Abstract
Tic disorders and Tourette syndrome are the most common movement disorders in children and are characterized by movements or vocalizations. Clinically, Tourette syndrome is frequently associated with comorbid psychiatric symptoms. Although dysfunction of cortical–striatal–thalamic–cortical circuits with aberrant neurotransmitter function has been considered the proximate cause of tics, the mechanism underlying this association is unclear. Recently, many studies have been conducted to elucidate the epidemiology, clinical course, comorbid symptoms, and pathophysiology of tic disorders by using laboratory studies, neuroimaging, electrophysiological testing, environmental exposure, and genetic testing. In addition, many researchers have focused on treatment for tics, including behavioral therapy, pharmacological treatment, and surgical treatment. Here, we provide an overview of recent progress on Tourette syndrome.
Keywords: Tourette syndrome, Tics, Tic disorders, Neuropsychology, Comorbidity
Introduction
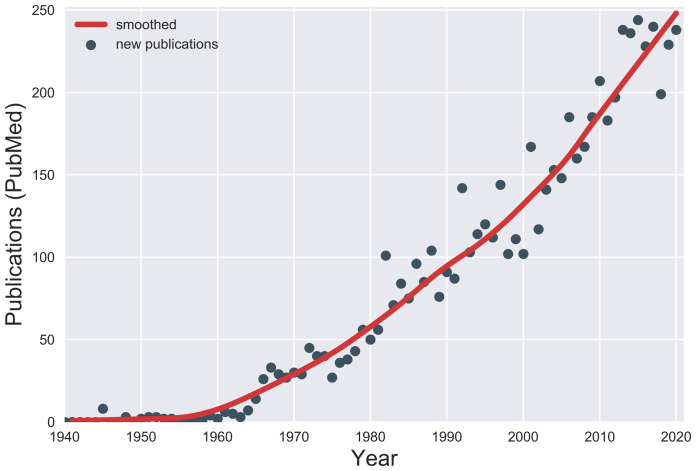
Tic disorders are characterized by sudden, rapid, recurrent movement (motor tics) or vocalization (vocal or phonic tics)1. Tourette syndrome (TS) indicates the presence of multiple motor and vocal tics spanning a period of more than 1 year, and onset is before the age of 18 years. The cardinal features of tics are a premonitory urge (an unpleasant sensation preceding tics)2, suppressibility (the ability to voluntarily suppress tics for variable periods)3, and suggestibility (more likely to experience a tic when it is mentioned)4. Tics can be associated with various psychiatric comorbidities, which can complicate the clinical picture. Since the most recent previous review in this journal5, the number of publications on tic disorders has been steadily increasing (Figure 1), leading to a need for this update6. Thus, we have reviewed recent progress on the clinical course, epidemiology, comorbidities, pathophysiology, and treatment of TS. We searched PubMed for articles published between 2018 and 2021 and used search terms such as tic disorders or Tourette. We then subjectively selected articles that met the objectives of this review. We also included articles that were recommended by colleagues.
Figure 1. Publications on Tourette syndrome.
The number of new publications on Tourette syndrome or other tic disorders each year was estimated from PubMed. PubMed was searched by using the search string “(“Tic Disorders”[MeSH] OR Tourette NOT Tourette[AU]) AND year[PDAT] NOT year+1[PDAT]” for each year from 1940 through 2020.
Clinical course and phenomenology
Recent years have seen changes to definitions of tic disorders. The Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision (DSM-IV-TR) required a year of tics, excluding any tic-free period of three months or longer, for the diagnosis of TS; however, the three-month requirement was removed in the DSM-51. The International Classification of Diseases 10th revision (ICD-10) required “multiple motor tics” and one or more vocal tics for the diagnosis of TS, but the requirement for more than one motor tic was removed in ICD-117.
Tic symptoms generally follow a fluctuating course in terms of severity and frequency, with a mixture of old and new tics, and may be exacerbated by psychological and physical strains (such as anxiety or fatigue) and environmental changes8,9. Traditionally, experts have asserted that a tic disorder that began only in the past few months would usually disappear within a year; however, a direct follow-up study revealed that at least 90% of children still exhibited tics after a year10. Among them, children who could suppress tics better under conditions of immediate reward showed less severe tics at follow-up11. A population-based longitudinal study of parents and children with TS showed that low socioeconomic status was a risk factor for TS and chronic tic disorders12. A longitudinal cohort study of children with TS at the Danish National Tourette Clinic identified that severity of tics, attention-deficit/hyperactivity disorder (ADHD), and obsessive–compulsive disorder (OCD) in childhood strongly predicted the corresponding severity scores in early adulthood13. In the same group, 18% of participants who were at least 16 years old were tic-free, 60% had minimal or mild tics, and 22% had moderate to severe tics, indicating an age-related decrease in tics14.
The Yale Global Tic Severity Scale (YGTSS) was invented to rate tic severity15. A large cross-sectional study of 617 adults and children with tic disorders found good internal consistency of the YGTSS between children and adults but suggested some modifications to improve its usability and value16. Similarly, the European Multicentre Tics in Children Study (EMTICS), which includes 16 clinical sites, examined the psychometric quality of the YGTSS and found overall good results but suggested some modifications, including that the total tic severity scale and the impairment rating be used separately17.
Both motor and vocal tics are categorized as simple or complex18. Tics can be further classified as clonic, tonic, or dystonic on the basis of their phenomenology19,20. Clonic tics are abrupt, brief jerking movements. Dystonic tics are sustained abnormal movements, whereas tonic tics are isometric contractions. Some authors add blocking tics, namely transient interruptions of ongoing motor activities or speech without loss of consciousness20. A prospective study identified tonic tics in 85% of adults and 64% of children with TS; tonic tics correlated with the number of tics, severity, and comorbidities21.
Functional tic-like movements, which are usually rare in clinical practice, have recently drawn attention. Clinical features of tics and functional tic-like movements overlap and can coexist, which can make it difficult to differentiate tics from functional tic-like movements22. Compared with TS, functional tics are thought to be characterized by lack of premonitory urge and suppressibility, female preponderance, the presence of other functional symptoms, resistance to tic medications, and lack of positive family history of tic disorders22. During the Covid-19 pandemic, we encountered many adolescents with sudden onset of severe tics and tic-like movements. Some of the cases may be explained by increased stress exacerbating pre-existing tic disorders in the context of widespread social stressors, but other cases were more consistent with a functional tic-like disorder23. Some patients before onset of their symptoms had watched videos of social media influencers demonstrating similar tic symptoms, suggesting probable social contagion23.
Epidemiology
The true prevalence of tic disorders is difficult to estimate owing in part to symptom fluctuation over time and heterogeneous presentations24. In a meta-analysis of school-based studies, the prevalences of transient tic disorder (now called provisional tic disorder, tics of less than 1 year), chronic vocal tics (phonic tics of more than 1 year but no motor tics), chronic motor tics (motor tics of more than 1 year but no phonic tics), and tic disorder not otherwise specified (tics that do not meet criteria for other tic disorders) were estimated to be 3.0%, 0.7%, 1.7%, and 0.8%, respectively25. However, this analysis excluded several studies that used direct observation to identify tics, and these studies generally reported much higher prevalence. A meta-analysis using data from population-based studies conducted in China reported overall prevalences of 1.7% for transient tic disorder and 1.2% for chronic motor or vocal tic disorders26. A systematic review of 21 population-based studies of children who were 4 to 18 years old estimated the prevalence of TS to be 0.52% (95% confidence interval [CI] 0.32‒0.85)27, although some high-quality studies find prevalence to be as much as 10-fold higher28. In contrast, prevalence is much lower in adults; a meta-analysis of three studies involving 2,356,485 adults estimated the prevalence to be 0.001% (118 cases per million)29. However, given that most children with recent onset of tics continue to have tics for over 1 year10, this result probably underestimates the true prevalence of TS among the general population. A possible explanation for this is that a substantial number of patients with tics do not seek medical attention or do not realize they have tics since their tics are subtle and not troublesome. Therefore, a large population-based observational study is needed to evaluate the true prevalence of TS.
Pathophysiology
The pathophysiology of tic disorders is complicated, and studies have implicated cortical–striatal–thalamic–cortical (CSTC) circuits and associated neurotransmitters30,31. Various methods, including neuroimaging, physiological, laboratory, animal, and genetic studies, have been used to unveil its pathophysiology.
Neuroimaging studies
Several neuroimaging studies have investigated anatomical changes as well as structural and functional connectivity32. A follow-up study using high-resolution magnetic resonance imaging (MRI) in 41 children with new-onset tics showed that larger hippocampal volume at the baseline visit predicted higher tic severity at 1-year follow-up33. A high-resolution MRI study using voxel-based cerebellar morphometry and seed-to-voxel structural covariance mapping (structural connectivity) demonstrated reduced cerebellar gray matter volume in patients with TS, which was considered to affect higher-order cognitive and motor processing34. The study also showed abnormal gray matter structural connectivity with frontal and cingulate cortices and sensorimotor networks, indicating that cerebellar involvement in tics is part of cortico–basal ganglia–cerebellar interactions.
