Abstract
A 48-year-old woman presented with sudden-onset altered sensorium 2 days after a snake bite (unidentified species) and was found to have a large right frontal intracerebral haemorrhage (ICH) with transtentorial herniation (TTH) causing brain stem compression. A day later, neurological examination revealed internuclear ophthalmoplegia (INO) fitting the clinical description of wall eyed bilateral INO syndrome. INO is a rare ocular motor sign, the most common causes being brain stem infarction, haemorrhage or demyelinating disease. It rarely acts as a false localising sign, such as in this case, and in an even rarer cause for ICH, that is, haemotoxic snake bite without initial evidence of coagulopathy. An emphasis needs to be laid on detailed physical examination, often considered a lost art nowadays, to help detect subtle clinical signs which could herald ominous complications of conditions like TTH and help in early diagnosis and treatment of the same.
Keywords: brain stem / cerebellum, neurology, emergency medicine, cranial nerves
Background
Wall eyed bilateral internuclear ophthalmoplegia (WEBINO) is a rare neurological sign and usually a localising sign in brain stem lesions, most commonly due to vascular (infarct or haemorrhage) or demyelinating disorders (multiple sclerosis (MS)). It involves abnormalities of horizontal gaze, which is controlled by a complex network of nuclei, connected by the medial longitudinal fasciculus (MLF) in the brain stem.
Rare causes of INO include transtentorial herniation (TTH), among which TTH due to non-traumatic causes is especially rare. Initial subtle ocular signs such as mild adduction paresis on horizontal gaze or mild exotropia on primary gaze can be easily missed during routine clinical evaluation, causing a delay in diagnosis of life-threatening conditions like TTH.
Case presentation
A 48-year-old woman presented to the emergency room (ER) late in the evening, with sudden-onset agitation and irrelevant talk for 4–5 hours. She was referred from a primary health centre (PHC) a few hours away, where she had been admitted 2 days prior for an alleged snake bite (unidentified species) on her right fourth finger. The only symptom at onset had been nausea and one episode of vomiting. She had no history of head trauma, no known comorbidities or significant medical, surgical or personal history and had not been on any medication for any illness (figure 1).
Figure 1.
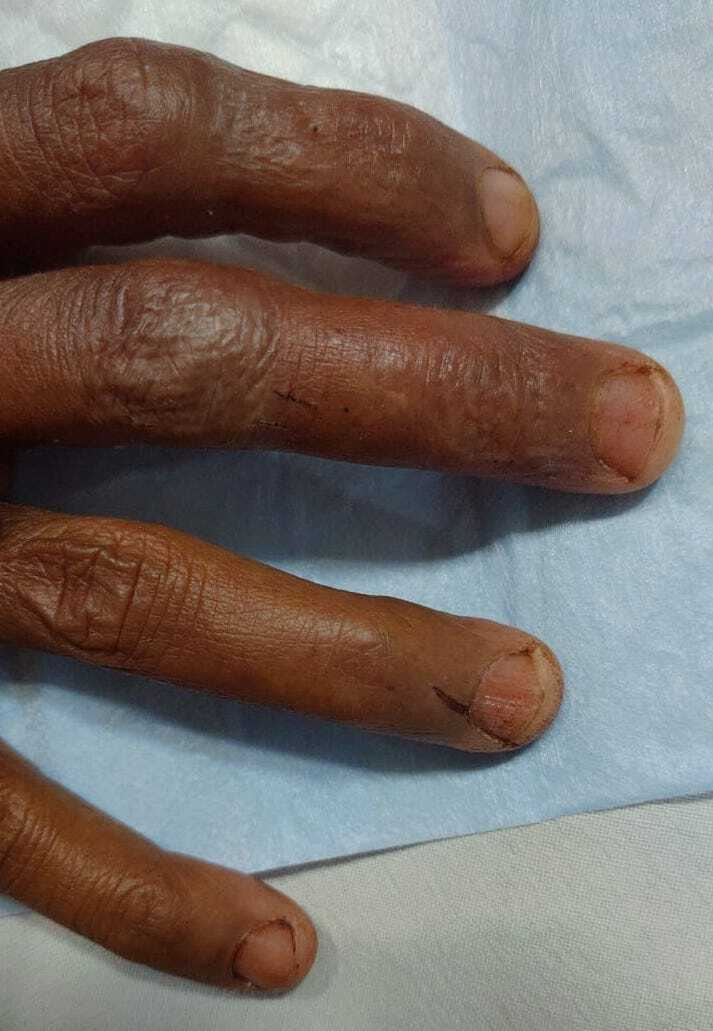
Fang marks (of an unidentified species of snake) with a linear abrasion, visible near the nail bed of the right fourth finger.
