Abstract
Dietary intake has been shown to change the composition of gut microbiota and some changes in microbiota (dysbiosis) have been linked to diabetes, hypertension, and obesity, which are established risk factors for atrial fibrillation (AF). In addition, intestinal dysbiosis generates microbiota-derived bioactive metabolites that might exert proarrhythmic actions. Although emerging preclinical investigations and clinical observational cohort studies suggest a possible role of gut dysbiosis in AF promotion, the exact mechanisms through which dysbiosis contributes to AF remain unclear. This Viewpoint article briefly reviews evidence suggesting that abnormalities in the intestinal microbiota play an important and little-recognized role in the pathophysiology of AF and that an improved understanding of this role may open up new possibilities in the management of AF.
Keywords: Arrhythmia, Atrial fibrillation, cardiometabolic, dysbiosis, gut microbiota, metabolites
Graphical abstract
Introduction
Multiple mechanisms contribute to the pathophysiology of atrial fibrillation (AF). Dietary intake has been shown to change the composition of gut microbiota and some changes in microbiota (dysbiosis) have been linked to diabetes, hypertension, and obesity, which are established risk factors for AF. The gut microbiota is a dynamic ecosystem containing symbiotic and pathogenic microorganisms that reside in the colon and produce bioactive metabolites.1 Although emerging preclinical investigations and clinical observational cohort studies suggest a possible role of dysbiosis in AF promotion, the potential mechanisms through which dysbiosis might contribute to AF are poorly understood.2 Here, we briefly review evidence suggesting that abnormalities in the intestinal microbiota play an important and little-recognized role in the pathophysiology of AF and that an improved understanding of this role may open up new therapeutic approaches for AF.
Gut microbiota-derived metabolites and their potential contribution to AF
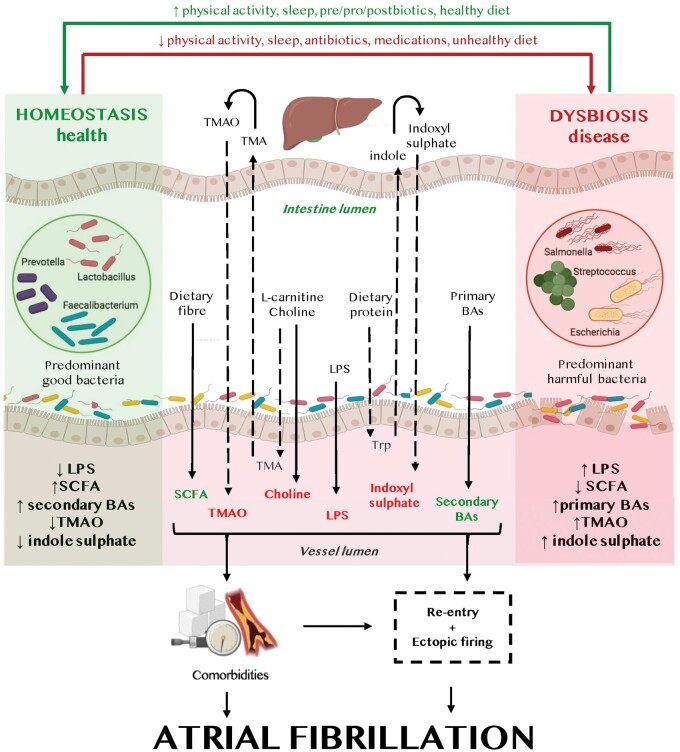
Trimethylamine N-oxide (TMAO), lipopolysaccharide (LPS), indoxyl sulfate, and bile acids are the most extensively studied detrimental microbial metabolites potentially involved in AF pathogenesis. In contrast, short-chain fatty acids (SCFAs), derived from the gastrointestinal fermentation of dietary fibres by commensal anaerobic bacteria, may be protective in AF (Graphical abstract).
Putative mechanisms of gut microbiota-associated atrial fibrillation. Lifestyle components and diet directly impact the gut microbiota composition (homeostasis left, dysbiosis right) and the gut microbiota-derived metabolites. Gut microbiota-derived metabolites (beneficial metabolites in green, detrimental metabolites in red) contribute to the development of atrial arrhythmogenic remodelling and atrial fibrillation-promoting comorbidities, leading to both ectopic firing and a re-entrant substrate for atrial fibrillation maintenance. BA, bile acids; LPS, lipopolysaccharide; SCFA, short-chain fatty acids; TMA, trimethylamine; TMAO, trimethylamine N-oxide; Trp, tryptophan.
