ABSTRACT
The Ouchterlony double immunodiffusion assay is a serological technique used in the detection of antibodies and antigens for diagnostic purposes and also used in immunology laboratory courses as a common teaching assay where students observe the geometrical precipitation line patterns that form in the agarose, elucidating degrees of homology between antigens. In this classical technique, students must wait several hours to days to obtain results when protein antigens and antibodies are used. Furthermore, these proteins degrade over time if not frozen or stored in the refrigerator and are the most expensive consumables of the laboratory exercise. In this study, inexpensive and commonly used inorganic ionic salt solutions that are stable and can be stored at room temperature for several years were used to mimic antigens and antibodies. The precipitation lines started to form in the agarose plates after 15 min and fully developed within an hour, showing different geometrical precipitation patterns and spur formations that could be identified by students as full identity, partial identity, and nonidentity between the simulated (inorganic) antigens. Students conducting this exercise in a combined lecture and laboratory immunology course were able to finish the exercise as well as record and discuss results within class time, and tvhey showed increased interest in the laboratory exercise and had a better understanding of antibody-antigen reactions. Thus, this simulated laboratory experiment is an inexpensive, safe, and fast exercise that allows students to observe precipitations reactions of the Ouchterlony assay within the class session time.
KEYWORDS: Ouchterlony assay, immunodiffusion, inorganic compounds, simulation, classroom activity, antibody, antigen
INTRODUCTION
The Ouchterlony immunodiffusion assay, developed by the Swedish physician Örjan Ouchterlony, is used for the detection of antigens and antibodies and determination of homologies between antigens (1, 2). The assay is also a common laboratory exercise in undergraduate immunology and microbiology classes for illustrating antigen-antibody precipitation reactions to students (3–5). In the Ouchterlony assay, a series of samples (the antigens) are placed in the outer wells of a gel plate, and antibodies (antiserum) are placed in the center well, after which they diffuse out and form different geometric precipitation lines in the gel (Fig. 1). The intersecting lines may produce full identity (no spurs), partial identity (with one spur), or a nonidentity where the two lines cross completely (two spurs), as shown in Fig. 1 (6, 7). One of the drawbacks of this assay in a teaching environment is the time it takes for high-molecular-weight protein antigens and antibodies to diffuse toward each other in the gel, as the assay can take up to 48 h to fully develop. Furthermore, proteins are unstable and degrade over time, and antibodies can lose specificity, especially if stored at room temperature. In addition, the antigens and antibodies are the costliest consumables for this assay. Conversely, inexpensive inorganic ionic salts have a long shelf life of several years, even at room temperature, and are of low molecular weight, which allows them to have faster diffusion rates in the agarose gel, resulting in quicker reactions that students can observe within a laboratory class time. Thus, in this study, we present a lab exercise for undergraduate immunology laboratory, and related, courses where various inorganic salt solutions are used as simulated antigens and antibodies (antiserum) to mimic the geometric patterns formed in the Ouchterlony assay.
FIG 1.

The Ouchterlony immunodiffusion assay and the different geometrical precipitation line patterns formed in the agarose gel. The antiserum is placed in the center well, and the antigens are placed in the outer wells. Precipitation lines form between the center well and outer wells, and depending on the homology between adjacent antigens, various geometrical spur patterns develop at the intersection, such as full identity (no spur), nonidentity (two spurs), and partial identity (one spur).
(This laboratory exercise was presented at the Microbe 2019 Annual Conference in San Francisco, CA [8].)
Learning objectives
From conducting this lab, immunology laboratory students will be able to:
-
1.
Detect antigen-antibody precipitation complexes in agarose gels.
-
2.
Observe the presence of a unique antigen or antibody.
-
3.
Understand the conditions resulting in the antigen-antibody precipitation line.
-
4.
Identify the homologies between antigens as fully identical, nonidentical, or partially identical by the precipitation spurs that are formed.
PROCEDURE
Safety issues
Students and instructors should follow manufacturer’s safety instructions when working with the salt solutions and agarose. All chemicals, experiments, and student activities in this study were approved by Florida Gulf Coast University’s Environmental Health and Safety office and institutional review boards. Furthermore, the ASM biosafety guidelines were followed during the creation and use of these protocols, and instructors can refer to those ASM guidelines for standard laboratory practices and personal protection (https://asm.org/Guideline/ASM-Guidelines-for-Biosafety-in-Teaching-Laborator).
Materials
The instructor can prepare the salt solutions in advance and store them at room temperature. Ionic salt solutions used in this exercise include 2.0 M BaCl2, 0.2 M AgNO3, 1.0 M MnSO4, 0.2 M NaCl, 1.0 M CuSO4, 1.5 M MgSO4, 0.2 M Na3PO4, and 0.2 M NH4Cl. All salt compounds can be purchased from Sigma-Aldrich (St. Louis, MO). A 1.2% agarose solution can be prepared by heating 1.2 g of pure agarose (Sigma-Aldrich) in 100 ml of deionized water using a microwave for 2 min on high power and can be kept in a 60°C water bath until ready to be poured into 60-mm by 15-mm petri dishes (Thermo Fisher Scientific, Waltham, MA) by the students.