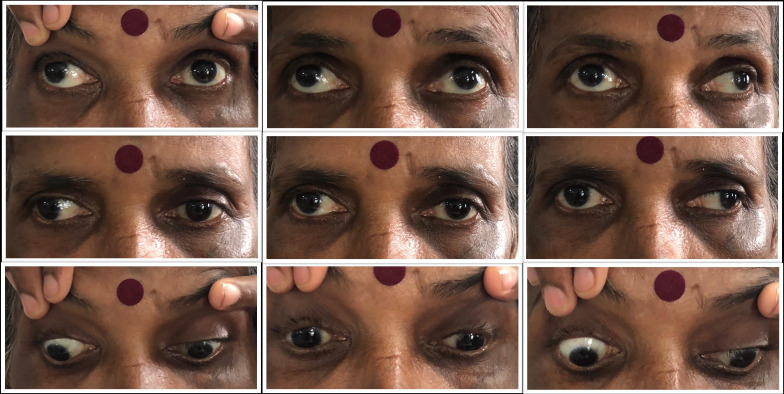
After remaining asymptomatic for 2 days, she developed sudden-onset altered mental status. On presentation in the ED, she was agitated, with irrelevant talk. She was vitally stable with a blood pressure (BP) of 140/90 mm Hg and subsequently was diagnosed with hypertension (systolic BP remained in the range of 150–160 mm of Hg) during her course of stay. Fang marks were visible on her right fourth finger with a small linear abrasion and no other local inflammatory signs. Initially, on examination, the left eye was deviated downward and outward with a mid-dilated pupil and intact light reflex (likely partial oculomotor palsy). No other focal neurological deficits were present (figure 2).
Figure 2.
Extraocular movements depicting bilateral exotropia (Right > Left) on primary gaze and adduction palsy of contralateral eye on horizontal gaze bilaterally, suggestive of wall eyed bilateral internuclear ophthalmoplegia.
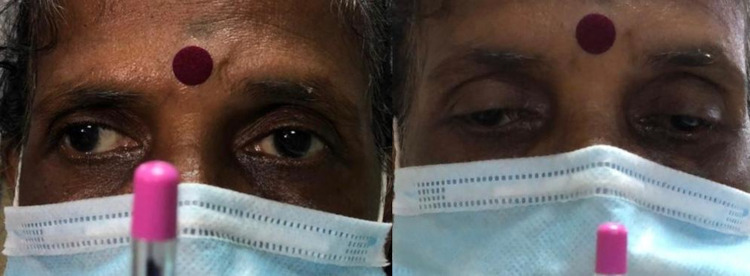
Her sensorium rapidly improved by the next day after initiating appropriate treatment and a subsequent neurological assessment revealed binocular diplopia with bilateral primary gaze exotropia (right more than left) with loss of bilateral adduction, slow saccades on horizontal conjugate eye movement, loss of convergence and intact bilateral pupillary light reflex, suggestive of wall eyed bilateral INO (WEBINO), that is, involvement of bilateral MLF, although nystagmus of the abducting eye was absent, which is a frequent finding in INO. The absence of abducting nystagmus, however, does not preclude the diagnosis of INO. The patient had no prior history of diplopia, squint or any other neurological symptoms (figure 3).
Figure 3.
Loss of convergence (as depicted in this case) may be a feature of WEBINO syndrome. WEBINO, wall eyed bilateral internuclear ophthalmoplegia.