Functional imaging studies have been providing new insights into the temporal correlation of activity between different brain regions (i.e., functional connectivity)32. To investigate changes of functional brain organization in TS with development, a resting-state functional connectivity MRI study of 172 children and adults with TS was performed35. Functional connectivity alteration was age-specific: brain networks in children with TS appeared “older” whereas brain networks in adults with TS appeared “younger” than those in age-matched controls35. A resting-state functional MRI (fMRI) study using graph theoretical measures focused on topological changes of basal ganglia–thalamocortical and cortico–cerebellar brain networks in TS36. Patients with TS showed increased basal ganglia–cortical and thalamo–cortical connectivity and reduced cortico–cerebellar connectivity. They also showed decreased connectivity within the precuneus, posterior cingulate, and right and left angular gyri and within the right and left insular cortex. The altered connectivity may suggest a lack of brain maturation and interoception involvement as neural correlates of tics and premonitory urges.
A functional imaging study using whole-brain structural MRI with voxel-based morphometry techniques and seed-to-voxel structural covariance mapping was conducted to investigate structural connectivity of tic severity and of premonitory urges37. Motor severity and premonitory urge severity were not correlated and were associated with anatomically separate regions of the right insular cortex. This result suggests that tics and premonitory urges have separate network connectivity. A resting-state fMRI with seed-based functional connectivity analysis was conducted to investigate neuronal functional connectivity of tics, OCD, and premonitory urge38. Greater connectivity between the putamen and sensorimotor cortex was noted in participants with TS, whereas less connectivity between the supplementary motor area (SMA) and thalamus and between the caudate and precuneus was noted in participants with obsessive–compulsive symptoms. Moreover, a case-control study using diffusion tensor imaging MRI and transcranial magnetic stimulation (TMS) over the right pre-SMA was conducted in children with chronic motor tics and healthy controls39. The study showed that pre-SMA-mediated motor cortex inhibition was impaired in participants with tic disorders; less inhibition correlated with worse tic suppressibility and more severe tics39. The functional connectivity within sensorimotor regions might play a role in tic severity, premonitory urges, suppressibility, and obsessive–compulsive symptoms.
Electrophysiology studies
Electrophysiological methods are well suited to studying temporal brain dynamics. A narrative review summarized that electrophysiological studies, including electroencephalogram (EEG) and event-related potential studies, could echo neuronal processes and parallel clinical characteristics of TS40. A case-control study using resting-state EEG of patients with TS showed decreased frontotemporal–occipital–parietal connectivity and a dysfunctional network of intrinsic long-range connectivity between the frontal and temporal–occipital–parietal lobes and proposed these as potential biomarkers for TS41. Local field potentials can be recorded during deep brain stimulation (DBS) implantation surgery. A retrospective clinical study of 17 patients with TS who underwent DBS of the thalamus showed that increased power in the low-frequency (5–15 Hz) band could be a biomarker for TS and correlated with the severity of tics42.
Laboratory studies
Recently, new evidence has supported dopaminergic involvement in the pathophysiology of TS. A case-control study measuring urine tetrahydroisoquinolines, which modulate dopaminergic neurotransmission and metabolism in the central nervous system, demonstrated a significant increase in the levels of norsalsolinol in patients with TS and those with TS and ADHD, suggesting dopaminergic hyperactivity in the pathophysiology of TS43. Another case-control study measuring cerebrospinal fluid (CSF) levels of endocannabinoids demonstrated significant alterations in the levels, suggesting an involvement of the endocannabinoid system in the pathophysiology of TS44. The study authors speculated that an increased level of endocannabinoids might be a compensation for dopaminergic hyperactivity.
Animal studies have also suggested CSTC circuit involvement in TS pathophysiology. Postsynaptic SAP90/PSD95-associated protein 3 (SAPAP3) is highly expressed in the striatum and plays an important role in cortico-striatal circuitry in OCD-like behaviors, as shown in a mouse experiment45. Sapap3-knockout mice exhibited tic-like movements with short, sudden, repetitive movements (e.g., rapid head twitching and body twitching)46. The tic-like movements in Sapap3-knockout mice were reduced significantly by treatment with aripiprazole, an atypical antipsychotic drug used to treat tics46. Another animal experiment was conducted using D1CT-7 transgenic TS mice47. D1CT-7 mice are a “transgenic line generated through the attachment of a neuropotentiating cholera toxin to the D1 receptor promoter” and have been considered a good animal model of TS48. The researchers administered DK-I-56-1, a selective positive allosteric modulator of GABA-A receptor containing alpha6 subunits, to D1CT-7 transgenic TS model mice and found that DK-I-56-1 was as effective as D1 and D2 receptor antagonists and significantly reduced tic-like movements47. MicroRNAs (miRNAs) have attracted attention as biomarkers for diseases, intercellular communication, and neural development49. A clinical study using serum miRNA expression profiles as molecular fingerprints for children with either TS or Arnold–Chiari malformation showed nine differentially expressed miRNAs as potential molecular tools for the diagnosis of TS50. These pathophysiological studies have converged to suggest the importance of CSTC circuits and their associated neurotransmitters and other brain regions but have not yet supported a clear, inclusive theory of tic pathophysiology.
Etiology
Environmental factors
Environmental influences such as prenatal and perinatal epigenetic factors and inflammatory factors have been investigated as underlying causes of tic disorders. A systematic meta-analysis found a 35% increase in the risk of tic disorders in offspring with maternal smoking during pregnancy (pooled relative risk 1.35, 95% CI 1.17‒1.56)51. Similarly, a prospective case-control study showed that maternal pro-inflammatory states were associated with tics and OCD in children, supporting a possible role of maternal inflammation52.
Inflammation has been extensively discussed in tic pathophysiology. A recent review article on immunological mechanisms in the pathophysiology of tic disorders argues that innate and adaptive systemic immune pathways and neuroinflammatory mechanisms play an important role in the pathogenesis of at least some patients with TS53. The article also postulated that hyper-reactive systemic immune pathways and neuroinflammation may help explain the natural fluctuations of tic disorder symptoms over time.
The prevalence and characteristics of tics in patients with encephalitis were reviewed in a systematic study, which found that sporadic cases of tics were associated with encephalitis, particularly during a post-encephalitis period, and with basal ganglia involvement54. A case-control autopsy study (of nine individuals with TS) using basal ganglia transcriptome by RNA sequencing in the caudate and putamen found disrupted basal ganglia neuronal signaling55. The study also found a significant increase in immune and inflammatory transcripts. These results suggest metabolic alterations and inflammatory involvement in TS pathophysiology. A comprehensive review of immune dysfunction in TS discussed clinical correlation with group A Streptococcus (GAS) infection, autoantibody analysis, and gene expression studies and identified some support for a hypothesis of autoimmune dysfunction as the pathophysiology of TS and its associated neuropsychiatric symptoms56.
CSF analyses have revealed inflammatory mechanisms in tic disorders. The longitudinal EMTICS study investigated the role of environmental exposure and immunology in the clinical course and comorbidities of TS57, and immunohistochemistry staining on rat brain sections failed to show evidence of specific neuronal surface antibodies (such as NMDA, CASPR2, LGI1, AMPAR, and GABAAR) in children with TS58. A prospective CSF analysis study demonstrated positive oligoclonal bands in 20% of participants (4 out of 20) but did not compare that rate with that of tic-free control participants and observed no specific surface autoantibodies, such as NMDA, CASPR2, LGI1, AMPA, and GABA1/B, and no specific binding pattern59. Although specific surface antibodies were not detected, the presence of oligoclonal bands in CSF analysis could suggest an autoimmune etiology in TS59. The authors suggested a pathological immune process of intrathecal IgG antibody production, considering that other studies find OCB positive in only 5% of healthy individuals and it is rarely seen in patients with non-inflammatory diseases.
On the other hand, the EMTICS study also examined whether exacerbations of tics and comorbid symptoms were associated with GAS exposure, which was determined by throat swabs and serum antibodies60. GAS exposure was not significantly associated with tic exacerbations (odds ratio 1.006‒1.235; P >0.3); however, it was associated with hyperactivity–impulsivity symptoms. The study authors concluded that GAS exposure was less likely to be a risk factor for tic exacerbations; therefore, the evaluation and treatment of GAS infections are not warranted in the context of worsening of tics. The authors also attempted to identify biomarkers of tics, examining hair cortisol concentration as a physiological marker of long-term stress in patients with tics, and found no association between hair cortisol concentration and tic severity61.
Genetics
Tic disorders are polygenic inherited disorders involving different genes. In a population-based cohort study in Sweden using the Genome-wide Complex Trait Analysis program, the heritability estimate of TS was 0.58 to 0.77, and the odds ratio for tic disorders of first-degree relatives of probands with tic disorders (18.7) was significantly higher than that of the second-degree (4.6) and third-degree (3.1) relatives62,63.
