Abstract
Background:
Lifestyle is important in type 2 diabetes mellitus (T2DM). This study’s aim was to investigate whether a healthy-lifestyle-supporting smartphone application could affect treatment outcomes at an endocrinology outpatient clinic.
Methods:
Consecutively invited patients were randomly assigned to an intervention or control group after age and gender stratification. In addition to standard care, intervention group participants used a smartphone application to access a lifestyle program (SidekickHealth) through which they received personalized recommendations and education about healthy lifestyles. Tests at baseline and every other month for six months included body weight and blood tests for glycated hemoglobin (HbA1c) and blood lipids, as well as questionnaires about distress related to diabetes, health-related quality of life, depression, and anxiety. Statistics included comparisons both within and between groups.
Results:
A total of 37 patients (23 women) were included, whereof 30 finished, 15 in each group (19% dropout); the average age was 51.2 ± 10.6 (25-70) years. No significant differences emerged between groups, but within the intervention group, there was a significant decrease in HbA1c from 61 ± 21.4 to 52.7 ± 15.2 mmol/mol, in disease-specific distress from 19.5 ± 16.5 to 11.7 ± 13.4, and in anxiety symptoms from 5.4 ± 4.0 to 4.1 ± 3.8. No significant changes occurred within the control group. The application usage was most frequent during the first months and differed interpersonally.
Conclusions:
Our results indicate that the SidekickHealth digital lifestyle program could potentially enhance outpatient treatment in T2DM, in terms of both glycemic control and psychological well-being but larger confirmative studies are needed.
Keywords: e-health, health-related quality of life, intervention, lifestyle, smartphone apps, type 2 diabetes
Introduction
Lifestyle changes along with early diagnosis and treatment of type 2 diabetes mellitus (T2DM) are essential to preventing or significantly delaying complications of the disease.1 According to clinical guidelines, all people with diabetes should participate in diabetes self-management education with a focus on self-care, empowerment, and support from healthcare professionals. The aim of such education is to enable people with T2DM to make informed decisions and to encourage self-care and increase active collaboration with professionals as well as to improve their health and quality of life.2 The education should be person-centered or tailored to personal preferences, values, and needs, where the main pillars are psychological and behavioral strategies on how to handle everyday life with diabetes, including lifestyle factors such as diet and physical activity.1,3 There is evidence supporting that diabetes self-management education increases diabetes-related knowledge and self-care,4 lowers glycated hemoglobin (HbA1c) levels,5 and reduces diabetes-related distress,4 while the effects on health-related quality of life (HRQoL) are more divergent.6-8
The field of technology solutions to support health management has evolved rapidly in recent years, and web-based support tools, distance learning, and even smartphone applications (apps) can be beneficial in lifestyle changes.9,10 Recent review papers report that smartphone apps can be useful tools for providing self-care support in diabetes, supporting lifestyle changes, and even improving glycemic control.11-13 The apps can assist with self-management, including by promoting a healthy diet, weight loss, an increase in physical activity, and regular blood glucose monitoring.11,14 The usage of apps could thus increase the effectivity of diabetes care without increasing the frequency of outpatient visits, which would be positive for the patients’ health and would save healthcare resources. Although both diabetes-specific and general lifestyle-supporting smartphone apps have been found effective in terms of glycemic control, the evidence of their effects on other variables, such as HRQoL and psychological well-being, is less robust.12,15,16
The aim of this study was to investigate whether the addition of a general lifestyle program (SidekickHealth) through a smartphone app could increase the effects of regular healthcare on blood glucose control, blood lipids, body mass index (BMI), diabetes-related distress, anxiety, depression, and HRQoL of people with T2DM, at a hospital-based endocrinology outpatient clinic.
Methods
Design
A randomized controlled trial of two parallel groups was conducted. Participants were randomly assigned to an intervention or control group in blocks of four, using blinded extracts of closed envelopes after stratification by gender and age (<60 vs ≥60 years).
Sample
The cohort comprised the clients of a hospital-based endocrine clinic. All who met the inclusion criteria were consecutively invited to participate when attending routine appointments.
The inclusion criteria were as follows: diagnosed with T2DM at least 6 months ago; able to write and speak the Icelandic language; own a smartphone; able to use the SidekickHealth app; age 18-75 years; have not undergone or planned bariatric surgery during the trial period. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Bioethics Committee of Akureyri Hospital (4/2017). All participants gave written informed consent.
Data Collection
Measurements were conducted four times for both groups: at baseline and after two, four, and six months. Information on diagnosed diseases and medication use was collected from medical records. The data collection lasted from March 2017 until September 2018.
Measurements
Physical measurements and blood tests, including body weight and blood tests for HbA1c and blood lipids, were performed according to routine monitoring at the clinic. Weight and waist circumference were measured by a nurse who was blinded to which group each participant belonged.