Preclinical investigations
Intestinal microbiota convert dietary l-carnitine (e.g. in red meat) and choline (e.g. in eggs) to trimethylamine, which is oxidated to TMAO by flavin-containing monooxygenases in the liver.3 In a canine model, injection of TMAO in the atrial autonomic ganglionated plexi increased the local expression of proinflammatory cytokines, in association with increased ganglionated plexi activity, enhanced atrial tachycardia-induced shortening of atrial refractoriness, and increased AF susceptibility.4 TMAO also activates the NACHT, LRR, and PYD domain-containing protein 3 (NLRP3) inflammasome, which is causally involved in AF pathogenesis.5 , 6
LPSs are components of the outer membrane of most Gram-negative bacteria. Translocation of gut bacteria or endotoxins occurs if the gut barrier function is compromised, for example by the hemodynamic changes caused by sympathetic nervous system activation. In the blood, LPS is transferred from circulating LPS-binding proteins to CD14 molecules on the surface of leukocytes. This process activates toll-like receptor 4, causing a strong innate immune response. In animal models, administration of LPS increases the atrial concentration of proinflammatory cytokines, downregulates the L-type calcium current, and abbreviates the effective refractory period. These are all AF-promoting actions. Aging-associated gut dysbiosis promotes AF via increased levels of circulating LPSs and glucose, along with increased activation of atrial NLRP3 inflammasomes, which leads to atrial fibrosis.7 Transplantation of faecal microbiota from young to old rats, or selective inhibition of LPS or NLRP3 inflammasome, attenuates atrial fibrosis and age-related AF susceptibility, pointing to gut dysbiosis-related systemic inflammation as the cause of the AF-promoting substrate in elderly rats.7 In isolated rabbit atria, indoxyl sulfate contributes to increased atrial inflammation and fibrosis, while promoting pulmonary vein triggering through oxidative stress and dysregulation of calcium handling.8
Primary bile acids like chenodeoxycholic acid can induce cardiac fibrosis via inflammatory processes. Taurine-conjugated primary bile acids might induce changes in membrane potential by activating muscarinic M2-receptors and associated currents in cardiomyocytes, causing atrial-refractoriness abbreviation that promotes AF. In turn, secondary bile acids like ursodeoxycholic acid prevent arrhythmias by stabilizing the cell membrane potential in human cardiomyocytes.2
Beneficial effects have been reported for SCFAs (acetate, butyrate, and propionate), which are derived from the fermentation of dietary fibre by commensal anaerobic bacteria in the gut. High SCFA levels modulate gene expression by inhibiting histone deacetylase activity through the regulation of chromatin structure, display beneficial effects by improving glucose homeostasis and insulin sensitivity, and might reduce ectopic lipid storage. Accordingly, high-fibre diets and acetate supplementation prevent the development of hypertension, ventricular hypertrophy, and fibrosis in hypertensive mice.3 Conversely, low blood SCFA concentrations might contribute to poor metabolite-sensing G-protein-coupled receptor engagement, which can compromise gut integrity and enhance the passage of substances like LPS into the bloodstream.
Clinical observational cohort studies
Circulating levels of gut microbiota-derived metabolites such as TMAO predict cardiovascular events9 and are associated with AF independently of traditional AF risk factors.10 The concentrations of the TMAO precursor choline are higher in atrial tissue and plasma samples in AF patients vs. non-AF subjects. In AF patients, the levels of circulating primary bile acids correlate with structural remodelling.11 AF patients with increased levels of LPS show a high risk of major adverse cardiovascular events,12 supporting the clinical relevance of gut dysbiosis-related inflammation in AF patients. Indoxyl sulfate concentrations independently predict the risk of AF recurrence after AF catheter ablation.13
Strategies to modify gut microbiota in patients
Prospective intervention studies targeting the gut microbiota in AF patients are still missing. Nevertheless, modulation of the microbiota composition and microbia-derived metabolites may represent an interesting approach to influence AF occurrence/progression and concomitant AF risk factors. One strategy to modify the microbiota is the oral consumption of acetate-producing bacteria (probiotics) or supplementation of food ingredients such as fermentable fibres that induce bacterial growth (prebiotics). Such food additives, including resveratrol, have been used to halt the development of the AF substrate in animal models. Combinations of pro- and prebiotics (synbiotics) may provide a better response to dietary interventions and reduce the impact of resistant microbial commensals. The most direct approach is application of protective postbiotics such as SCFAs.