Several candidate susceptibility genes for TS have been identified but have not been confirmed and this is likely due to the small sample size of each study and genetic and phenotypic heterogeneity64. The SLITRK1 gene on chromosome 13q31.1 encoding a single-pass transmembrane protein in the central nervous system was reported to be associated with TS65. The IMMP2L gene encoding the inner mitochondrial membrane peptidase subunit 2 was implicated in TS66, but a recent study using skin fibroblasts from adults with the IMMP2L deletions and TS failed to show evidence of mitochondrial dysfunction67. Expression analysis, genotyping, and methylation analysis of SLC6A4 in 57 patients with TS showed a significantly higher expression of SLC6A4 mRNA, which encodes serotonin transporter, compared with healthy controls68. SLC6A4 overexpression may contribute to TS pathophysiology by increasing serotonin clearance. Whole-exome sequencing studies identified the CELSR3 gene on chromosome 3p21.3169, the ASH1L gene on chromosome 1q2270, and possibly disrupted variants of the OPRK1 gene on chromosome 8q11.23, encoding the opioid kappa receptor71 as high-risk genes for TS.
A study of 802 TS trios found that novel mutations were more common in simplex but not multiplex families and identified a greater-than-expected number of mutations related to cell polarity69. That study also identified shared genetic variance with OCD and autism spectrum disorder. The Brainstorm Consortium Genome-wide association study data on 3581 patients with TS and 7682 controls identified three significant gene sets involving ligand-gated ion channel signaling, lymphocytic and cell adhesion, and trans-synaptic signaling processes72.
Comorbidities
TS is frequently accompanied by ADHD, OCD, anxiety, depression, autism spectrum disorder, sleep disorders, migraine, rage attacks, and self-injurious behavior (SIB)73–77. Aggressive behavior measured by the Overt Aggression Scale is associated with the severity of comorbid ADHD78. According to a systematic literature review, 35% of patients with TS had SIB, and obsessive–compulsive behaviors were correlated with SIB in patients with TS74. Similarly, a cohort study of Polish patients with TS showed that SIB was associated with tic severity, OCD, and ADHD79. To understand the prevalence of psychiatric comorbidities, a cross-sectional interview study of 1374 adolescents and adults with TS and 1142 TS-unaffected family members was conducted. The study found that 86% of patients with TS had a lifetime prevalence of any psychiatric symptoms (excluding tics) and 58% of them had more than two psychiatric illnesses80. The severity of tics as well as symptoms of the two most common comorbidities—ADHD and OCD—declined during adolescence in a 6-year Danish cohort study of 314 children and adolescents with TS14.
Non-psychiatric comorbidities have been increasingly reported. A study using data from the National Health Insurance Research Database of Taiwan showed an increased risk of traumatic brain injury (TBI) in patients with previously diagnosed TS (hazard ratio [HR] 1.59, 95% CI 1.37–1.85)81. Moreover, patients with TS undergoing antipsychotic treatment had a lower risk of TBI than infrequent users (HR 0.76, 95% CI 0.57–0.99). The study authors speculated that the improvement of tics with antipsychotics protected against TBI or that antipsychotics reduced their impulsivity (or SIB). A large population-based cohort study using the Swedish National Patient Register has shown that individuals with tic disorders have a higher risk of transportation-related injuries and death than the general population (adjusted HR 1.50, 95% CI 1.33‒1.69); however, TS individuals without ADHD did not have a significantly elevated risk, suggesting that comorbid ADHD was the contributor to the automotive injuries62,82. The same group showed a higher risk of metabolic and cardiovascular disorders such as obesity (adjusted HR 2.76, 95% CI 2.47‒3.09), type 2 diabetes (adjusted HR 1.67, 95% CI 1.42‒1.96), and circulatory system diseases (adjusted HR 1.76, 95% CI 1.67‒1.86) in patients with TS compared with the general population83. Surprisingly, use of antipsychotics for more than 1 year significantly decreased the risk of metabolic and cardiovascular disorders83. The risk of substance misuse (alcohol, drugs, and substance-related crimes) in individuals with TS was higher than that in the general population (adjusted HR 3.11, 95% CI 2.94‒3.29)84.
Although it is generally assumed that most patients with tic disorders have normal intelligence, the association between TS and cognition is unclear85. A case-control study on learning ability in TS revealed impairment of visual associative learning (a style of learning where one learns things by associating them with a stimulus), but retrieval and generalization were not affected86. Another case-control study using an automatic imitation task showed that participants with TS responded faster than controls but had higher error rates, suggesting a different control mechanism of their motor responses to sensory stimuli from observed actions87. Yet another case-control study examined the executive function and psychomotor speed of TS children with ADHD, TS children without ADHD, ADHD children without tics, and controls and showed that the severity of ADHD possibly affects executive dysfunction88. ADHD appears to play a role in learning, and ADHD treatment may improve learning and executive function, but more research is necessary. A case-control study in Sweden showed that treatment-seeking individuals with tic disorders experienced academic underachievement from primary school through university89.
Owing to heterogeneous symptoms, several groups have attempted to categorize or view tic disorders and TS as a spectrum. Using a cluster dendrogram of hierarchical ascendant clustering based on comorbidities, a prospective clinical study of 174 patients with TS demonstrated three clusters: TS with no other neurodevelopmental comorbidities; TS with higher intelligence, attention deficit, and handwriting problems; and TS with neurodevelopmental comorbidities, learning disabilities, and academic impairments90. A retrospective analysis of 1018 patients with TS and chronic motor tic disorders (motor tics for more than 1 year but no phonic tics) found that TS and chronic tic disorders were not distinct in clinical or demographic variables and suggested that “tic spectrum disorders” with TS were more severe and were associated with more comorbidities whereas chronic motor tics were less severe91. A very compelling data analysis and review concludes that TS and chronic motor or phonic tic disorder are the same illness or at least that they occur on a spectrum with TS at the more severe end92. In clinic settings, the term TS can occasionally provoke avoidance from patients and their guardians because of myths and misconceptions created by social media. These findings will allow clinicians to advise patients on the expected clinical course of tics and other comorbidities, and the concept of “tic spectrum disorder” may help patients and guardians accept the diagnosis.
Although a large interview study across nine academic TS and OCD specialty clinics showed a correlation between tic severity and tic impairment93, non-tic-related symptoms can be more problematic than tics themselves94. Paying attention to comorbid symptoms and treatment is often more important than addressing tics themselves. Continual assessment of comorbidities by using a scale or assessment tool is warranted. For example, the mini-child Tourette syndrome impairment scale, a parent- and child-reported tic impairment scale, was invented to quantify tic-related and non-tic-related impairment across the school, home, and social domains and was shown to be well correlated with tic and comorbid symptom severity95,96.
Treatment
The European Society for the Study of Tourette Syndrome (ESSTS) is about to release an updated version of its 2011 guidelines for TS, and the revised guidelines will cover in detail many of the points we touch on in this section97.
Behavior therapy
Behavioral therapies are recommended as the first-line treatment for tics by the American Academy of Neurology practice guidelines98. These therapies consist of exposure and response prevention (ERP), habit reversal therapy (HRT), or its descendant comprehensive behavioral interventions for tics (CBIT)99. A randomized control trial evaluating the long-term effect of both HRT and ERP in children and adults with tic disorders showed that the benefit of the therapies persisted at the 1-year follow-up visit in 74% of participants100. Despite robust evidence of the behavioral therapies to reduce tic frequency and severity and diminish the urges, finding a trained therapist can be challenging. To improve access to the therapies, internet-based training programs, which have shown improvement in parent-rated tic severity, tic-related impairment, and quality of life, have been developed101,102. Group-based behavioral therapies have also been investigated because group therapies are more cost-effective, treating more patients at once. They can also provide an opportunity for patients to meet in person, share their experiences, and support each other. An open-label controlled clinical trial of combined HRT and ERP was conducted in adolescents with TS103. The participants were randomly assigned to either individual or group therapy. In total, 67% of participants were considered responders, and there was no significant difference between individual and group therapies. Furthermore, group-based CBIT for children and adolescents showed a significant reduction in tics and severity of comorbid symptoms such as anxiety, behavioral problems, and aggressive behaviors104,105.
Pharmacological therapy
Pharmacological treatment is considered when behavioral interventions fail or are unavailable or when urgent benefit is required. Various medicines, including alpha-2 adrenergic agonists, antiepileptic drugs, and dopamine receptor blocking agents, are commonly used for the treatment of tics98. Most patients with tics respond to behavioral therapy or tic-suppressing medications. However, some do not, and others experience problematic side effects, so new treatment modalities are being investigated. Recently, novel drugs have been investigated for the treatment of tics. Tiapride, a dopamine receptor blocking agent, is used mainly in Europe for the treatment of tics. A clinical study of children with TS showed that 83% of participants responded to tiapride and no patients had serious adverse reactions106. Ecopipam, a selective D1 receptor agonist, was studied in a randomized, placebo-controlled crossover study of children and adolescents with TS107. Ecopipam significantly reduced tic severity at 16 days (95% CI −6.5 to −0.9; P = 0.011) and 30 days (95% CI −6.1 to −0.3; P = 0.033) and was well tolerated without serious side effects. Lurasidone, a dopamine and serotonin receptor antagonist, is an atypical antipsychotic used for the treatment of schizophrenia and bipolar disorder108. Six children and adolescents with treatment-refractory TS, aggressive behavior, and obsessive symptoms responded significantly to lurasidone as an add-on therapy to risperidone or aripiprazole109.
