level is increased in T. gondii- and P. berghei ANKA (PbA)-infected mice29,30. Deckert-Schlüter et al. reported that TNF-α induction in microglia during CT was dependent on signals downstream of the interferon gamma (IFN-γ) receptor31. Depletion of CD8+ T cells, a major source of IFN-γ, results in impaired production of microglial cytokines, including TNF-α in T. gondii–infected brain, highlighting a regulatory role for T cells and IFN-γ signaling on microglia cytokine production32. Furthermore, production of IFN-γ by microglia themselves33 may serve as an autocrine signal for TNF-α production.
Given the potent effects of TNF-α on CNS physiology, such as the regulation of synaptic activity34, and CNS remyelination35, it will be important to interrogate the effects of increased levels of TNF-α on neuronal and astroglia functions during CT and CM. For example, impairment of neurotransmitter uptake occurs in TNF-α–activated astrocytes36 and may result in excessive neuronal excitatory stimulation and death. Moreover, neutralization of TNF-α in IL-10–deficient mice infected with Plasmodium chabaudi reduces astrocyte activation and disease severity37.
The phagocytic function of microglia represents an important aspect of the intimate microglia–neuron interaction, as it is crucial for elimination of undesired synapses and apoptotic neurons38. A recent study by Carrillo et al. showed that chronic CT caused the loss of perisomatic inhibitory synapses and ensheathment of neurons by activated microglia in hippocampus and neocortex39. Li et al. further advanced our understanding about molecular mechanisms underlying microglia–neuron interaction during CT40. The authors reported that degenerating neurons marked by complement proteins (C1q and C3) and elevated levels of fractalkine chemokine (CX3CL1) were surrounded by activated microglia. As the fractalkine receptor (CX3CR1), among CNS cells, is exclusively expressed on microglia41, fractalkine signaling may regulate the recruitment of microglia to the site of tissue injury and initiate microglia–neuron communication during CT. Furthermore, the presence of complement protein deposits on damaged neurons makes them susceptible to clearance by surrounding microglia40. Collectively, these findings suggest that destruction of inhibitory synapse and neuronal structures by phagocytic microglia may cause neuronal dysfunction leading to the emergence of neurological problems.
Astrocyte responses in cerebral toxoplasmosis and cerebral malaria
Astrocytes, the most abundant glial cell type in the CNS, constitute a heterogenous cell population that maintains neuronal homeostasis. Astrocytes perform a plethora of functions, including regulation of energy metabolism42, supporting synaptic structure and plasticity43,44, and maintenance and regulation of the BBB45.
Disruption of the BBB integrity is a critical step in the pathogenesis of CM leading to brain edema that damages neuronal structure46. Medana et al. reported that retinal astrocytes exhibited an uneven distribution and ensheathment of retinal blood vessels before the expression of neurological signs47. Furthermore, in the late stages of ECM, retinal astrocytes lost their contact with the blood–retinal barrier (BRB)47. These findings suggest that loss of astrocytic support on BRB may be involved in the BRB compromise, hemorrhage, and edema.
Astrocytes respond to noxious insults through rapid morphological changes that contain and restrict the spread of tissue injury. Upregulation of the astrocytic cytoskeletal glial fibrillary acidic protein (GFAP) and the subsequent reactive astrogliosis limit pathogen distribution in the CNS48. Activated astrocytes with increased GFAP levels have been observed in the brain during chronic CT49 and ECM13. The protective role of GFAP upregulation during CT was demonstrated by Stenzel et al.49. The authors showed that GFAP knockout (KO) mice infected with T. gondii were unable to control parasite growth and confine inflammatory lesions caused by the parasite49. These findings emphasize that activation of astrocytes is critical for a strong protective anti-Toxoplasma response. It is not yet clear whether GFAP upregulation is associated with protective or detrimental outcomes in CM. Mice lacking both astrocytic intermediate filaments—GFAP and vimentin—have been shown to exhibit impaired astrocyte activation and larger infarct size after ischemic brain injury50. Given the presence of sequestered iRBCs, occlusion of the BBB, and induction of hypoxia described in ECM, it is critical to investigate the role of astrocyte reactivity in this disease.