Investigations
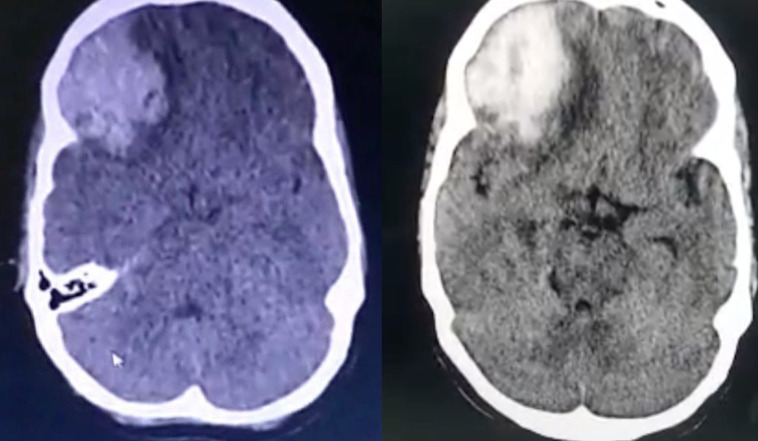
As per the letter from the referring hospital, she had no documented coagulopathy at the onset (the bedside whole blood clotting time was less than 10 min), but, had mild thrombocytopaenia (Platelet count=0.93×103/mm³) and hypokalaemia (serum Potassium=3.0 mEq/L) with normal renal function. A non-contrast CT (NCCT) of the brain, done at the PHC immediately after the patient developed altered sensorium, had revealed a large acute haematoma (intracerebral haemorrhage (ICH) or ICH) measuring 43×39 mm in the right frontal lobe with surrounding oedema, causing significant mass effect and midline shift to left by ~5 to 6 mm. Thus, she was referred to a tertiary centre for further care.
In the ER, her bedside whole blood clotting time was 4 min and she had no visible bleeding manifestations. A repeat NCCT brain at our hospital revealed no significant increase in the size of ICH but there was additional dilatation of left lateral ventricle and effacement of basal cisterns suggestive of descending TTH. A CT angiogram was done 3 days later and revealed similar findings with no evidence of any ischaemia, vascular insufficiency, thrombus, aneurysm or vascular malformation. Routine blood investigations revealed mild thrombocytopaenia which resolved within 2 days and normal coagulation parameters (prothrombin time and activated partial thromboplastin time). A peripheral smear revealed normocytic normochromic picture with schistocytes and elevated serum LDH (Lactate Dehydrogenase) (471 IU/L) and CPK-NAC (Creatine Phosphokinae, N- Acetyl Cysteine activated) (3957 IU/L) suggestive of venom induced haemolysis. Other laboratory parameters, ECG, chest radiograph and ultrasonography of the abdomen and pelvis were unremarkable.
Differential diagnosis
The ICH was primarily attributed to the haemotoxic effects of snake venom rather than the newly diagnosed hypertension. Although hypertension has been known to be the most common cause of spontaneous ICH, due to chronic degenerative changes in the arterioles,1 the atypical site of bleed and minimally elevated BP recordings with no other evidence of uncontrolled chronic hypertension (such as retinopathy or left ventricular hypertrophy) and in view of the temporal relationship with the snake bite, the ICH was likely triggered by the haemotoxic effects of snake venom, with chronic arteriolar damage due to previously undiagnosed hypertension as only a contributory factor. Snake venom can cause ICH due to coagulopathy, but also has many enzymes which can rarely cause or contribute to bleeding manifestations without causing overt coagulopathy.2 3 Some snake venoms even produce both coagulant and anticoagulant effects with a theoretical risk of simultaneous infarction and haemorrhage.4
The most likely cause for ophthalmoplegia (ie, WEBINO) could be ascribed to the descending TTH evident on the CT scan. An MRI to exclude any demyelinating disease or brain stem infarct could not be performed due to financial constraint. However, a subsequent CT which was repeated almost 9 days after the onset of symptoms, did not reveal any evidence of ischaemic changes in the brain stem.
Although it cannot be definitively ruled out without an MRI, the onset of INO associated with a history of snake bite and ICH with acutely altered sensorium which rapidly improved with antioedema measures, and undiagnosed hypertension (likely contributory factor in ICH), makes the possibility of demyelinating illness like MS, very unlikely. Moreover, the diagnosis of MS requires neurological deficits or lesions to be disseminated in time or space, which the patient did not have.
Treatment
At the PHC, she had been treated empirically with antiemetics, proton pump inhibitors, intravenous antibiotics (gentamicin and cefotaxime) and corrective measures for hypokalaemia (magnesium sulfate and potassium chloride drip). Antisnake venom or transfusion of blood products had not been given.
In the ER, immediate empiric treatment with Anti-Snake Venom, transfusion of fresh frozen plasma to correct the suspected venom induced consumptive coagulopathy (in view of thrombocytopaenia with evidence of haemolysis and rhabdomyolysis) and 20% mannitol to reduce intracranial pressure was given. Her sensorium improved to near normal by 24 hours. BP was well controlled with a low dose calcium channel blocker. She was admitted for observation in the ICU for 2 days and then transferred to the general ward.