Dopamine-depleting agents block the vesicular monoamine transporter type 2 and are used to treat hyperkinetic movement disorders such as chorea, tardive dyskinesia, and tics110. Tetrabenazine, a dopamine-depleting agent, was found to be effective in an open-label study of 120 patients with tics111. Subsequently, an open-label study of 28 children and adolescents with TS investigated valbenazine, which is a purified parent drug of the (+)-α-isomer of tetrabenazine, but it failed to show statistically significant efficacy112. A trial of deutetrabenazine, a deuterated form of tetrabenazine, also failed to show a significant benefit (ARTISTS1).
The use of cannabis and cannabis-derived products has been reported to improve tics. A study of TS model rodents demonstrated that delta-9-tetrahydrocannabinol might reduce tic behaviors and premonitory urges in young adult mice but might also exacerbate tic behaviors in juvenile mice113. A retrospective data analysis of adults with TS who received cannabis-based medicine showed a subjective improvement in tics, comorbidities, and quality of life, although adverse effects were noted in half the patients114. A randomized clinical trial to evaluate the safety and efficacy of cannabis in adults with TS was launched in 2018, but it was terminated because the recruitment and enrollment were prolonged (ClinicalTrials.gov Identifier: NCT03247244). A multicenter, randomized, double-blind, placebo-controlled trial of patients with tic disorders is being conducted to investigate the use of the cannabis extract nabiximols for the treatment of tics115.
Complementary and alternative medicines—including dietary or nutritional supplements (calcium, magnesium, coenzyme Q10, fish oil, gastrodin, and vitamins B, C, D, and E), chiropractic manipulations, meditation, acupuncture, hypnosis, homeopathy, and biofeedback—have been reported for the treatment of tics116,117; however, the evidence is limited because of a lack of randomized control studies. The efficacy and safety of a Chinese herbal medicine (5-Ling granule) in the treatment of TS were evaluated in a multicenter, double-blind randomized controlled trial, finding it as effective as tiapride in improving tic symptoms118.
TMS is a non-invasive brain stimulation treatment to modulate neural plasticity and has been attracting considerable attention119. An open-label clinical trial (of 10 children with TS) using low-frequency repetitive TMS to the bilateral SMA for 15 sessions showed a statistically significant decrease in tic severity120. These results are promising but a large randomized trial is warranted to validate the findings.
Surgical therapy
DBS may be a promising neurosurgical treatment for tics121. Patient selection criteria and an algorithm for DBS treatment for TS, consisting of five pillars, have been proposed: high tic severity, tic-related impact on quality of life, failure of behavioral and pharmacological treatment, stability of comorbid symptoms, and age of 18 years or more122. The International Deep Brain Stimulation Database and Registry reports an overall adverse event rate of 35%, and medically serious complications—intracranial hemorrhage (1.3%), infection (3.2%), and lead explantation (0.6%)—were less common than stimulation-induced adverse effects, including dysarthria (6.3%) and paresthesia (8.2%)121. Tic-like behaviors have also been reported as adverse effects of DBS. A patient who underwent DBS of the ventral internal capsule and ventral striatum for treatment-resistant major depressive disorder developed stimulation-dependent TS-like behaviors such as motor tic-like movements in the arms as well as coprolalia and stuttered speech123.
There have been many reports of patients with TS who underwent DBS in various targets121. A double-blind randomized controlled trial of bilateral anterior globus pallidus pars interna (GPi) DBS was conducted in 19 patients with TS; one group received active stimulation and the other group received sham stimulation124. No significant difference in tic severity was noted three months after surgery. However, in an open-label, follow-up study, 75% of patients had an average 70% tic reduction in the YGTSS total score after 48 months125. Posteroventral GPi DBS has also been reported126. In addition to the GPi, the thalamus, globus pallidus externus, anterior limb of the internal capsule, and nucleus accumbens have been suggested as targets for DBS for tics127. An adult with TS who underwent bilateral dual-targeted DBS to the centromedian–parafascicular complex (CM-Pf) and the ventral capsule/ventral striatum reported benefits in terms of motor and non-motor TS symptoms128. A prospective clinical study of 25 patients with TS who underwent DBS to the CM-Pf showed improvement of tics by 45% at the 1-year follow-up129. Another, retrospective, study focused on DBS with a target of either the ventralis oralis (Voi) or the CM-Pf of the thalamus (41 patients) and the anteromedial GPi (am-GPi) (14 patients)130. They found possible superiority of the am-GPi to the Voi/CM-Pf for the treatment of obsessive–compulsive symptoms in TS; however, target selection was not random, as the authors note the CM-Pf was preferred for patients with impairment mostly from tics rather than comorbidities. The International Tourette Syndrome DBS Database and Registry conducted a retrospective, probabilistic tractography study of DBS targeting the GPi or CM131. Stimulus-dependent connectivity to specific regions was shown to be likely to mediate improvement in tics131. Specifically, the improvement of tics by DBS in GPi can be attributed to modulation of the limbic and associative networks by optimally positioned stimulation contacts, while the improvement of tics by DBS in the medial thalamus can be attributed to modulation of the sensorimotor and parietal–temporal–occipital networks. This finding could be used to refine the neuromodulation targets and stimulation parameters for tic disorders. Currently, it is common to select targets on the basis of clinical symptoms. However, considerable debate remains about the optimal DBS target (or targets) in TS, and there are no evidence-based guidelines to assist in the selection of DBS target in TS. Most studies that reported successful DBS results for the treatment of TS are case reports or open-label studies with various targets, and randomized control studies have shown inconsistent results124,132–134. Some investigators have interpreted the greater benefit in open-label trials as evidence of our difficulty predicting ideal lead location and pulse characteristics for treating tics. In fact, considerable debate remains about the optimal DBS target (or targets) in TS, and there are no evidence-based guidelines to assist in the selection of DBS target in TS. However, in other movement disorders, the stringent requirements of randomized controlled trials have not prevented DBS from producing relatively dramatic results (e.g., in dystonia135 or Parkinson disease136). Alternatively, the better results in open-label studies may indicate expectation or placebo effects, even in patients with treatment-resistant TS treated with DBS. Placebo benefit can be demonstrated directly in DBS for Parkinson disease137. Thus, further studies are needed to identify the optimum target, benefits, risks, and stimulation parameters for DBS in TS138, and in our current state of knowledge, selection of patients for DBS surgery, including patients with severe TS, should be done quite cautiously122,139.
Conclusions
Although tics are a common movement disorder in children, there are still many unanswered questions, including on the causes and natural history of tics and their relationship with comorbid symptoms. Increasingly many research studies are published each year and have provided new insights into the pathophysiology and etiology of tics. Recent years have seen increasing information about the relationship between tics and comorbidities and about new genetic findings. Inflammatory processes have also been a topic of continued interest.
We believe the near future for Tourette research may include more large-scale collaborative studies, which can provide more powerful results. Advances in imaging technology are also likely to enhance our understanding of tics. Treatment of TS is becoming more standardized with the recent American Academy of Neurology and forthcoming ESSTS guidelines. Behavioral therapy interventions are becoming more widely accepted but still have limitations on availability. However, alternative approaches, including internet-delivered and group therapy, are being explored. Furthermore, new medications are still needed and likely will lead to improved treatments. Substantial debate continues about DBS therapy, its effectiveness, and optimal targets, and the international DBS registry will continue to move the field forward. We remain optimistic about improved care for TS that future research in all these areas is likely to produce.
The peer reviewers who approve this article are:
James F. Leckman, Departments of Psychiatry, Pediatrics and Psychology, Yale University, New Haven, CT, USA.
Natalia Szejko, Yale University, New Haven, CT, USA
Tammy Hedderly, TANDeM Clinic, Evelina London Children’s Hospital, Guy's and St Thomas NHS Foundation Trust, King's College London, UK
Joohi Jimenez-Shahed, Movement Disorders Neuromodulation & Brain Circuit Therapeutics, Neurology and Neurosurgery, Icahn School of Medicine at Mount Sinai, New York, USA.