Astrocytes also mount diverse immunological responses, including production of specific pro- and anti-inflammatory mediators during CT and ECM. IFN-γ plays a critical role in the destruction of T. gondii in the host cells through different mechanisms51. One of the mechanisms is the activation of signal transducer STAT1, which induces transcription of IFN-γ–dependent genes such as IRF-152. Using transgenic mice with specific deletion of STAT1 in astrocytes, Hidano et al. showed that a more severe form of CT developed with a higher mortality and cerebral parasite load, suggesting that the astrocytic IFN-γ signaling response to the parasite has protective outcomes53. Moreover, loss of STAT1 signaling in astrocytes caused a shift in parasite tropism from neurons to astrocytes as higher numbers of parasite cysts appeared in STAT1 KO astrocytes53. The protective function of astrocytes in response to IFN-γ and clearance of parasites could be one explanation of why there are no T. gondii astrocytic cysts in wild-type (WT) mice. IFN-γ production during ECM, in contrast to CT, contributes to cerebral increase of Plasmodium biomass since IFN-γ–deficient mice exhibit lower parasite loads and iRBCs in the brain54. Of note, IFN-γ produced by CD4+ T cells induces expression of CXCL9 and CXCL10 in cerebral endothelial cells, leading to firm attachment of T cells to brain vasculature and ultimately infiltration of cytotoxic CD8+ T cells55–57. Although the sources of CXCL9 and CXCL10 during ECM have not been completely determined, astrocytes may represent a potential source for these chemokines as PbA-iRBC–stimulated astrocytes displayed increased levels of CXCL1013.
The signaling pathway downstream of glycoprotein 130 (gp130), the ubiquitous signal transducer for members of the IL-6 cytokine family, plays a critical role in the establishment of protective and detrimental responses during CT and ECM. IL-6 signaling regulates various functions in astrocytes, such as activation of JAK/STAT3 signaling pathway, regulation of cell proliferation, and expression of GFAP and vimentin58,59. It has been demonstrated that IL-6 is required for the restriction of T. gondii proliferation in the brain and the development of protective response against CT60. In vivo deletion of astrocytic gp130 resulted in reduced astrocyte activation leading to impaired parasite confinement and ultimately the development of extensive necrotic lesions61. Conversely, in ECM, IL-6 production has been associated with neurotoxic sequelae. Administration of anti-IL-6 neutralizing antibodies in PbA-infected mice resulted in reduced astrocyte activation associated with decreased glial nodules, neuronal death, and longer survival62, suggesting neurotoxic roles of astrocytes driven by IL-6 signaling in ECM.
IL-33 is released from necrotic cells as a cellular alarmin and interacts with its receptor, the orphan IL-1 receptor family member ST2. It has been demonstrated that ST2 is upregulated during CT in the brain, and IL-33/ST2 signaling is found to prevent T. gondii growth and decrease neural tissue destruction63. Still et al. showed that oligodendrocytes and astrocytes were the major sources of IL-33 in T. gondii–infected brain64. Importantly, IL-33 signals on astrocytes through ST2 receptor leading to the production of inflammatory chemokines such as CCL2 and CXCL10 and infiltration of leukocytes which results in the control of infection64. As discussed above, astrocytes in addition to oligodendrocytes also produce IL-33 during ECM20. In turn, IL-33 production by astrocytes likely leads to enhanced synaptic engulfment by microglia65 and increases the formation of excitatory synapses by neurons66. Therefore, it will be important to know how synapse structure and neuronal function are dysregulated by IL-33 produced during both CT and ECM, even though IL-33 has a clear protective effect by controlling Toxoplasma infection in the brain.