Outcome and follow-up
She was admitted for a duration of 10 days, over the course of which, her sensorium rapidly improved. At the time of discharge, her sensorium had completely recovered with ophthalmoplegia (WEBINO) as the only residual deficit and a repeat NCCT scan at discharge revealed a resolving ICH with significant reduction in cerebral oedema and effacement of basal cisterns (figure 4). She was discharged and asked to follow up for periodic assessment. Prognosis was expected to be good due to rapid improvement in sensorium after appropriate treatment and the residual INO was expected to resolve in a few months with extraocular muscle exercises and intermittent eye patching for symptomatic relief of diplopia. She followed up 2 weeks later and had significantly reduced primary gaze exotropia, with mild recovery of adduction in the left eye.
Figure 4.
Non-contrast CT of the brain depicting a large acute intracranial haematoma with surrounding intracerebral oedema in the right frontoparietal cortex with midline shift to left and effacement of basal cisterns suggestive of transtentorial herniation, at admission (left) and 10 days later, at discharge (right), showing reduction of oedema with more clearly defined midline structures.
Discussion
INO was first described in 1921 by Paton, due to lesions of the MLF.5
The pathway for horizontal gaze involves the frontal eye field (FEF), paramedian pontine reticular formation (PPRF), MLF, abducens nuclei and oculomotor nerve. Fibres from FEF innervate the contralateral PPRF and abducens nucleus, which in turn provide inputs to the ipsilateral lateral rectus and contralateral medial rectus (via the contralateral MLF and oculomotor nerve).
There are variations of horizontal gaze abnormalities, depending on the involvement of structures in the pathway, such as: conjugate horizontal gaze palsy, paralytic pontine exotropia, INO, one-and-a-half syndrome, eight-and-a-half syndrome and the WEBINO syndrome (first described by Dr Martin Lubow in 1971, wherein the lesion was presumed to be in the midbrain).6 They can be distinguished from each other by careful clinical examination, thus helping in approximate localisation of the lesion. WEBINO is a rare syndrome characterised by bilateral INO, comprising of bilateral exotropia on primary gaze and bilateral adduction palsy due to bilateral MLF damage, with likely abnormalities of the medial rectus subnuclei of the ventral oculomotor nuclear complex.7
Here, it is important to throw light on the role of the cortical centres of gaze, since a frontal lobe ICH could cause ocular motor deficits. In humans, the cortical centres involved in the preparation and triggering of eye movements, that is, gaze control, lie in the frontal lobe, namely—the FEF, along with the supplementary eye field (SEF), pre-SEF, the dorsolateral prefrontal cortex, the cingulate eye field, the parietal eye field and areas of the posterior parietal cortex and subcortical structures like the superior colliculus in the midbrain. The human FEF, located in the superior precentral sulcus near the caudal end of the superior frontal sulcus (Brodmann’s area 6), is responsible for reflexive, voluntary, memory-guided and predictive saccades, as well as antisaccades, smooth pursuit and optokinetic nystagmus. The role of FEF in gaze has been demonstrated by mainly clinical cases involving frontal lobe lesions and transcranial magnetic stimulation of FEF.8 9
Therefore, although a frontal lobe ICH can cause slow saccades on horizontal conjugate eye movements, it cannot directly cause primary gaze exotropia, loss of adduction and convergence associated with INO, for which, the lesion would have to involve the network of nuclei responsible for horizontal gaze in the midbrain and pons. Hence, brain stem compression due to TTH is the most plausible explanation for bilateral INO in this case.
In WEBINO syndrome, the most important feature is impaired adduction of the eye ipsilateral to the MLF lesion. Abduction nystagmus of the eye contralateral to the MLF lesion is frequently associated with INO as per Hering’s law of equal innervation wherein increased innervation to the ipsilateral (to the MLF lesion) medial rectus, is associated with increased innervation to the contralateral lateral rectus (yoke muscle) causing overshooting of target during contralateral horizontal gaze resulting in abduction nystagmus. Alternatively, it could also be simply due to gaze-evoked nystagmus.10
Other associated features in INO may be—loss of horizontal saccades, skew deviation, up-beat nystagmus, loss of convergence, impaired vertical pursuit and an abnormal vestibulo-ocular reflex.11
Vertical eye movement abnormalities are more common in bilateral INO,10 may be due to a higher likelihood of involvement of structures in and around the MLF such as the interstitial nucleus of Cajal, trochlear nucleus, oculomotor nucleus and vestibular nuclei.
