Abstract
Background
While some recent studies have reported the development of tuberculosis (TB) in patients exposed to immune checkpoint inhibitors (ICIs), there is limited evidence to date. Therefore, we evaluated the risk of TB in patients with cancer exposed to ICIs using the National Health Insurance claims data in South Korea.
Methods
Patients with diagnostic codes for non-small cell lung cancer, urothelial carcinoma or melanoma between August 2017 and June 2019 were identified. The incidence rate and standardized incidence ratio (SIR) of TB were calculated for both the ICI exposure and non-exposure groups. The risk of TB according to ICI exposure was assessed using a multivariable Cox regression model.
Results
During the study period, 141 550 patients with cancer and 916 new TB cases were identified. Among the 5037 patients exposed to ICIs, 20 were diagnosed with TB at a median of 2.2 months after the ICI was initiated. The crude incidence rate of TB per 100,000 person-years was 675.8 (95% CI 412.8 to 1043.8) for the ICI exposure group and 599.1 (95% CI 560.5 to 639.6) for the non-exposure group. The SIR for TB was 8.1 (95% CI 8.0 to 8.2) in the ICI exposure group. After adjusting for potential confounding factors, ICI treatment was not significantly associated with an increased risk of TB (HR: 0.73; 95% CI 0.47 to 1.14).
Conclusions
While the incidence of TB in cancer patients exposed to ICIs was eightfold higher than in the general population, the risk of patients with cancer developing TB did not significantly differ according to ICI exposure.
Keywords: tuberculosis, immunotherapy, programmed cell death 1 receptor
Background
Inhibiting the immune checkpoint pathway has been shown to be effective in treating patients with advanced solid cancers.1–3 Since immune checkpoint inhibitors (ICIs) were introduced, the use of these drugs to treat various types of cancer has increased, and a better understanding of adverse events, such as immune-related adverse events, is increasingly necessary.4 Recently, several studies have reported the development of tuberculosis (TB) in patients during or after ICI therapy.5–12 The development of active TB in patients with advanced cancer entails significant risks, including delayed antineoplastic therapy and death. The risk of developing active TB is two to three times higher in patients with solid cancer than in the general population.13–16 In addition, antineoplastic therapy was reported as an independent risk factor for active TB in patients with cancer.17 However, there are no specific guidelines for screening or treating latent tuberculosis infection (LTBI) in patients with cancer.18 19 Notably, the Society for Immunotherapy of Cancer (SITC) guideline recommends LTBI screening prior to initiating ICI20; however, this recommendation was made considering the potential use of corticosteroids or tumor necrosis factor (TNF) inhibitors for the treatment of immune-related adverse events during ICI use, rather than the direct association between ICI and the development of active TB. Therefore, it is necessary to evaluate the burden of active TB in patients with cancer receiving ICI as well as the association between ICI exposure and the subsequent development of active TB.
Estimating the incidence of active TB in patients with cancer poses significant challenges in terms of the need for longitudinal studies with large sample sizes and long observation periods, especially in high-income countries where the burden of active TB is low (<30 cases/100,000 persons per year).21 South Korea is a high-income country with an intermediate TB burden (55/100,000 persons in 2017) and thus has a considerably higher incidence of TB than other developed countries.22 In this respect, South Korea provides a unique and promising study setting including: (1) accessibility to the database of the National Health Insurance (NHI) program covering 50 million people, (2) nationwide TB notification system, and (3) an intermediate TB burden; therefore, we presumed that the analysis of the NHI claims data of South Korea could provide an important answer to the clinicoepidemiologic question about the risk of TB in patient with cancer receiving ICIs.
Methods
Study design, population, and database
This population-based retrospective cohort study was conducted using the nationwide claims database in South Korea. The NHI system in South Korea provides universal healthcare coverage to the entire population residing in the country. The healthcare providers are required to claim their medical services for reimbursement, and all these claims are collected and reviewed by the Health Insurance and Review Assessment Service (HIRA).23 The data collected by the HIRA encompasses all the claimed healthcare records, including medical visits, prescriptions, procedures and surgeries. The HIRA database is available for research purposes after the encryption and deidentification process is complete. Using the HIRA data repository, the burden of TB was estimated in patients with cancer according to ICI exposure. To determine the incidence of TB in patients receiving ICIs compared with the general population, annual statements regarding reported cases of TB and the official statistical database of the annual population census in South Korea were used.22 24
In South Korea, NHI coverage for ICIs have been approved for the following: nivolumab and pembrolizumab for advanced non-small cell lung cancer (NSCLC) since August 2017; atezolizumab for advanced NSCLC or urothelial carcinoma since January 2018; and nivolumab and pembrolizumab for advanced melanoma since February 2018. Insurance coverage for ICIs in patients with NSCLC and urothelial carcinoma are approved for patients who were previously treated with platinum-containing chemotherapy. We conducted an analysis on adult patients with cancer (aged ≥18 years) with diagnostic codes for NSCLC, urothelial carcinoma, or melanoma from August 2017 to June 2019. The exclusion criteria were as follows: (1) history of active TB, (2) HIV infection, (3) solid organ transplant, (4) inflammatory bowel disease, (5) hematologic malignancy, (6) past medication history of immunosuppressants, or (7) no available records after the first visit (lost to follow-up). The need for written or verbal consent was waived by the review board due to the observational nature of the study and the fact that the patient identifiers were fully encrypted prior to analysis.