Transforming growth factor beta 1 (TGF-β1) is a pleiotropic cytokine that negatively regulates immunopathological responses during neuroinflammatory disorders. Cekanaviciute et al. reported that TGF-β1 signaling with anti-inflammatory effects was induced in astrocytes in response to T. gondii infection67. That study showed that in vivo inhibition of astrocytic TGF-β1 signaling did not affect T. gondii burden in the brain but did result in increased activation of nuclear factor kappa B (NF-κB) pathway and subsequently upregulation of CCL5, accompanied by increased T-cell infiltration and neuronal death67. NF-κB activation in astrocytes regulates inflammatory responses involved in pathological outcomes of neurodegenerative diseases (reviewed in 68). For example, activation of astroglia NF-κB leads to overexpression of complement protein C3, reduced synaptogenesis, and disruption of dendrite morphology contributing to impairment of neuronal activity69. In vitro inhibition of NF-κB in astrocytes exposed to T. gondii antigens consistently decreased the levels of neurotoxic markers, including complement protein C3, suggesting that astrocytic NF-κB signaling may contribute to neurological deficits of CT70. However, by inducing IL-1, NF-κB signaling itself is critical for instigating TGF-β counter-regulation71. Negative feedback regulation by TGF-β may represent an important mechanism for minimizing the detrimental effects of the pro-inflammatory response while maintaining its parasite restrictive functions.
In addition to mediating immunoregulatory TGF-β signaling, astrocytes perform neuroprotective functions, including maintenance of brain water homeostasis and antioxidant defense. Expression of the water channel aquaporin-4 (AQP4) by astrocytes is critical for preventing excessive brain edema during ECM72,73. Upregulation of the antioxidant protein neuroglobin by astrocytes during ECM74 may also minimize tissue injury.
Concluding remarks
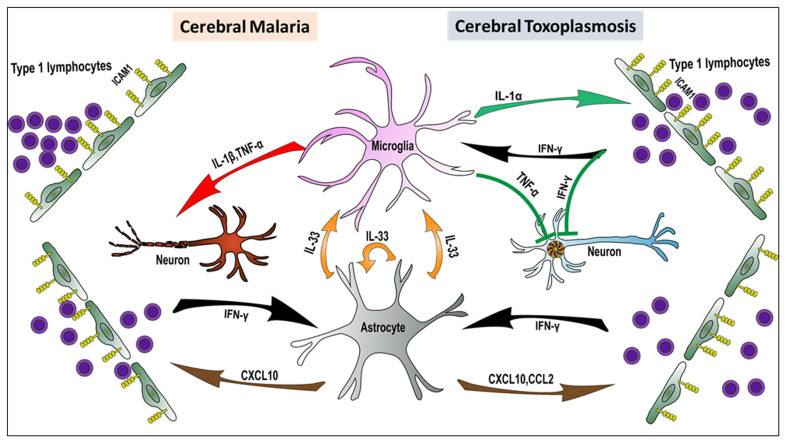
Here, we have discussed the experimental evidence indicating that microglia and astrocytes are critical regulators of CNS immune responses during Toxoplasma and Plasmodium infection. Reflecting the distinct cellular tropisms of these two infections, these immune responses are primarily protective in CT because they drive effector mechanisms critical for controlling T. gondii replication in the CNS. By contrast, in CM, the TH1 response is largely detrimental, negatively affecting neurological function. However, it is likely that collateral neurotoxic effects occur during CT when the extent of glial activation exceeds what is required for parasite removal. As shown in Figure 1, tissue-resident astrocytes and microglia act as sentinel innate cells that trigger the infiltration/sequestration of the CNS by the peripheral immune cells. Crosstalk between astrocytes (also oligodendrocytes) and microglia is mediated by the alarmins IL-33 and IL-1. It will be important to investigate what parasite-derived versus endogenous signals drive alarmin upregulation and release.
Figure 1. Alarmin-mediated microglia–astrocyte crosstalk during cerebral toxoplasmosis (right) and cerebral malaria (left).
Activated astrocytes and microglia initiate signaling pathways required for peripheral immune cell infiltration/sequestration. In cerebral toxoplasmosis, these immune pathways result in the control of Toxoplasma gondii replication and partial restoration of tissue homeostasis. Conversely, in cerebral malaria, immune responses elicited in microglia and astrocytes lead to impairment of neuronal function and exacerbation of neurological symptoms. ICAM1, intercellular adhesion molecule 1; IFN-γ, interferon gamma; IL, interleukin; TNF-α, tumor necrosis factor alpha.
