Abstract
Background:
Limited data exist regarding diabetes technology use among adults with type 1 diabetes (T1D) in urban racially/ethnically diverse safety-net hospitals. We examined racial/ethnic differences in the use of continuous glucose monitor (CGM) and continuous subcutaneous insulin infusion (CSII) in this setting.
Methods:
A retrospective review of 227 patients ≥ 18 years of age with T1D seen in an urban, safety-net endocrinology clinic during 2016-2017 was completed (mean age: 39; 80% English-speaking; 50% had public insurance). Diabetes technology use, defined as either CGM or CSII or both CGM and CSII, and clinical outcomes were examined by race/ethnicity.
Results:
Overall, 30% used CGM and 26% used CSII. After adjusting for age, language, insurance, and annual income, diabetes technology use in non-White patients was significantly lower than in White patients, predominantly lower in Black (aOR 0.25 [95% CI 0.11-0.56]) and patients identified as other race/ethnicity (aOR 0.30 [95% CI 0.11-0.77]). At the highest household income level (≥$75,000/y), Black and Hispanic individuals were significantly less likely than White individuals to use diabetes technology (P < .0007). Mean hemoglobin A1c (HbA1c) was lower in patients using any diabetes technology compared with patients using no technology (P < .0001). Use of CGM and CSII together was associated with the lowest HbA1c across all racial/ethnic groups.
Conclusions:
Racial/ethnic disparities in diabetes technology use and glycemic control were observed even after adjusting for sociodemographic factors. Further research should explore barriers to accessing diabetes technology in non-White populations.
Keywords: diabetes technology, health care disparities, safety-net hospital, type 1 diabetes mellitus
Introduction
For individuals living with type 1 diabetes mellitus (T1D), diabetes technologies—defined as continuous subcutaneous insulin infusion (CSII) and continuous glucose monitor (CGM)—are associated with improved glycemic control, reduced rates of severe hypoglycemia, and improved quality of life.1-6 Diabetes technologies, in particular CGM and sensor-augmented pump therapy, provide benefit at the individual and societal level through increase in quality-adjusted life years, reduction in complications, and a suggestion of extending the life expectancy of patients with T1D. Furthermore, they have been shown to be cost-effective, decreasing the future economic burden of T1D.7-9 Due to the potential improvement in glycemic control and reductions in incidence of hypoglycemia, it is recommended that clinicians consider use of CGM in all patients with T1D and CSII in select patients with T1D.10-12
Given evidence of benefit from randomized controlled trials, the use of CSII and CGM has increased significantly in recent years.13 However, despite overall increases in diabetes technology use, racial and ethnic disparities exist in the use of these technologies. These racial disparities may contribute to disparities in health outcomes; to date this has been most robustly demonstrated in pediatric populations.14-17 It is well established that racial and ethnic minority youth with T1D have lower rates of diabetes technology use than their non-Hispanic White counterparts.13,18,19 A recent study demonstrated that despite overall increases in insulin pump use, non-Hispanic Black and Spanish-speaking pediatric patients are about half as likely to use insulin pump therapy as their non-Hispanic White and English-speaking counterparts.18 Although these disparities are well documented in youth and adolescent T1D populations as well as in adults with type 2 diabetes,20 access to diabetes technology and health outcomes for adults of racial and ethnic minority groups with T1D remains understudied.
The incidence of T1D is increasing fastest in racial and ethnic minority populations.21 As such, it is essential to examine and understand variation in treatment methods and outcomes in institutions that care for high proportions of racial and ethnic minorities in an effort to mitigate disparities, promote equitable health care access and delivery, and improve health outcomes. Racial and ethnic minority communities have always primarily received their care from urban safety-net institutions. Safety-net hospitals are defined as institutions that deliver health care and other health-related services to patients who are un- or under-insured, including those covered by Medicaid, and regardless of a patient’s ability to pay.22 Urban safety-net hospitals demonstrate higher proportions of care for racial and ethnic minorities and non-English speaking patients than other urban hospitals.23 However, much of what is known about the current state of diabetes technology use and clinical outcomes in US adults with T1D comes from the T1D Exchange Clinic Registry, a predominantly white, privately insured cohort that may not reflect the changing demographic landscape of T1D.13,21 For this reason, there is need for further research to examine diabetes technology use in racially and ethnically diverse patient populations such as those cared for by urban safety-net hospital systems.