Funding Statement
This work was supported by National Institutes of Health grant R01MH104030. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.American Psychiatric Association: Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. 2013. Reference Source [Google Scholar]
- 2.Bliss J, Cohen DJ, Freedman DX: Sensory experiences of Gilles de la Tourette syndrome. Arch Gen Psychiatry. 1980; 37(12): 1343–7. 10.1001/archpsyc.1980.01780250029002 [DOI] [PubMed] [Google Scholar]
- 3.Ueda K, Kim S, Greene DJ, et al. : Correlates and clinical implications of tic suppressibility. Curr Dev Disord Rep. 2021; 8(2): 112–20. 10.1007/s40474-021-00230-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganos C, Münchau A, Bhatia KP: The Semiology of Tics, Tourette's, and Their Associations. Mov Disord Clin Pract. 2014; 1(3): 145–53. 10.1002/mdc3.12043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thenganatt MA, Jankovic J: Recent Advances in Understanding and Managing Tourette Syndrome [version 1; peer review: 3 approved]. F1000Res. 2016; 5: F1000 Faculty Rev-152. 10.12688/f1000research.7424.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richards CA, Black KJ: Tourette Syndrome research highlights 2014 [version 2; peer review: 1 approved, 2 approved with reservations]. F1000Res. 2015; 4: 69. 10.12688/f1000research.6209.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization: International Statistical Classification of Diseases and related health problems (11th ed.). 2019.Reference Source [Google Scholar]
- 8.Horesh N, Zimmerman S, Steinberg T, et al. : Is onset of Tourette syndrome influenced by life events? J Neural Transm (Vienna). 2008; 115(5): 787–93. 10.1007/s00702-007-0014-3 [DOI] [PubMed] [Google Scholar]
- 9.Leckman JF, Zhang H, Vitale A, et al. : Course of tic severity in Tourette syndrome: The first two decades. Pediatrics. 1998; 102(1 Pt 1): 14–9. 10.1542/peds.102.1.14 [DOI] [PubMed] [Google Scholar]
- 10.Kim S, Greene DJ, Bihun EC, et al. : Provisional Tic Disorder is not so transient. Sci Rep. 2019; 9(1): 3951. 10.1038/s41598-019-40133-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S, Greene DJ, Robichaux-Viehoever A, et al. : Tic Suppression in Children With Recent-Onset Tics Predicts 1-Year Tic Outcome. J Child Neurol. 2019; 34(12): 757–64. 10.1177/0883073819855531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller LL, Scharf JM, Mathews CA, et al. : Tourette syndrome and chronic tic disorder are associated with lower socio-economic status: Findings from the Avon Longitudinal Study of Parents and Children cohort. Dev Med Child Neurol. 2014; 56(2): 157–63. 10.1111/dmcn.12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groth C, Skov L, Lange T, et al. : Predictors of the Clinical Course of Tourette Syndrome: A Longitudinal Study. J Child Neurol. 2019; 34(14): 913–21. 10.1177/0883073819867245 [DOI] [PubMed] [Google Scholar]
- 14.Groth C, Mol Debes N, Rask CU, et al. : Course of Tourette Syndrome and Comorbidities in a Large Prospective Clinical Study. J Am Acad Child Adolesc Psychiatry. 2017; 56(4): 304–12. 10.1016/j.jaac.2017.01.010 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 15.Leckman JF, Riddle MA, Hardin MT, et al. : The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989; 28(4): 566–73. 10.1097/00004583-198907000-00015 [DOI] [PubMed] [Google Scholar]
- 16.McGuire JF, Piacentini J, Storch EA, et al. : A multicenter examination and strategic revisions of the Yale Global Tic Severity Scale. Neurology. 2018; 90(19): e1711–e1719. 10.1212/WNL.0000000000005474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas M, Jakubovski E, Fremer C, et al. : Yale Global Tic Severity Scale (YGTSS): Psychometric Quality of the Gold Standard for Tic Assessment Based on the Large-Scale EMTICS Study. Front Psychiatry. 2021; 12: 626459. 10.3389/fpsyt.2021.626459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueda K, Black KJ: A Comprehensive Review of Tic Disorders in Children. J Clin Med. 2021; 10(11): 2479. 10.3390/jcm10112479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jankovic J: Tourette's syndrome. N Engl J Med. 2001; 345(16): 1184–92. 10.1056/NEJMra010032 [DOI] [PubMed] [Google Scholar]
- 20.Jankovic J, Kurlan R: Tourette syndrome: Evolving concepts. Mov Disord. 2011; 26(6): 1149–56. 10.1002/mds.23618 [DOI] [PubMed] [Google Scholar]
- 21.Kaczyńska J, Janik P: Tonic Tics in Gilles de la Tourette Syndrome. Neuropediatrics. 2021. 10.1055/s-0040-1722689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganos C, Martino D, Espay AJ, et al. : Tics and functional tic-like movements: Can we tell them apart? Neurology. 2019; 93(17): 750–8. 10.1212/WNL.0000000000008372 [DOI] [PubMed] [Google Scholar]
- 23.Heyman I, Liang H, Hedderly T: COVID-19 related increase in childhood tics and tic-like attacks. Arch Dis Child. 2021; archdischild-2021-321748. 10.1136/archdischild-2021-321748 [DOI] [PubMed] [Google Scholar]
- 24.Cubo E: Review of Prevalence Studies of Tic Disorders: Methodological Caveats. Tremor Other Hyperkinet Mov (N Y). 2012; 2: tre-02-61-349-1. 10.7916/D8445K68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knight T, Steeves T, Day L, et al. : Prevalence of tic disorders: A systematic review and meta-analysis. Pediatr Neurol. 2012; 47(2): 77–90. 10.1016/j.pediatrneurol.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 26.Yang C, Zhang L, Zhu P, et al. : The prevalence of tic disorders for children in China: A systematic review and meta-analysis. Medicine (Baltimore). 2016; 95(30): e4354. 10.1097/MD.0000000000004354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scharf JM, Miller LL, Gauvin CA, et al. : Population prevalence of Tourette syndrome: A systematic review and meta-analysis. Mov Disord. 2015; 30(2): 221–8. 10.1002/mds.26089 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 28.Cubo E, Gabriel y Galán JMT, Villaverde VA, et al. : Prevalence of tics in schoolchildren in central Spain: A population-based study. Pediatr Neurol. 2011; 45(2): 100–8. 10.1016/j.pediatrneurol.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 29.Levine JLS, Szejko N, Bloch MH: Meta-analysis: Adulthood prevalence of Tourette syndrome. Prog Neuropsychopharmacol Biol Psychiatry. 2019; 95: 109675. 10.1016/j.pnpbp.2019.109675 [DOI] [PubMed] [Google Scholar]
- 30.Albin RL, Mink JW, et al. : Recent advances in Tourette syndrome research. Trends Neurosci. 2006; 29(3): 175–82. 10.1016/j.tins.2006.01.001 [DOI] [PubMed] [Google Scholar]
- 31.Augustine F, Singer HS: Merging the Pathophysiology and Pharmacotherapy of Tics. Tremor Other Hyperkinet Mov (N Y). 2020; 8: 595. 10.7916/D8H14JTX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greene DJ, Schlaggar BL, Black KJ, et al. : Neuroimaging in Tourette Syndrome: Research Highlights From 2014-2015. Curr Dev Disord Rep. 2015; 2(4): 300–8. 10.1007/s40474-015-0062-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S, Greene DJ, D'Andrea CB, et al. : Hippocampal Volume in Provisional Tic Disorder Predicts Tic Severity at 12-Month Follow-up. J Clin Med. 2020; 9(6): 1715. 10.3390/jcm9061715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sigurdsson HP, Jackson SR, Jolley L, et al. : Alterations in cerebellar grey matter structure and covariance networks in young people with Tourette syndrome. Cortex. 2020; 126: 1–15. 10.1016/j.cortex.2019.12.022 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 35.Nielsen AN, Gratton C, Church JA, et al. : Atypical Functional Connectivity in Tourette Syndrome Differs Between Children and Adults. Biol Psychiatry. 2020; 87(2): 164–73. 10.1016/j.biopsych.2019.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramkiran S, Heidemeyer L, Gaebler A, et al. : Alterations in basal ganglia-cerebello-thalamo-cortical connectivity and whole brain functional network topology in Tourette's syndrome. Neuroimage Clin. 2019; 24: 101998. 10.1016/j.nicl.2019.101998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson SR, Loayza J, Crighton M, et al. : The role of the insula in the generation of motor tics and the experience of the premonitory urge-to-tic in Tourette syndrome. Cortex. 2020; 126: 119–33. 10.1016/j.cortex.2019.12.021 [DOI] [PubMed] [Google Scholar]
- 38.Bhikram T, Arnold P, Crawley A, et al. : The functional connectivity profile of tics and obsessive-compulsive symptoms in Tourette Syndrome. J Psychiatr Res. 2020; 123: 128–35. 10.1016/j.jpsychires.2020.01.019 [DOI] [PubMed] [Google Scholar]