The primary consequence of immune infiltration by type 1 lymphocytes is the differentiation of microglia and astrocytes into M1-like and A1-like type cells75,76, that have antiparasitic effector functions but also possess neurotoxic potential. As discussed above, immunoregulation by TGF-β is essential for curtailing this inherently destructive potential. What signals trigger the transitioning of astrocyte and microglial phenotypes from neurotoxic to reparative is another area for future studies. Deployment of single-cell RNA-sequencing technology will be helpful in defining the cellular heterogeneity, dynamics, and regulatory circuits that govern this important transition.
The peer reviewers who approve this article are:
Robin Stephens, Department of Internal Medicine, Division of Infectious Diseases, University of Texas Medical Branch, Galveston, TX, USA
Alexander W Pfaff, Institut de Parasitologie et Pathologie Tropicale, Fédération de Médecine Translationnelle, Université de Strasbourg, Strasbourg, France
Tajie H. Harris, Center for Brain Immunology and Glia, Department of Neuroscience, University of Virginia, Charlottesville, Virginia, USA
Funding Statement
This work was supported by the grant R21AI129870 from the US National Institute of Health.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Amor S, Puentes F, Baker D, et al. : Inflammation in neurodegenerative diseases. Immunology. 2010; 129(2): 154–69. 10.1111/j.1365-2567.2009.03225.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arling TA, Yolken RH, Lapidus M, et al. : Toxoplasma gondii antibody titers and history of suicide attempts in patients with recurrent mood disorders. J Nerv Ment Dis. 2009; 197(12): 905–8. 10.1097/NMD.0b013e3181c29a23 [DOI] [PubMed] [Google Scholar]
- 3.Torrey EF, Yolken RH: Toxoplasma gondii and schizophrenia. Emerging Infect Dis. 2003; 9(11): 1375–80. 10.3201/eid0911.030143 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 4.Flegr J, Horáček J: Negative Effects of Latent Toxoplasmosis on Mental Health. Front Psychiatry. 2019; 10: 1012. 10.3389/fpsyt.2019.01012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt NH, Ball HJ, Hansen AM, et al. : Cerebral malaria: Gamma-interferon redux. Front Cell Infect Microbiol. 2014; 4: 113. 10.3389/fcimb.2014.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hearn J, Rayment N, Landon DN, et al. : Immunopathology of cerebral malaria: Morphological evidence of parasite sequestration in murine brain microvasculature. Infect Immun. 2000; 68(9): 5364–76. 10.1128/IAI.68.9.5364-5376.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belnoue E, Kayibanda M, Vigario AM, et al. : On the pathogenic role of brain-sequestered alphabeta CD8+ T cells in experimental cerebral malaria. J Immunol. 2002; 169(11): 6369–75. 10.4049/jimmunol.169.11.6369 [DOI] [PubMed] [Google Scholar]
- 8.Idro R, Marsh K, John CC, et al. : Cerebral malaria: Mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr Res. 2010; 68(4): 267–74. 10.1203/PDR.0b013e3181eee738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q, Barres BA: Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2018; 18(4): 225–42. 10.1038/nri.2017.125 [DOI] [PubMed] [Google Scholar]
- 10.Crotti A, Ransohoff RM: Microglial Physiology and Pathophysiology: Insights from Genome-wide Transcriptional Profiling. Immunity. 2016; 44(3): 505–15. 10.1016/j.immuni.2016.02.013 [DOI] [PubMed] [Google Scholar]
- 11.Liu LR, Liu JC, Bao JS, et al. : Interaction of Microglia and Astrocytes in the Neurovascular Unit. Front Immunol. 2020; 11: 1024. 10.3389/fimmu.2020.01024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dellacasa-Lindberg I, Fuks JM, Arrighi RBG, et al. : Migratory activation of primary cortical microglia upon infection with Toxoplasma gondii. Infect Immun. 2011; 79(8): 3046–52. 10.1128/IAI.01042-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shrivastava SK, Dalko E, Delcroix-Genete D, et al. : Uptake of parasite-derived vesicles by astrocytes and microglial phagocytosis of infected erythrocytes may drive neuroinflammation in cerebral malaria. Glia. 2017; 65(1): 75–92. 10.1002/glia.23075 [DOI] [PubMed] [Google Scholar]