Experiment
Ouchterlony plate preparation by the students. Students can make their own Ouchterlony plates by pouring 5 ml of melted 1.2% agarose (kept at 60°C) into 60-mm by 15-mm petri dishes, after which the plates can be left at room temperature for 5 min for the agarose to fully harden. Students can use a Feinberg agar gel cutter (Shandon Scientific Co., London, England) or the ends of glass Pasteur pipets to cut equidistant 6.5-mm-diameter circular outer wells and one 9.0-mm center well into the hardened agarose (Fig. 1). A printed diagram outlining the Ouchterlony plate with the hole markings can be used by the students as a guide for cutting the holes if glass Pasteur pipets are used for cutting the agarose (see Appendix 1 in the supplemental material). Toothpicks work well to lift the cut agarose plugs from the Ouchterlony plates.
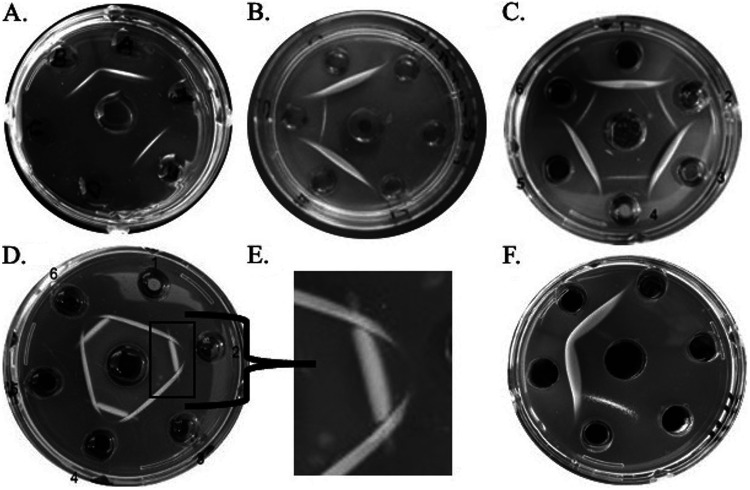
Results obtained from the Ouchterlony assay using inorganic salt solutions. As shown in Fig. 1, the simulated antigens are added to the outer wells (50 μl/well) and the antiserum (antibodies) to the center well (70 μl/well). Table 1 shows different combinations of salt chemical reactions (simulated antigen-antibody reactions) that will produce the precipitation lines and spur formation showing full identity, partial identity, and nonidentity. Deionized water can be used as a nonreacting simulated antigen. Precipitation and spur lines start developing in the gel after 15 min and will fully develop within 60 min. Results should be recorded by the students during this time frame, as the lines will slowly thicken and become distorted as time passes. An example datasheet table to record results along with a set of discussion and quiz questions are contained in Appendix 1 in the supplemental material. The instructor can modify the precipitin line development time range by slightly changing the agarose concentration, since higher agarose concentrations slow the salt diffusion rates and, conversely, lower agarose concentrations increase salt diffusion rates (9). Furthermore, the concentration of salt solutions can also be modified to affect the precipitation line thickness. Figure 2A shows an example of full identity (continuous precipitation line); the adjacent simulated antigens are NaCl in the outer wells, and they react with the antiserum (AgNO3) placed in the center well. Partial identity can be observed between AgNO3 and CuSO4 (the simulated antigens) in Fig. 2C; one spur is formed on each intersection after reacting with the antiserum (BaCl2), placed in the center well. To generate nonidentity spur formation (2 spurs), as shown in Fig. 2D and E, AgNO3 and MnSO4 are added to the center well (simulated antiserum) and Na3PO4 and NH4Cl are added to alternating adjacent outer wells as the simulated antigens. Additional partial and full identities are shown in Fig. 2B and F, outlining the chemical reactions from Table 1. The list of chemical precipitation reactions shown in Table 1 is not exhaustive, as other ionic salt compounds not listed may also produce similar precipitation lines and spur formation.
TABLE 1.
Precipitation reactions of inorganic salt solutions used for simulating antigen-antibody reactions of the Ouchterlony double immunodiffusion assay
| Spur identity | Center well (simulated antibody) | Outer wells (simulated antigen) | Precipitation reaction at the equivalence zone (antibody + antigen → immune complex product) | Example figure |
|---|---|---|---|---|
| Full identity | AgNO3 | NaCl | AgNO3 (aq) + NaCl (aq) → NaNO3 (aq) + AgCl (s) | Fig. 2A |
| Partial identity | BaCl2 | AgNO3 | 2AgNO3 (aq) + BaCl2 (aq) → Ba(NO3)2 (aq) + 2AgCl (s) | Fig. 2B |
| MgSO4 | MgSO4 (aq) + BaCl2 (aq) → MgCl2 (aq) + BaSO4 (s) | |||
| Partial identity | BaCl2 | AgNO3 | 2AgNO3 (aq) + BaCl2 (aq) → Ba(NO3)2 (aq) + 2AgCl (s) | Fig. 2C |
| CuSO4 | CuSO4 (aq) + BaCl2 (aq) → CuCl2 (aq) + BaSO4 (s) | |||
| Partial identity | BaCl2 | AgNO3 | 2AgNO3 (aq) + BaCl2 (aq) → Ba(NO3)2 (aq) + 2AgCl (s) | Fig. 2F |
| MnSO4 | MnSO4 (aq) + BaCl2 (aq) → MnCl2 (aq) + BaSO4 (s) | |||
| Nonidentity | AgNO3 | Na3PO4 | NH4Cl (aq) + AgNO3 (aq) → NH4NO3 (aq) + AgCl (s) | Fig. 2D and E |
| MnSO4 | NH4Cl | 2Na3PO4 (aq) +3MnSO4 (aq) → 3Na2SO4 (aq) + Mn3(PO4)2 (s) | ||
| No precipitation | Any salt | H2O | No precipitation reactions occur with water | Fig. 2A, B and F |
FIG 2.