Definitions and outcomes
Cancer patients with NSCLC, urothelial carcinoma, and melanoma were identified using International Classification of Diseases-10 (ICD-10) codes (C34, C66, C67, and C43) as primary or secondary diagnoses. ICI exposure was defined as the presence of a prescription for ICIs at least once during the study period. The remaining patients were categorized into the non-exposure group. The index date was defined as the date of the first administration of the ICI for the exposure group and the date of the first visit with diagnostic codes for cancer during the study period for the non-exposure group. The date of the last follow-up was determined by the last documented claim record for each individual during the study period. The main outcome of interest was the development of active TB requiring treatment. New cases of TB were identified by the presence of relevant diagnostic codes for active TB (ICD-10 code A15, A16, A17, A18, or A19) and prescription records for anti-TB medications as described in our previous study.25
In addition, the following potential confounding factors in the development of TB were identified based on claims records present during the study period or in the preceding 12 months: age, sex, hypertension, diabetes, dyslipidemia, chronic lung disease, chronic kidney disease, chronic liver disease, rheumatic disease, previous history of latent TB infection (LTBI) treatment, active chemotherapy, and concomitant use of corticosteroids or immunosuppressants. The use of corticosteroids was defined as the presence of prescription records for prednisone equivalents ≥15 mg/day for at least 14 days. The diagnostic and drug codes used in this study are summarized in online supplemental table 1.
jitc-2021-002960supp001.pdf (60.6KB, pdf)
Statistical analysis
Categorical data were compared using the χ2 test, and continuous variables were analyzed using the independent t-test. Incidence rates were presented as the number of TB episodes per 100,000 person-years (PYs) of follow-up. The CIs for the incidence rates were estimated under the assumption that the number of TB cases followed a Poisson distribution. To estimate the burden of TB in patients with cancer relative to the general population, standardized incidence ratios (SIRs) and 95% CIs were calculated. The risk factors for TB were identified using the Cox proportional hazard model. In the multivariable Cox regression model, baseline covariates including age, sex, health insurance type, type of cancer, diabetes, hypertension, chronic lung disease, chronic kidney disease, chronic liver disease, rheumatic disease, history of treatment for latent TB infection, active chemotherapy, concomitant use of corticosteroids, and concomitant use of immunosuppressants were included. A number of sensitivity analyses were performed to test the robustness of our findings. First, we repeatedly measured the outcome of TB as defined by diagnostic codes regardless of receiving anti-TB agents. In addition, we reanalyzed the study cohort after excluding TB cases diagnosed within 30 days after cancer diagnosis. We performed analyses in subgroups defined according to age (<50 years vs ≥50 years), sex, and cancer type. The presence of interactions in these subgroups was evaluated. Considering the comprehensive nature of the claims data, we assumed that the database has minimal or no missing values. All reported p values are two sided, and a p value less than 0.05 was considered statistically significant. Data processing and statistical analyses were conducted using SAS Enterprise Guide V.7.1 (SAS Institute).
Results
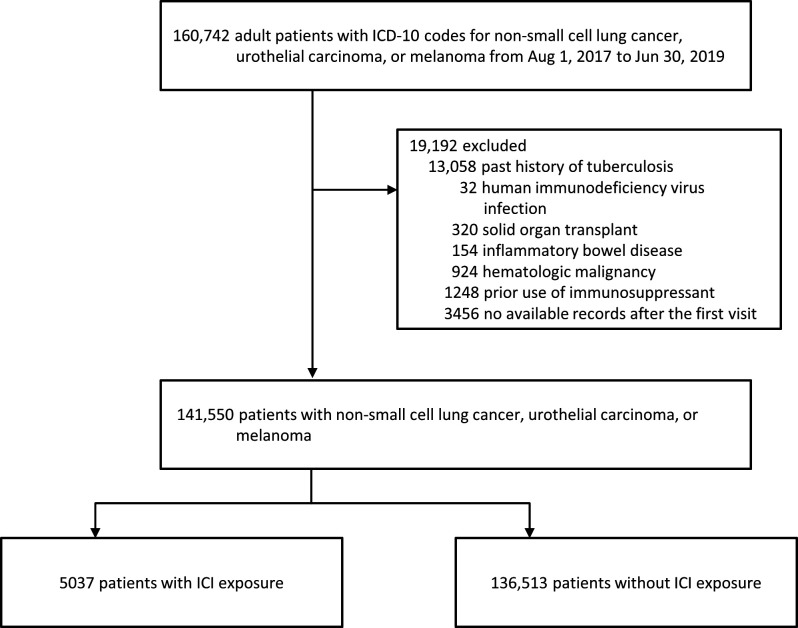
During the study period, a total of 160,742 adult patients (aged >18 years) with diagnostic codes for NSCLC, urothelial carcinoma, or melanoma were identified (figure 1). After excluding patients according to the exclusion criteria, 141,550 patients with NSCLC, urothelial carcinoma, or melanoma were included in the analysis. Of these, 5037 (3.6%) were in the ICI exposure group, and the remaining 136,513 (96.4%) patients were in the ICI non-exposure group. Among the 5037 patients in the exposure group, 1972 (39.2%), 2593 (51.5%) and 472 (9.4%) received nivolumab, pembrolizumab, and atezolizumab, respectively. The baseline characteristics of the study population are shown in table 1. We found that most of the cancer patients included in this study were male, were elderly, and had lung cancer. Patients in the ICI exposure group differed significantly from those in the ICI non-exposure group in terms of age, sex, type of cancer, comorbidities, active chemotherapy, and concomitant use of corticosteroids. No differences concerning a previous history of LTBI treatment were found between the ICI exposure and non-exposure groups.
Figure 1.
Flow chart of study population. ICD-10, International Classification of Diseases-10; ICI, immune checkpoint inhibitor.
Table 1.