- 14.Bhandage AK, Kanatani S, Barragan A: Toxoplasma- Induced Hypermigration of Primary Cortical Microglia Implicates GABAergic Signaling. Front Cell Infect Microbiol. 2019; 9: 73. 10.3389/fcimb.2019.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 15.Nacer A, Movila A, Sohet F, et al. : Experimental cerebral malaria pathogenesis--hemodynamics at the blood brain barrier. PLoS Pathog. 2014; 10(12): e1004528. 10.1371/journal.ppat.1004528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaur C, Rathnasamy G, Ling EA: Roles of activated microglia in hypoxia induced neuroinflammation in the developing brain and the retina. J Neuroimmune Pharmacol. 2013; 8(1): 66–78. 10.1007/s11481-012-9347-2 [DOI] [PubMed] [Google Scholar]
- 17.Wang WY, Tan MS, Yu JT, et al. : Role of pro-inflammatory cytokines released from microglia in Alzheimer's disease. Ann Transl Med. 2015; 3(10): 136. 10.3978/j.issn.2305-5839.2015.03.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hickman S, Izzy S, Sen P, et al. : Microglia in neurodegeneration. Nat Neurosci. 2018; 21(10): 1359–69. 10.1038/s41593-018-0242-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batista SJ, Still KM, Johanson D, et al. : Gasdermin-D-dependent IL-1α release from microglia promotes protective immunity during chronic Toxoplasma gondii infection. Nat Commun. 2020; 11(1): 3687. 10.1038/s41467-020-17491-z [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 20.Reverchon F, Mortaud S, Sivoyon M, et al. : IL-33 receptor ST2 regulates the cognitive impairments associated with experimental cerebral malaria. PLoS Pathog. 2017; 13(4): e1006322. 10.1371/journal.ppat.1006322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reverchon F, de Concini V, Larrigaldie V, et al. : Hippocampal interleukin-33 mediates neuroinflammation-induced cognitive impairments. J Neuroinflammation. 2020; 17(1): 268. 10.1186/s12974-020-01939-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Besnard AG, Guabiraba R, Niedbala W, et al. : IL-33-mediated protection against experimental cerebral malaria is linked to induction of type 2 innate lymphoid cells, M2 macrophages and regulatory T cells. PLoS Pathog. 2015; 11(2): e1004607. 10.1371/journal.ppat.1004607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strangward P, Haley MJ, Albornoz MG, et al. : Targeting the IL33-NLRP3 axis improves therapy for experimental cerebral malaria. Proc Natl Acad Sci U S A. 2018; 115(28): 7404–9. 10.1073/pnas.1801737115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deckert-Schlüter M, Bluethmann H, Rang A, et al. : Crucial role of TNF receptor type 1 (p55), but not of TNF receptor type 2 (p75), in murine toxoplasmosis. J Immunol. 1998; 160(7): 3427–36. [PubMed] [Google Scholar]
- 25.Gazzinelli RT, Eltoum I, Wynn TA, et al. : Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-alpha and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993; 151(7): 3672–81. [PubMed] [Google Scholar]
- 26.Lucas R, Lou JN, Juillard P, et al. : Respective role of TNF receptors in the development of experimental cerebral malaria. J Neuroimmunol. 1997; 72(2): 143–8. 10.1016/s0165-5728(96)00185-3 [DOI] [PubMed] [Google Scholar]
- 27.Mbagwu SI, Lannes N, Walch M, et al. : Human Microglia Respond to Malaria-Induced Extracellular Vesicles. Pathogens. 2019; 9(1): 21. 10.3390/pathogens9010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer HG, Nitzgen B, Reichmann G, et al. : Cytokine responses induced by Toxoplasma gondii in astrocytes and microglial cells. Eur J Immunol. 1997; 27(6): 1539–48. 10.1002/eji.1830270633 [DOI] [PubMed] [Google Scholar]