- 39.Bruce AB, Yuan W, Gilbert DL, et al. : Altered frontal-mediated inhibition and white matter connectivity in pediatric chronic tic disorders. Exp Brain Res. 2021; 239(3): 955–65. 10.1007/s00221-020-06017-0 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 40.Rothenberger A, Heinrich H: Electrophysiology Echoes Brain Dynamics in Children and Adolescents With Tourette Syndrome-A Developmental Perspective. Front Neurol. 2021; 12: 587097. 10.3389/fneur.2021.587097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duan K, Wu Q, Liao Y, et al. : Discrimination of Tourette Syndrome Based on the Spatial Patterns of the Resting-State EEG Network. Brain Topogr. 2021; 34(1): 78–87. 10.1007/s10548-020-00801-5 [DOI] [PubMed] [Google Scholar]
- 42.Marceglia S, Prenassi M, Galbiati TF, et al. : Thalamic Local Field Potentials Are Related to Long-Term DBS Effects in Tourette Syndrome. Front Neurol. 2021; 12: 578324. 10.3389/fneur.2021.578324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capetian P, Roessner V, Korte C, et al. : Altered urinary tetrahydroisoquinoline derivatives in patients with Tourette syndrome: Reflection of dopaminergic hyperactivity? J Neural Transm (Vienna). 2021; 128(1): 115–20. 10.1007/s00702-020-02289-6 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 44.Müller-Vahl KR, Bindila L, Lutz B, et al. : Cerebrospinal fluid endocannabinoid levels in Gilles de la Tourette syndrome. Neuropsychopharmacology. 2020; 45(8): 1323–9. 10.1038/s41386-020-0671-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welch JM, Lu J, Rodriguiz RM, et al. : Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007; 448(7156): 894–900. 10.1038/nature06104 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 46.Lamothe H, Schreiweis C, Mallet L, et al. : The Sapap3-knockout mouse model manifests a spectrum of repetitive behaviours. bioRxiv. 2020; 2020.01.22.915215. 10.1101/2020.01.22.915215 [DOI] [Google Scholar]
- 47.Cadeddu R, Knutson DE, Mosher LJ, et al. : The α6 GABA A Receptor Positive Allosteric Modulator DK-I-56-1 Reduces Tic-Related Behaviors in Mouse Models of Tourette Syndrome. Biomolecules. 2021; 11(2): 175. 10.3390/biom11020175 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 48.Godar SC, Mosher LJ, Strathman HJ, et al. : The D1CT-7 mouse model of Tourette syndrome displays sensorimotor gating deficits in response to spatial confinement. Br J Pharmacol. 2016; 173(13): 2111–21. 10.1111/bph.13243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Brien J, Hayder H, Zayed Y, et al. : Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol (Lausanne). 2018; 9: 402. 10.3389/fendo.2018.00402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mirabella F, Gulisano M, Capelli M, et al. : Enrichment and Correlation Analysis of Serum miRNAs in Comorbidity Between Arnold-Chiari and Tourette Syndrome Contribute to Clarify Their Molecular Bases. Front Mol Neurosci. 2021; 13: 608355. 10.3389/fnmol.2020.608355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ayubi E, Mansori K, Doosti-Irani A: Effect of maternal smoking during pregnancy on Tourette syndrome and chronic tic disorders among offspring: A systematic review and meta-analysis. Obstet Gynecol Sci. 2021; 64(1): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 52.Jones HF, Han VX, Patel S, et al. : Maternal autoimmunity and inflammation are associated with childhood tics and obsessive-compulsive disorder: Transcriptomic data show common enriched innate immune pathways. Brain Behav Immun. 2021; 94: 308–17. 10.1016/j.bbi.2020.12.035 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 53.Martino D, Johnson I, Leckman JF: What Does Immunology Have to Do With Normal Brain Development and the Pathophysiology Underlying Tourette Syndrome and Related Neuropsychiatric Disorders? Front Neurol. 2020; 11: 567407. 10.3389/fneur.2020.567407 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 54.Badenoch J, Searle T, Watson I, et al. : Tics in patients with encephalitis. Neurol Sci. 2021; 42(4): 1311–23. 10.1007/s10072-021-05065-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lennington JB, Coppola G, Kataoka-Sasaki Y, et al. : Transcriptome Analysis of the Human Striatum in Tourette Syndrome. Biol Psychiatry. 2016; 79(5): 372–82. 10.1016/j.biopsych.2014.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsu CJ, Wong LC, Lee WT: Immunological Dysfunction in Tourette Syndrome and Related Disorders. Int J Mol Sci. 2021; 22(2): 853. 10.3390/ijms22020853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schrag A, Martino D, Apter A, et al. : European Multicentre Tics in Children Studies (EMTICS): Protocol for two cohort studies to assess risk factors for tic onset and exacerbation in children and adolescents. Eur Child Adolesc Psychiatry. 2019; 28(1): 91–109. 10.1007/s00787-018-1190-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baglioni V, Coutinho E, Menassa DA, et al. : Antibodies to neuronal surface proteins in Tourette Syndrome: Lack of evidence in a European paediatric cohort. Brain Behav Immun. 2019; 81: 665–9. 10.1016/j.bbi.2019.08.008 [DOI] [PubMed] [Google Scholar]
- 59.Baumgaertel C, Skripuletz T, Kronenberg J, et al. : Immunity in Gilles de la Tourette-Syndrome: Results From a Cerebrospinal Fluid Study. Front Neurol. 2019; 10: 732. 10.3389/fneur.2019.00732 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 60.Martino D, Schrag A, Anastasiou Z, et al. : Association of Group A Streptococcus Exposure and Exacerbations of Chronic Tic Disorders: A Multinational Prospective Cohort Study. Neurology. 2021; 96(12): e1680–e1693. 10.1212/WNL.0000000000011610 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 61.Buse J, Rothe J, Uhlmann A, et al. : Hair cortisol-a stress marker in children and adolescents with chronic tic disorders? A large European cross-sectional study. Eur Child Adolesc Psychiatry. 2021. 10.1007/s00787-020-01714-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mataix-Cols D, Isomura K, Pérez-Vigil A, et al. : Familial Risks of Tourette Syndrome and Chronic Tic Disorders. A Population-Based Cohort Study. JAMA Psychiatry. 2015; 72(8): 787–93. 10.1001/jamapsychiatry.2015.0627 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 63.Davis LK, Yu D, Keenan CL, et al. : Partitioning the heritability of Tourette syndrome and obsessive compulsive disorder reveals differences in genetic architecture. PLoS Genet. 2013; 9(10): e1003864. 10.1371/journal.pgen.1003864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Georgitsi M, Willsey AJ, Mathews CA, et al. : The Genetic Etiology of Tourette Syndrome: Large-Scale Collaborative Efforts on the Precipice of Discovery. Front Neurosci. 2016; 10: 351. 10.3389/fnins.2016.00351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abelson JF, Kwan KY, O'Roak BJ, et al. : Sequence variants in SLITRK1 are associated with Tourette's syndrome. Science. 2005; 310(5746): 317–20. 10.1126/science.1116502 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 66.Patel C, Cooper-Charles L, McMullan DJ, et al. : Translocation breakpoint at 7q31 associated with tics: Further evidence for IMMP2L as a candidate gene for Tourette syndrome. Eur J Hum Genet. 2011; 19(6): 634–9. 10.1038/ejhg.2010.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bjerregaard VA, Schönewolf-Greulich B, Juel Rasmussen L, et al. : Mitochondrial Function in Gilles de la Tourette Syndrome Patients With and Without Intragenic IMMP2L Deletions. Front Neurol. 2020; 11: 163. 10.3389/fneur.2020.00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hildonen M, Levy AM, Dahl C, et al. : Elevated Expression of SLC6A4 Encoding the Serotonin Transporter (SERT) in Gilles de la Tourette Syndrome. Genes (Basel). 2021; 12(1): 86. 10.3390/genes12010086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang S, Mandell JD, Kumar Y, et al. : De Novo Sequence and Copy Number Variants Are Strongly Associated with Tourette Disorder and Implicate Cell Polarity in Pathogenesis. Cell Rep. 2018; 24(13): 3441–3454.e12. 10.1016/j.celrep.2018.08.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu S, Tian M, He F, et al. : Mutations in ASH1L confer susceptibility to Tourette syndrome. Mol Psychiatry. 2020; 25(2): 476–90. 10.1038/s41380-019-0560-8 [DOI] [PubMed] [Google Scholar]
- 71.Depienne C, Ciura S, Trouillard O, et al. : Association of Rare Genetic Variants in Opioid Receptors with Tourette Syndrome. Tremor Other Hyperkinet Mov (N Y). 2019; 9. 10.7916/tohm.v0.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsetsos F, Yu D, Sul JH, et al. : Synaptic processes and immune-related pathways implicated in Tourette syndrome. Transl Psychiatry. 2021; 11(1): 56. 10.1038/s41398-020-01082-z [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 73.Robertson MM: A personal 35 year perspective on Gilles de la Tourette syndrome: Prevalence, phenomenology, comorbidities, and coexistent psychopathologies. Lancet Psychiatry. 2015; 2(1): 68–87. 10.1016/S2215-0366(14)00132-1 [DOI] [PubMed] [Google Scholar]