Baseline characteristics of patients with cancer in the immune checkpoint inhibitor (ICI) exposure and non-exposure groups
| ICI exposure | P value* | |||||
| Nivolumab (n=1972) |
Pembrolizumab (n=2593) |
Atezolizumab (n=472) |
Total ICI exposure (n=5037) |
ICI non-exposure (n=136,513) |
||
| Age, year, mean (SD) | 65.8 (9.7) | 65.5 (10.2) | 67.7 (9.9) | 65.8 (10.0) | 68.5 (11.1) | <0.001 |
| Sex | <0.001 | |||||
| Male | 1522 (77.2) | 1858 (71.7) | 372 (78.8) | 3752 (74.5) | 92 050 (67.4) | |
| Female | 450 (22.8) | 735 (28.3) | 100 (21.2) | 1285 (25.5) | 44 463 (32.6) | |
| Type of insurance | <0.001 | |||||
| National Health Insurance | 1779 (90.2) | 2380 (91.8) | 425 (90.0) | 4584 (91.0) | 127 201 (93.2) | |
| Medical aids† | 156 (7.9) | 193 (7.4) | 45 (9.5) | 394 (7.8) | 8495 (6.2) | |
| Veterans | 37 (1.9) | 20 (0.8) | 2 (0.4) | 59 (1.2) | 817 (0.6) | |
| Type of cancer | <0.001 | |||||
| NSCLC | 1796 (91.1) | 2038 (78.6) | 154 (32.6) | 3988 (79.2) | 94 208 (69.0) | |
| Urothelial carcinoma | 9 (0.5) | 10 (0.4) | 318 (67.4) | 337 (6.7) | 39 268 (28.8) | |
| Melanoma | 167 (8.5) | 545 (21.0) | 0 (0) | 712 (14.1) | 4086 (3.0) | |
| Underlying diseases | ||||||
| Diabetes mellitus | 641 (32.5) | 804 (31.0) | 145 (30.7) | 1590 (31.6) | 39 015 (28.6) | <0.001 |
| Hypertension | 968 (49.1) | 1220 (47.0) | 251 (53.2) | 2439 (48.4) | 71 349 (52.3) | <0.001 |
| Chronic lung disease | 1163 (59.0) | 1392 (294.9) | 210 (44.5) | 2765 (54.9) | 55 289 (40.5) | <0.001 |
| Chronic kidney disease | 72 (3.7) | 72 (15.3) | 55 (11.7) | 199 (4.0) | 6346 (4.6) | 0.02 |
| Chronic liver disease | 37 (1.9) | 34 (7.2) | 6 (1.3) | 77 (1.5) | 1506 (1.1) | 0.01 |
| Rheumatic disease | 49 (2.5) | 73 (15.5) | 17 (3.6) | 139 (2.8) | 2873 (2.1) | 0.002 |
| Predisposing factors | ||||||
| History of LTBI treatment | 19 (1.0) | 28 (1.1) | 2 (0.4) | 49 (1.0) | 1686 (1.2) | 0.10 |
| Active chemotherapy | 569 (28.9) | 675 (26.0) | 70 (14.8) | 1314 (26.1) | 31 870 (23.3) | <0.001 |
| Concomitant use of corticosteroid | 459 (23.3) | 533 (20.6) | 71 (15.0) | 1063 (21.1) | 12 603 (9.2) | <0.001 |
| Concomitant use of immunosuppressant | 14 (0.7) | 34 (1.3) | 26 (5.5) | 74 (1.5) | 1889 (1.4) | 0.69 |
Data are n (%).
*Comparison of total ICI exposure group to non-exposure group.
†Medical aid is a public assistance program that targets impoverished people in need of medical assistance as part of the South Korean social welfare program.
LTBI, latent tuberculosis infection; NSCLC, non-small cell lung cancer.
There were 916 cases of TB infections in the 141,550 patients included in this study, 20 of which occurred in the ICI exposure group. Detailed characteristics of these 20 cases of TB are summarized in table 2. Most of the patients were male (80% (16/20)) and most had NSCLC (95% (19/20)). Of the 20 cases of TB, 12 occurred in patients who had received pembrolizumab, and eight occurred in patients who had received nivolumab. No cases of TB occurred in patients who received atezolizumab or patients with urothelial carcinoma. TB developed a median of 2.2 months (range: 0.4–16.5) after ICI treatment was initiated, and most of the cases of TB (80% (16/20)) were pulmonary infections. Eight (40%) patients were taking corticosteroids concomitantly.
Table 2.
Characteristics of the 20 patients with tuberculosis following ICI exposure
| Patient number | Age, years | Sex | Cancer type | Other comorbidities | Type of ICI | Total ICI cycles received | Interval* (months) | Type of TB | Concomitant corticosteroid | Concomitant immunosuppressant |
| 1 | 73 | Male | Lung cancer | None | Nivolumab | 5 | 5.7 | Pulmonary | No | No |
| 2 | 80 | Male | Lung cancer | Hypertension and chronic lung disease | Nivolumab | 1 | 1.3 | Pulmonary | No | No |
| 3 | 65 | Male | Lung cancer | Chronic lung disease | Nivolumab | 8 | 1.7 | Miliary | No | No |
| 4 | 82 | Male | Lung cancer | Chronic lung disease | Pembrolizumab | 6 | 1.3 | Pulmonary | No | No |
| 5 | 74 | Female | Lung cancer | Hypertension, diabetes and chronic kidney disease | Pembrolizumab | 2 | 0.4 | Meningitis | Yes | No |
| 6 | 81 | Female | Melanoma | Hypertension and chronic lung disease | Pembrolizumab | 2 | 0.7 | Pulmonary | No | No |
| 7 | 65 | Male | Lung cancer | None | Pembrolizumab | 3 | 2.8 | Vertebra | Yes | No |
| 8 | 44 | Female | Lung cancer | Hypertension | Nivolumab | 2 | 1.5 | Pulmonary | No | No |
| 9 | 61 | Male | Lung cancer | Chronic lung disease | Pembrolizumab | 7 | 4.7 | Pericarditis | No | No |
| 10 | 64 | Male | Lung cancer | Hypertension and diabetes | Pembrolizumab | 7 | 5.3 | Pulmonary | Yes | No |
| 11 | 58 | Male | Lung cancer | None | Pembrolizumab | 2 | 2.3 | Pulmonary | No | No |
| 12 | 59 | Male | Lung cancer | Hypertension and chronic lung disease | Pembrolizumab | 1 | 0.9 | Pulmonary | Yes | No |
| 13 | 71 | Male | Lung cancer | Hypertension and chronic lung disease | Nivolumab | 2 | 0.6 | Pulmonary | No | No |
| 14 | 53 | Male | Lung cancer | Chronic lung disease | Nivolumab | 10 | 7.5 | Pulmonary | Yes | No |
| 15 | 54 | Male | Lung cancer | Diabetes and chronic lung disease | Pembrolizumab | 2 | 2.8 | Pulmonary | No | No |
| 16 | 79 | Male | Lung cancer | Chronic lung disease | Pembrolizumab | 3 | 2.0 | Pulmonary | Yes | No |
| 17 | 67 | Female | Lung cancer | Hypertension and diabetes | Nivolumab | 10 | 16.5 | Pulmonary | Yes | No |
| 18 | 78 | Male | Lung cancer | Chronic lung disease | Pembrolizumab | 10 | 13.6 | Pulmonary | No | Yes |
| 19 | 61 | Male | Lung cancer | Hypertension, diabetes and chronic lung disease | Pembrolizumab | 2 | 0.6 | Pulmonary | Yes | No |
| 20 | 83 | Male | Lung cancer | Chronic lung disease | Nivolumab | 10 | 9.1 | Pulmonary | No | No |
*Interval from the first administration of the ICI to the development of TB
ICI, immune checkpoint inhibitor; TB, tuberculosis.