- 29.Medana IM, Hunt NH, Chaudhri G: Tumor necrosis factor-alpha expression in the brain during fatal murine cerebral malaria: Evidence for production by microglia and astrocytes. Am J Pathol. 1997; 150(4): 1473–86. [PMC free article] [PubMed] [Google Scholar]
- 30.Deckert M, Sedgwick JD, Fischer E, et al. : Regulation of microglial cell responses in murine Toxoplasma encephalitis by CD200/CD200 receptor interaction. Acta Neuropathol. 2006; 111(6): 548–58. 10.1007/s00401-006-0062-z [DOI] [PubMed] [Google Scholar]
- 31.Deckert-Schlüter M, Bluethmann H, Kaefer N, et al. : Interferon-gamma Receptor-Mediated but Not Tumor Necrosis Factor Receptor Type 1- or Type 2-Mediated Signaling Is Crucial for the Activation of Cerebral Blood Vessel Endothelial Cells and Microglia in Murine Toxoplasma Encephalitis. Am J Pathol. 1999; 154(5): 1549–61. 10.1016/s0002-9440(10)65408-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlüter D, Meyer T, Strack A, et al. : Regulation of microglia by CD4+ and CD8+ T cells: Selective analysis in CD45-congenic normal and Toxoplasma gondii-infected bone marrow chimeras. Brain Pathol. 2001; 11(1): 44–55. 10.1111/j.1750-3639.2001.tb00380.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki Y, Claflin J, Wang X, et al. : Microglia and macrophages as innate producers of interferon-gamma in the brain following infection with Toxoplasma gondii. Int J Parasitol. 2005; 35(1): 83–90. 10.1016/j.ijpara.2004.10.020 [DOI] [PubMed] [Google Scholar]
- 34.Rizzo FR, Musella A, de Vito F, et al. : Tumor Necrosis Factor and Interleukin-1 β Modulate Synaptic Plasticity during Neuroinflammation. Neural Plast. 2018; 2018: 8430123. 10.1155/2018/8430123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnett HA, Mason J, Marino M, et al. : TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001; 4(11): 1116–22. 10.1038/nn738 [DOI] [PubMed] [Google Scholar]
- 36.Trindade P, Loiola EC, Gasparotto J, et al. : Short and long TNF-alpha exposure recapitulates canonical astrogliosis events in human-induced pluripotent stem cells-derived astrocytes. Glia. 2020; 68(7): 1396–409. 10.1002/glia.23786 [DOI] [PubMed] [Google Scholar]
- 37.Wilson KD, Ochoa LF, Solomon OD, et al. : Elimination of intravascular thrombi prevents early mortality and reduces gliosis in hyper-inflammatory experimental cerebral malaria. J Neuroinflammation. 2018; 15(1): 173. 10.1186/s12974-018-1207-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galloway DA, Phillips AEM, Owen DRJ, et al. : Phagocytosis in the Brain: Homeostasis and Disease. Front Immunol. 2019; 10: 790. 10.3389/fimmu.2019.00790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carrillo GL, Ballard VA, Glausen T, et al. : Toxoplasma infection induces microglia-neuron contact and the loss of perisomatic inhibitory synapses. Glia. 2020; 68(10): 1968–86. 10.1002/glia.23816 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 40.Li Y, Severance EG, Viscidi RP, et al. : Persistent Toxoplasma Infection of the Brain Induced Neurodegeneration Associated with Activation of Complement and Microglia. Infect Immun. 2019; 87(8): e00139–19. 10.1128/IAI.00139-19 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 41.Cardona AE, Pioro EP, Sasse ME, et al. : Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006; 9(7): 917–24. 10.1038/nn1715 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 42.Morita M, Ikeshima-Kataoka H, Kreft M, et al. : Metabolic Plasticity of Astrocytes and Aging of the Brain. Int J Mol Sci. 2019; 20(4): 941. 10.3390/ijms20040941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allen NJ, Eroglu C: Cell Biology of Astrocyte-Synapse Interactions. Neuron. 2017; 96(3): 697–708. 10.1016/j.neuron.2017.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 44.Perez-Catalan NA, Doe CQ, Ackerman SD: The role of astrocyte-mediated plasticity in neural circuit development and function. Neural Dev. 2021; 16(1): 1. 