- 74.Stafford M, Cavanna AE: Prevalence and clinical correlates of self-injurious behavior in Tourette syndrome. Neurosci Biobehav Rev. 2020; 113: 299–307. 10.1016/j.neubiorev.2020.03.022 [DOI] [PubMed] [Google Scholar]
- 75.Kwak C, Vuong KD, Jankovic J: Migraine headache in patients with Tourette syndrome. Arch Neurol. 2003; 60(11): 1595–8. 10.1001/archneur.60.11.1595 [DOI] [PubMed] [Google Scholar]
- 76.Barbanti P, Fabbrini G: Migraine and Tourette syndrome. Arch Neurol. 2004; 61(4): 606–7; author reply 607. 10.1001/archneur.61.4.606-b [DOI] [PubMed] [Google Scholar]
- 77.Ghosh D, Rajan PV, Das D, et al. : Headache in children with Tourette syndrome. J Pediatr. 2012; 161(2): 303–7.e6. 10.1016/j.jpeds.2012.01.072 [DOI] [PubMed] [Google Scholar]
- 78.Benaroya-Milshtein N, Shmuel-Baruch S, Apter A, et al. : Aggressive symptoms in children with tic disorders. Eur Child Adolesc Psychiatry. 2020; 29(5): 617–24. 10.1007/s00787-019-01386-6 [DOI] [PubMed] [Google Scholar]
- 79.Szejko N, Jakubczyk A, Janik P: Prevalence and Clinical Correlates of Self-Harm Behaviors in Gilles de la Tourette Syndrome. Front Psychiatry. 2019; 10: 638. 10.3389/fpsyt.2019.00638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirschtritt ME, Lee PC, Pauls DL, et al. : Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry. 2015; 72(4): 325–33. 10.1001/jamapsychiatry.2014.2650 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 81.Chen SF, Su YC, Wang LY, et al. : Tourette's syndrome is associated with an increased risk of traumatic brain injury: A nationwide population-based cohort study. Parkinsonism Relat Disord. 2019; 63: 88–93. 10.1016/j.parkreldis.2019.02.033 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 82.Mataix-Cols D, Brander G, Chang Z, et al. : Serious Transport Accidents in Tourette Syndrome or Chronic Tic Disorder. Mov Disord. 2021; 36(1): 188–95. 10.1002/mds.28301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brander G, Isomura K, Chang Z, et al. : Association of Tourette Syndrome and Chronic Tic Disorder With Metabolic and Cardiovascular Disorders. JAMA Neurol. 2019; 76(4): 454–61. 10.1001/jamaneurol.2018.4279 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 84.Virtanen S, Sidorchuk A, Fernández de la Cruz L, et al. : Association of Tourette Syndrome and Chronic Tic Disorder With Subsequent Risk of Alcohol- or Drug-Related Disorders, Criminal Convictions, and Death: A Population-Based Family Study. Biol Psychiatry. 2021; 89(4): 407–14. 10.1016/j.biopsych.2020.09.014 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 85.Morand-Beaulieu S, Leclerc JB, Valois P, et al. : A Review of the Neuropsychological Dimensions of Tourette Syndrome. Brain Sci. 2017; 7(8): 106. 10.3390/brainsci7080106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eördegh G, Pertich A, Tárnok Z, et al. : Impairment of visually guided associative learning in children with Tourette syndrome. PLoS One. 2020; 15(6): e0234724. 10.1371/journal.pone.0234724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Quadrelli E, Bartoli B, Bolognini N, et al. : Automatic imitation in youngsters with Gilles de la Tourette syndrome: A behavioral study. Child Neuropsychol. 2021; 27(6): 782–798. 10.1080/09297049.2021.1892050 [DOI] [PubMed] [Google Scholar]
- 88.Openneer TJC, Forde NJ, Akkermans SEA, et al. : Executive function in children with Tourette syndrome and attention-deficit/hyperactivity disorder: Cross-disorder or unique impairments? Cortex. 2020; 124: 176–87. 10.1016/j.cortex.2019.11.007 [DOI] [PubMed] [Google Scholar]
- 89.Pérez-Vigil A, Fernández de la Cruz L, Brander G, et al. : Association of Tourette Syndrome and Chronic Tic Disorders With Objective Indicators of Educational Attainment: A Population-Based Sibling Comparison Study. JAMA Neurol. 2018; 75(9): 1098–105. 10.1001/jamaneurol.2018.1194 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 90.Cravedi E, Deniau E, Giannitelli M, et al. : Disentangling Tourette syndrome heterogeneity through hierarchical ascendant clustering. Dev Med Child Neurol. 2018; 60(9): 942–50. 10.1111/dmcn.13913 [DOI] [PubMed] [Google Scholar]
- 91.Müller-Vahl KR, Sambrani T, Jakubovski E: Tic disorders revisited: Introduction of the term "tic spectrum disorders". Eur Child Adolesc Psychiatry. 2019; 28(8): 1129–35. 10.1007/s00787-018-01272-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Claudio-Campos K, Stevens D, Koo SW, et al. : Is Persistent Motor or Vocal Tic Disorder a Milder Form of Tourette Syndrome? Mov Disord. 2021; 36(8): 1829–1910. 10.1002/mds.28593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McGuire JF, Piacentini J, Storch EA, et al. : Defining tic severity and tic impairment in Tourette Disorder. J Psychiatr Res. 2021; 133: 93–100. 10.1016/j.jpsychires.2020.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stiede JT, Alexander JR, Wellen B, et al. : Differentiating tic-related from non-tic-related impairment in children with persistent tic disorders. Compr Psychiatry. 2018; 87: 38–45. 10.1016/j.comppsych.2018.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Storch EA, Lack CW, Simons LE, et al. : A measure of functional impairment in youth with Tourette's syndrome. J Pediatr Psychol. 2007; 32(8): 950–9. 10.1093/jpepsy/jsm034 [DOI] [PubMed] [Google Scholar]
- 96.Garris JF, Huddleston DA, Jackson HS, et al. : Implementation of the Mini-Child Tourette Syndrome Impairment Scale: Relationships to Symptom Severity and Treatment Decisions. J Child Neurol. 2021; 36(4): 288–95. 10.1177/0883073820967518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Müller-Vahl KR, Szejko, N, Verdellen C, et al. : European clinical guidelines for Tourette Syndrome and other tic disorders: Summary statement. Eur Child Adolesc Psychiatry. 2021; In press. 10.1007/s00787-021-01832-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pringsheim T, Okun MS, Müller-Vahl K, et al. : Practice guideline recommendations summary: Treatment of tics in people with Tourette syndrome and chronic tic disorders. Neurology. 2019; 92(19): 896–906. 10.1212/WNL.0000000000007466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Verdellen C, van de Griendt J, Hartmann A, et al. : European clinical guidelines for Tourette syndrome and other tic disorders. Part III: Behavioural and psychosocial interventions. Eur Child Adolesc Psychiatry. 2011; 20(4): 197–207. 10.1007/s00787-011-0167-3 [DOI] [PubMed] [Google Scholar]
- 100.Nissen JB, Carlsen AH, Thomsen PH: One-year outcome of manualised behavior therapy of chronic tic disorders in children and adolescents. Child Adolesc Psychiatry Ment Health. 2021; 15(1): 9. 10.1186/s13034-021-00362-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Conelea CA, Wellen BCM: Tic Treatment Goes Tech: A Review of TicHelper.com. Cogn Behav Pract. 2017; 24(3): 374–81. 10.1016/j.cbpra.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Andrén P, Aspvall K, Fernández de la Cruz L, et al. : Therapist-guided and parent-guided internet-delivered behaviour therapy for paediatric Tourette's disorder: A pilot randomised controlled trial with long-term follow-up. BMJ Open. 2019; 9(2): e024685. 10.1136/bmjopen-2018-024685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nissen JB, Kaergaard M, Laursen L, et al. : Combined habit reversal training and exposure response prevention in a group setting compared to individual training: A randomized controlled clinical trial. Eur Child Adolesc Psychiatry. 2019; 28(1): 57–68. 10.1007/s00787-018-1187-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zimmerman-Brenner S, Pilowsky-Peleg T, Rachamim L, et al. : Group behavioral interventions for tics and comorbid symptoms in children with chronic tic disorders. Eur Child Adolesc Psychiatry. 2021. 10.1007/s00787-020-01702-5 [DOI] [PubMed] [Google Scholar]