Incidence rate and standardized incidence ratio of TB in the ICI exposure and non-exposure groups
The total observation time was 152,521 PY for all the patients with cancer included in this study. Of this, 2959 PY was attributed to the ICI exposure group, and 149,562 PY to the ICI non-exposure group, with average follow-up periods of 0.59 and 1.10 years, respectively. The crude TB incidence per 100,000 PY was 675.8 in the ICI exposure group and 599.1 in the ICI non-exposure group (table 3). The SIR of TB in the ICI exposure group was 8.1 (95% CI 8.0 to 8.2), 6.0 (95% CI 5.9 to 6.1), and 10.9 (95% CI 10.7 to 11.1) for the total, male, and female participants, respectively (table 4). The SIR estimates of TB for the total, male, and female participants in the non-exposure group were 8.2 (95% CI 8.1 to 8.3), 8.4 (95% CI 8.2 to 8.4), and 7.9 (95% CI 7.8 to 8.1), respectively.
Table 3.
The incidence rate of TB in patients with cancer exposed to ICIs
| ICI exposure | ICI non-exposure | |||||||
| n (%) | TB events | Person-years | Incidence* (95% CI) | n (%) | TB events | Person-years | Incidence* (95% CI) | |
| Total | 5037 (100.0) | 20 | 2959 | 675.8 (412.8 to 1043.8) | 136 513 (100.0) | 896 | 149 562 | 599.1 (560.5 to 639.6) |
| Sex | ||||||||
| Male | 3752 (74.5) | 16 | 2173 | 736.5 (421.0 to 1196.0) | 92 050 (67.4) | 676 | 98 733 | 684.7 (634.0 to 738.3) |
| Female | 1285 (25.5) | 4 | 787 | 508.3 (138.5 to 1301.6) | 44 463 (32.6) | 220 | 50 829 | 432.8 (377.5 to 494.0) |
| Age (years) | ||||||||
| 20–29 | 8 (0.2) | 0 | 5 | 288 (0.2) | 3 | 319 | 939.7 (193.8 to 2746.1) | |
| 30–39 | 40 (0.8) | 0 | 26 | 1278 (0.9) | 6 | 1464 | 409.9 (150.4 to 892.2) | |
| 40–49 | 269 (5.3) | 1 | 184 | 544.4 (13.8 to 3033.0) | 5805 (4.3) | 28 | 6992 | 400.5 (266.1 to 578.8) |
| 50–59 | 976 (19.4) | 4 | 607 | 658.7 (179.5 to 1686.6) | 20 749 (15.2) | 86 | 25 013 | 343.8 (275.0 to 424.6) |
| 60–69 | 1793 (35.6) | 6 | 1058 | 567.3 (208.2 to 1234.7) | 40 364 (29.6) | 258 | 47 363 | 544.7 (480.3 to 615.4) |
| 70–79 | 1615 (32.1) | 5 | 914 | 546.8 (177.5 to 1276.0) | 46 003 (33.7) | 335 | 49 406 | 678.1 (607.4 to 754.7) |
| ≥80 | 336 (6.7) | 4 | 165 | 2422.3 (660.0 to 6202.1) | 22 026 (16.1) | 180 | 19 006 | 947.1 (813.8 to 1096.1) |
| Type of cancer | ||||||||
| NSCLC | 3988 (79.2) | 19 | 2336 | 813.2 (489.6 to 1269.9) | 94 208 (69.0) | 752 | 96 624 | 778.3 (723.6 to 836.0) |
| Urothelial carcinoma | 337 (6.7) | 0 | 173 | 38 931 (28.5) | 135 | 49 047 | 275.3 (230.8 to 325.8) | |
| Melanoma | 712 (14.1) | 1 | 450 | 222.0 (5.6 to 1237.0) | 3374 (2.5) | 9 | 3891 | 231.3 (105.8 to 439.0) |
*Indicates incidence rate per 100,000 person-years.
ICI, immune checkpoint inhibitor; NSCLC, non-small cell lung cancer; TB, tuberculosis.
Table 4.
The standardized incidence ratio of TB in patients with cancer exposed to ICIs
| Standardized TB incidence rate* | 95% CI | TB incidence rate in general population* | 95% CI | Standardized incidence ratio | 95% CI | P value | |
| ICI exposure | |||||||
| Total | 494.4 | 492.3 to 496.5 | 61.4 | 60.6 to 62.1 | 8.1 | 8.0 to 8.2 | <0.001 |
| Male | 428.2 | 425.3 to 431.0 | 71.6 | 70.4 to 72.2 | 6.0 | 5.9 to 6.1 | <0.001 |
| Female | 559.3 | 556.1 to 562.5 | 51.4 | 50.4 to 52.3 | 10.9 | 10.7 to 11.1 | <0.001 |
| ICI non-exposure | |||||||
| Total | 502.6 | 500.5 to 504.8 | 61.4 | 60.6 to 62.1 | 8.2 | 8.1 to 8.3 | <0.001 |
| Male | 600.0 | 596.7 to 603.4 | 71.6 | 70.4 to 72.2 | 8.4 | 8.2 to 8.5 | <0.001 |
| Female | 407.1 | 404.4 to 409.9 | 51.4 | 50.4 to 52.3 | 7.9 | 7.8 to 8.1 | <0.001 |
*Indicates incidence rate per 100,000 person-years.
ICI, immune checkpoint inhibitor; TB, tuberculosis.
Risk factors for TB in patients with cancer
The univariable Cox analysis (table 5) revealed that the following risk factors were significantly associated with TB: increasing age (HR 1.02; 95% CI 1.02 to 1.03), male sex (HR 1.54; 95% CI 1.33 to 1.79), medical aids (HR 1.40; 95% CI 1.10 to 1.78), lung malignancy (HR 3.35; 95% CI 1.80 to 6.25), diabetes (HR 1.22; 95% CI 1.07 to 1.41), hypertension (HR 1.18; 95% CI 1.04 to 1.34), chronic lung disease (HR 1.77; 95% CI 1.55 to 2.01), active chemotherapy (HR 1.72; 95% CI 1.50 to 1.98), and the concomitant use of corticosteroids (HR 1.56; 95% CI 1.29 to 1.88). As a risk factor, ICI treatment was not found to be significantly associated with the development of TB in the univariable analysis (HR 0.85; 95% CI 0.55 to 1.33) and in the multivariable Cox regression analysis (HR 0.73; 95% CI 0.47 to 1.14).
Table 5.
Risk of tuberculosis in patients with cancer
| Total, (n) |
TB cases, (n) | Person-years | Unadjusted | Adjusted | |||
| HR (95% CI) | P value | HR (95% CI) | P value | ||||
| Age, year | 1.02 (1.02 to 1.03) | <0.001 | 1.03 (1.02 to 1.03) | <0.001 | |||
| Sex | |||||||
| Male | 95,802 | 692 | 100 905 | 1.54 (1.33 to 1.79) | <0.001 | 1.61 (1.39 to 1.89) | <0.001 |
| Female | 45,748 | 224 | 51,616 | 1 (reference) | 1 (reference) | ||
| Health insurance type | |||||||
| National health insurance | 127,201 | 837 | 142,716 | 1 (reference) | 0.02 | 1 (reference) | |
| Medical aids* | 8495 | 73 | 8544 | 1.40 (1.10 to 1.78) | 0.01 | 1.28 (1.01 to 1.63) | 0.04 |
| Veterans | 8147 | 6 | 1262 | 0.90 (0.40 to 2.01) | 0.80 | 0.77 (0.34 to 1.72) | 0.52 |
| Type of cancer | |||||||
| NSCLC | 98,196 | 771 | 98,960 | 3.35 (1.80 to 6.25) | <0.001 | 2.43 (1.30 to 4.54) | 0.01 |
| Urothelial carcinoma | 39,268 | 135 | 49,220 | 1.27 (0.67 to 2.41) | 0.47 | 0.91 (0.48 to 1.73) | 0.76 |
| Melanoma | 4086 | 10 | 4342 | 1 (reference) | <0.001 | 1 (reference) | |
| Underlying diseases | |||||||
| Diabetes mellitus | 40,605 | 295 | 42,149 | 1.22 (1.07 to 1.41) | 0.004 | 1.07 (0.93 to 1.24) | 0.34 |
| Hypertension | 73,788 | 509 | 78,074 | 1.18 (1.04 to 1.34) | 0.01 | 0.99 (0.86 to 1.14) | 0.86 |
| Chronic lung disease | 58,054 | 491 | 59,344 | 1.77 (1.55 to 2.01) | <0.001 | 1.32 (1.15 to 1.51) | <0.001 |
| Chronic kidney disease | 6545 | 53 | 6988 | 1.28 (0.97 to 1.69) | 0.08 | 1.29 (0.97 to 1.72) | 0.08 |
| Chronic liver disease | 1583 | 15 | 1560 | 1.56 (0.94 to 2.60) | 0.88 | 1.38 (0.83 to 2.31) | 0.22 |
| Rheumatic disease | 3012 | 27 | 3159 | 1.42 (0.97 to 2.09) | 0.07 | 1.31 (0.89 to 1.93) | 0.17 |
| Predisposing factors | |||||||
| History of LTBI treatment | 1735 | 11 | 1903 | 0.97 (0.54 to 1.76) | 0.93 | 0.97 (0.54 to 1.76) | 0.92 |
| Active chemotherapy | 33,184 | 306 | 32,353 | 1.72 (1.50 to 1.98) | <0.001 | 1.45 (1.24 to 1.69) | <0.001 |
| Concomitant use of corticosteroid | 13,666 | 125 | 13,196 | 1.56 (1.29 to 1.88) | <0.001 | 1.09 (0.89 to 1.34) | 0.4 |
| Concomitant use of immunosuppressant | 1963 | 14 | 2273 | 1.03 (0.61 to 1.75) | 0.90 | 0.98 (0.58 to 1.68) | 0.95 |
| ICI treatment | 5037 | 20 | 2959 | 0.85 (0.55 to 1.33) | 0.48 | 0.73 (0.47 to 1.14) | 0.17 |
*Medical aid is a public assistance program that targets impoverished people in need of medical assistance as part of the South Korean social welfare program.
ICI, immune checkpoint inhibitor; LTBI, latent tuberculosis infection; NSCLC, non-small cell lung cancer; TB, tuberculosis.
Subgroup and sensitivity analyses
In the subgroup analyses, no significant difference in incidence for TB was observed between the ICI exposure and ICI non-exposure groups, regardless of age group, sex, and type of cancer (all p values for interaction >0.05) (table 6). The results were robust in sensitivity analyses in which the definition of TB was made by diagnostic codes regardless of anti-TB medication. In the sensitivity analysis conducted in the study cohort, excluding TB cases diagnosed within 30 days of cancer diagnosis, no significant difference in TB incidence was observed between the ICI exposure and ICI non-exposure groups (table 6).
Table 6.
Subgroup and sensitivity analyses
| TB events in non-exposure group, (n) | TB events in ICI exposure group, (n) | HR (95% CI) | P value | |
| Subgroup analyses* | ||||
| Age | 0.98† | |||
| <50 | 37 | 1 | 0.62 (0.09 to 4.55) | 0.64 |
| ≥50 | 859 | 19 | 0.69 (0.44 to 1.09) | 0.11 |
| Sex | 0.56† | |||
| Male | 676 | 16 | 0.71 (0.43 to 1.16) | 0.17 |
| Female | 220 | 4 | 0.89 (0.33 to 2.42) | 0.83 |
| Cancer subtype | 0.98† | |||
| NSCLC | 752 | 19 | 0.76 (0.48 to 1.19) | 0.23 |
| Urothelial carcinoma | 135 | 0 | 0.93 (0.06 to 15.15) | 0.96 |
| Melanoma | 9 | 1 | 0.84 (0.14 to 5.15) | 0.85 |
| Sensitivity analyses* | ||||
| Defining TB by ICD-10 codes only | 1765 | 44 | 0.83 (0.61 to 1.12) | 0.21 |
| Excluding TB cases within 30days following cancer diagnosis | 637 | 15 | 0.89 (0.53 to 1.50) | 0.67 |
*All subgroup and sensitivity analyses were adjusted for age, sex, health insurance type, type of cancer, diabetes, hypertension, chronic lung disease, chronic kidney disease, chronic liver disease, rheumatic disease, active chemotherapy, concomitant immunosuppressant use, concomitant use of corticosteroid, and history of treatment for latent TB infection.
†P value for interaction.
ICD-10, International Classification of Diseases code version 10; ICI, immune checkpoint inhibitor; NSCLC, non-small cell lung cancer; PS, propensity matching; TB, tuberculosis.
Discussion
In this nationwide population-based study, the incidence of TB in the patients exposed to ICIs was approximately eightfold higher relative to the general population. However, there were no significant differences in risk for TB in patients with cancer according to ICI exposure. These findings suggest that the development of TB in patients with cancer receiving ICIs was associated with the underlying malignancy rather than the exposure to ICIs. To the best of our knowledge, this is the first study illustrating the burden of TB in cancer patients with or without ICI exposure.
Similar to findings in several chronic infections, including HIV, immune checkpoint proteins (programmed death-1 [PD-1] and programmed death-ligand 1 [PD-L1]) were overexpressed in the monocytes or natural killer cells collected from patients with active TB.26 27 An enhanced PD1/PD-L1 pathway can contribute to chronic and persistent infections through the dysfunction of immune effector cells. Inhibiting the immune checkpoint pathway may therefore prevent TB reactivation by interfering with the ability of mycobacterium to exploit the PD1/PD-L1 pathway for immune evasion. In animal models, however, PD-1 deficient mice were more susceptible to mycobacterium than wild-type mice, and they showed more evidence of fulminant infectious processes.28–30 Thus, the PD-1/PD-L1 pathway may aid in controlling tissue damage by preventing the excessive production of IFN-gamma in effector T cells activated by TB antigens.31 However, whether TB susceptibility in patients with cancer could be clinically affected by the use of ICIs has not yet been clarified.
We found that there was no significant difference in the TB incidence among cancer patients according to ICI exposure. This finding was consistently observed before and after adjusting for multiple variables including age, sex, cancer type, underlying comorbidities, previous treatment of LTBI, active chemotherapy, and concomitant use of corticosteroids. Therefore, the high rate of TB in the ICI exposure group in our study was likely due to the underlying malignancy rather than exposure to ICIs, given that 19 out of 20 TB cases previously exposed to ICI were patients with lung cancer. In addition, the use of immunosuppressive medications such as corticosteroids to manage immune-related adverse events following ICI administration may also have contributed to the development of TB in this patient population.32 Considering the increased risk for TB with corticosteroid use, there may be an additive risk of developing TB in patients with cancer receiving concomitant corticosteroids.33 Therefore, prescreening for LTBI should be considered in patients with cancer using ICI as recommended by the SITC guideline regarding the possibility of immune-related adverse events requiring the use of corticosteroids or TNF inhibitors.20 32
It has been reported that patients with solid cancers have a risk of developing active TB that is two to three times greater that of the general population.13–15 In particular, the incidence of TB in patients with lung cancer was reported to be six to nine times that of the general population.15 16 Consistent with these findings, our study showed that patients with cancer (including those with lung, urothelial, and skin cancers) had about an eightfold higher rate of developing active TB compared with the general population in South Korea. Given the greater risk of TB in patients with lung cancer compared with those with other types of cancer, the high TB incidence observed in our study was likely due to the large proportion of patients with lung cancer in the study population. Considering the high risk for TB in patients with cancer regardless of ICI exposure, further targeted study is needed to evaluate the cost-effectiveness of prescreening and treatment for LTBI in patients with cancer.
There were several strengths to this study. To the best of our knowledge, this is the first population-based study reporting the risk of TB following ICI exposure. By identifying those exposed to ICIs in a nationwide sample from a country with an intermediate burden of TB, we were able to effectively determine the incidence of the disease in those exposed to ICIs and thus assess whether ICIs were associated with TB occurrence. In addition, an SIR was accurately calculated based on annual statistics of reported TB cases and population census data published by the Korean government. We also excluded individuals with suppressed cellular immunity, which is a known risk factor for the development of TB. In the risk factor analysis, a multivariable analysis was conducted to control for potential confounders after adjusting for a range of variables. In addition, we performed several subgroup and sensitivity analyses to confirm the robustness of our results.
There were several limitations to our study. First, due to the observational nature of the claims database, it cannot be ruled out that TB cases may have been misclassified. The methods used to identify cases of TB in the national claims data and the national reported data were not the same. However, healthcare providers are required to report new cases of TB through a web-based notification system in South Korea,22 34 and the completeness of the TB reporting data has been found to be over 90% in comparison with the reimbursement data from the NHI.35 Therefore, misclassification bias is expected to be low in this study. Second, data on TB severity were not assessed. Since concerns have been raised about the more fulminant course of TB after ICI treatment, as seen in animal studies, further studies are needed to address the severity of TB following ICI exposure. Third, only a limited number of ICIs (nivolumab, atezolizumab, and pembrolizumab) and types of cancer (lung cancer, urothelial carcinoma, and skin cancer) were assessed in this study. Therefore, the risk of TB in patients with other types of cancers, as well as in those receiving other types of ICIs, such as CTLA-4 inhibitors, were not evaluated in this study. Fourth, detailed information on PD-1 expression, cancer stage, performance status, surgical history, and prior radiation therapy in patients with cancer were not collected as raw data and therefore not included in the analysis. Fifth, the observation period in the ICI exposure group was relatively short compared with the ICI non-exposure group. Due to the nature of the slow-growing mycobacteria and the shorter observation period in the ICI exposure group, the number of TB events may have been underestimated in current study. Further studies with a longer follow-up periods may provide better answers in the future. Sixth, because the claims data did not contain the information on exposure history to the index TB patient, it was not possible to distinguish between recently transmitted TB and reactivation of remote infection among the cases of active TB. Therefore, the results of this study should be interpreted with caution, especially in areas with low TB burden where remote infection rather than recent transmission substantially contributes to the burden of active TB.36
In our study, the incidence of TB in patients with cancer receiving ICIs was eight times higher than in the general population. However, no significant differences were observed among patients with cancer according to ICI exposure. These findings suggest that patients with cancer undergoing chemotherapy are at risk for developing TB regardless of the type of treatment they are receiving. Further studies are needed to evaluate the cost-effectiveness of testing for and treating LTBI in patients with cancer receiving systemic chemotherapy.
Acknowledgments
This study used Health Insurance Review & Assessment (HIRA) research data (M20200217323) compiled by the HIRA.
Footnotes
SB and Y-JK contributed equally.
Contributors: All authors critically revised the manuscript for important intellectual content. S-OL, S-HK, S-HC, YSK, SB, and S-CY contributed to the study conceptualization. SB, JHK, JJ, MJK, YPC, S-HK, S-HC, YSK, and S-OL contributed to study design, literature search, and drafting of the manuscript. SB and Y-JK verified the underlying data. SB, Y-JK, and MK carried out the statistical analysis. Interpretation of data was performed by S-CY, SB, Y-JK, and MK. SB and Y-JK equally contributed to the work. All authors revised the manuscript critically.
Funding: This study was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, which is funded by the Ministry of Health & Welfare, Republic of Korea (grant number. HW20C2062).
Disclaimer: The views expressed are those of the authors and not necessarily those of the HIRA and the Ministry of Health and Welfare.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available at https://opendata.hira.or.kr/ with permission from the Health Insurance Review and Assessment Service (HIRA). Data are provided by the HIRA after reviewing the researcher's request for academic purposes. The data used in this article were provided by the Health Insurance Review and Assessment Service of Korea at the request of the authors [https://opendata.hira.or.kr].
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study was approved by the Institutional Review Board of the Asan Medical Center (approval number: 2020-0088), and the need for written or verbal consent was waived due to the observational nature of the study and the fact that the patient identifiers were fully encrypted prior to analysis.
References
- 1.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909–20. 10.1016/S0140-6736(16)00561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 4.Wang DY, Salem J-E, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 2018;4:1721–8. 10.1001/jamaoncol.2018.3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujita K, Terashima T, Mio T. Anti-Pd1 antibody treatment and the development of acute pulmonary tuberculosis. J Thorac Oncol 2016;11:2238–40. 10.1016/j.jtho.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 6.Lee JJX, Chan A, Tang T. Tuberculosis reactivation in a patient receiving anti-programmed death-1 (PD-1) inhibitor for relapsed Hodgkin's lymphoma. Acta Oncol 2016;55:519–20. 10.3109/0284186X.2015.1125017 [DOI] [PubMed] [Google Scholar]
- 7.Chu Y-C, Fang K-C, Chen H-C, et al. Pericardial tamponade caused by a hypersensitivity response to tuberculosis reactivation after anti-PD-1 treatment in a patient with advanced pulmonary adenocarcinoma. J Thorac Oncol 2017;12:e111–4. 10.1016/j.jtho.2017.03.012 [DOI] [PubMed] [Google Scholar]
- 8.Picchi H, Mateus C, Chouaid C, et al. Infectious complications associated with the use of immune checkpoint inhibitors in oncology: reactivation of tuberculosis after anti PD-1 treatment. Clin Microbiol Infect 2018;24:216–8. 10.1016/j.cmi.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 9.Jensen KH, Persson G, Bondgaard A-L, et al. Development of pulmonary tuberculosis following treatment with anti-PD-1 for non-small cell lung cancer. Acta Oncol 2018;57:1127–8. 10.1080/0284186X.2018.1433877 [DOI] [PubMed] [Google Scholar]
- 10.Elkington PT, Bateman AC, Thomas GJ, et al. Implications of tuberculosis reactivation after immune checkpoint inhibition. Am J Respir Crit Care Med 2018;198:1451–3. 10.1164/rccm.201807-1250LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He W, Zhang X, Li W, et al. Activated pulmonary tuberculosis in a patient with melanoma during PD-1 inhibition: a case report. Onco Targets Ther 2018;11:7423–7. 10.2147/OTT.S178246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Eeden R, Rapoport BL, Smit T, et al. Tuberculosis infection in a patient treated with nivolumab for non-small cell lung cancer: case report and literature review. Front Oncol 2019;9:659. 10.3389/fonc.2019.00659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simonsen DF, Farkas DK, Horsburgh CR, et al. Increased risk of active tuberculosis after cancer diagnosis. J Infect 2017;74:590–8. 10.1016/j.jinf.2017.03.012 [DOI] [PubMed] [Google Scholar]
- 14.Seo GH, Kim MJ, Seo S, et al. Cancer-specific incidence rates of tuberculosis: a 5-year nationwide population-based study in a country with an intermediate tuberculosis burden. Medicine 2016;95:e4919. 10.1097/MD.0000000000004919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobler CC, Cheung K, Nguyen J, et al. Risk of tuberculosis in patients with solid cancers and haematological malignancies: a systematic review and meta-analysis. Eur Respir J 2017;50:1700157. 10.1183/13993003.00157-2017 [DOI] [PubMed] [Google Scholar]
- 16.Cheng MP, Abou Chakra CN, Yansouni CP, et al. Risk of active tuberculosis in patients with cancer: a systematic review and meta-analysis. Clin Infect Dis 2017;64:635–44. 10.1093/cid/ciw838 [DOI] [PubMed] [Google Scholar]
- 17.Kim H-R, Hwang SS, Ro YK, et al. Solid-Organ malignancy as a risk factor for tuberculosis. Respirology 2008;13:413–9. 10.1111/j.1440-1843.2008.01282.x [DOI] [PubMed] [Google Scholar]
- 18.Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American thoracic Society/Infectious diseases Society of America/Centers for disease control and prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis 2017;64:e1–33. 10.1093/cid/ciw694 [DOI] [PubMed] [Google Scholar]
- 19.Anon . Targeted tuberculin testing and treatment of latent tuberculosis infection. this official statement of the American thoracic Society was adopted by the ats board of directors, July 1999. this is a joint statement of the American thoracic Society (ats) and the centers for disease control and prevention (CDC). this statement was endorsed by the Council of the infectious diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med 2000;161:S221–47. 10.1164/ajrccm.161.supplement_3.ats600 [DOI] [PubMed] [Google Scholar]
- 20.Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for immunotherapy of cancer (SITC) toxicity management Working group. J Immunother Cancer 2017;5:95. 10.1186/s40425-017-0300-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization . Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. Geneva: World Health Organization, 2018. [PubMed] [Google Scholar]
- 22.Korea Centers for Disease Control and Prevention . 2019 annual report on the notified tuberculosis in Korea. Cheongju: Korea Centers for Diseases Control and Prevention, 2020: 44–9. [Google Scholar]
- 23.Kim JA, Yoon S, Kim LY, et al. Towards Actualizing the value potential of Korea health insurance review and assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci 2017;32:718–28. 10.3346/jkms.2017.32.5.718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korean Statistical Information Service (KOSIS) . Report on the National population and housing census 2015~2019.: statistics Korea, 2020. Available: https://kosis.kr/statisticsList/statisticsListIndex.do?menuId=M_01_01&vwcd=MT_ZTITLE&parmTabId=M_01_01&statId=1962001&themaId=A#A_4.2 [Accessed 05 Apr 2021].
- 25.Kim M-C, Yun S-C, Lee S-O, et al. Association between tuberculosis, statin use, and diabetes: a propensity score-matched analysis. Am J Trop Med Hyg 2019;101:350–6. 10.4269/ajtmh.18-0983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jurado JO, Alvarez IB, Pasquinelli V, et al. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J Immunol 2008;181:116–25. 10.4049/jimmunol.181.1.116 [DOI] [PubMed] [Google Scholar]
- 27.Alvarez IB, Pasquinelli V, Jurado JO, et al. Role played by the programmed death-1-programmed death ligand pathway during innate immunity against Mycobacterium tuberculosis. J Infect Dis 2010;202:524–32. 10.1086/654932 [DOI] [PubMed] [Google Scholar]
- 28.Lázár-Molnár E, Chen B, Sweeney KA, et al. Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc Natl Acad Sci U S A 2010;107:13402–7. 10.1073/pnas.1007394107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barber DL, Mayer-Barber KD, Feng CG, et al. CD4 T cells promote rather than control tuberculosis in the absence of PD-1-mediated inhibition. J Immunol 2011;186:1598–607. 10.4049/jimmunol.1003304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tousif S, Singh Y, Prasad DVR, et al. T cells from programmed death-1 deficient mice respond poorly to Mycobacterium tuberculosis infection. PLoS One 2011;6:e19864. 10.1371/journal.pone.0019864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakai S, Kauffman KD, Sallin MA, et al. Cd4 T cell-derived IFN-γ plays a minimal role in control of pulmonary Mycobacterium tuberculosis infection and must be actively repressed by PD-1 to prevent lethal disease. PLoS Pathog 2016;12:e1005667. 10.1371/journal.ppat.1005667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv119–42. 10.1093/annonc/mdx225 [DOI] [PubMed] [Google Scholar]
- 33.Jick SS, Lieberman ES, Rahman MU, et al. Glucocorticoid use, other associated factors, and the risk of tuberculosis. Arthritis Rheum 2006;55:19–26. 10.1002/art.21705 [DOI] [PubMed] [Google Scholar]
- 34.Go U, Park M, Kim U-N, et al. Tuberculosis prevention and care in Korea: evolution of policy and practice. J Clin Tuberc Other Mycobact Dis 2018;11:j.jctube.2018.04.006:28–36. 10.1016/j.jctube.2018.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang H-Y, Yoo H, Park W, et al. Tuberculosis notification completeness and timeliness in the Republic of Korea during 2012-2014. Osong Public Health Res Perspect 2016;7:j.phrp.2016.08.002:320–6. 10.1016/j.phrp.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Behr MA, Edelstein PH, Ramakrishnan L. Revisiting the timetable of tuberculosis. BMJ 2018;362:k2738. 10.1136/bmj.k2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2021-002960supp001.pdf (60.6KB, pdf)
Data Availability Statement
Data are available at https://opendata.hira.or.kr/ with permission from the Health Insurance Review and Assessment Service (HIRA). Data are provided by the HIRA after reviewing the researcher's request for academic purposes. The data used in this article were provided by the Health Insurance Review and Assessment Service of Korea at the request of the authors [https://opendata.hira.or.kr].