Pharmacological modulation of the gastrointestinal intraluminal milieu by selective pharmacological inhibition of intestinal absorption of dietary ions, glucose, fat, or proteins might offer an alternative approach. Inhibition of intestinal sodium-proton-exchanger subtype 3 using an orally non-absorbable inhibitor attenuates the development of an arrhythmogenic substrate for AF in hypertensive rats.14 In addition, pharmacological suppression of detrimental gut microbiota-derived metabolites (e.g. TMAO) has been used to assess the effect of gut dysbiosis on AF mechanisms in experimentally animal models. Oral administration of antibiotics and other drugs such as statins can enhance or reduce the growth rate of gut microbiota and impact the formation of microbial metabolites. Conversely, gut microbiota may also impact drug metabolism as exemplified by digoxin and calcium antagonists (Supplementary material online, Table S1). However, the interactions between drug use and gut microbiota in AF patients require determination in future studies.
As diet, sleep habits, and physical activity influence the intestinal microbial community, lifestyle interventions may represent an upstream approach to primary and secondary prevention of AF within risk factor management programmes. Where gut dysbiosis cannot be corrected, pharmacological inhibition of common downstream profibrotic/inflammatory pathways (e.g. the NLRP3 inflammasome system) might represent an approach to attenuating dysbiosis-related AF-promoting atrial remodelling.
Gaps in knowledge and further directions
To dissect the precise role of gut microbiota/metabolites, an unbiased multiomics approach will likely be needed. Microbial-phenotype triangulation approaches and the use of germ-free mice is important approaches in the investigation of the impact of gut microbiota in AF pathophysiology. Although several preclinical studies clearly show that gut microbiota can contribute to AF initiation and maintenance under experimental conditions, it remains unclear whether these findings can be translated into the clinical setting and whether modification of gut microbiota may provide novel therapeutic opportunities in AF management. Well-designed prospective intervention studies are required to assess whether abnormalities in gut microbiota are just a marker of other AF risk factors or an independent contributor to AF and thus a potential therapeutic target. There is still no agreement on how best to assess gut microbiota composition, diet patterns, and the response to targeted interventions. As a result of dynamic habitual diet patterns, sleep habits, and physical activity, the intestinal microbial community exhibits significant daily fluctuations.15 Therefore, a longitudinal assessment of lifestyle components should be implemented in future studies. Dietary composition (micro- and macronutrients) is difficult to control but can be assessed by mobile health applications or detailed diaries. It also remains uncertain whether AF patient may benefit from which intervention at which time point, making individualized treatment decisions currently impossible. Moreover, the majority of studies of the gut microbiota in AF focuses on the identification of bacteria only and ignores other important nonbacterial components of gut microbiota. In addition, the investigation of the role of selected individual bacterial species compared to whole bacterial communities development warrants further investigation in AF. Finally, clinical implementation of gut microbiota-modifying interventions for AF patients will require multidisciplinary collaboration between cardiologists/electrophysiologists and gastroenterologists, best organized in an integrated care team.
Conclusions
Emerging preclinical and clinical evidence suggests a relationship between dysbiosis and AF, although the precise causal factors are still poorly understood. To move this complex field forward, preclinical studies should focus on the role of gut microbial dysbiosis in well-established experimental AF models, the mechanisms mediating microbiome effects, and the effectiveness of clinically translatable interventions like dietary manipulation (including probiotics) and faecal transplantation. Clinical intervention studies in AF patients will be required to definitively establish whether gut microbiota is a modifiable risk factor for AF occurrence and progression. While there are many unknown elements of AF pathophysiology, the role of gut microbiota requires particular attention because of the extensive evidence pointing to an important contribution, the lack of definitive studies to clarify the role and underlying mechanisms, and the availability of clinically-effective interventions to beneficially alter the microbiome.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
D.L., M.G., P.S., J.P., and N.L. have no funding to disclose. S.N. is supported by the Canadian Institutes of Health Research (148401). D.D. is supported by grants from National Institutes of Health (R01HL136389, R01HL131517, and R01HL089598), the DFG (Do 769/4-1), and the European Union (large-scale integrative project MEASTRIA, No. 965286 to D.D.).