Loss of convergence is a variable finding. Two types of INO had been postulated earlier—anterior (at the level of oculomotor nuclei) and posterior (below the level of oculomotor nuclei)—the former being implicated in loss of convergence.12 Subsequent studies have rejected this rule, based on imaging and lesioning experiments.13 Dissociation of convergence may be present, which refers to the ability to converge during accommodation, but inability to adduct the eye ipsilateral to the MLF lesion during horizontal gaze. This is absent in other syndromes which may be confused with INO, such as myasthenia gravis, oculomotor palsy and medial rectus palsy.10
Various cases of INO have been published so far, the largest of which was a case series by Keane in 2005, comprising 410 cases, out of which 188 were bilateral. Infarction was the most common cause (predominantly unilateral INO), whereas MS was found to be the second most common cause (predominant cases being bilateral). MS has been found to be the most common brain stem lesion associated with bilateral INO or WEBINO syndrome, substantiated by many case series.12 14–17
Rare causes have been implicated, also described in the above case series—114 out of 410 were due to unusual causes, such as head trauma, infection, tumour, TTH, brainstem haemorrhage, vasculitis, postsurgical/ procedural and some miscellaneous causes. Only 3 out of 20 cases of TTH were due to ICH; rest were due to subdural or extradural haematoma (SDH/EDH).17
Ophthalmoplegia has been frequently observed in TTH, usually unilateral oculomotor palsy, and INO is relatively rare. However, if present, INO is usually bilateral. In a study (in 1986) analysing 25 cases of TTH with ocular motor signs, 10 out of 13 cases associated with INO were bilateral. Twenty out of 25 cases of TTH were due to traumatic intracranial haemorrhage (18 SDH/EDH and 2 large haemorrhagic contusions). The remaining five cases of TTH were due to non-traumatic causes, out of which one was due to an apparent spontaneous ICH and the rest were secondary to non traumatic SDH.18 Multiple cases of INO due to traumatic intracranial haemorrhage and even some cases of trauma with no apparent findings on CT have been reported.19–22
Bilateral INO is very rare and usually missed during examination. If detected, increased intracranial pressure and brain stem compression are rarely considered by physicians in the initial list of differentials, until development of typical signs such as headache, vomiting and altered sensorium, especially in cases with no history of trauma.
Learning points.
Internuclear ophthalmoplegia (INO) is usually a helpful localising sign (most commonly associated with demyelinating diseases or stroke) and usually unilateral. It is very rarely bilateral or a false localising sign (where the actual lesion is distant from the expected anatomical location causing a neurological deficit, such as in cases of intracranial haemorrhage).
Subtle signs of ophthalmoplegia, such as gaze evoked adduction paresis in INO, are rarely detected. A detailed initial examination is important, with periodic reassessment and an emphasis on detection of ocular signs, which are frequently missed during routine evaluation.
In cases with low suspicion of infarction or demyelinating disease, INO could help in diagnosing rare causes like transtentorial herniation (TTH) leading to early and appropriate management, preventing prolonged hospitalisation and fatal complications like coma and death.
INO associated with TTH has been seen to be usually bilateral,18 and increased intracranial pressure with early TTH could be an important differential diagnosis in wall eyed bilateral INO syndrome. However, more studies are needed to decide the sensitivity and reproducibility of the above correlation.
The case, not only rare, throws light on the importance of physical diagnosis in a world with rapidly emerging diagnostic modalities, which has replaced the art of keen clinical observation and diagnosis.
Acknowledgments
I would like to acknowledge Dr Virti Shah, a neurologist at MGM Medical College and Hospital, Navi Mumbai, who played a key role in guiding us to a diagnosis.