Examples of student Ouchterlony plates showing different geometrical patterns. (A) Full identity, where a continuous line develops with no spur formation. The center well contains AgNO3 (the simulated antiserum) and the two adjacent outer wells contain NaCl (the simulated antigens). (B) Partial identity between MgSO4 and AgNO3 reacting with BaCl2 from the center well. (C) Another partial identity, where there is one spur at the intersections. The alternating adjacent outer wells contain CuSO4 and AgNO3 and the center well contains BaCl2. (D) Nonidentity, where two spurs are formed at the intersection of the precipitation lines formed by Na3PO4 and NH4Cl antigens from the outer wells reacting with antibodies AgNO3 and MnSO4 diffusing out from the center well. (E) Magnification of the bracketed section of panel D showing nonidentity at the intersection. (F) Full and partial identity in the same Ouchterlony plate, where two adjacent antigen wells have AgNO3 (thicker continuous lines) and one adjacent well has MnSO4 (thinner line) reacting with BaCl2 as the antiserum in the center well. The product (precipitation line) of each chemical reaction is shown in Table 1.
CONCLUSION
In this laboratory exercise, inexpensive salts solutions that are highly stable at room temperature are used to mimic the antigen-antibody precipitation reaction of the Ouchterlony assay. Diffusion of low-molecular-weight inorganic salts as the simulated antigens and antibodies on an agarose gel produces precipitation reactions that are faster than those of high-molecular-weight protein antigens and antibodies. Students in a combined lecture and laboratory immunology course were able to finish this laboratory exercise within class time (2 h and 15 min), which also included a lecture that was intercalated into the laboratory exercise. Students showed an increased interest in the laboratory exercise and had a good grasp of antibody-antigen reactions and determination of antigen homology (identity), as they performed well in the post-lab discussion and quiz (see Appendix 1). Thus, this simulated laboratory exercise is a time-saving, inexpensive, and efficient way to depict reactions of the Ouchterlony assay to students in the classroom.
ACKNOWLEDGMENTS
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
We have no conflicts of interest to declare, including competing financial or personal interests, that could have appeared to influence the work reported in this paper.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Ouchterlony O. 1949. Antigen-antibody reactions in gels. Acta Pathol Microbiol Scand 26:507–515. doi: 10.1111/j.1699-0463.1949.tb00751.x. [DOI] [PubMed] [Google Scholar]
- 2.Ouchterlony O. 1962. Diffusion-in-gel methods for immunological analysis. II. Prog Allergy 6:30–154. doi: 10.1159/000313795. [DOI] [PubMed] [Google Scholar]
- 3.Kindt T, Goldsby R, Osborne B. 2007. Kuby immunology. W.H. Freeman and Co., New York, NY. [Google Scholar]
- 4.Turgeon ML. 1996. Immunology & serology in laboratory medicine, 2nd ed. Mosby, St. Louis, MO. [Google Scholar]
- 5.Armstrong B. 2008. Antigen–antibody reactions. ISBT Science Series 3:21–32. doi: 10.1111/j.1751-2824.2008.00185.x. [DOI] [Google Scholar]
- 6.Hornbeck P. 2017. Double-immunodiffusion assay for detecting specific antibodies (Ouchterlony). Curr Protoc Immunol 116:2.3.1–2.3.4. doi: 10.1002/0471142735.im0203s00. [DOI] [PubMed] [Google Scholar]
- 7.Bailey GS. 1996. Ouchterlony double immunodiffusion BT, p 749–752. In Walker JM (ed), The protein protocols handbook. Humana Press, Totowa, NJ. [Google Scholar]
- 8.Mujtaba MG, Bhagu J, Herrera M, Baliban T. 2019. Simulating antibody and antigen reactions of the ouchterlony double immunodiffusion assay using inorganic compounds. Poster presented at the Microbe 2019 Conference, San Francisco, CA, June 20–24.
- 9.Serwer P. 1983. Agarose gels: properties and use for electrophoresis. Electrophoresis 4:375–382. doi: 10.1002/elps.1150040602. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: Student laboratory exercise background, materials and methods, datasheet, discussion questions, instructor’s salt precipitation chart, and quiz questions. Download JMBE00103-21_Supp_1_seq2.docx, DOCX file, 0.2 MB (270.8KB, docx)