The primary objective of this study is to examine variation in diabetes technology utilization and outcomes in a diverse population of patients with T1D cared for at an academic, urban safety-net hospital.
Research Design and Methods
Study Design and Participants
In this retrospective cohort study, clinical data were collected for individuals with T1D seen in the adult endocrinology and diabetes clinic at Boston Medical Center in Boston, Massachusetts, the largest, urban safety-net hospital in New England between October, 2016, and September, 2017. Nearly 60% of patients seen at Boston Medical Center are racial/ethnic minorities and public insurance beneficiaries and one third do not speak English as their primary language.
Patients were identified for inclusion in the study if they were seen for a diabetes care visit within the adult endocrinology clinic, identified as those receiving a point-of-care hemoglobin A1c (HbA1c). Diagnosis of T1D was determined through review of the endocrinologist’s documentation in the clinical record. Patients were not excluded on the basis of missing demographic or other clinical data. A total of 227 adult patients (age ≥18 years) with T1D were included.
This study was reviewed and determined exempt by the Boston University Institutional Review Board.
Data Collection
Information on age, race/ethnicity, language spoken, diabetes duration, insurance status, CSII use, CGM use, non-insulin glucose-lowering medication use, and HbA1c levels obtained as part of usual care was collected from the medical records. Zip code was utilized as a proxy for socioeconomic status using median household income data from the 2017 American Community Survey as obtained from the US Census Bureau.24
Outcomes
The primary outcome of interest was the binary (yes/no) use of diabetes technology, defined as CSII and/or CGM. Glycemic control as indicated by HbA1c was assessed as a secondary outcome.
Covariates
Age was treated as a categorical variable with age groups as follows to standardize our reporting with the T1D exchange data: 18 to <26 years, 26 to <50 years, and ≥50 years.13 Race/ethnicity data were collected from the medical record which is categorized according to the United States Office of Management and Budget standards for classification. Due to low numbers of those of certain racial groups the data were combined as follows: non-Hispanic White (White); non-Hispanic Black (Black); Hispanic; and other race/ethnicity which includes Asian, American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, multiple, other, or unknown. Insurance status was reported as private, public (Medicare or Medicaid), or none. Language spoken was categorized as English, Spanish, or other. Socioeconomic status was categorized based on median annual household income as follows <$50,000, $50,000 to <$75,000, and ≥$75,000. Diabetes duration, measured in years, was determined by chart review. The patients’ most recent HbA1c was used and treated as a continuous variable (hereafter referred to as HbA1c).
Statistical Analysis
T tests, analysis of variance (ANOVA), and Fisher exact tests were used to assess differences in patient characteristics by race/ethnicity and use of diabetes technology. Univariate logistic regression models were constructed to test the association of age, gender, race/ethnicity, language spoken, socioeconomic status, insurance status, and diabetes duration with use of diabetes technology. Through manual selection of independent variables, a multivariable logistic regression model was constructed for the use of diabetes technology utilizing predictor variables for which univariate logistic regression demonstrated an odds ratio ≤0.75 or ≥1.5.
For continuous variables, results are expressed as mean and standard deviation for normally distributed variables or median (interquartile range) for non-normally distributed variables and number and percentage for categorical variables. All P-values are two-sided, level of significance 0.05. Data analyses were performed using SAS® OnDemand for Academics (SAS Institute, Cary, NC).
Results
A total of 227 patients with T1D were included in the study: 97 White (43%), 57 Black (25%), 35 Hispanic (15%), and 38 other (17%). Demographic characteristics according to race/ethnicity are shown in Table 1. Overall, mean age was 38.8 ± 13.0 years, 59% were male, mean duration of diabetes was 20.9 ± 12.8 years, and most recent mean HbA1c was 8.9% ± 2.0% (74 ± 22 mmol/mol). Eighty percent of the sample spoke English as their primary language. Notably, only one third of Hispanic patients and two thirds of those categorized as other race/ethnicity spoke English. Differences in socioeconomic status distribution were observed among racial/ethnic groups; 50% of White patients had an annual household income of more than $75,000 compared with only 19% Black, 17% Hispanic, and 27% of other. Public health insurance was held by 50% of the sample with the highest proportion of individuals covered by public health insurance among Hispanic patients (71%) as compared with other groups. Only two patients (1%) had no health insurance coverage.