Instruments
Diabetes-specific emotional distress was measured by the Problem Areas in Diabetes Scale (PAID),17 which has 20 items and is scored from 0 to 100; a lower score indicates less distress. The PAID is amply validated18,19 and is widely used in the United States and Europe.20 In clinical practice, the use of PAID is recommended to measure diabetes-related distress.18 No cutoff score has yet been established for PAID, although some findings point toward 33 as a possible cutoff score.19 The psychometric properties of the Icelandic version of PAID have been tested and found to be reliable and valid.21
Anxiety and depression were measured by the Hospital Anxiety and Depression Scale (HADS),22 a 14-item scale with 7 items in each component originally designed to measure anxiety and depression in hospitalized people. Each component is scored from 0 to 21, and a lower score indicates less anxiety and depression. Studies have demonstrated the reliability and validity of HADS for screening purposes, with a cutoff score of 8 or higher for both anxiety and depression.23 The psychometric properties of the Icelandic translation have been confirmed.24,25
HRQoL was measured by the Icelandic health-related Quality of Life scale (IQL-test), a 32-item scale that gives a total HRQoL score as well as 12 subscores. The normal score for this scale is 50, and lower scores indicate less HRQoL. The IQL-test has been found to be reliable and valid, with an internal validity of 0.91 for the total score.26
Intervention
In addition to standard care, based on guidelines from American Diabetes Association,1,2 the intervention group received the SidekickHealth smartphone app.27 It includes a digital lifestyle program grounded in behavioral research and economics.28 The app was designed by the SidekickHealth company,27 to help people increase their frequency of healthy behaviors through goal setting, self-monitoring, and the completion of health-related tasks in three main categories: nutrition, physical activity, and stress management. Lifestyle change enhancement in the SidekickHealth app is based, among other things, on the US National Diabetes Prevention Program.29 It is a gamified technology that awards healthy behaviors with health points that mount up and result in water donations to UNICEF as an extra reward. It also encourages goal setting and self-monitoring through the registration of certain tasks, as well as allowing for competition with other users. Everything is presented in a simple visually illustrated way. The app’s main registration categories are nutrition (servings a day of fruits, vegetables, nuts, seeds and water, as well as setting goals about avoiding sugared beverages, candy, junk food, or late snacks), physical activity (distance and/or time walking, running, bicycling, stair walking, strength exercises, and total step count), stress management (relaxation exercises, yoga, mindfulness, and subjective estimation of stress, energy level, and quality of sleep), and clinic (weight, blood glucose, blood pressure, blood test results, and medication). Users must manually enter their own readings and activities, except for the pedometer. The SidekickHealth app has been used for general health promotion,27 and among obese adults30 but so far, few publications exist. The SidekickHealth app was not designed specifically for people with diabetes and has not been tested in a clinical population with T2DM.
At the first visit, the participants in the intervention group were taught to use the SidekickHealth app to support their lifestyle changes. Each participant received a keyword-protected account, in accordance with SidekickHealth privacy policy.27 Every week during the first 16 weeks, the intervention group automatically received standard, general guidance and support for a healthy lifestyle from the SidekickHealth software, and equally often, the first author sent short individualized encouragement through the app, based on registered activity in the app. Examples of such encouragement were “Keep up the good work,” “How about setting goals about diet?,” and “I encourage you to do some relaxation today.” After the first 16 weeks, both types of messages were received every other week for two more months. The SidekickHealth platform automatically gathered information on the participants’ registered activity, and the platform administrators forwarded the information to the first author. No information was available about whether the participants watched the standard guidance and encouragements, but at each visit at the clinic, they were encouraged to watch and use the information from the SidekickHealth platform.
Statistical Analyses
Comparisons within the ratio-scale variables from baseline to six months within and between the groups were calculated using the dependent and independent-samples Student’s t-test, respectively, and an analysis of variance (ANOVA, repeated measures) for the development over all four measurement points, along with Tukey’s post hoc test. For the variables on an ordinal or interval scale, the Mann–Whitney U-test was used for the independent comparisons, and Friedman’s ANOVA and Wilcoxon’s sign rank test were used for the dependent comparisons. A chi-squared test was used for the nominal variables. All analyses were according to the intention-to-treat approach, and the level of significance was set at P <.05. Power calculations based on an estimated intervention-induced intergroup difference of 0.8 in HbA1c% showed that 20 participants were needed in each group to yield 80% power with α = 0.05 (G*Power). Statistica 12 (StatSoft) was used for all the analyses except the power analysis.
Results
Thirty-seven participants, 23 of them women (62.1%), were included in the trial. Fifteen participants completed the trial in each group (Figure 1). The dropout rates in the intervention and control groups were 16.7% and 21.1%, respectively.
