Abstract
Introduction:
Twenty to 40% of Guillain Barré syndrome (GBS) patients will not be able to walk independently despite effective treatment. Older patients carry additional risks for worse outcomes.
Methods:
A single center, ambispective cohort study was performed. Only subjects ≥18 years with a 3-month follow-up were included. Elderly patients were considered as a whole if ≥ 60 years. Demographics, CSF and nerve conduction studies were compared. A binomial logistic regression and Kaplan-Meier analyses were carried out to estimate good prognosis (Hugues ≤2) at 3-month follow-up.
Results:
From 130 patients recruited, 27.6% were elderly adults. They had a more severe disease, higher mEGOS and more cranial nerve involvement. Age ≥70 years, invasive mechanical ventilation and axonal subtype, portrayed an unfavorable 3-month outcome. Further analysis demonstrated an earlier recovery in independent walk at 3 months for patients <70 years.
Conclusions:
Elderly patients with GBS have a more severe disease at admission and encounter worse prognosis at 3-month follow-up, especially those above 70 years.
Keywords: Guillain-Barre syndrome, elderly adult, outcome
Introduction
Guillain Barré syndrome (GBS) is an acute inflammatory polyradiculoneuropathy, considered to be the most common cause of acute flaccid paralysis nowadays.1,2 The global incidence is about 1 to 2 per 100,000 inhabitants per year.3 It affects all age groups with 2 incidence peaks at the fourth and sixth decades of life.2
The overall outcome is favorable, although 20-40% of patients with GBS will not be able to walk independently at 6 months despite effective and prompt treatment.3 Invasive mechanical ventilation (IMV) is required in 20-30% of the patients, considerably increasing mortality in the acute phase.1,4
Advanced age has been associated to severity and poor prognosis, it increases both prolonged hospital stay and probability of IMV and further intensive care unit admission. Furthermore, autonomic dysfunction and electrolyte imbalance have been directly linked to poor outcomes, both prevalent among elderly patients.5,6 Even though there is a high incidence of patients with GBS over 60 years, evidence is scarce regarding clinical features and functional outcomes in this vulnerable subset of patients.
Over the past few years, we have witnessed a dramatic worldwide rise in life expectancy; unfortunately, this is not always accompanied with better quality of life, elderly patients´ functionality gradually decreases instead.7 The aging process is non-linear, it implies several biochemical and structural changes that produce irreversible neuronal damage due to loss of repairing mechanisms8 For the aforementioned reasons, this specific population should be evaluated individually for a correct decision-making process.
We aimed to compare clinical and electrophysiological characteristics between adults and elderly patients with GBS, in addition to functional outcomes assessment.
Material and Methods
A single center, ambispective cohort study of patients with GBS was carried out. GBS diagnosis was established according to Asbury criteria.9 Only subjects ≥18 years with a 3-month follow-up were included. Elderly patients were those ≥ 60 years; in this group those between 60-69 years were considered as young-old and those ≥ 70 years as old-old. Data was collected between January 2015 to May 2020. The following clinical characteristics were described: age, gender, comorbidities, previous infection, time from clinical onset to admission, muscle strength grade according to the Medical Research Council (MRC) scale at admission,10 Hughes score at admission,11 modified Erasmus GBS Outcome Score (mEGOS) at admission,12 cranial nerve involvement, IMV requirement, duration and related complications, dysautonomia (defined by heart rate and blood pressure alterations not explained by infection or other causes), treatment modality: intravenous immunoglobulin (IVIG) at 2g/kg, plasma exchange (PE) or conservative treatment. Hospital length of stay, tracheostomy and gastrostomy requirement and the presence of delirium according to DSM-5 criteria were included.13 Patients were classified into specific clinical subtypes according to Wakerley definitions.14
Nerve conduction studies (NCS) from at least 2 motor nerves in the upper extremities (median and ulnar), 2 in the lower extremities (common peroneal and tibial) and one sensory nerve in the upper and lower extremities (median and sural, respectively) were performed. Distal compound muscle action potential (dCMAP), distal latency and nerve conduction velocities (NCV) were recorded, also sensory nerve action potential (SNAP) in the Median and Sural sensory nerves. Electrophysiological classification was established according to Hadden criteria.15
Albuminocytological dissociation in cerebrospinal fluid (CSF) was defined as protein levels >45mg/dl and cell count ≤50cells/µL.16 Short-term poor functional outcome was defined as dependent walking (Hughes ≥3) at 3-month follow-up.
Katz Index of Independence in Activities of Daily Living (ADL) was applied in telephone survey to assess long-term functionality in the old-old group. Patients were considered independent when scoring 5-6 points or achieving category A (Independent), B (Independent in all BADL, except for one), C (Dependent for bathing and one other BADL) or D (Dependent for bathing, dressing and one other BADL).17
All subjects gave written informed consent to participate in the study. The study protocol was approved by the local Ethics Committee.
Statistical Analysis
Demographic data was analyzed with descriptive statistics. Kolmogorov-Smirnov test was used to assess normal distribution. Ordinal data was compared with chi-square test and Fisher´s test when appropriate. Quantitative data was compared with Mann-Whitney U test. TRIPOD statement was used to identify poor functional outcome risk factors in the elderly as well as detailed review of published literature.18 A univariate logistic analysis and a multivariate logistic regression model were performed. The following covariates were included into the univariate model: Age, preceding diarrhea, IMV, MRC at admission, mEGOS, lower cranial nerve involvement, in-hospital delirium, GBS subtype and the presence of dysautonomia. Multivariate model included: age ≥70 years, IMV requirement and the electrophysiological axonal variant. We assessed the goodness-of-fit with the Homer-Lemeshow test and the Area Under the Curve (AUC) was reported. Results were reported as odds ratios (OR) with 95% confidence intervals (CI). A survival analysis with a Kaplan-Meir test for independent walking at 3 months was performed for the young-old and old-old groups. A p value of < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 22 (IBM SPSS Statistics, SPSS Inc, Chicago, IL).
Results
Demographics in the Elderly
From the 130 patients with GBS included, a total of 36 (27.6%) met the definition of elderly. Mean age was 69.4 ± 7.1 years, with a male predominance of 64%. Ten patients (27.7%) had a previous history of an upper respiratory tract infection and 16 (44.4%) of diarrhea. A Hughes score ≥3 was more prevalent in the elderly group (96.1% vs 69.1%, p = 0.007) as well as mEGOS score (6.5 vs 5, p < 0.001) at admission. There was no difference in days from clinical onset to admission (5 vs 5, p = 0.83). Cranial nerve involvement was present in 22 patients (61.1%), with facial nerve (47.2%) being the most common. Furthermore, bulbar nerve involvement was more prevalent in the elderly (41.6% vs 23.4%, p = 0.034). Classical sensory-motor GBS variant was present in half of the patients (Table 1). Albuminocytological dissociation was more prevalent in the elderly group (50% vs 27.6%, p = 0.007).
Table 1.
Clinical and Epidemiological Characteristics of Patients With GBS.
| Elderly N = 36 |
Adult N = 94 |
p value | |
|---|---|---|---|
| Demographics | |||
| Age (years), mean, SD (min-max) | 69.4 ± 7.1 (60-86) | 38.4 ± 12.9 (16-59) | <0.001 |
| Gender (male), (%) | 23 (63.8) | 64 ( | 0.39 |
| Comorbidities | |||
| Mellitus Diabetes, n (%) | 2 (5.5) | 6 (6.3) | 0.61 |
| Smoking, n (%) | 11 (30.5) | 27 (28.7) | 0.09 |
| High blood pressure, n (%) | 2 (5.5) | 2 (2.1) | 0.86 |
| Previous history | |||
| Upper respiratory tract infection, n (%) | 10 (27.7) | 27 (28.7) | 0.54 |
| Diarrhea, n (%) | 16 (44.4) | 34 (36.1) | 0.25 |
| Clinical features | |||
| Days from clinical onset to admission, median (IQR) | 5 (2.35-9.5) | 5 (3-80) | 0.83 |
| Hughes ≥3 at admission, n (%) | 33 (96.1) | 65 (69.1) | 0.007 |
| MRC at admission, mean ± SD | 34.7 ± 13.6 | 35.8 ± 18.2 | 0.75 |
| mEGOS, median (IQR) | 6.5 (4-8) | 5 (2-7) | <0.001 |
| Cranial nerve involvement: | |||
| Facial nerve, n (%) | 17 (47.2) | 39 (41.4) | 0.34 |
| Oculomotor nerves, n (%) | 10 (27.7) | 22 (23.4) | 0.38 |
| Lower cranial nerves, n (%) | 15 (41.6) | 22 (23.4) | 0.034 |
| Dysautonomia, n (%) | 10 (27.7) | 26 (27.6) | 0.57 |
| Blood pressure variability, n (%) | 5 (13.8) | 13 (13.8) | 0.59 |
| Heart rate variability, n (%) | 4 (11.1) | 20 (21.2) | 0.13 |
| IVM, n (%) | 15 (41.6) | 31 (32.9) | 0.23 |
| Days of IVM, median (IQR) | 37 (9-50) | 18 (10-37.5) | 0.50 |
| IVM-related complications: | |||
| Pneumonia, n (%) | 12 (33.3) | 19 (20.2) | 0.17 |
| Lung atelectasis, n (%) | 3 (8.3) | 3 (3.1) | 0.29 |
| GBS clinical variants | |||
| Sensory-motor, n (%) | 19 (52.7) | 45 (47.8) | 0.38 |
| Pure motor, n (%) | 12 (33.3) | 28 (29.7) | 0.42 |
| MFS/ Overlap, n (%) | 3 (8.3) | 17 (18.0) | 0.12 |
| Pharyngeal-cervical-brachial weakness, n (%) | 2 (5.5) | 2 (2.1) | 0.29 |
| Bifacial weakness with distal paraesthesias, n (%) | 0 (0) | 2 (2.1) | 0.52 |
| CSF analysis | |||
| Days from clinical onset to lumbar puncture, median (IQR) | 6 (4-12) | 6 (4-8) | 0.67 |
| Albuminocytologic dissociation, n (%) | 18 (50) | 26 (27.6) | 0.007 |
| CSF proteins (mg/dl), median (IQR) | 67 (35.2-113.7) | 39 (26-60) | 0.002 |
| Treatment | |||
| Conservative, n (%) | 7 (19.4) | 25 (26.5) |
0.25 |
| IV Immunoglobulin, n (%) | 20 (55.5) | 37 (28.7) | |
| Plasma exchange, n (%) | 9 (25) | 32 (35.1) | |
| Traqueostomy, n (%) | 10 (27.7) | 21 (22.3) | 0.39 |
| Gastrostomy, n (%) | 10 (27.7) | 20 (21.2) | 0.34 |
| Days of hospital stay, median(IQR) | 16 (9-44) | 9 (5-21.5) | 0.047 |
| In-hospital delirium, n (%) | 11 (30.5) | 9 (9.5) | 0.006 |
| Independent gait at 3 month follow-up, n (%) | 15 (41.6) | 32 (34) | 0.54 |
GBS: Guillain-Barré syndrome, mEGOS: Modified Erasmus GBS Outcome Scores, MRC: medical research council, IVM: Invasive mechanical ventilation, MFS: Miller Fisher syndrome, CSF: Cerebrospinal fluid, IV: Intravenous.
Twenty elderly patients (55.5%) received IV immunoglobulin and 9 (25%) received plasma exchange. According to our hospital protocols, subjects with mild, sparing cranial nerve involvement and non-progressive forms of the disease (Hughes <2) were conservatively managed. The median days of hospital stay were significantly greater in the elderly group (16 vs 9, p = 0.047).
Seasonal Distribution
Seasonal distribution of GBS with demyelinating features was observed from early fall to early spring (September to March), whereas axonal subtype was more prevalent in late spring and summer (April to September). Peak incidence months of GBS in March, April and June were observed in adult patients, conversely, the elderly presented commonly in July and September.
Electrophysiological Features
Nerve conduction studies were performed in all elderly patients and in 110 (84.6%) adults with GBS. Demyelination was more prevalent in the elderly group (58.8% vs 35.5%, p = 0.03) (Table 2). There were no differences on dCMAP amplitudes of all the explored motor nerves, but in the sensory nerves, the elderly group had decrease SNAP of the median and sural, (10.3 vs 28 µV), p ≤ 0.001) and (8.2 vs 10.5 µV, p ≤ 0.001) respectively (Table 2). However, when comparison between young-old and old-old, we observed a significant decrease in dCMAP for the median nerve (0.4 mV vs 3.1 mV, p = 0.047) and tibial nerve (0.7 mV vs 4.5 mV, p = 0.043) in the old-old group.
Table 2.
Electrophysiological Characteristics of Patients With GBS.
| Elderly N = 34 | Adult N = 76 | p value | |
|---|---|---|---|
| Days from clinical onset to NCS, median (IQR) | 7 (5-13) | 7 (5-9.75) | 0.38 |
| Demyelinating subtype, n (%) | 20 (58.8) | 27 (35.5) | 0.03 |
| Axonal subtype, n (%) | 11 (32.3) | 43 (56.5) | 0.05 |
| Equivocal, n (%) | 3 (8.8) | 6 (7.8) | 0.66 |
| Cubital nerve dCMAP amplitude, median (IQR) | 2.4 (1.2-4.8) | 2.4 (0.6-5.5) | 0.83 |
| Median nerve dCMAP amplitude, median (IQR) | 2.9 (2.0-5.6) | 1.5 (0.3-5.6) | 0.24 |
| Tibial nerve dCMAP amplitude, median (IQR) | 1.5 (0.1-3.2) | 1.3 (0.1-3.4) | 0.23 |
| Peroneal nerve dCMAP amplitude, median (IQR) | 2.0 (0.34-5.7) | 1.5 (0.2-5.0) | 0.39 |
| Median SNAP amplitude(µV), median (IQR) | 10.3 (0-17.9) | 28 (15.9-40.6) | <0.001 |
| Sural SNAP amplitude(µV), median (IQR) | 8.2 (0.0-13.8) | 19.5 (10.7-25.9) | <0.001 |
GBS: Guillain-Barré syndrome, NCS: Nerve conduction study, dCMAP: Distal compound muscle action potential, SNAP: Sensory nerve action potential.
Prognostic Outcomes
No difference was observed between elderly and adult patients in independent walking at 3 months (41.6% vs 34%, p = 0.54). When young-old vs old-old groups were compared, inability to walk was more prevalent in the later (23.8% vs 66.6%, p = 0.017; respectively).
No deaths were reported across study groups during the 3-month period. Patients ≥70 years (OR 10.3 [1.3-77], p = 0.023), invasive mechanical ventilation (OR 7.3 [1.0-51.4], p = 0.044) and axonal subtype (OR 9.2 [1.3-63.9], p = 0.024) portrayed an unfavorable outcome at 3 months (unable to walk) in the multivariate analysis (Table 3). Kaplan-Meier analysis showed no difference in early walking recovery between adults and elderly patients with GBS (Figure 1, log rank = 0.88) but did so between young-old and old-old comparison (Figure 1, log rank = 0.008).
Table 3.
Prognostic Factors Related to Poor Outcome in the Elderly at 3 Month Follow-Up.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variable | Poor outcome N = 15 | Favorable Outcome N = 21 | OR (CI 95%) | p value | OR (CI 95%) | p value |
| Age (years), mean, SD | 73.7 ± 7.1 | 66.3 ± 5.5 | 1.2 (1.0-1.3) | 0.007 | ||
| Age 60-69, n (%) | 5 (33.3) | 16 (76.1) | 0.15 (0.03-0.6) | 0.01 | ||
| Age ≥70 years, n (%) | 10 (66.6) | 5 (23.8) | 6.4 (1.4-27.8) | 0.017 | 10.3 (1.3-77) | 0.023 |
| Diarrhea, n (%) | 8 (53.3) | 8 (38) | 1.8 (0.4-7.1) | 0.50 | ||
| IVM, n (%) | 10 (66.6) | 5 (23.8) | 6.4 (1.4-27.8) | 0.017 | 7.3 (1.0-51.4) | 0.044 |
| MRC at admission, mean ± SD | 27.3 ± 13.1 | 40 ± 11.5 | 0.91 (0.85-0.98) | 0.013 | ||
| mEGOS, median (IQR) | 7 (6-9) | 6 (4-7.5) | 1.4 (1-2.1) | 0.041 | ||
| Lower cranial nerves involvement, n (%) | 7 (46.6) | 8 (38) | 1.4 (0.3-5.4) | 0.73 | ||
| In-Hospital delirium | 7 (46.6) | 4 (19) | 3.7 (0.8-16.4) | 0.141 | ||
| Pure motor clinical variant, n (%) | 6 (40) | 6 (28.5) | 1.6 (0.41-6.7) | 0.49 | ||
| Sensitive-motor clinical variant, n (%) | 7 (46.6) | 12 (57.1) | 0.6 (0.17-2.4) | 0.73 | ||
| Demyelinating subtype, n (%) | 6/15 | 13/20 | 0.35 (0.9-1.4) | 0.018 | ||
| Axonal subtype, n (%) | 9/15 | 3/20 | 8.5 (1.7-42.2) | 0.01 | 9.2 (1.3-63.9) | 0.024 |
| Dysautonomia, n (%) | 4 (26.6) | 6 (28.5) | 0.9 (0.2-4.0) | 0.99 | ||
IVM: Invasive mechanical ventilation, MRC: medical research council, mEGOS: Modified Erasmus GBS Outcome Scores, IV: Intravenous.
Description of the Multivariate Regression Model.
Overall model fit: Chi-square 18.9, DGL, 3, p-value = 0.0001.
Goodness of Fit Test: r2 = 0.417; Hosmer & Lemeshow test, Chi-square, 2.144, GL5, p-value = 0.82.
Model performance: AUC 0.868, 95% CI 0.75-0.98, p = 0.001.
Figure 1.
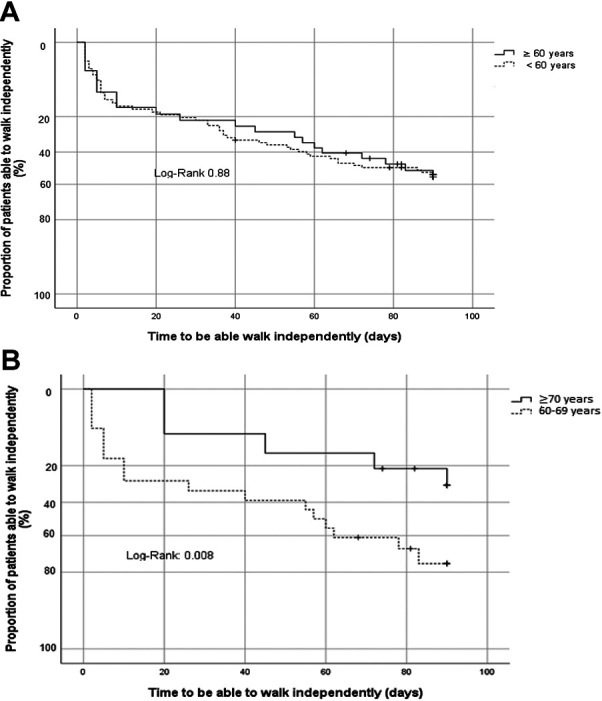
Kaplan Meier analysis for: a) proportion of patients able to walk independently at 3 months according to age, b) proportion of elderly patients able to walk independently at 3 months (cut point 70 years).
Katz Index was applied in 7 patients (46.6%) in the old-old group who were locatable at the time of the survey. The mean time from acute illness to the evaluation was 816±533 days; 5/7 (71.4%) achieved independency in their ADL. One of the 2 dependent patients had a previous history of atypical parkinsonism, which contributes significantly to his disability.
Discussion
Older population has risen worldwide dramatically over the years. In the last decade, we have seen a growth rate of 3.6% in the Mexican elderly, and almost 9% of the total population is above 60 years. Overall Mexican healthy life expectancy is 65.8 years and general life expectancy is 74.7 years.19 Worldwide statistics from the United Nations report a similar aging pattern, projecting an increase for 2050 that will exceed the number of young people.7 An exponentially increasing incidence with age has been acknowledged in patients with GBS, with an estimated rate of 2.66 cases per 100,000 person-year in the elderly, compared with 0.75 cases in the younger. This suggests a 20% incidence increment for every 10-year increase in age.20
Among patients with GBS, prevalence in adults under 60 years has been reported in 15-51% and 10-16% in subjects older than 70 years.2,20 We found similar results in our study, with an elderly prevalence of 38% and 15% over 70 years. This modest elderly prevalence might be related to an overall lower life expectancy in Latin America.21 Additionally, some authors state that GBS might be underdiagnosed in this age group, as GBS-related symptoms can be easily confused with more common diseases in the elderly.2
Acute motor axonal neuropathy (AMAN) is the most frequent subtype of GBS in both Mexico and Asian countries, with a seasonal peak in the summer due to gastrointestinal infections.22-25 However, acute inflammatory demyelinating polyneuropathy (AIDP) was more prevalent in the elderly Mexican and presented with a high incidence in winter months, supporting the well-known seasonal association with demyelinating subtypes.22,23
Elderly patients with GBS presented with more severe symptoms, reflected in greater Hughes scores at admission. Interestingly, there was no difference between MRC scores in the elderly and adults with GBS, however, bulbar cranial nerve involvement was more prevalent in the former. This greater Hughes score at admission might be explained by the predisposition to ventilatory failure in patients with early involvement of bulbar muscles rather than limb weakness.17
Although CSF albuminocytological dissociation does not discern a particular GBS variant, it is more common in demyelinating compared to axonal subtypes, at any given age.23,26 We observed an undoubtedly higher prevalence of albuminocytological dissociation in the elderly coupled with a tendency to demyelinating features in nerve conduction studies. More studies are further required to elucidate if Latin American elderly patients with GBS differ in pathophysiology from adult patients with more common axonal subtypes in these countries.
It has been reported that the lower the distal CMAP in the NCS, the worse the functional outcome.27 Although no significant difference was observed in the distal CMAPs between adults and the elderly, the old-old group manifested with extremely low amplitudes (<20% of LLN) when compared to the young-old group. Some age-specific factors, such as neurodegenerative changes in peripheral nerves, may play an important role in the functional prognosis of patients with GBS.8
Delirium is a common and often underdiagnosed condition in elderly patients in acute care settings, it has proven to prolong hospital stays, increase mortality and medical costs.28 Elderly GBS patients are particularly frail and predisposed to this medical condition, observed in almost a double increment in days of hospital stay in our study. Further complications related to IMV and its duration, such as pneumonia and atelectasis, can delay hospital discharge.
Hughes score is the most used scale for severity assessment in GBS.11 It evaluates walking capacity and assisted ventilation requirement, however, the former is subjected to strength evaluation of the lower extremities and it may not be the most appropriate severity stratification for elderly patients with GBS, since this frail population have other pre-existing comorbidities that directly impact in prognosis. Moreover, mEGOS score was greater in our elderly patients, although we have to bear in mind that 2 points are added in patients older than 60 years, and we could not replicate this poor prognosis established only by mEGOS score in this particular population.12 Conversely, we found patients with GBS above 70 years to have a significant poor prognosis.
The geriatric population must be assessed multidimensionally and multidisciplinary. ADL and instrumental activities of daily living (IADL) evaluate daily self-care activities and those for living independently, respectively. Both must be taken into consideration when assessing this frail population.29 Loss of 3 or more items in ADL or IADL in less than 2 years is considered a catastrophic loss of functionality, with hip fractures and stroke the leading causes.30 Complementary scales that measure ADL and IADL, such as Katz index17 and Barthel´s scale should be considered in the evaluation of elderly patients with GBS, to make a more accurate functional prognosis.
At the 27.7 ± 17.7 months follow-up, almost all patients in the old-old group achieved independent living. Although these patients are particularly vulnerable with worse short-term prognosis and slower recovery, adequate care and rehabilitation can restore functionality in the long-term.
To our knowledge, this is the first Mexican study to compare elderly and adult patients with Guillain-Barré, and to assess prognostic factors such as independent walking at 3 months. Furthermore, data can be extrapolated to other Latin American countries; similar life expectancy and health conditions have been observed among them.21
One of our major limitations was the tertiary referral center recruitment, where the complexity of patients with GBS differ from the general population.1
Conclusions
Mexican elderly patients with GBS have a more severe disease at admission and encounter worse prognosis at 3 months, especially those above 70 years. Demyelinating subtype appears to be more common in this subset of patients although axonal subtype confers a worse functional prognosis. Elderly patients must be of special interest as its growth rate is now faster than that of the total population. The clinician must be aware of the correct clinical assessment when GBS is encountered in this vulnerable population. We recommend assessment tools to evaluate ADL for implementation of better rehabilitation programs.
Footnotes
Author Contributions: Maria Eugenia Briseño-Godínez: planning, writing, editing and reviewing. Angel Antonio Arauz-Góngora: editing and reviewing. Juan Carlos López-Hernández: editing, analyzing and reviewing. Adib Jorge de Saráchaga: editing and reviewing. Esther Y. Pérez-Valdez: editing and reviewing. Raúl Nathanael May-Más: editing and reviewing. Gabriela López-Hernández: editing and reviewing. Lisette Bazán-Rodriguez: editing and reviewing. Javier Andrés Galnares-Olalde: editing and reviewing. Elizabeth León-Manríquez: editing and reviewing. Edwin Steven Vargas-Cañas: editing and reviewing.
Availability of Supporting Data: Supporting data is available if required.
Consent for Publication: Each patient signed a consent for publication in the present article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval and Consent to Participate: The present article was approved by the Ethics committee.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Javier Andrés Galnares-Olalde, MD  https://orcid.org/0000-0003-3004-6221
https://orcid.org/0000-0003-3004-6221
References
- 1.Wu X, Li C, Zhang B, et al. Predictors for mechanical ventilation and short-term prognosis in patients with Guillain-Barré syndrome. Crit Care. 2015;19(1):310. doi:10.1186/s13054-015-1037-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peric S, Berisavac I, Stojiljkovic Tamas O, et al. Guillain-Barré syndrome in the elderly. J Peripher Nerv Syst. 2016;21(2):105–110. doi:10.1111/jns.12163 [DOI] [PubMed] [Google Scholar]
- 3.Leonhard SE, Mandarakas MR, Gondim FAA, et al. Diagnosis and management of Guillain–Barré syndrome in ten steps. Nat Rev Neurol. 2019;15(11):671–683. doi:10.1038/s41582-019-0250-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet. 2016;388(10045):717–727. doi:10.1016/S0140-6736(16)00339-1 [DOI] [PubMed] [Google Scholar]
- 5.Zhang B, Wu X, Shen D, et al. The clinical characteristics and short-term prognosis in elderly patients with Guillain-Barre syndrome. Med (United States). 2017;96(1):e5848. doi:10.1097/MD.0000000000005848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagappa M, Rahul W, Sinha S, et al. Guillain Barre Syndrome in the elderly: experience from a tertiary-care hospital in India. J Clin Neurosci. 2017;46:45–49. doi:10.1016/j.jocn.2017.08.048 [DOI] [PubMed] [Google Scholar]
- 7.United Nations. Department of Economic and Social Affairs. Population Division. World Population 2017; 2017. [Google Scholar]
- 8.Verdú E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5(4):191–208. doi:10.1046/j.1529-8027.2000.00026.x [DOI] [PubMed] [Google Scholar]
- 9.Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol. 1990;27(1S):S21–S24. doi:10.1002/ana.410270707 [DOI] [PubMed] [Google Scholar]
- 10.Ruud PK, Frans G, Meche M, Paul S. Interobserver agreement in the assessment of muscle strength Guillain-Barre syndrome. Muscle Nerv. 1991;14(11):1103–1109. [DOI] [PubMed] [Google Scholar]
- 11.Hughes RAC, Newsom-Davis JM, Perkin GD, Pierce JM. Controlled trial of prednisolone in acute polyneuropathy. Lancet. 1978;312(8093):750–753. doi:10.1016/S0140-6736(78)92644-2 [DOI] [PubMed] [Google Scholar]
- 12.Walgaard C, Lingsma HF, Ruts L, Van Doorn PA, Steyerberg EW, Jacobs BC. Early recognition of poor prognosis in Guillain-Barré syndrome. Neurology. 2011;76(11):968–975. doi:10.1212/WNL.0b013e3182104407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.López Ibor Aliño VM. DSM-V-TR: manual diagnóstico y estadístico de los trastornos mentales. Am Psychiatr Assoc. 2002;1:205. https://books.google.com.pe/books?hl=es&lr=&id=m6Wdcfn80DwC&oi=fnd&pg=PR9&dq=dsm+iv&ots=P8JjQYCYCn&sig=qldk-PuHbTk32Lk5ochaV04hW7M#v=onepage&q=dsmiv&f=false [Google Scholar]
- 14.Wakerley BR, Uncini A, Yuki N. Guillain-Barré and Miller Fisher Syndromes—New diagnostic classification. Nat Rev Neurol. 2014;10(9):537–544. doi:10.1038/nrneurol.2014.138 [DOI] [PubMed] [Google Scholar]