Table 1.
Demographic Characteristics by Race/Ethnicity.
| Characteristics | Overall (N = 227) | White (n = 97) | Black (n = 57) | Hispanic (n = 35) | Othera (n = 38) | P |
|---|---|---|---|---|---|---|
| Age, years | 38.8±13.0 | 40.7±12.7 | 37.2±14.1 | 35.1±10.1 | 39.7±13.9 | .12 |
| 18-25 | 38 (17) | 14 (14) | 15 (26) | 6 (17) | 3 (8) | .08 |
| 26-49 | 138 (61) | 57 (59) | 29 (51) | 26 (74) | 26 (68) | |
| >50 | 51 (22) | 26 (27) | 13 (23) | 3 (9) | 9 (24) | |
| Gender | ||||||
| Male | 134 (59) | 61 (63) | 33 (58) | 17 (49) | 23 (61) | .52 |
| Duration of diabetes, years | 20.9±12.8 | 23.5±13.8 | 19±13 | 17.5±10.8 | 20.4±10.5 | .05 |
| Most recent HbA1c, % | 8.9±2.0 | 8.1±1.5 | 10.1±2.2 | 9.2±2 | 8.9±1.9 | <.0001 |
| Most recent HbA1c, mmol/mol | 74±22 | 65±16 | 87±24 | 77±22 | 74±21 | <.0001 |
| Technology use | <.0001 | |||||
| Any technology useb | 85 (37) | 54 (55) | 12 (21) | 10 (28) | 9 (24) | |
| None | 142 (63) | 43 (45) | 45 (79) | 25 (72) | 29 (76) | |
| Language | <.0001 | |||||
| English | 182 (80) | 95 (98) | 51 (89) | 11 (31) | 25 (66) | |
| Spanish | 25 (11) | 0 | 0 | 24 (69) | 1 (3) | |
| Otherc | 20 (9) | 2 (2) | 6 (11) | 0 | 12 (31) | |
| Health insurance | .07 | |||||
| Private | 111 (49) | 56 (58) | 26 (46) | 10 (29) | 19 (50) | |
| Publicd | 114 (50) | 40 (41) | 31 (54) | 25 (71) | 18 (47) | |
| No insurance | 2 (1) | 1 (1) | 0 | 0 | 1 (3) | |
| Annual household income, $ | .0002 | |||||
| <50,000 | 40 (18) | 8 (8) | 16 (28) | 7 (20) | 9 (24) | |
| 50,000-<75,000 | 111 (49) | 41 (42) | 30 (53) | 22 (63) | 18 (49) | |
| >75,000 | 75 (33) | 48 (50) | 11 (19) | 6 (17) | 10 (27) | |
Data are presented as mean ± SD or n (%). Fisher’s exact P values are reported.
Race or ethnic group was reported by the patients. The designation “other” includes Asian, American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, multiple, other, or unknown.
Technology use included patients who used CGM alone, CSII alone, and both CGM and CSII.
Other language includes Portuguese, Haitian Creole, Tigrinya, Albanian, German, Cape Verdean, Ethiopian, Chinese, Amharic.
Public insurance included Medicare and Medicaid.
Abbreviations: HbA1c, hemoglobin A1c; CGM, continuous glucose monitor; CSII, continuous subcutaneous insulin infusion.