- 105.Heijerman-Holtgrefe AP, Verdellen CWJ, van de Griendt JMTM, et al. : Tackle your Tics: Pilot findings of a brief, intensive group-based exposure therapy program for children with tic disorders. Eur Child Adolesc Psychiatry. 2021; 30(3): 461–73. 10.1007/s00787-020-01532-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fekete S, Egberts K, Preissler T, et al. : Estimation of a preliminary therapeutic reference range for children and adolescents with tic disorders treated with tiapride. Eur J Clin Pharmacol. 2021; 77(2): 163–70. 10.1007/s00228-020-03000-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gilbert DL, Murphy TK, Jankovic J, et al. : Ecopipam, a D1 receptor antagonist, for treatment of tourette syndrome in children: A randomized, placebo-controlled crossover study. Mov Disord. 2018; 33(8): 1272–80. 10.1002/mds.27457 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 108.Bawa R, Scarff JR: Lurasidone: A new treatment option for bipolar depression-a review. Innov Clin Neurosci. 2015; 12(1–2): 21–3. [PMC free article] [PubMed] [Google Scholar]
- 109.Colizzi M, Bortoletto R, Zoccante L: The Effectiveness of Lurasidone Add-On for Residual Aggressive Behavior and Obsessive Symptoms in Antipsychotic-Treated Children and Adolescents with Tourette Syndrome: Preliminary Evidence from a Case Series. Children (Basel). 2021; 8(2): 121. 10.3390/children8020121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jankovic J: Dopamine depleters in the treatment of hyperkinetic movement disorders. Expert Opin Pharmacother. 2016; 17(18): 2461–70. 10.1080/14656566.2016.1258063 [DOI] [PubMed] [Google Scholar]
- 111.Porta M, Sassi M, Cavallazzi M, et al. : Tourette's syndrome and role of tetrabenazine: Review and personal experience. Clin Drug Investig. 2008; 28(7): 443–59. 10.2165/00044011-200828070-00006 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 112.Farber RH, Angelov A, Kim K, et al. : Clinical development of valbenazine for tics associated with Tourette syndrome. Expert Rev Neurother. 2021; 21(4): 393–404. 10.1080/14737175.2021.1898948 [DOI] [PubMed] [Google Scholar]
- 113.Gorberg V, McCaffery P, Anavi-Goffer S: Different responses of repetitive behaviours in juvenile and young adult mice to Δ 9 -tetrahydrocannabinol and cannabidiol may affect decision making for Tourette syndrome. Br J Pharmacol. 2021; 178(3): 614–25. 10.1111/bph.15302 [DOI] [PubMed] [Google Scholar]
- 114.Milosev LM, Psathakis N, Szejko N, et al. : Treatment of Gilles de la Tourette Syndrome with Cannabis-Based Medicine: Results from a Retrospective Analysis and Online Survey. Cannabis Cannabinoid Res. 2019; 4(4): 265–74. 10.1089/can.2018.0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jakubovski E, Pisarenko A, Fremer C, et al. : The CANNA-TICS Study Protocol: A Randomized Multi-Center Double-Blind Placebo Controlled Trial to Demonstrate the Efficacy and Safety of Nabiximols in the Treatment of Adults With Chronic Tic Disorders. Front Psychiatry. 2020; 11: 575826. 10.3389/fpsyt.2020.575826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kumar A, Duda L, Mainali G, et al. : A Comprehensive Review of Tourette Syndrome and Complementary Alternative Medicine. Curr Dev Disord Rep. 2018; 5(2): 95–100. 10.1007/s40474-018-0137-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Y, Zhao L, Li AY: Gastrodin - A potential drug used for the treatment of Tourette Syndrome. J Pharmacol Sci. 2021; 145(3): 289–95. 10.1016/j.jphs.2021.01.005 [DOI] [PubMed] [Google Scholar]
- 118.Zheng Y, Zhang ZJ, Han XM, et al. : A proprietary herbal medicine (5-Ling Granule) for Tourette syndrome: A randomized controlled trial. J Child Psychol Psychiatry. 2016; 57(1): 74–83. 10.1111/jcpp.12432 [DOI] [PubMed] [Google Scholar]
- 119.Lefaucheur JP, Aleman A, Baeken C, et al. : Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014-2018). Clin Neurophysiol. 2020; 131(2): 474–528. 10.1016/j.clinph.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 120.Kahl CK, Kirton A, Pringsheim T, et al. : Bilateral transcranial magnetic stimulation of the supplementary motor area in children with Tourette syndrome. Dev Med Child Neurol. 2021; 63(7): 808–15. 10.1111/dmcn.14828 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 121.Martinez-Ramirez D, Jimenez-Shahed J, Leckman JF, et al. : Efficacy and Safety of Deep Brain Stimulation in Tourette Syndrome: The International Tourette Syndrome Deep Brain Stimulation Public Database and Registry. JAMA Neurol. 2018; 75(3): 353–9. 10.1001/jamaneurol.2017.4317 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 122.Martino D, Deeb W, Jimenez-Shahed J, et al. : The 5 Pillars in Tourette Syndrome Deep Brain Stimulation Patient Selection: Present and Future. Neurology. 2021; 96(14): 664–76. 10.1212/WNL.0000000000011704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Camprodon JA, Chou T, Testo AA, et al. : Case Report: Deep Brain Stimulation to the Ventral Internal Capsule/Ventral Striatum Induces Repeated Transient Episodes of Voltage-Dependent Tourette-Like Behaviors. Front Hum Neurosci. 2020; 14: 590379. 10.3389/fnhum.2020.590379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Welter ML, Houeto JL, Thobois S, et al. : Anterior pallidal deep brain stimulation for Tourette's syndrome: A randomised, double-blind, controlled trial. Lancet Neurol. 2017; 16(8): 610–9. 10.1016/S1474-4422(17)30160-6 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 125.Welter ML, Houeto JL, Worbe Y, et al. : Long-term effects of anterior pallidal deep brain stimulation for tourette's syndrome. Mov Disord. 2019; 34(4): 586–8. 10.1002/mds.27645 [DOI] [PubMed] [Google Scholar]
- 126.Canaz H, Karalok I, Topcular B, et al. : DBS in pediatric patients: Institutional experience. Childs Nerv Syst. 2018; 34(9): 1771–6. 10.1007/s00381-018-3839-1 [DOI] [PubMed] [Google Scholar]
- 127.Viswanathan A, Jimenez-Shahed J, Baizabal Carvallo JF, et al. : Deep brain stimulation for Tourette syndrome: Target selection. Stereotact Funct Neurosurg. 2012; 90(4): 213–24. 10.1159/000337776 [DOI] [PubMed] [Google Scholar]
- 128.Kakusa B, Saluja S, Tate WJ, et al. : Robust clinical benefit of multi-target deep brain stimulation for treatment of Gilles de la Tourette syndrome and its comorbidities. Brain Stimul. 2019; 12(3): 816–8. 10.1016/j.brs.2019.02.026 [DOI] [PubMed] [Google Scholar]
- 129.Kimura Y, Iijima K, Takayama Y, et al. : Deep Brain Stimulation for Refractory Tourette Syndrome: Electrode Position and Clinical Outcome. Neurol Med Chir (Tokyo). 2021; 61(1): 33–9. 10.2176/nmc.oa.2020-0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Servello D, Galbiati TF, Balestrino R, et al. : Deep Brain Stimulation for Gilles de la Tourette Syndrome: Toward Limbic Targets. Brain Sci. 2020; 10(5): 301. 10.3390/brainsci10050301 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 131.Johnson KA, Duffley G, Anderson DN, et al. : Structural connectivity predicts clinical outcomes of deep brain stimulation for Tourette syndrome. Brain. 2020; 143(8): 2607–23. 10.1093/brain/awaa188 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 132.Ackermans L, Duits A, van der Linden C, et al. : Double-blind clinical trial of thalamic stimulation in patients with Tourette syndrome. Brain. 2011; 134(Pt 3): 832–44. 10.1093/brain/awq380 [DOI] [PubMed] [Google Scholar]
- 133.Schoenberg MR, Maddux BN, Riley DE, et al. : Five-months-postoperative neuropsychological outcome from a pilot prospective randomized clinical trial of thalamic deep brain stimulation for Tourette syndrome. Neuromodulation. 2015; 18(2): 97–104. 10.1111/ner.12233 [DOI] [PubMed] [Google Scholar]
- 134.Kefalopoulou Z, Zrinzo L, Jahanshahi M, et al. : Bilateral globus pallidus stimulation for severe Tourette's syndrome: A double-blind, randomised crossover trial. Lancet Neurol. 2015; 14(6): 595–605. 10.1016/S1474-4422(15)00008-3 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 135.Kupsch A, Benecke R, Müller J, et al. : Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006; 355(19): 1978–90. 10.1056/NEJMoa063618 [DOI] [PubMed] [Google Scholar]
- 136.Deuschl G, Schade-Brittinger C, Krack P, et al. : A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006; 355(9): 896–908. 10.1056/NEJMoa060281 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 137.Keitel A, Wojtecki L, Hirschmann J, et al. : Motor and cognitive placebo-/nocebo-responses in Parkinson's disease patients with deep brain stimulation. Behav Brain Res. 2013; 250: 199–205. 10.1016/j.bbr.2013.04.051 [DOI] [PubMed] [Google Scholar]
- 138.Coulombe MA, Elkaim LM, Alotaibi NM, et al. : Deep brain stimulation for Gilles de la Tourette syndrome in children and youth: A meta-analysis with individual participant data. J Neurosurg Pediatr. 2018; 23(2): 236–46. 10.3171/2018.7.PEDS18300 [DOI] [PubMed] [Google Scholar]
- 139.Muller-Vahl KR: Deep brain stimulation in Tourette syndrome: The known and the unknown. J Neurol Neurosurg Psychiatr. 2019; 90(10): 1076–7. 10.1136/jnnp-2019-321008 [DOI] [PMC free article] [PubMed] [Google Scholar]