Footnotes
Twitter: @bhaimangesh
Contributors: SS: submitting author-primary role in evaluation, diagnosis and management of patient along with detailed literature review and construction of case report. BBN: guided in diagnosis and decision making as part of the medical team caring for the patient. Reviewed the manuscript in detail and helped edit and revise it. JG: head of the primary team caring for the patient. Scientific advisor and helped with literature search and editing the case report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Qureshi AI, Tuhrim S, Broderick JP, et al. Spontaneous intracerebral hemorrhage. N Engl J Med 2001;344:1450–60. 10.1056/NEJM200105103441907 [DOI] [PubMed] [Google Scholar]
- 2.Menon G, Kongwad LI, Nair RP, et al. Spontaneous intracerebral bleed post snake envenomation. J Clin Diagn Res 2017;11:PD03-PD04. 10.7860/JCDR/2017/25095.9517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalra SP, Varma PP, Chatterji RS. Experience with VIPERINE envenomation. Med J Armed Forces India 1998;54:204–7. 10.1016/S0377-1237(17)30543-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denson KW. Coagulant and anticoagulant action of snake venoms. Toxicon 1969;7:5–11. 10.1016/0041-0101(69)90154-8 [DOI] [PubMed] [Google Scholar]
- 5.McGettrick P, Eustace P. The w.e.b.i.n.o. syndrome. Neuroophthalmology 1985;5:109–15. 10.3109/01658108509014426 [DOI] [Google Scholar]
- 6.Hoyt WF, Daroff RB. Supranuclear disorder of ocular control systems in man. the control of eye movements. New York: Academic Press, 1971: 175–235. [Google Scholar]
- 7.Beh SC, Frohman EM. Webino and the return of the King's speech. J Neurol Sci 2012;315:153–5. 10.1016/j.jns.2011.11.035 [DOI] [PubMed] [Google Scholar]
- 8.Vernet M, Quentin R, Chanes L, et al. Frontal eye field, where art thou? anatomy, function, and non-invasive manipulation of frontal regions involved in eye movements and associated cognitive operations. Front Integr Neurosci 2014;8:66. 10.3389/fnint.2014.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivaud S, Müri RM, Gaymard B, et al. Eye movement disorders after frontal eye field lesions in humans. Exp Brain Res 1994;102:110–20. 10.1007/BF00232443 [DOI] [PubMed] [Google Scholar]
- 10.Virgo JD, Plant GT. Internuclear ophthalmoplegia. Pract Neurol 2017;17:149–53. 10.1136/practneurol-2016-001428 [DOI] [PubMed] [Google Scholar]
- 11.Liu DTL, Li C-L, Lee VYW. Internuclear ophthalmoplegia. Arch Neurol 2006;63:626. 10.1001/archneur.63.4.626-a [DOI] [PubMed] [Google Scholar]
- 12.Cogan DG. Internuclear ophthalmoplegia, typical and atypical. Arch Ophthalmol 1970;84:583–9. 10.1001/archopht.1970.00990040585005 [DOI] [PubMed] [Google Scholar]
- 13.Leigh RJ, Zee DS. Ocular Motor Syndromes Caused by Lesions of the Midbrain. In: The neurology of eye movements. 4 edn. New York: Oxford University Press, 2015. [Google Scholar]
- 14.Smith JW, Cogan DG. Internuclear ophthalmoplegia; a review of fifty-eight cases. AMA Arch Ophthalmol 1959;61:687–94. [PubMed] [Google Scholar]
- 15.Fötzsch R. Die internukleare Ophthalmoplegie. Ophthalmologica 1971;162:331–42. 10.1159/000306302 [DOI] [PubMed] [Google Scholar]
- 16.Gonyea EF. Bilateral internuclear ophthalmoplegia. association with occlusive cerebrovascular disease. Arch Neurol 1974;31:168–73. 10.1001/archneur.1974.00490390050004 [DOI] [PubMed] [Google Scholar]
- 17.Keane JR. Internuclear ophthalmoplegia: unusual causes in 114 of 410 patients. Arch Neurol 2005;62:714–7. 10.1001/archneur.62.5.714 [DOI] [PubMed] [Google Scholar]
- 18.Keane JR. Bilateral ocular motor signs after tentorial herniation in 25 patients. Arch Neurol 1986;43:806–7. 10.1001/archneur.1986.00520080050019 [DOI] [PubMed] [Google Scholar]
- 19.Chen K-T, Lin T-K, Hsieh T-C. Isolated internuclear ophthalmoplegia after massive supratentorial epidural hematoma: a case report and review of the literature. World Neurosurg 2017;100:712.e5–712.e13. 10.1016/j.wneu.2017.01.071 [DOI] [PubMed] [Google Scholar]
- 20.Chon J, Kim M. Bilateral internuclear ophthalmoplegia following head trauma. Indian J Ophthalmol 2017;65:246–7. 10.4103/ijo.IJO_236_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh WP, Hafner JW, Kattah JC. Bilateral internuclear ophthalmoplegia following minor head trauma. J Emerg Med 2003;24:19–22. 10.1016/S0736-4679(02)00662-5 [DOI] [PubMed] [Google Scholar]
- 22.Baker RS. Internuclear ophthalmoplegia following head injury. Case report. J Neurosurg 1979;51:552–5. 10.3171/jns.1979.51.4.0552 [DOI] [PubMed] [Google Scholar]