10.1186/s13064-020-00151-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daneman R, Prat A: The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015; 7(1): a020412. 10.1101/cshperspect.a020412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tunon-Ortiz A, Lamb TJ: Blood brain barrier disruption in cerebral malaria: Beyond endothelial cell activation. PLoS Pathog. 2019; 15(6): e1007786. 10.1371/journal.ppat.1007786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medana IM, Chan-Ling T, Hunt NH: Redistribution and degeneration of retinal astrocytes in experimental murine cerebral malaria: Relationship to disruption of the blood-retinal barrier. Glia. 1996; 16(1): 51–64. [DOI] [PubMed] [Google Scholar]
- 48.Sofroniew MV: Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009; 32(12): 638–47. 10.1016/j.tins.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stenzel W, Soltek S, Schlüter D, et al. : The intermediate filament GFAP is important for the control of experimental murine Staphylococcus aureus-induced brain abscess and Toxoplasma encephalitis. J Neuropathol Exp Neurol. 2004; 63(6): 631–40. 10.1093/jnen/63.6.631 [DOI] [PubMed] [Google Scholar]
- 50.Li L, Lundkvist A, Andersson D, et al. : Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab. 2008; 28(3): 468–81. 10.1038/sj.jcbfm.9600546 [DOI] [PubMed] [Google Scholar]
- 51.Könen-Waisman S, Howard JC: Cell-autonomous immunity to Toxoplasma gondii in mouse and man. Microbes Infect. 2007; 9(14–15): 1652–61. 10.1016/j.micinf.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 52.Krause CD, He W, Kotenko S, et al. : Modulation of the activation of Stat1 by the interferon-gamma receptor complex. Cell Res. 2006; 16(1): 113–23. 10.1038/sj.cr.7310015 [DOI] [PubMed] [Google Scholar]
- 53.Hidano S, Randall LM, Dawson L, et al. : STAT1 Signaling in Astrocytes Is Essential for Control of Infection in the Central Nervous System. mBio. 2016; 7(6): e01881–16. 10.1128/mBio.01881-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Claser C, Malleret B, Gun SY, et al. : CD8+ T cells and IFN-γ mediate the time-dependent accumulation of infected red blood cells in deep organs during experimental cerebral malaria. PLoS One. 2011; 6(4): e18720. 10.1371/journal.pone.0018720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Villegas-Mendez A, Greig R, Shaw TN, et al. : IFN-γ-producing CD4+ T cells promote experimental cerebral malaria by modulating CD8+ T cell accumulation within the brain. J Immunol. 2012; 189(2): 968–79. 10.4049/jimmunol.1200688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sorensen EW, Lian J, Ozga AJ, et al. : CXCL10 stabilizes T cell-brain endothelial cell adhesion leading to the induction of cerebral malaria. JCI Insight. 2018; 3(8): e98911 10.1172/jci.insight.98911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghazanfari N, Gregory JL, Devi S, et al. : CD8+ and CD4+ T Cells Infiltrate into the Brain during Plasmodium berghei ANKA Infection and Form Long-Term Resident Memory. J Immunol. 2021; ji2000773. 10.4049/jimmunol.2000773 [DOI] [PubMed] [Google Scholar]
- 58.Selmaj KW, Farooq M, Norton WT, et al. : Proliferation of astrocytes in vitro in response to cytokines. A primary role for tumor necrosis factor. J Immunol. 1990; 144(1): 129–35. [PubMed] [Google Scholar]
- 59.Ben Haim L, Carrillo-de Sauvage MA, Ceyzériat K, et al. : Elusive roles for reactive astrocytes in neurodegenerative diseases. Front Cell Neurosci. 2015; 9: 278. 10.3389/fncel.2015.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suzuki Y, Rani S, Liesenfeld O, et al. : Impaired resistance to the development of toxoplasmic encephalitis in interleukin-6-deficient mice. Infect Immun. 1997; 65(6): 2339–45. 10.1128/iai.65.6.2339-2345.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drögemüller K, Helmuth U, Brunn A, et al. : Astrocyte gp130 expression is critical for the control of Toxoplasma encephalitis. J Immunol. 2008; 181(4): 2683–93. 10.4049/jimmunol.181.4.2683 [DOI] [PubMed] [Google Scholar]