Figure 1.
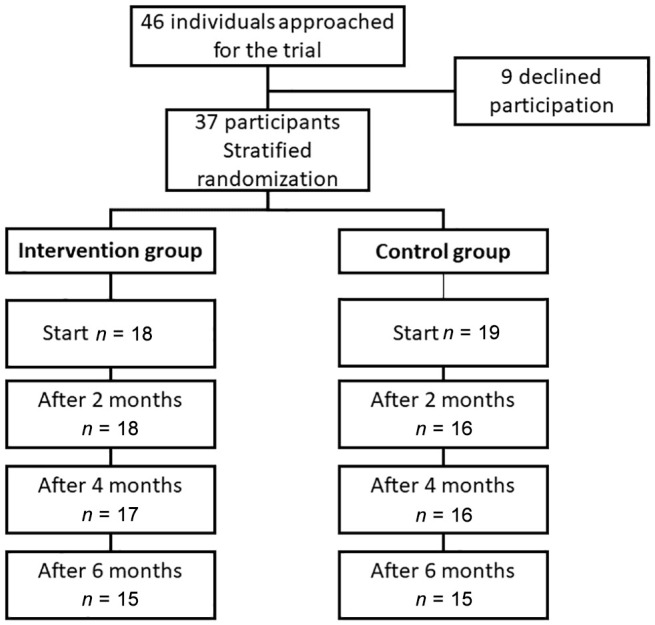
A flowchart showing the inclusion, randomization, and participation throughout the study.
Participants who dropped out were older (P = .04), and had more diagnosed diseases (P = .02) and higher triglycerides (P < .001) than the others. Table 1 shows the participants’ background characteristics. The age range was 25-70 years, but few (n = 5) were ≥60 years. On average, participants had almost five other diagnosed diseases; only one was diagnosed with T2DM alone. The most common diagnoses were (in descending order) obesity, high blood pressure, elevated blood lipids, and fibromyalgia. On average, the participants used more than six medications, and a majority had mixed treatment for their diabetes. There was no difference between the groups in any background characteristics (P > .05).
Table 1.
Characteristics of the Participants.
| Intervention group*
(n = 15) |
Control group*
(n = 15) |
|
|---|---|---|
| Gender (women/men) | 9 / 6 | 10 / 5 |
| Age (years) | 50.9 ± 11.8 (25-68) |
51.5 ± 9.5 (32-70) |
| Time from diagnosis (years) | 4.9 ± 5.1 (0.5-15) | 7.4 ± 4.4 (0.5-16) |
| Number of other diagnosesa | 4.8 ± 3.2 | 4.7 ± 2.6 |
| Medical treatments | ||
| Total number of medicationsb | 6.1 ± 2.6 | 6.5 ± 3.3 |
| Insulin and GLP-1c | 1 (7%) | 2 (13%) |
| Oral diabetic medication | 4 (27%) | 5 (33%) |
| Mixed treatmentd | 10 (67%) | 8 (53%) |
Mean ± standard deviation (range) or numbers (%); n = 30.
There was no difference between the groups in any variable.
Number of diagnoses other than type 2 diabetes per individual. bNumber of all medications per individual. cOnly injection therapy (i.e., insulin or insulin and GLP-1). dMixed therapy: can be oral and insulin, oral and GLP-1, or oral, insulin, and GLP-1. GLP-1: glucagon-like peptide 1.
Usage of the app was most frequent during the first months, and there was a large interpersonal difference. Ten participants used the app regularly the first four months, whereof seven remained regular users and three used the app more sporadically the last two months. One participant was a sporadic user the first four months but became a regular user the last two months. Two participants never activated the app and the remaining two were sporadic users throughout the study. Table 2 shows the registered activity in the SidekickHealth app. Most participants recorded diet and physical activity, but fewer used the stress management or clinic factors. No correlation was found between app usage (number of entry days nor collected health points) and change in HbA1c level, anxiety (HADS), or diabetes distress (PAID).
Table 2.
Recorded Entries in the App Throughout the Study Period (Six Months), n = 15.
| Mean | Standard deviation | Min-Max | |
|---|---|---|---|
| Number of entry days | 71.9 | 66.1 | 0-167 |
| Health points earned | 8,756.6 | 10,644.1 | 0-20,163 |
| Total number of inputs | 370.6 | 358.6 | 0-1,052 |
| Nutritiona | 146.9 | 195.4 | 0-601 |
| Physical activityb | 126.5 | 141.1 | 0-462 |
| Mindc | 33.1 | 49.8 | 0-150 |
| Clinicd | 23.3 | 51.9 | 0-204 |
Options for marking when participants ate fruits, vegetables, nuts, and seed, and when they drank water, skipped soda and sweets, or used the hunger meter.
Pedometer, location used on the phone to track walking and cycling. Ideas and directions for exercises. Sports, chores, and other activities.
Relaxation and mindfulness exercises. Assessment of stress, sleep, and energy. Meditation exercises.
Options for recording blood pressure, pulse, weight, medication, blood test results, and measurement of work-related stress.
Options for marking: aNutrition; servings a day of fruit, vegetables, nuts, seeds, and water, as well as avoiding sugared beverages, candy, junk food, or late snacks. bPhysical activity: distance and/or time walking, running, bicycling, stair walking, strength exercises, sport activities, and total step count. cMind: relaxation exercises, yoga, mindfulness, and subjective estimation of stress, energy level, and quality of sleep. dClinic: options for recording weight, blood sugar, blood pressure, medication, and blood test results.
Table 3 shows the results at baseline and after six months in both groups. The average BMI was above 30 kg/m2 in both groups and did not change over time. No significant differences emerged between the groups, but within the intervention group, there was a statistically significant decrease in HbA1c level, diabetes distress (PAID), and anxiety symptoms (HADS). No significant changes occurred within the control group over the research period.
Table 3.
Results at Baseline and After Six Months.
| Intervention
group n = 15 |
Control
group n = 15 |
|||
|---|---|---|---|---|
| Baseline | 6 months | Baseline | 6 months | |
| Weight (kg) | 112.5 ± 28.3 | 111.9 ± 28.4 | 94.3 ± 27.7 | 93.9 ± 27.7 |
| BMI (kg/m2) | 38 ± 9.2 | 37.7 ± 9.4 | 32.7 ± 9.1 | 33.1 ± 9.3 |
| Waist circumference (cm) | 123.5 ± 21.8 | 121.5 ± 22.0 | 110.2 ± 21.6 | 107.5 ± 21.5 |
| Systolic (mmHg) | 135.1 ± 19.0 | 133.6 ± 19.2 | 133.8 ± 18.0 | 127.8 ± 19.0 |
| Diastolic (mmHg) | 79.8 ± 9.1 | 80.3 ± 7.4 | 81.8 ± 8.9 | 80.8 ± 6.1 |
| HbA1c (%) | 7.7 ± 2.0 | 7.0 ± 1.4 * | 7.8 ± 1.9 | 7.7 ± 1.4 |
| HbA1c (mmol/mol) | 61.0 ± 21.4 | 52.7 ± 15.2 * | 61.4 ± 20.8 | 60.9 ± 14.0 |
| Triglycerides | 1.4 ± 1.1 | 1.5 ± 0.7 | 2.1 ± 1.1 | 2.3 ± 2.0 |
| Cholesterol | 4.2 ± 1.3 | 4.4 ± 0.7 | 4.7 ± 1.3 | 4.5 ± 1.0 |
| HDL | 1.2 ± 0.3 | 1.1 ± 0.2 | 1.2 ± 0.3 | 1.2 ± 0.2 |
| LDLx | 2.7 ± 1.0 | 2.6 ± 1.0 | 2.4 ± 0.9 | 2.5 ± 0.9 |
| HRQoL | 48.4 ± 11.2 | 48.5 ± 11.8 | 46.4 ± 10.9 | 46.0 ± 13.0 |
| HADS anxiety | 5.4 ± 4.0 | 4.1 ± 3.8 * | 4.9 ± 3.7 | 5.5 ± 4.7 |
| HADS depression | 3.2 ± 4.2 | 3.3 ± 3.0 | 3.7 ± 3.9 | 4.2 ± 4.6 |
| PAID | 19.5 ± 16.5 | 11.7 ± 13.4 * | 17.5 ± 16.2 | 18.5 ± 23.0 |
Mean ± standard deviation, n = 30.
Bold face values has significant difference within the group, P < .05.
LDL was not calculated for four participants. No significant differences between the groups. BMI, body mass index; HADS, Hospital Anxiety and Depression Scale; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; HRQoL, health-related quality of life; LDL, low-density lipoprotein; PAID, Problem Areas in Diabetes Scale.
The development for both groups throughout the study time in HbA1c level, diabetes distress (PAID), and anxiety (HADS), respectively, is shown in Figures 2–4. A significant difference within the intervention group was already evident at four months and remained at a similar level for HbA1c (Figure 2) and PAID (Figure 3) for the next two months. Anxiety, however, did not decrease significantly until after six months (Figure 4). No significant difference between the groups was seen at any given time, but for PAID, the repeated-measures pattern over time differed between the groups (Figure 3).
Figure 2.
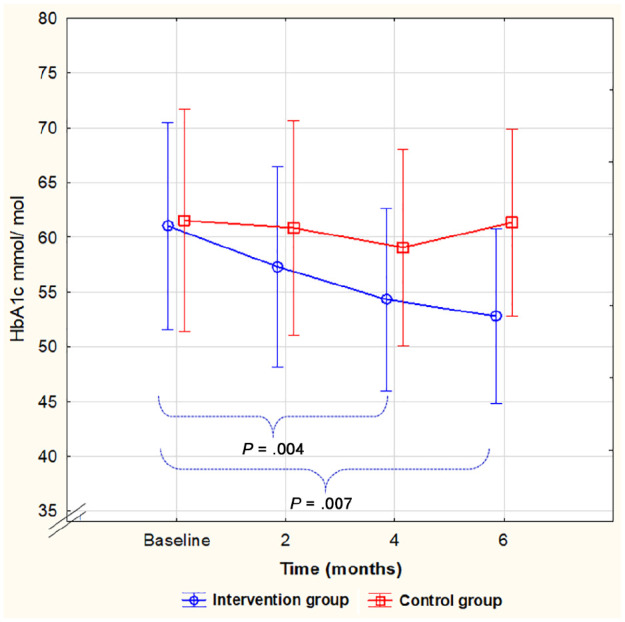
HbA1c level during the study, mean, and 95% confidence interval. A significant decrease from baseline to four and six months within the intervention group only. No significant differences between the groups (F(3, 78) = 1.625, P = .190).
HbA1c, glycated hemoglobin.
Figure 3.
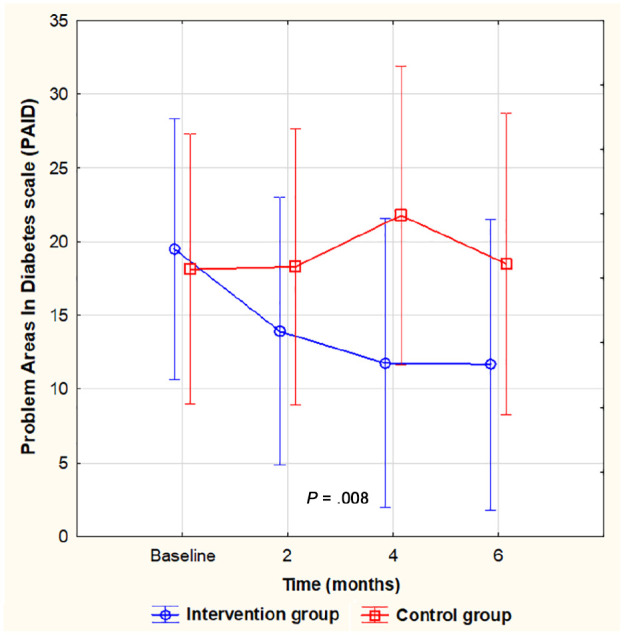
Diabetes distress (PAID) during the study, mean, and 95% confidence interval. A significant decrease from baseline to four and six months within the intervention group only. No significant differences between the groups at any given time, but a difference in the pattern of repeated measures between the groups (F(3, 81) = 3.009, P = .035).
PAID, Problem Areas in Diabetes Scale.
Figure 4.
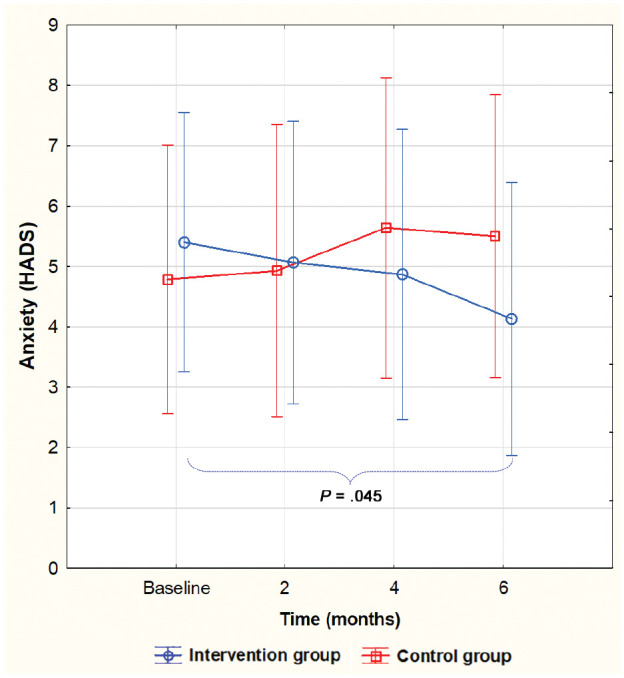
Level of anxiety during the study, mean, and 95% confidence interval. A significant decrease from baseline to six months in the intervention group only. No significant differences between the groups (F(3, 81) = 2.117, P = .104).
HADS, Hospital Anxiety and Depression Scale.
Discussion
Our results indicate that adding the SidekickHealth app to regular outpatient support can be more efficient in achieving a reduction in HbA1c level, anxiety, and diabetes-related distress over four to six months than outpatient support alone, as significant improvement was seen only in the intervention group. However, as there was no significant difference between the groups at any given time, this must be viewed with caution.
The mean HbA1c reduction in the intervention group in our study was slightly larger than the reduction (0.4%) in the intervention group in a six-month multicenter randomized study by Kim et al, even though they used a diabetes-specific app in their intervention.10 The baseline HbA1c level in this study was very similar to the baseline level in their study.10 Hou et al found a mean reduction of 0.49% in HbA1c level compared with controls in their systematic review and meta-analysis of 10 intervention studies using smartphone apps in people with T2DM. They also found that apps including three or more self-monitoring tasks demonstrated similar results as apps with more self-monitoring tasks. Furthermore, if the app included feedback from healthcare providers, the reduction in HbA1c level in the intervention groups compared to controls was 0.56%, although the difference in the reduction in HbA1c levels between the healthcare provider support groups and the automatic support groups was not significant.11 This indicates that the possibility of adding healthcare provider support through the smartphone app in our study could be a valuable factor in increasing the total effect of the intervention. At six months, the HbA1c level in our intervention group was close to what has been found to be beneficial to reduce the incidence of diabetes-related microvascular complications.1 The reduction in HbA1c level of the participants in the intervention group is also noteworthy, as previous studies claim that if the initial HbA1c level is above 8%, the HbA1c reduction is greater with an educational intervention than when the initial HbA1c is below 8%,10,31 as was the case at baseline in both our groups.
The reduction within the intervention group in both diabetes-related distress and anxiety from baseline to six months confirms previous findings that increased support can reduce diabetes-related distress and anxiety.4,32 Which is interesting as both distress and anxiety scores for both groups at baseline were relatively low.19,23 HRQoL and depression were however unchanged; the results are in line with the findings of two recent review studies including, in total, 15 articles on app use in T2DM,12,33 although HRQoL has been found to increase in some other smartphone-app intervention studies.10 Other outcomes such as BMI, lipid profile, and blood pressure were unchanged after 24 weeks of using the SidekickHealth app, although the HbA1c level decreased, which is in accordance with other studies on lifestyle changes16,32 but BMI has been found to change in some studies.10,32
Most participants in our intervention group used the app. Some used it steadily in the beginning (10 out of 15), but as the time passed, it was used less, and 2 participants never activated the app. This is in accordance with the results from a qualitative study by Torbjørnsen et al,34 where around half of their 24 participants regularly used a diabetes-specific diary app over 1 year, and the other half used it either occasionally (n = 6) or stopped using it soon after it was obtained (n = 6). Some participants in the study by Torbjørnsen found it challenging to use the app, but others highlighted that the diabetes app was easy to use and always at hand.34 The SidekickHealth app was developed for general support and motivation for a healthy lifestyle, especially for people with lifestyle-mediated chronic diseases. In the app, there are opportunities to manually record blood sugar levels, but few participants in our study did so: only 2 out of the 10 regular users. One of the most valued features of the diabetes app in Torbjørnsen et al’s study was the automatic transmission of blood glucose measurements into the app, whereas the participants found it demanding to manually record data on diet and physical activity into their app.34 Such a barrier to manually recording blood glucose measurements into the SidekickHealth app could thus explain why only two T2DM patients used that option, while many more used the features that required minimal manual effort. In our study, more people at or above 60 years dropped out of the study, but the two people in the intervention group who never used the app were younger than 60 years. Our participants were too few for us to conclude anything about the effect of age on app usage, but in a study from Norway with around 100 participants in app-intervention groups, people above 60 years were more frequent app users than their younger counterparts.35
Modern healthcare standards for diabetes list both diabetes education and support from healthcare providers as important parts of care in T2DM. Support is generally considered more informal in assisting the patient in implementing and sustaining a certain behavior than diabetes education.2 We assume that by receiving automatic information through the SidekickHealth app along with encouragement from the endocrine clinic once a week and every other week for the last two months, the intervention group participants gained increased support to sustain certain healthy behaviors, such as maintaining diet and physical activity, as shown by their most used entries in the app. The support might have assisted in reducing their HbA1c levels as well as their diabetes distress and anxiety, as mentioned above, and it only took 20-30 minutes per week to provide the supportive messages for the whole group. A review on technologies in diabetes claims that devices facilitating interaction with healthcare providers enhance feelings of support, and that some people also enjoy supplementing their usual therapy with technical devices.36
The SidekickHealth app is graphically designed with its gamified technology, which requires no writing but includes only marking features that apply to behaviors or goals. The app has thus been considered user friendly, a feature that is shown to be very important for usage in a systematic review by McMillan et al.37 However, access to the SidekickHealth platform including encouragement and education is not free, whereas a simpler version of the app with only user registration is available for free.
Limitations and Strengths
The main limitation of this study is the few participants, as we did not reach the planned power, and thus cannot exclude a type 2 error in between the groups comparisons. The main reason for there being fewer participants than planned was unforeseen long-term illness of staff at the clinic. As there was no follow-up after the intervention, any long-term effects are unknown.
The randomization and stratification of the participants according to age and gender, a single blinded measurement procedure, and the results reported according to intention to treat can, however, be considered a strength. The low dropout rate increases the internal validity of the study. In addition, it could be considered a strength that the study included both biomedical and psychosocial variables.
Conclusion
The addition of the SidekickHealth app to clinical practice has potential to positively influence glycemic control and diabetes distress and anxiety in patients with T2DM, but larger studies are needed to confirm the effects of its use. Including the app in clinical practice is not burdensome for the healthcare provider, but it requires commitment from the users. It can thus be considered feasible as an add-on support in specialized outpatient T2DM care for patients who are motivated to use technical solutions.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by grants from the Akureyri Hospital Research Fund, the University of Akureyri Research Fund, and the Icelandic Nurse Association Science Fund.
ORCID iD: Árún K. Sigurðardóttir  https://orcid.org/0000-0003-0670-8248
https://orcid.org/0000-0003-0670-8248
References
- 1.American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl 1):S66-S76. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. 5. Facilitating behavior change and well-being to improve health outcomes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl 1):S48-S65. [DOI] [PubMed] [Google Scholar]
- 3.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2012;55(6):1577-1596. [DOI] [PubMed] [Google Scholar]
- 4.Sigurdardottir AK, Benediktsson R, Jonsdottir H. Instruments to tailor care of people with type 2 diabetes. J Adv Nurs. 2009;65(10):2118-2130. [DOI] [PubMed] [Google Scholar]
- 5.Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943. [DOI] [PubMed] [Google Scholar]
- 6.Christoffersen LA, Hansen AK, Pals RA, Willaing I, Siersma V, Olesen K. Effect of a participatory patient education programme (NExt EDucation) in group-based patient education among Danes with type 2 diabetes. Chronic Illn. 2018;18:1742395318799843. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham AT, Crittendon DR, White N, Mills GD, Diaz V, LaNoue MD. The effect of diabetes self-management education on HbA1c and quality of life in African-Americans: a systematic review and meta-analysis. BMC Health Serv Res. 2018;16;18(1):367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan LSM, Khoo EYH, Tan CS, et al. Sensitivity of three widely used questionnaires for measuring psychological distress among patients with type 2 diabetes mellitus. Qual Life Res. 2015;24(1):153-162. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association. 7. Diabetes technology: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl 1):S77-S88. [DOI] [PubMed] [Google Scholar]
- 10.Kim EK, Kwak SH, Jung HS, et al. The effect of a smartphone-based, patient-centered diabetes care system in patients with type 2 diabetes: a randomized, controlled trial for 24 weeks. Diabetes Care. 2019;42(1):3-9. [DOI] [PubMed] [Google Scholar]
- 11.Hou C, Carter B, Hewitt J, Francisa T, Mayor S. Do mobile phone applications improve glycemic control (HbA1c) in the self-management of diabetes? A systematic review, meta-analysis, and GRADE of 14 randomized trials. Diabetes Care. 2016;39(11):2089-2095. [DOI] [PubMed] [Google Scholar]
- 12.Lunde P, Nilsson BB, Bergland A, Kværner KJ, Bye A. The effectiveness of smartphone apps for lifestyle improvement in noncommunicable diseases: systematic review and meta-analyses. J Med Internet Res. 2018;20(5):e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu X, Guo X, Zhang Z. The efficacy of mobile phone apps for lifestyle modification in diabetes: systematic review and meta-analysis. JMIR Mhealth Uhealth. 2019;7(1):e12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamprinos I, Demski H, Mantwill S, Kabak Y, Hildebrand C, Ploessnig M. Modular ICT-based patient empowerment framework for self-management of diabetes: design perspectives and validation results. Int J Med Inform. 2016;91:31-43. [DOI] [PubMed] [Google Scholar]
- 15.Bonoto BC, de Araújo VE, Godói IP, et al. Efficacy of mobile apps to support the care of patients with diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. JMIR Mhealth Uhealth. 2017;5(3):e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carpenter R, DiChiacchio T, Barker K. Interventions for self-management of type 2 diabetes: an integrative review. Int J Nurs Sci. 2019;6(1):70-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polonsky W, Anderson B, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18(6):754-760. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Lee E, Kim C, Moon SH. Diabetes-related emotional distress instruments: a systematic review of measurement properties. Int J Nurs Stud. 2015;52(12):1868-1878. [DOI] [PubMed] [Google Scholar]
- 19.Hermanns N, Kulzer B, Krichbaum M, Kubiak T, Haak T. How to screen for depression and emotional problems in patients with diabetes: comparison of screening characteristics of depression questionnaires, measurement of diabetes-specific emotional problems and standard clinical assessment. Diabetologia. 2006;49(3):469-477. [DOI] [PubMed] [Google Scholar]
- 20.Speight J, Reaney MD, Barnard KD. Not all roads lead to Rome-a review of quality of life measurement in adults with diabetes. Diabet Med. 2009;26(4):315-327. [DOI] [PubMed] [Google Scholar]
- 21.Sigurdardottir AK, Benediktsson R. Reliability and validity of the Icelandic version of the Problem Area in Diabetes (PAID) Scale. Int J Nurs Stud. 2008;45(4):526-533. [DOI] [PubMed] [Google Scholar]
- 22.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361-370. [DOI] [PubMed] [Google Scholar]
- 23.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69-77. [DOI] [PubMed] [Google Scholar]
- 24.Agustsdottir S, Kristinsdottir A, Jonsdottir K, Larusdottir SO, Smari J, Valdimarsdottir HB. The impact of dispositional emotional expressivity and social constraints on distress among prostate cancer patients in Iceland. Br J Health Psychol. 2010;15(1):51-61. [DOI] [PubMed] [Google Scholar]
- 25.Smári J, Valtýsdóttir H. Dispositional coping, psychological distress and disease-control in diabetes. Pers Individ Dif. 1997;22(2):151-156. [Google Scholar]
- 26.Helgason T, Björnsson JK, Tómasson K, Grétarsdóttir E. Health-related quality of life among Icelanders. Laeknabladid. 2000;86(4):251-257. [PubMed] [Google Scholar]
- 27.SidekickHealth.com [Internet]: Reykjavík: We are unique [cited 2020 Jan 27]. https://sidekickhealth.com/solution/.
- 28.Thorgeirsson T, Kawachi I. Behavioral economics: merging psychology and economics for lifestyle interventions. Am J Prev Med. 2013;44(2):185-189. [DOI] [PubMed] [Google Scholar]
- 29.Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3(11):866-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oddsson S. Effects of a gamified mobile application to support a lifestyle-change program in adults: a controlled pilot. ADA 77th Scientific Sessions. Diabetes. 2017;66(suppl 1):A1-A100. [Google Scholar]
- 31.Sigurdardottir AK, Jonsdottir H, Benediktsson R. Outcomes of educational interventions in type 2 diabetes: WEKA data-mining analysis. Patient Educ Couns. 2007;67(1-2):21-31. [DOI] [PubMed] [Google Scholar]
- 32.Hernández-Jiménez S, García-Ulloa AC, Bello-Chavolla OY, Aguilar-Salinas CA, Kershenobich-Stalnikowitz D. Long-term effectiveness of a type 2 diabetes comprehensive care program. The CAIPaDi model. Diabetes Res Clin Pract. 2019;151:128-137. [DOI] [PubMed] [Google Scholar]
- 33.Veazie S, Winchell K, Gilbert J, et al. Rapid evidence review of mobile applications for self-management of diabetes. J Gen Intern Med. 2018;33(7):1167-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torbjørnsen A, Ribu L, Rønnevig M, Grøttland A, Helseth S. Users’ acceptability of a mobile application for persons with type 2 diabetes: a qualitative study. BMC Health Serv Res. 2019;19(1):641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmen H, Torbjørnsen A, Wahl AK, et al. A mobile health intervention for self-management and lifestyle change for persons with type 2 diabetes, part 2: one-year results from the Norwegian randomized controlled trial RENEWING HEALTH. JMIR Mhealth Uhealth. 2014;2(4):e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrison S, Stadler M, Ismail K, Amiel S, Herrmann-Werner A. Are patients with diabetes mellitus satisfied with technologies used to assist with diabetes management and coping?: a structured review. Diabetes Technol Ther. 2014;16(11):771-783. [DOI] [PubMed] [Google Scholar]
- 37.McMillan KA, Kirk A, Hewitt A, MacRury S. A systematic and integrated review of mobile-based technology to promote active lifestyles in people with type 2 diabetes. J Diabetes Sci Technol. 2017;11(2):299-307. [DOI] [PMC free article] [PubMed] [Google Scholar]