Conflict of interest: D.L., M.G., P.S., J.P., N.L., and S.N. have no conflict of interest. D.D. is a member of the Scientific Advisory Boards of Omeicos Therapeutics GmbH and Acesion Pharma and obtained honoraria for educational lectures from Novartis, Boston Scientific, and Bristol Meyrs Squibb.
Data availability
No new data were generated or analysed in support of this research.
Supplementary Material
Contributor Information
Dominik Linz, Department of Cardiology, Maastricht University Medical Centre and Cardiovascular Research Institute Maastricht, Universiteitssingel 50, 6229 ER Maastricht, the Netherlands; Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Blegdamsvej 3B, 2200 Copenhagen, Denmark; Centre for Heart Rhythm Disorders, Royal Adelaide Hospital and University of Adelaide, 1 Port Road, SA 5000 Adelaide, Australia; Department of Cardiology, Radboud University Medical Centre, Geert Grooteplein Zuid 10, 6525 GA Nijmegen, the Netherlands.
Monika Gawałko, Department of Cardiology, Maastricht University Medical Centre and Cardiovascular Research Institute Maastricht, Universiteitssingel 50, 6229 ER Maastricht, the Netherlands; Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Blegdamsvej 3B, 2200 Copenhagen, Denmark; 1st Department of Cardiology, Medical University of Warsaw, Banacha 1A, 02-197 Warsaw, Poland; Institute of Pharmacology, West German Heart and Vascular Center, University Duisburg-Essen, Hufelandstraße 55, Essen 45147,Germany.
Prashanthan Sanders, Centre for Heart Rhythm Disorders, Royal Adelaide Hospital and University of Adelaide, 1 Port Road, SA 5000 Adelaide, Australia.
John Penders, Department of Medical Microbiology, Care and Public Health Research Institute (Caphri) and School for Nutrition and Translational Research in Metabolism (NUTRIM), Maastricht University Medical Centre, Universiteitssingel 40, 6229 ER Maastricht, the Netherlands.
Na Li, Department of Medicine (Section of Cardiovascular Research), Baylor College of Medicine, 1 Baylor Plaza, Houston, TX 77030, USA.
Stanley Nattel, Institute of Pharmacology, West German Heart and Vascular Center, University Duisburg-Essen, Hufelandstraße 55, Essen 45147,Germany; Montréal Heart Institute and University de Montréal, Medicine and Research Center and Department of Pharmacology McGill University, 3655 Promenade Sir William Osler, Montreal QC, H3G 1Y6, Canada; IHU LIRYC and Fondation Bordeaux Université Bordeaux, , Avenue du Haut Lévêque, 33600 Pessac, Bordeaux, France.
Dobromir Dobrev, Institute of Pharmacology, West German Heart and Vascular Center, University Duisburg-Essen, Hufelandstraße 55, Essen 45147,Germany; Montréal Heart Institute and University de Montréal, Medicine and Research Center and Department of Pharmacology McGill University, 3655 Promenade Sir William Osler, Montreal QC, H3G 1Y6, Canada; Department of Molecular Physiology and Biophysics, Baylor College of Medicine, 1 Baylor Plaza, Houston, TX 77030, USA.