- 62.Sarkar S, Keswani T, Sengupta A, et al. : Differential modulation of glial cell mediated neuroinflammation in Plasmodium berghei ANKA infection by TGF β and IL 6. Cytokine. 2017; 99: 249–59. 10.1016/j.cyto.2017.07.026 [DOI] [PubMed] [Google Scholar]
- 63.Jones LA, Roberts F, Nickdel MB, et al. : IL-33 receptor (T1/ST2) signalling is necessary to prevent the development of encephalitis in mice infected with Toxoplasma gondii. Eur J Immunol. 2010; 40(2): 426–36. 10.1002/eji.200939705 [DOI] [PubMed] [Google Scholar]
- 64.Still KM, Batista SJ, O'Brien CA, et al. : Astrocytes promote a protective immune response to brain Toxoplasma gondii infection via IL-33-ST2 signaling. PLoS Pathog. 2020; 16(10): e1009027. 10.1371/journal.ppat.1009027 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 65.Vainchtein ID, Chin G, Cho FS, et al. : Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science. 2018; 359(6381): 1269–73. 10.1126/science.aal3589 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 66.Wang Y, Fu WY, Cheung K, et al. : Astrocyte-secreted IL-33 mediates homeostatic synaptic plasticity in the adult hippocampus. Proc Natl Acad Sci U S A. 2021; 118(1): e2020810118. 10.1073/pnas.2020810118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cekanaviciute E, Dietrich HK, Axtell RC, et al. : Astrocytic TGF-β signaling limits inflammation and reduces neuronal damage during central nervous system Toxoplasma infection. J Immunol. 2014; 193(1): 139–49. 10.4049/jimmunol.1303284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shih RH, Wang CY, Yang CM: NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front Mol Neurosci. 2015; 8: 77. 10.3389/fnmol.2015.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lian H, Yang L, Cole A, et al. : NFκB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer's disease. Neuron. 2015; 85(1): 101–15. 10.1016/j.neuron.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jin Y, Yao Y, El-Ashram S, et al. : The Neurotropic Parasite Toxoplasma gondii Induces Astrocyte Polarization Through NFκB Pathway. Front Med (Lausanne). 2019; 6: 267. 10.3389/fmed.2019.00267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rameshwar P, Narayanan R, Qian J, et al. : NF-kappa B as a central mediator in the induction of TGF-beta in monocytes from patients with idiopathic myelofibrosis: an inflammatory response beyond the realm of homeostasis. J Immunol. 2000; 165(4): 2271–7. 10.4049/jimmunol.165.4.2271 [DOI] [PubMed] [Google Scholar]
- 72.Szu JI, Binder DK: The Role of Astrocytic Aquaporin-4 in Synaptic Plasticity and Learning and Memory. Front Integr Neurosci. 2016; 10: 8. 10.3389/fnint.2016.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Promeneur D, Lunde LK, Amiry-Moghaddam M, et al. : Protective role of brain water channel AQP4 in murine cerebral malaria. Proc Natl Acad Sci U S A. 2013; 110(3): 1035–40. 10.1073/pnas.1220566110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.DellaValle B, Hempel C, Kurtzhals JA, et al. : In vivo expression of neuroglobin in reactive astrocytes during neuropathology in murine models of traumatic brain injury, cerebral malaria, and autoimmune encephalitis. Glia. 2010; 58(10): 1220–7. 10.1002/glia.21002 [DOI] [PubMed] [Google Scholar]
- 75.Liddelow SA, Guttenplan KA, Clarke LE, et al. : Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017; 541(7638): 481–7. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 76.Zamanian JL, Xu L, Foo LC, et al. : Genomic analysis of reactive astrogliosis. J Neurosci. 2012; 32(18): 6391–410. 10.1523/JNEUROSCI.6221-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation