Abstract
The long non-coding RNA, urothelial cancer-associated 1 (UCA1) is an important regulator in several tumors. However, to the best of our knowledge, the clinical roles of UCA1 in cervical cancer remain unclear. Thus, the present study aimed to investigate the function and mechanism of UCA1 in cervical cancer. Reverse transcription-quantitative PCR analysis was performed to detect UCA1 and microRNA (miR)-299-3p expression in cervical cancer tissues and cell lines. The Cell Counting Kit-8 and Transwell assays were performed to assess cell proliferation and invasion, respectively. Furthermore, the dual-luciferase reporter assay was performed to confirm the association between UCA1 and miR-299-3p. Rescue experiments were performed to determine the mechanism of the UCA1/miR-299-3p axis. The results demonstrated that UCA1 expression was upregulated in cervical cancer tissues and cell lines. Furthermore, overexpression of UCA1 enhanced the proliferation and invasion of cervical cancer cells, the effects of which were reversed following UCA1 knockdown. Notably, UCA1 interacted with miR-299-3p and negatively regulated miR-299-3p expression. In addition, miR-299-3p expression was downregulated in cervical cancer tissues and cell lines. Overexpression of miR-299-3p suppressed the proliferation and invasion of cervical cancer cells, reversing the effects of UCA1 knockdown on cervical cancer cell proliferation. Taken together, the results of the present study suggest that UCA1 promotes cell proliferation and invasion by regulating miR-299-3p expression in cervical cancer.
Keywords: urothelial cancer-associated 1, microRNA-299-3p, proliferation, invasion, cervical cancer
Introduction
Cervical cancer is one of the most common types of cancer worldwide and a leading cause of cancer-associated mortality in women (1,2). In 2012, the incidence of cervical cancer was 527,600 cases and nearly 265,700 mortalities worldwide (3). According to its heterogeneity, cervical cancer is histologically divided into three subtypes, adenosquamous carcinoma, squamous cell carcinoma and adenocarcinoma (4). Currently, the main treatment strategies for patients with cervical cancer include pelvic lymph node dissection, hysterectomy and radiotherapy combined with chemotherapy (5). Although a certain degree of progress has been made in the early diagnosis, surgical treatment and application of the human papillomavirus (HPV)16, 18, 31, 33, 45, 52 and 58 vaccine for cervical cancer, the survival rate of patients with advanced cervical cancer remains low (6,7). The 5-year survival rate for cervical cancer in China from 2012–2015 was only 59.8% (8). Thus, it is important to identify and develop biomarkers for cervical cancer and determine its disease mechanism.
Non-coding RNAs (ncRNAs) are distinctive RNA molecules that are not translated into proteins (9). ncRNAs are divided into two types, including housekeeping and regulatory ncRNAs (10). The regulatory ncRNAs include microRNAs (miRNAs/miRs), circular RNAs (circRNAs) and long ncRNAs (lncRNAs) (11). miRNAs are short ncRNAs, ~22 nucleotides in length that regulate gene expression by binding to mRNAs (12–14) or by adjusting their stability through sponging circRNAs (15,16). lncRNAs are a group of ncRNAs with a length of >200 nucleotides (17). lncRNAs function as sponges that target miRNAs and subsequently regulate gene expression (18,19). Several studies have reported that miRNAs and lncRNAs play important roles in certain types of cancer (20–22).
Increasing evidence suggest that lncRNAs are involved in the occurrence of multiple tumors (23–25). Several lncRNAs, such as growth arrest-specific 5 (26), nuclear paraspeckle assembly transcript 1 (27), metastasis-associated lung adenocarcinoma transcript 1 (28) and small nucleolar host gene 12 (29), have been identified as important regulatory factors in cervical cancer. The lncRNA, urothelial carcinoma-associated 1 (UCA1) is located on human 19p13.12 (30) and has been reported to play an important role in different types of cancer, including colorectal (31), prostate (32), gastric (33) and bladder cancer (34). Previous studies have reported that UCA1 regulates the proliferation, migration and invasion of cervical cancer cells (35–37). However, the mechanism of UCA1 in the progression of cervical cancer remains unclear.
miRNAs have been reported to act as facilitators or suppressors in different types of cancer (38–40). According to previous studies, miR-299-3p participates in multiple tumor processes. In hepatocellular carcinoma, miR-299-3p acts as a tumor suppressor by regulating Sirtuin 5 (41). It also plays a tumor suppressive role by targeting SHOC2 leucine-rich repeat scaffold protein in thyroid cancer (42). miR-299-3p inhibits cell proliferation and invasion by targeting vascular endothelial growth factor A in human colon carcinoma (43). In cervical cancer, miR-299-3p suppresses cell proliferation and invasion by binding to transcription factor 4 (TCF4) (44). However, the function of miR-299-3p, and the association between UCA1 and miR-299-3p in cervical cancer remain unknown.
The present study aimed to investigate the role and potential mechanism of UCA1 in cervical cancer. The association between UCA1 and miR-299-3p in the occurrence of cervical cancer was also investigated.
Materials and methods
Clinical specimens
Cervical cancer and paired adjacent normal tissues (n=30) were surgically collected from the Department of Obstetrics and Gynecology, Sanya People's Hospital (Sanya, China) from May 2018 to May 2020. The average age of all patients was 49 years (age range, 36–69 years). The clinical information of patients with cervical cancer is presented in Table SI. No patients received adjuvant treatment prior to surgery. In addition, the patients with other tumors or a history of treatments for other gynecological tumors were excluded from the present study. All tissue samples were immediately preserved in liquid nitrogen and stored at −80°C until RNA extraction. The present study was approved by the Ethics Committee of the Sanya People's Hospital (approval no. 2017.106; Sanya, China) and written informed consent was provided by all patients prior to the study start.
Cell lines
The Ect1/E6E7 normal human cervical epithelial cell line was purchased from the American Type Culture Collection, while the SiHa, HeLa, CaSki and ME180 human cervical cancer cell lines were purchased from the The Cell Bank of Type Culture Collection of The Chinese Academy of Sciences. All cells were maintained in DMEM (cat. no. SH30022.01B; Cytiva) supplemented with 10% fetal bovine serum (cat. no. 16140071; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin (cat. no. 15140148; Thermo Fisher Scientific, Inc.) and 100 µg/ml streptomycin (cat. no. 15140122; Thermo Fisher Scientific, Inc.), at 37°C with 5% CO2 and 100% humidity.
Cell transfection
The overexpression vector pcDNA 3.1 was purchased from Thermo Fisher Scientific, Inc. All sequences were synthesized by Shanghai GenePharma Co., Ltd. The primers for the amplification of the full-length UCA1 were as follows: Forward, 5′-CCGGAATTCTGACATTCTTCTGGACAATG-3′ and reverse, 5′-CCGCTCGAGCTGACTCTTTTAGGAAGATTTCT-3′. The overexpression vector pcDNA-UCA1 was produced by cloning the full-length UCA1 into pcDNA3.1. For the knockdown of UCA1, the small interfering (si)RNAs targeting UCA1 (si-UCA1) were designed. The sequences was as follows: 5′-GGACAACAGUACACGCAUATT-3′. The sequences used to increase or decrease miR-299-3p expression were as follows: miR-299-3p mimics, 5′-UCGCCAAAUGGUAGGGUGUAU-3′; miR-299-3p inhibitor, 5′-AAGCGGUUUACCAUCCCACAU-3′; miR-NC mimics, 5′-UUCUCCGAACGUGUCACGUG-3′ and miR-NC inhibitor, 5′-CAGUACUUUUGUGUAGUACA-3′. The UCA1 overexpression vector pcDNA-UCA1, specific siRNAs targeting UCA1 (si-UCA1), negative control siRNA (si-NC), miR-299-3p mimics, NC mimics, miR-299-3p inhibitor and NC inhibitor were transfected into SiHa cells. For cell transfection, SiHa cells were seeded into a 6-well plate wat a density of 1×105 cells/well. 1.5 ml of serum-free medium containing 500 µl of Lipofectamine™ 3000 transfection solution (cat. no. L3000015; Thermo Fisher Scientific, Inc.) were added in each well at 37°C for 48 h. After cell culture for 1–2 weeks, the positive colonies were harvested for amplification culture.
Reverse transcription-quantitative (RT-q)PCR
Total RNA was extracted from normal and tumor tissues and cell lines using TRIzol® reagent (cat. no. 15596018; Thermo Fisher Scientific, Inc.). The reaction mixture containing 1 µg of total RNA was reverse transcribed into cDNA using the PrimeScript™ RT reagent kit (cat. no. RR036A; Takara Bio, Inc.). The temperature protocols for RT were as follows: 50°C for 15 min and 85°C for 5 sec. The reactions were performed using an ABI 7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.), and the RNA expression levels were measured using a SYBR™ Green Master Mix (cat. no. RR430S; Takara Bio, Inc.). The thermocycling conditions of qPCR were as follows: Initial denaturation at 95°C for 3 min, followed by 45 cycles of denaturation at 95°C for 15 sec and annealing/elongation at 60°C for 20 sec. The following primer sequences were used for qPCR: UCA1 forward, 5′-GCCAGCCTCAGCTTAATCCA-3′ and reverse, 5′-CCCTGTTGCTAAGCCGATGA-3′; miR-299-3p forward, 5′-ACACTCCAGCTGGGTATGTGGGATGGTAAAC-3′ and reverse, 5′-GTGCAGGGTCCGAG-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′ reverse; and GAPDH forward, 5′-ACCCACATCCCTCAGACAC-3′ and reverse, 5′-CCCCAATACGACCAAATCC-3′. GAPDH and U6 were used as the internal controls. Relative expression levels were calculated using the 2−ΔΔCq method (45). The experiments were performed in triplicate.
Cell Counting Kit-8 (CCK-8) assay
Cervical cancer cell proliferation was assessed via the CCK-8 assay (cat. no. C0039; Beyotime Institute of Biotechnology). The transfected cells were collected by centrifugation at 1,000 × g for 5 min at 4°C, then the transfected cells (2×103 cells/100 µl) were cultured in 96-well plates. Following incubation for 0, 24, 48 and 72 h, 10 µl CCK-8 solution was added into each well and incubated for an additional 2 h at 37°C. Cell proliferation was subsequently analyzed at a wavelength of 450 nm. The experiments were performed in triplicate.
Invasion assay
Cell invasion was assessed via the Transwell assay. The Transwell chambers were pre-coated with 100 µl Matrigel at 37°C for 1 h. Transfected cells were collected (1,000 × g for 5 min at 4°C), then the cells (1×105 cells/well) were seeded into the upper chambers of Matrigel-coated Transwell plates (8-µm pore size; cat. no. 354483; Corning, Inc.) in serum-free DMEM. The DMEM (cat. no. SH30022.01B; Cytiva) medium with 10% fetal bovine serum (cat. no. 16140071; Thermo Fisher Scientific, Inc.) was plated in the lower chambers. Following incubation for 24 h at 37°C, cells in the lower chambers were fixed with 5% glutaraldehyde and stained with 0.1% crystal violet dye (cat. no. 548-62-9; MilliporeSigma) for 30 min at room temperature, respectively. Stained cells were counted in five randomly selected fields using a BX63 microscope (magnification, ×100, Olympus Corporation).
Dual-luciferase reporter assay
LncBase Predicted v.2 software (46) was used to predict the potential binding sites between UCA1 and miR-299-3p.
The dual-luciferase reporter plasmids were constructed by inserting a UCA1 wild-type (UCA1-WT) or UCA1 mutant (UCA1-MUT) sequence into the psiCHECK-2 luciferase vector (Promega Corporation). SiHa cells were co-transfected with UCA1-WT or -MUT reporter and miR-299-3p or NC mimics, using Lipofectamine® 3000 transfection reagent (cat. no. L3000015; Thermo Fisher Scientific, Inc.). Transfected cells were subsequently cultured in 24-well plates. Following incubation at 37°C for 24 h, luciferase activities were detected using a Dual-Luciferase Reporter assay system (cat. no. E1910; Promega Corporation). Firefly luciferase activity was normalized to Renilla luciferase activity (cat. no. E2810; Promega Corporation).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8 (GraphPad Software, Inc.). All experiments were performed in triplicate and data are presented as the mean ± SD. Paired Student's t-test was used to compare differences between tumor tissues and adjacent normal tissues, while unpaired Student's t-test was used to compare differences between unpaired groups. One-way ANOVA followed by Tukey's or Dunnett's post hoc tests were used to compare differences between multiple groups. Pearson's correlation coefficient analysis was performed to assess the correlation between UCA1 and miR-299-3p expression. P<0.05 was considered to indicate a statistically significant difference.
Results
UCA1 expression is downregulated in cervical cancer tissues and cell lines
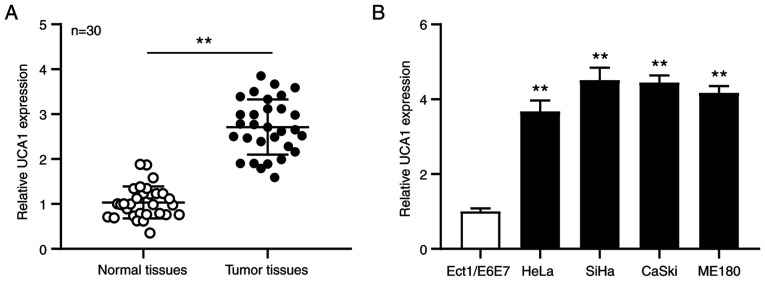
RT-qPCR analysis was performed to detect UCA1 expression in cervical cancer tissues and adjacent normal tissues (n=30). The results demonstrated that UCA1 expression was significantly upregulated in cervical cancer tissues compared with adjacent normal tissues (P<0.01; Fig. 1A). RT-qPCR analysis was also performed to detect UCA1 expression in the SiHa, HeLa, CaSki and ME180 cervical cancer cell lines and Ect1/E6E7 human normal cervical epithelial cell line. The results demonstrated that UCA1 expression was significantly upregulated in the cervical cancer cell lines compared with the normal cell line (all P<0.01; Fig. 1B). Taken together, these results suggest that UCA1 plays a key role in the development of cervical cancer.
Figure 1.
UCA1 expression is upregulated in cervical cancer tissues and cell lines. Reverse transcription-quantitative PCR analysis was performed to detect UCA1 expression in (A) cervical cancer tissues (n=30) and adjacent normal tissues (n=30), as well as (B) cervical cancer cell lines and Ect1/E6E7 cells. **P<0.01 vs. Ect1/E6E7 cells. UCA1, urothelial cancer-associated 1.
UCA1 positively regulates cervical cancer cell proliferation and invasion
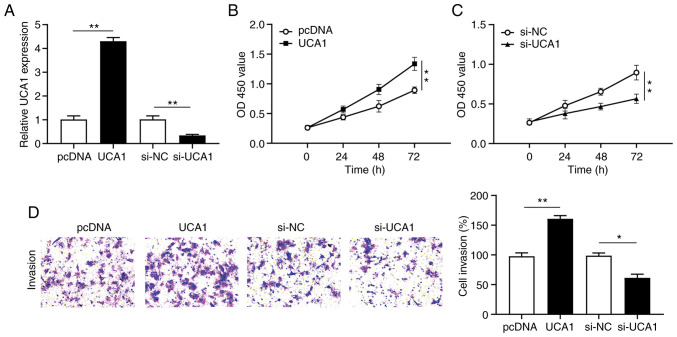
To further investigate the effect of UCA1 in cervical cancer cell proliferation and invasion, SiHa cells were transfected with UCA1 or si-UCA1 to construct a UCA1 overexpression and knockdown model, respectively. The expression of UCA1 in transfected SiHa cells was detected via RT-qPCR analysis. The results demonstrated that UCA1 expression significantly increased (P<0.01) following transfection with UCA1 and significantly decreased (P<0.01) following transfection with si-UCA1 (Fig. 2A).
Figure 2.
Overexpression of UCA1 promotes cervical cancer cell proliferation and invasion. (A) UCA1 expression was detected in SiHa cells transfected with pcDNA, UCA1, si-NC or si-UCA1. (B and C) The Cell Counting Kit-8 assay was performed to assess cell proliferation following transfection. (D) The Transwell assay was performed to assess cell invasion following transfection. *P<0.05; **P<0.01. UCA1, urothelial cancer-associated 1; si, small interfering; NC, negative control; OD, optical density.
The CCK-8 assay was performed to assess the proliferation of cervical cancer cells. The results demonstrated that overexpression of UCA1 significantly promoted the proliferation of SiHa cells (P<0.01; Fig. 2B), the effects of which were reversed following UCA1 knockdown (P<0.01; Fig. 2C). Furthermore, the Transwell assay demonstrated that overexpression of UCA1 significantly promoted the invasive ability of SiHa cells (P<0.01; Fig. 2D), the effects of which were reversed following UCA1 knockdown. Collectively, these results suggest that UCA1 is involved in cervical cancer cell proliferation and invasion.
miR-299-3p can bind with UCA1, and its expression is negatively regulated by UCA1
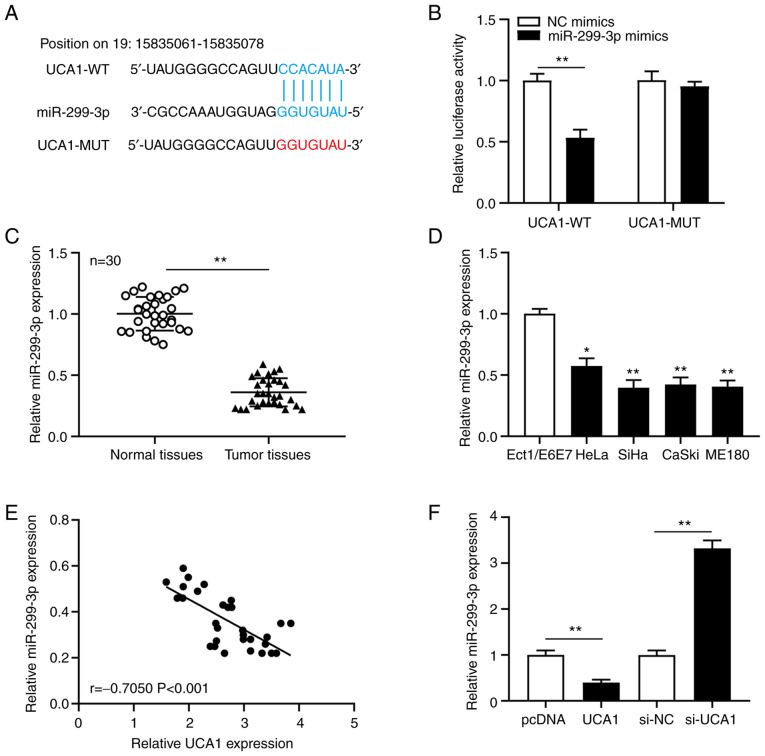
To clarify the molecular mechanism of UCA1 in cervical cancer, LncBase software was used to predict the binding sites between miRNAs and UCA1. The results revealed a potential binding site between UCA1 and miR-299-3p (Fig. 3A). The dual-luciferase reporter assay was subsequently performed to confirm the association between UCA1 and miR-299-3p in SiHa cells. The results demonstrated that transfection with miR-299-3p mimics significantly decreased (P<0.01) the luciferase activity of UCA1-WT, but not UCA1-MUT in SiHa cells (Fig. 3B). RT-qPCR analysis was performed to detect miR-299-3p expression in cervical cancer tissues and cell lines. The results demonstrated that miR-299-3p expression was significantly downregulated in cervical cancer tissues (P<0.01; Fig. 3C) and cell lines (all P<0.01; Fig. 3D). Pearson's correlation analysis was performed to determine the correlation between UCA1 and miR-299a-3p expression in cervical cancer tissues. The results demonstrated that miR-299-3p expression was negatively correlated with UCA1 expression (Fig. 3E). SiHa cells were transfected with pcDNA, UCA1, si-NC or si-UCA1, and miR-299-3p expression was detected. The results demonstrated that overexpression of UCA1 significantly decreased miR-299-3p expression (P<0.01), while UCA1 knockdown significantly increased miR-299-3p expression (P<0.01) in SiHa cells (Fig. 3F). Taken together, these results suggest that UCA1 binds to miR-299-3p and negatively regulates its expression in cervical cancer.
Figure 3.
miR-299-3p directly targets the 3′-untralsated region of UCA1 and is downregulated in cervical cancer tissues and cell lines. (A) Binding sites between UCA1 and miR-299-3p were predicted using LncBase software. (B) The association between UCA1 and miR-299-3p was confirmed via the dual-luciferase reporter assay. RT-qPCR analysis was performed to detect miR-299-3p expression in (C) cervical cancer tissues and normal tissues, and (D) cervical cancer cell lines and Ect1/E6E7. (E) UCA1 expression was negatively correlated with miR-299-3p expression in cervical cancer. (F) RT-qPCR analysis was performed to detect miR-299-3p expression in SiHa cells transfected with pcDNA, UCA1, si-NC or si-UCA1. *P<0.05, **P<0.01 vs. Ect1/E6E7 cells. miR, microRNA; UCA1, urothelial cancer-associated 1; RT-qPCR, reverse transcription-quantitative PCR; si, small interfering; NC, negative control; WT, wild-type; MUT, mutant.
miR-299-3p suppresses cervical cancer cell proliferation and invasion
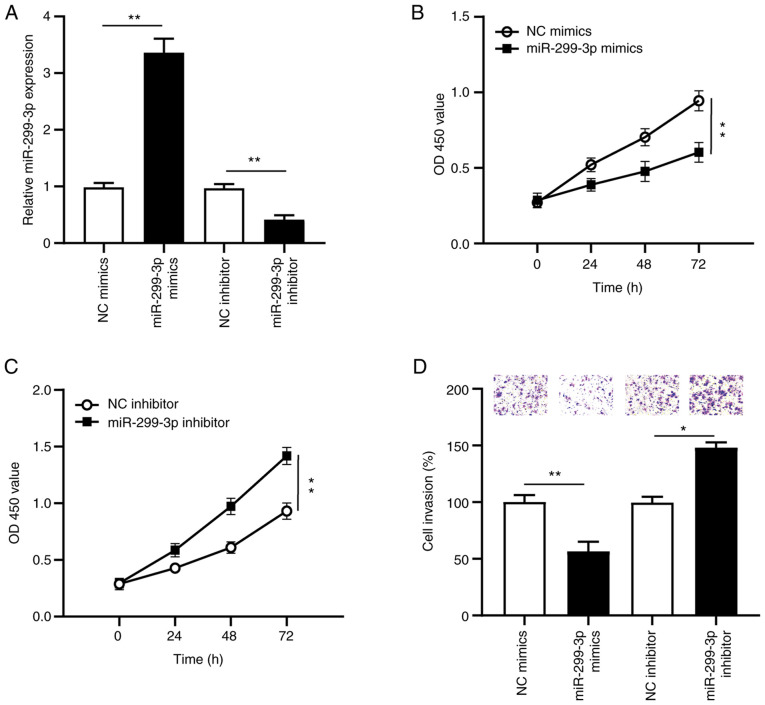
To determine the biological function of miR-299-3p in the proliferation and invasion of cervical cancer cells, SiHa cells were transfected with miR-299-3p mimics, NC mimics, miR-299-3p inhibitor or NC inhibitor. RT-qPCR analysis was performed to detect miR-299-3p expression. The results demonstrated that miR-229-3p expression significantly increased (P<0.01) following transfection with miR-299-3p mimics, the effects of which were reversed following transfection with miR-299-3p inhibitor (P<0.01), compared with the NC groups (Fig. 4A).
Figure 4.
Overexpression of miR-299-3p suppresses cervical cancer cell proliferation and invasion in vitro. SiHa cells were transfected with miR-299-3p mimics, NC mimics, miR-299-3p inhibitor or NC inhibitor. (A) miR-299-3p expression was detected in transfected SiHa cells. (B and C) The Cell Counting Kit-8 assay was performed to assess cell proliferation following transfection. (D) The Transwell assay was performed to assess cell invasion following transfection. *P<0.05; **P<0.01. miR, microRNA; NC, negative control; OD, optical density.
The proliferation and invasion of cervical cancer cells were assessed via the CCK-8 and Transwell assays, respectively. The results confirmed that overexpression of miR-299-3p significantly decreased cervical cancer cell proliferation (P<0.01; Fig. 4B), while miR-299-3p knockdown significantly increased cervical cancer cell proliferation (P<0.01; Fig. 4C). The Transwell assay demonstrated that transfection with miR-299-3p mimics significantly decreased cervical cancer cell invasion (P<0.01; Fig. 4D), while transfection with miR-299-3p inhibitor significantly promoted cervical cancer cell invasion (P<0.05; Fig. 4D). Collectively, these results suggest that miR-299-3p acts as a tumor suppressor in cervical cancer.
Overexpression of UCA1 promotes cell proliferation and invasion by suppressing miR-299-3p expression
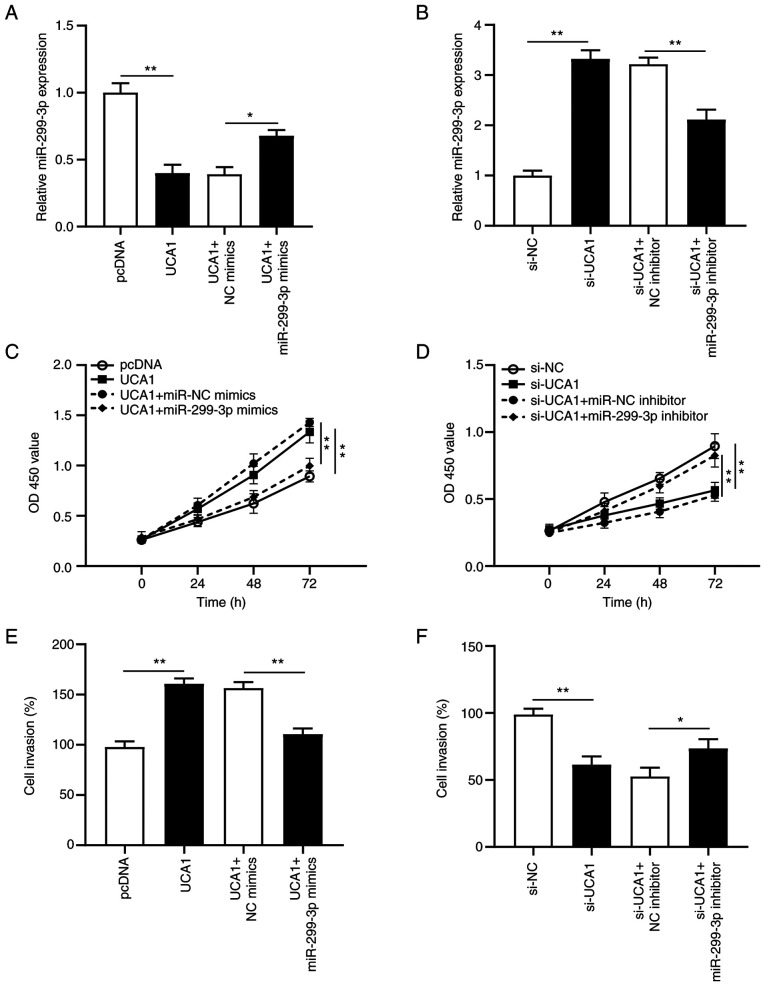
To further clarify the effect of the UCA1/miR-299-3p axis in cervical cancer tumorigenesis, rescue experiments were performed on SiHa cells. miR-299-3p mimics + UCA1 or miR-299-3p inhibitor + si-UCA1 were transfected into SiHa cells. RT-qPCR analysis demonstrated that overexpression of miR-299-3p partially reversed the inhibitory effects of overexpressing UCA1 on SiHa cells (P<0.05; Fig. 5A), while transfection with miR-299-3p inhibitor reversed the promoting effects of si-UCA1 on SiHa cells (P<0.01; Fig. 5B). Furthermore, transfection with miR-299-3p mimics inhibited the proliferative ability of UCA1-transfected SiHa cells (P<0.01; Fig. 5C), and si-UCA1-induced suppression in cell proliferation was recovered following transfection with miR-299-3p inhibitor (P<0.01; Fig. 5D). Furthermore, the invasive ability of UCA1-transfected SiHa cells decreased following transfection with miR-299-3p mimics (P<0.01; Fig. 5E), the effects of which were reversed following transfection with miR-299-3p inhibitor (P<0.05; Fig. 5F). Collectively, these results suggest that UCA1 promotes cervical cancer progression by regulating miR-299-3p expression.
Figure 5.
UCA1 enhances proliferation and invasion by inhibiting miR-299-3p expression in SiHa cells. SiHa cells were transfected with pcDNA, UCA1, UCA1 + NC mimics, UCA1 + miR-299-3p mimics, si-NC, si-UCA1, si-UCA1 + NC inhibitor and si-UCA1 + miR-299-3p inhibitor. (A and B) Reverse transcription-quantitative PCR analysis was performed to detect miR-299-3p expression following transfection. (C and D) The Cell Counting Kit-8 assay was performed to assess cell proliferation following transfection. (E and F) The Transwell assay was performed to assess cell invasion following transfection. *P<0.05; **P<0.01. UCA1, urothelial cancer-associated 1; miR, microRNA; NC, negative control; si, small interfering; OD, optical density.
Discussion
Cervical cancer is a common gynecological malignancy and the second most serious threat to women's health worldwide (47). Most cases of cervical cancer are caused by HPV infection (48). Although surgical treatment, chemotherapy and radiotherapy have a therapeutic effect on cervical cancer, this effect is insufficient (49). Recent studies have reported that lncRNAs play important regulatory roles in cervical cancer (50–52). Although several studies have clarified the role of UCA1 in different tumors (31–33), to the best of our knowledge, the molecular mechanism of UCA1 in cervical cancer remains unclear. In the present study, the role of UCA1 and its molecular mechanism in cervical cancer were further explored.
The lncRNA, UCA1 acts as an oncogene in different types of cancer, including pancreatic (53), gastric (54) and colorectal (26) cancers. Previous studies have demonstrated that UCA1 is pregulated in cervical cancer, which promotes cell proliferation, invasion and migration by regulating miR-145 or miR-204 expression (35,36). Consistent with these findings, the results of the present study demonstrated that UCA1 was upregulated in cervical cancer tissues and cell lines. Furthermore, overexpression of UCA1 promoted cervical cancer cell proliferation and invasion, the effects of which were reversed following UCA1 knockdown. Taken together, these findings suggest that UCA1 may play a key role in the proliferation and invasion of cervical cancer cells.
A recent study reported that miRNAs play important roles in cervical cancer. For example, miR-216a-3p inhibits cervical cancer cell proliferation and invasion by regulating actin-like 6A (55). Furthermore, miR-195-5p suppresses migration and invasion in cervical cancer by targeting ADP ribosylation factor-like GTPase 2 (ARL2) (56). Overexpression of miR-139-5p promotes cell proliferation and migration in cervical cancer by targeting TCF4 (57). miR-299-3p has been reported to act as a tumor suppressor in hepatocellular carcinoma, thyroid cancer and colon carcinoma (41–43). In addition, miR-299-3p inhibits cell proliferation and invasion by regulating TCF4 in cervical cancer (44). In the present study, miR-299-3p expression was significantly downregulated in cervical cancer, and its overexpression inhibited cervical cancer cell proliferation and invasion. Collectively, the results of the present study suggest that miR-299-3p plays an inhibitory role in cervical cancer.
Previous studies have reported that lncRNAs bind to specific miRNAs to regulate cancer progression (58,59). lncRNA UCA1 has been reported to regulate the progression of several tumors by targeting specific miRNAs. For example, UCA1 promotes cell proliferation in gastric cancer by regulating the miR-495/phosphatase of regenerating liver 3 axis (54) and mitochondrial function in bladder cancer via the miR-195/ARL2 signaling pathway (34). To further clarify the potential molecular mechanism of UCA1 in cervical cancer, the target genes of UCA1 were predicted using LncBase. The results revealed that UCA1 can bind to miR-299-3p. In addition, miR-299-3p expression was negatively correlated with UCA1 expression in cervical cancer tissues. Notably, overexpression of UCA1 decreased miR-299-3p expression in SiHa cervical cancer cells. Furthermore, transfection with miR-299-3p mimics inhibited cell proliferation and invasion, and transfection with miR-299-3p inhibitors reversed the effects of UCA1 knockdown on SiHa cells. Taken together, these results suggest that UCA1 acts as an oncogene in cervical cancer and regulates cell proliferation and invasion by inhibiting miR-299-3p expression.
The results of the present study provide novel insight into the treatment of cervical cancer. Notably, a novel target miRNA of UCA1 in cervical cancer, which has not been previously reported, was identified in the present study. In addition, the UCA1/miR-299-3p axis was revealed to regulate cell proliferation and invasion in cervical cancer, providing a novel target site for the treatment of cervical cancer. However, the present study is not without limitations. The sample size assessed was too small; thus, further studies with a larger sample size are required to investigate the mechanism of the regulatory effect of the UCA1/miR-299-3p axis on cell proliferation and invasion in cervical cancer. In addition, the present only performed in vitro experiments, and needs to be further investigated in vivo.
In conclusion, the results of the present study demonstrated that UCA1 promoted cell proliferation and invasion by targeting miR-299-3p expression in cervical cancer. These results suggest that the UCA1/miR-299-3p axis may present a potential therapeutic target for cervical cancer.
Supplementary Material
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Authors' contributions
MA and XX conceived and designed the present study, analyzed the data and drafted the initial manuscript. TC contributed to data collection, statistical analysis and manuscript preparation. MA and XX confirmed the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Sanya People's Hospital (approval no. 2017.106; Sanya, China) and written informed consent was provided by all patients prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393:169–182. doi: 10.1016/S0140-6736(18)32470-X. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Huang X, Liu X, Du B, Liu X, Xue M, Yan Q, Wang X, Wang Q. lncRNA LINC01305 promotes cervical cancer progression through KHSRP and exosome-mediated transfer. Aging (Albany NY) 2021;13:19230–19242. doi: 10.18632/aging.202565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal R, Kaye SB. Ovarian cancer: Strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502–516. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 6.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 7.Burki TK. Cervical cancer: Screening and risk with age. Lancet Oncol. 2014;15:e107. doi: 10.1016/S1470-2045(14)70032-4. [DOI] [PubMed] [Google Scholar]
- 8.Zeng H, Chen W, Zheng R, Zhang S, Ji J, Zou X, Xia C, Sun K, Yang Z, Li H, et al. Changing cancer survival in China during 2003–15: A pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6:e555–e567. doi: 10.1016/S2214-109X(18)30127-X. [DOI] [PubMed] [Google Scholar]
- 9.Gong Z, Zhang S, Zhang W, Huang H, Li Q, Deng H, Ma J, Zhou M, Xiang J, Wu M, et al. Long non-coding RNAs in cancer. Sci China Life Sci. 2012;55:1120–1124. doi: 10.1007/s11427-012-4413-9. [DOI] [PubMed] [Google Scholar]
- 10.Hemberg M, Gray JM, Cloonan N, Kuersten S, Grimmond S, Greenberg ME, Kreiman G. Integrated genome analysis suggests that most conserved non-coding sequences are regulatory factor binding sites. Nucleic Acids Res. 2012;40:7858–7869. doi: 10.1093/nar/gks477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anastasiadou E, Jacob LS, Slack FJ. Non-coding RNA networks in cancer. Nat Rev Cancer. 2018;18:5–18. doi: 10.1038/nrc.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang M, Wang C, Mu L. miR-148a induces apoptosis by upregulating BIM expression in gastric cancer cells. Int J Clin Exp Med. 2017;10:2791–2799. [Google Scholar]
- 13.Shen J, Zhang J, Xiao M, Yang J, Zhang N. miR-203 suppresses bladder cancer cell growth and targets Twist1. Oncol Res. 2018;26:1155–1165. doi: 10.3727/096504017X15041934685237. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Tang L, Yang B, Cao X, Li Q, Jiang L, Wang D. MicroRNA-377-3p inhibits growth and invasion through sponging JAG1 in ovarian cancer. Genes Genomics. 2019;41:919–926. doi: 10.1007/s13258-019-00822-w. [DOI] [PubMed] [Google Scholar]
- 15.Huang X, Li Z, Zhang Q, Wang W, Li B, Wang L, Xu Z, Zeng A, Zhang X, Zhang X, et al. Circular RNA AKT3 upregulates PIK3R1 to enhance cisplatin resistance in gastric cancer via miR-198 suppression. Mol Cancer. 2019;18:71. doi: 10.1186/s12943-019-0969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu G, Wan Q, Li J, Hu X, Gu X, Xu S. Circ_0038467 regulates lipopolysaccharide-induced inflammatory injury in human bronchial epithelial cells through sponging miR-338-3p. Thorac Cancer. 2020;11:1297–1308. doi: 10.1111/1759-7714.13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bill M, Papaioannou D, Karunasiri M, Kohlschmidt J, Pepe F, Walker CJ, Walker AE, Brannan Z, Pathmanathan A, Zhang X, et al. Expression and functional relevance of long non-coding RNAs in acute myeloid leukemia stem cells. Leukemia. 2019;33:2169–2182. doi: 10.1038/s41375-019-0429-5. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Huang D, Huang J, Nie K, Li X, Yang X. lncRNA TMPO-AS1 exerts oncogenic roles in HCC through regulating miR-320a/SERBP1 axis. Onco Targets Ther. 2020;13:6539–6551. doi: 10.2147/OTT.S250355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W, Zhang B, Jia Y, Shi H, Wang H, Guo Q, Li H. lncRNA LOXL1-AS1 regulates the tumorigenesis and development of lung adenocarcinoma through sponging miR-423-5p and targeting MYBL2. Cancer Med. 2020;9:689–699. doi: 10.1002/cam4.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan SY, Chen MM, Li GM, Wang YQ, Fan JG. miR-32 induces cell proliferation, migration, and invasion in hepatocellular carcinoma by targeting PTEN. Tumour Biol. 2015;36:4747–4755. doi: 10.1007/s13277-015-3124-9. [DOI] [PubMed] [Google Scholar]
- 21.Sempere LF, Christensen M, Silahtaroglu A, Bak M, Heath CV, Schwartz G, Wells W, Kauppinen S, Cole CN. Altered MicroRNA expression confined to specific epithelial cell subpopulations in breast cancer. Cancer Res. 2007;67:11612–11620. doi: 10.1158/0008-5472.CAN-07-5019. [DOI] [PubMed] [Google Scholar]
- 22.Xie H, Liao X, Chen Z, Fang Y, He A, Zhong Y, Gao Q, Xiao H, Li J, Huang W, Liu Y. lncRNA MALAT1 inhibits apoptosis and promotes invasion by antagonizing miR-125b in bladder cancer cells. J Cancer. 2017;8:3803–3811. doi: 10.7150/jca.21228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang X, Wang L, Xie S, Chen Y, Song S, Lu Y, Lu D. Long noncoding RNA MEG3 blocks telomerase activity in human liver cancer stem cells epigenetically. Stem Cell Res Ther. 2020;11:518. doi: 10.1186/s13287-020-02036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma P, Pan Y, Yang F, Fang Y, Liu W, Zhao C, Yu T, Xie M, Jing X, Wu X, et al. KLF5-modulated lncRNA NEAT1 contributes to tumorigenesis by acting as a scaffold for BRG1 to silence GADD45A in gastric cancer. Mol Ther Nucleic Acids. 2020;22:382–395. doi: 10.1016/j.omtn.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen SN, Li K, Liu Y, Yang CL, He CY, Wang HR. Silencing lncRNAs PVT1 upregulates miR-145 and confers inhibitory effects on viability, invasion, and migration in EC. Mol Ther Nucleic Acids. 2020;19:668–682. doi: 10.1016/j.omtn.2019.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Yang W, Xu X, Hong L, Wang Q, Huang J, Jiang L. Upregulation of lncRNA GAS5 inhibits the growth and metastasis of cervical cancer cells. J Cell Physiol. 2019;234:23571–23580. doi: 10.1002/jcp.28926. [DOI] [PubMed] [Google Scholar]
- 27.Yuan LY, Zhou M, Lv H, Qin X, Zhou J, Mao X, Li X, Xu Y, Liu Y, Xing H. Involvement of NEAT1/miR-133a axis in promoting cervical cancer progression via targeting SOX4. J Cell Physiol. 2019;234:18985–18993. doi: 10.1002/jcp.28538. [DOI] [PubMed] [Google Scholar]
- 28.Shen F, Zheng H, Zhou L, Li W, Xu X. Overexpression of MALAT1 contributes to cervical cancer progression by acting as a sponge of miR-429. J Cell Physiol. 2019;234:11219–11226. doi: 10.1002/jcp.27772. [DOI] [PubMed] [Google Scholar]
- 29.Lai SY, Guan HM, Liu J, Huang LJ, Hu XL, Chen YH, Wu YH, Wang Y, Wu Q, Zhou JY. Long noncoding RNA SNHG12 modulated by human papillomavirus 16 E6/E7 promotes cervical cancer progression via ERK/Slug pathway. J Cell Physiol. 2020;235:7911–7922. doi: 10.1002/jcp.29446. [DOI] [PubMed] [Google Scholar]
- 30.Wang XS, Zhang Z, Wang HC, Cai JL, Xu QW, Li MQ, Chen YC, Qian XP, Lu TJ, Yu LZ, et al. Rapid identification of UCA1 as a very sensitive and specific unique marker for human bladder carcinoma. Clin Cancer Res. 2006;12:4851–4858. doi: 10.1158/1078-0432.CCR-06-0134. [DOI] [PubMed] [Google Scholar]
- 31.Luan Y, Li X, Luan Y, Zhao R, Li Y, Liu L, Hao Y, Oleg Vladimir B, Jia L. Circulating lncRNA UCA1 promotes malignancy of colorectal cancer via the miR-143/MYO6 axis. Mol Ther Nucleic Acids. 2020;19:790–803. doi: 10.1016/j.omtn.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu Y, Gao F, He Q, Li G, Ding G. lncRNA UCA1 functions as a ceRNA to promote prostate cancer progression via sponging miR143. Mol Ther Nucleic Acids. 2020;19:751–758. doi: 10.1016/j.omtn.2019.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He X, Wang J, Chen J, Han L, Lu X, Miao D, Yin D, Geng Q, Zhang E. lncRNA UCA1 predicts a poor prognosis and regulates cell proliferation and migration by repressing p21 and SPRY1 expression in GC. Mol Ther Nucleic Acids. 2019;18:605–616. doi: 10.1016/j.omtn.2019.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li HJ, Sun XM, Li ZK, Yin QW, Pang H, Pan JJ, Li X, Chen W. lncRNA UCA1 promotes mitochondrial function of bladder cancer via the miR-195/ARL2 signaling pathway. Cell Physiol Biochem. 2017;43:2548–2561. doi: 10.1159/000484507. [DOI] [PubMed] [Google Scholar]
- 35.Wei H, Qiu YQ, Zeng QS, Wang SF, Yi CJ. lncRNA UCA1 regulates proliferation, migration and invasion of cervical cancer cells by targeting miR-145. Eur Rev Med Pharmacol Sci. 2020;24:3555–3564. doi: 10.26355/eurrev_202004_20816. [DOI] [PubMed] [Google Scholar]
- 36.He Q, Meng J, Liu S, Zeng Q, Zhu Q, Wei Z, Shao Y. Long non-coding RNA UCA1 upregulates KIF20A expression to promote cell proliferation and invasion via sponging miR-204 in cervical cancer. Cell Cycle. 2020;19:2486–2495. doi: 10.1080/15384101.2020.1807666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang TJ, Wang L, Zhang Y, Zheng JD, Liu L. lncRNA UCA1 regulates cervical cancer survival and EMT occurrence by targeting miR-155. Eur Rev Med Pharmacol Sci. 2020;24:9869–9879. doi: 10.26355/eurrev_202010_23197. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Pan Q, Shao Z. Tumor-suppressive role of microRNA-202-3p in hepatocellular carcinoma through the KDM3A/HOXA1/MEIS3 pathway. Front Cell Dev Biol. 2021;8:556004. doi: 10.3389/fcell.2020.556004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Qin H. miR-338-3p targets RAB23 and suppresses tumorigenicity of prostate cancer cells. Am J Cancer Res. 2018;8:2564–2574. [PMC free article] [PubMed] [Google Scholar]
- 40.Wang R, Zuo X, Wang K, Han Q, Zuo J, Ni H, Liu W, Bao H, Tu Y, Xie P. MicroRNA-485-5p attenuates cell proliferation in glioma by directly targeting paired box 3. Am J Cancer Res. 2018;8:2507–2517. [PMC free article] [PubMed] [Google Scholar]
- 41.Dang S, Zhou J, Wang Z, Wang K, Dai S, He S. miR-299-3p functions as a tumor suppressor via targeting Sirtuin 5 in hepatocellular carcinoma. Biomed Pharmacother. 2018;106:966–975. doi: 10.1016/j.biopha.2018.06.042. [DOI] [PubMed] [Google Scholar]
- 42.Chen X, Qi M, Yang Q, Li J. miR-299-3p functions as a tumor suppressor in thyroid cancer by regulating SHOC2. Eur Rev Med Pharmacol Sci. 2019;23:232–240. doi: 10.26355/eurrev_201901_16769. [DOI] [PubMed] [Google Scholar]
- 43.Wang JY, Jiang JB, Li Y, Wang YL, Dai Y. MicroRNA-299-3p suppresses proliferation and invasion by targeting VEGFA in human colon carcinoma. Biomed Pharmacother. 2017;93:1047–1054. doi: 10.1016/j.biopha.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 44.Yu Y, Zhao JD, Yang H. miR-299-3p inhibits proliferation and invasion of cervical cancer cell via targeting TCF4. Eur Rev Med Pharmacol Sci. 2019;23:5621–5627. doi: 10.26355/eurrev_201907_18296. [DOI] [PubMed] [Google Scholar]
- 45.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 46.Das T, Deb A, Parida S, Mondal S, Khatua S, Ghosh Z. LncRBase V.2: An updated resource for multispecies lncRNAs and ClinicLSNP hosting genetic variants in lncRNAs for cancer patients. RNA Biol. 2021;18:1136–1151. doi: 10.1080/15476286.2020.1833529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 48.Waggoner SE. Cervical cancer. Lancet. 2003;361:2217–2225. doi: 10.1016/S0140-6736(03)13778-6. [DOI] [PubMed] [Google Scholar]
- 49.Roik E, Sharashova E, Kharkova O, Nieboer E, Postoev V, Odland JØ. Sociodemographic characteristics, sexual behaviour and knowledge about cervical cancer prevention as risk factors for high-risk human papillomavirus infection in Arkhangelsk, North-West Russia. Int J Circumpolar Health. 2018;77:1498681. doi: 10.1080/22423982.2018.1498681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Q, Yan SP, Chu DX, Xie Y, Wang CF, Zhang JY, Li WC, Guo RX. Silencing of long non-coding RNA RP1-93H18.6 acts as a tumor suppressor in cervical cancer through the blockade of the PI3K/Akt axis. Mol Ther Nucleic Acids. 2020;19:304–317. doi: 10.1016/j.omtn.2019.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Chen DZ, Wang TF, Dai WC, Xu X, Chen PF. lncRNA FOXD2-AS1 accelerates the progression of cervical cancer via downregulating CDX1. Eur Rev Med Pharmacol Sci. 2019;23:10234–10240. doi: 10.26355/eurrev_201912_19660. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Q, Zheng J, Liu L. The long noncoding RNA PCGEM1 promotes cell proliferation, migration and invasion via targeting the miR-182/FBXW11 axis in cervical cancer. Cancer Cell Int. 2019;19:304. doi: 10.1186/s12935-019-1030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo Z, Wang X, Yang Y, Chen W, Zhang K, Teng B, Huang C, Zhao Q, Liu Z. Hypoxic tumor-derived exosomal long noncoding RNA UCA1 promotes angiogenesis via miR-96-5p/AMOTL2 in pancreatic cancer. Mol Ther Nucleic Acids. 2020;22:179–195. doi: 10.1016/j.omtn.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao Y, Xiong JB, Zhang GY, Liu Y, Jie ZG, Li ZR. Long noncoding RNA UCA1 regulates PRL-3 expression by sponging microRNA-495 to promote the progression of gastric cancer. Mol Ther Nucleic Acids. 2020;19:853–864. doi: 10.1016/j.omtn.2019.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Zhao J, Li L, Yang T. miR-216a-3p suppresses the proliferation and invasion of cervical cancer through downregulation of ACTL6A-mediated YAP signaling. J Cell Physiol. 2020;235:9718–9728. doi: 10.1002/jcp.29783. [DOI] [PubMed] [Google Scholar]
- 56.Pan SS, Zhou HE, Yu HY, Xu LH. miR-195-5p inhibits the cell migration and invasion of cervical carcinoma through suppressing ARL2. Eur Rev Med Pharmacol Sci. 2019;23:10664–10671. doi: 10.26355/eurrev_201912_19764. [DOI] [PubMed] [Google Scholar]
- 57.Ji X, Guo H, Yin S, Du H. miR-139-5p functions as a tumor suppressor in cervical cancer by targeting TCF4 and inhibiting Wnt/β-catenin signaling. Onco Targets Ther. 2019;12:7739–7748. doi: 10.2147/OTT.S215796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang D, Hu Y. Long non-coding RNA PVT1 competitively binds microRNA-424-5p to regulate CARM1 in radiosensitivity of non-small-cell lung cancer. Mol Ther Nucleic Acids. 2019;16:130–140. doi: 10.1016/j.omtn.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Zhang G, He X, Ren C, Lin J, Wang Q. Long noncoding RNA PCA3 regulates prostate cancer through sponging miR-218-5p and modulating high mobility group box 1. J Cell Physiol. 2019;234:13097–13109. doi: 10.1002/jcp.27980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.