References
- 1. Tang WH, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res 2017;120:1183–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mishima RS, Elliott AD, Sanders P, Linz D. Microbiome and atrial fibrillation. Int J Cardiol 2018;255:103–104. [DOI] [PubMed] [Google Scholar]
- 3. Marques FZ, Mackay CR, Kaye DM. Beyond gut feelings: how the gut microbiota regulates blood pressure. Nat Rev Cardiol 2018;15:20–32. [DOI] [PubMed] [Google Scholar]
- 4. Yu L, Meng G, Huang B, Zhou X, Stavrakis S, Wang M, Li X, Zhou L, Wang Y, Wang M, Wang Z, Deng J, Po SS, Jiang H. A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int J Cardiol 2018;255:92–98. [DOI] [PubMed] [Google Scholar]
- 5. Yao C, Veleva T, Scott L Jr, Cao S, Li L, Chen G, Jeyabal P, Pan X, Alsina KM, Abu-Taha I Dr, Ghezelbash S, Reynolds CL, Shen YH, LeMaire SA, Schmitz W, Müller FU, El-Armouche A, Tony Eissa N, Beeton C, Nattel S, Wehrens XHT, Dobrev D, Li N. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation 2018;138:2227–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scott L Jr, Fender AC, Saljic A, Li L, Chen X, Wang X, Linz D, Lang J, Hohl M, Twomey D, Pham TT, Diaz-Lankenau R, Chelu MG, Kamler M, Entman ML, Taffet GE, Sanders P, Dobrev D, Li N. NLRP3 inflammasome is a key driver of obesity-induced atrial arrhythmias. Cardiovasc Res 2021;117:1746–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang Y, Zhang S, Li B, Luo Y, Gong Y, Zhang JX, Zhou J, Zhuo Y, Wang X, Zhao Z, Han X, Gao X, Yu Y, Liang H, Zhao D, Sun S, Wang D, Xu D, Qu W, Bo G, Li W, Wu D, Li Y. Gut microbiota dysbiosis promotes age-related atrial fibrillation by lipopolysaccharide and glucose-induced activation of NLRP3-inflammasome. Cardiovasc Res 2021. Mar 23; doi: 10.1093/cvr/cvab114 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8. Chen WT, Chen YC, Hsieh MH, Huang SY, Kao YH, Chen YA, Lin YK, Chen SA, Chen YJ. The uremic toxin indoxyl sulfate increases pulmonary vein and atrial arrhythmogenesis. J Cardiovasc Electrophysiol 2015;26:203–210. [DOI] [PubMed] [Google Scholar]
- 9. Li XS, Obeid S, Wang Z, Hazen BJ, Li L, Wu Y, Hurd AG, Gu X, Pratt A, Levison BS, Chung YM, Nissen SE, Tang WHW, Mach F, Räber L, Nanchen D, Matter CM, Lüscher TF, Hazen SL. Trimethyllysine, a trimethylamine N-oxide precursor, provides near- and long-term prognostic value in patients presenting with acute coronary syndromes. Eur Heart J 2019;40:2700–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Büttner P, Okun JG, Hauke J, Holzwirth E, Obradovic D, Hindricks G, Thiele H, Kornej J. Trimethylamine N-oxide in atrial fibrillation progression. Int J Cardiol Heart Vasc 2020;29:100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang XH, Li Z, Zang MH, Yao TB, Mao JL, Pu J. Circulating primary bile acid is correlated with structural remodeling in atrial fibrillation. J Interv Card Electrophysiol 2020;57:371–377. [DOI] [PubMed] [Google Scholar]
- 12. Pastori D, Carnevale R, Nocella C, Novo M, Santulli M, Cammisotto V, Menichelli D, Pignatelli P, Violi F. Gut-derived serum lipopolysaccharide is associated with enhanced risk of major adverse cardiovascular events in atrial fibrillation: effect of adherence to mediterranean diet. J Am Heart Assoc 2017;6:e005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamagami F, Tajiri K, Doki K, Hattori M, Honda J, Aita S, Harunari T, Yamasaki H, Murakoshi N, Sekiguchi Y, Homma M, Takahashi N, Aonuma K, Nogami A, Ieda M. Indoxyl sulphate is associated with atrial fibrillation recurrence after catheter ablation. Sci Rep 2018;8:17276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Linz B, Hohl M, Mishima R, Saljic A, Lau DH, Jespersen T, Schotten U, Sanders P, Linz D. Pharmacological inhibition of sodium-proton-exchanger subtype 3-mediated sodium absorption in the gut reduces atrial fibrillation susceptibility in obese spontaneously hypertensive rats. Int J Cardiol Heart Vasc 2020;28:100534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, Abramson L, Katz MN, Korem T, Zmora N, Kuperman Y, Biton I, Gilad S, Harmelin A, Shapiro H, Halpern Z, Segal E, Elinav E. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014;159:514–529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analysed in support of this research.