- 15.Hadden RDM, Cornblath DR, Hughes RAC, et al. Electrophysiological classification of Guillain-Barre syndrome: clinical associations and outcome. Ann Neurol. 1998;44(5):780–788. doi:10.1002/ana.410440512 [DOI] [PubMed] [Google Scholar]
- 16.Doets AY, Verboon C, van den Berg B, et al. Regional variation of Guillain-Barre syndrome. Brain. 2018;141(10):2866–2877. doi:10.1093/brain/awy232 [DOI] [PubMed] [Google Scholar]
- 17.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged the index of ADL: a standardized measure of biological and psychosocial function downloaded from: by a university of Adelaide library user on 10/08/2017 table 1.—index of independence in activities of daily living. JAMA. 1963;185(12):914–919. [DOI] [PubMed] [Google Scholar]
- 18.Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD Statement. BMC Med. 2015;13(1):1–10. doi:10.1186/s12916-014-0241-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.INEGI. Perfil Sociodemográfico de Adultos Mayores. Vol 1; 2014. [Google Scholar]
- 20.Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain-Barre syndrome: a systematic review and meta-analysis. Neuroepidemiology. 2011;36(2):123–133. doi:10.1159/000324710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.OECD/The World Bank. Health at a Glance: Latin America and the Caribbean 2020. OECD Publishing; 2020. doi:10.1787/6089164f-en [Google Scholar]
- 22.Webb AJS, Brain SAE, Wood R, Rinaldi S, Turner MR. Seasonal variation in Guillain-Barré syndrome: a systematic review, meta-analysis and Oxfordshire cohort study. J Neurol Neurosurg Psychiatry. 2015;86(11):1196–1201. doi:10.1136/jnnp-2014-309056 [DOI] [PubMed] [Google Scholar]
- 23.López-Hernández JC, Colunga-Lozano LE, Garcia-Trejo S, et al. Electrophysiological subtypes and associated prognosis factors of Mexican adults diagnosed with Guillain-Barré syndrome, a single center experience. J Clin Neurosci. 2020;80:292–297. doi:10.1016/j.jocn.2020.04.059 [DOI] [PubMed] [Google Scholar]
- 24.Van Den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, Van Doorn PA. Guillain-Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. 2014;10(8):469–482. doi:10.1038/nrneurol.2014.121 [DOI] [PubMed] [Google Scholar]
- 25.Domínguez-moreno R, Tolosa-tort P, Patiño-tamez A, et al. Mortalidad asociada al diagnóstico de síndrome de Guillain-Barré en adultos ingresados en instituciones del sistema sanitario mexicano. Rev Neurol. 2014;58(1):4–10. doi:10.33588/rn.5801.2013370 [PubMed] [Google Scholar]
- 26.Nomani AZ, Iqbal M, Majeed H, et al. Albuminocytological dissociation in different electrophysiological GBS variants. Pakistan J Neurol Sci. 2015;10(4):32–36. http://ecommons.aku.edu/pjnshttp://ecommons.aku.edu/pjns/vol10/iss4/9 [Google Scholar]
- 27.Cornblath DR, Mellits ED, Griffin JW, et al. Motor conduction studies in Guillain-Barré syndrome: description and prognostic value. Ann Neurol. 1988;23(4):354–359. doi:10.1002/ana.410230407 [DOI] [PubMed] [Google Scholar]
- 28.Collier R. Hospital-induced delirium hits hard. CMAJ. 2012;184(1):23–24. doi:10.1503/cmaj.109-4069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meskers CGM, Reijnierse EM, Numans ST, et al. Association of handgrip strength and muscle mass with dependency in (instrumental) activities of daily living in hospitalized older adults—the empower study. J Nutr Heal Aging. 2019;23(3):232–238. doi:10.1007/s12603-019-1170-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guralnik JM, Ferrucci L, Balfour JL, Volpato S, Di Iorio A. Progressive versus catastrophic loss of the ability to walk: implications for the prevention of mobility loss. J Am Geriatr Soc. 2001;49(11):1463–1470. doi:10.1046/j.1532-5415.2001.4911238.x [DOI] [PubMed] [Google Scholar]