Diabetes Technology Use
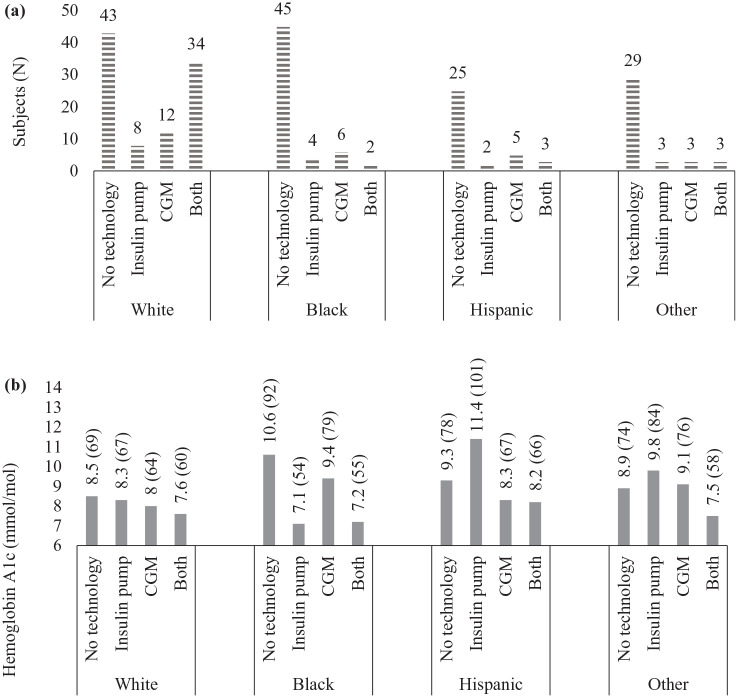
Of the 227 patients in the cohort, 85 patients (37%) used any diabetes technology. CGM was the most common form of diabetes technology used, documented in 68 patients (30%). CSII use was documented in 59 patients (26%). White patients were found to have a higher rate of diabetes technology use as compared with patients of other races or ethnicities (Table 1). Among White patients, 55% used technology compared with 21% of Black, 28% of Hispanic, and 24% of other race/ethnicity patients (P < .0001). Although rates of isolated CGM or CSII use was similar among groups, only 4% of Black patients, 9% of Hispanic, and 8% of those of other race/ethnicity used both CGM and CSII together in comparison to 35% of White patients (Figure 1).
Figure 1.
Frequency of technology use and hemoglobin A1c (HbA1c) by race/ethnicity. (a) Represents the frequency of technology use by race/ethnicity. (b) Represents most recent HbA1c in % (mmol/mol) by technology use and race/ethnicity.
While there were no statistically significant differences in diabetes technology use among patients who had an annual household income less than $50,000 across all racial/ethnic groups (P = .48) (Table 2), rates of technology use were highest in White individuals. Statistically significant racial/ethnic differences in diabetes technology use were observed among patients who had an annual household income between $50,000-$75,000 and ≥ $75,000. Overall, rates of technology use were highest among those with the highest annual income (≥ $75,000/y), however, within this income bracket, rates of technology use were lowest for Black and Hispanic patients.
Table 2.
Technology Use by Race/Ethnicity and Annual Household Income.
| Overall (N = 227) | White (N = 97) | Black (N = 57) | Hispanic (N = 35) | Other (N = 38) | P value | |
|---|---|---|---|---|---|---|
| Annual household income <$50,000 | .48 | |||||
| Any technology usea | 12 (30) | 4 (50) | 4 (25) | 1 (14) | 3 (33) | |
| No technology use | 28 (70) | 4(50) | 12 (75) | 6 (86) | 6 (67) | |
| Annual household income $50,000-<$75,000 | .002 | |||||
| Any technology use | 39 (35) | 22 (54) | 8 (27) | 8 (36) | 1 (5) | |
| No technology use | 72 (65) | 19 (46) | 22 (73) | 14 (64) | 18 (95) | |
| Annual household income ≥$75,000 | .0007 | |||||
| Any technology use | 34 (45) | 28 (58) | 0 | 1 (17) | 5 (50) | |
| No technology use | 41 (55) | 20 (42) | 11 (100) | 5 (83) | 5 (50) |
Data are presented as mean ± SD or N (%). Fisher’s exact P values reported.
Technology use included patients who used CGM alone, insulin pump alone, and both CGM and CSII.
Abbreviations: CGM, continuous glucose monitor; CSII, continuous subcutaneous insulin infusion.
Factors Associated with Diabetes Technology Use
Multivariable logistic regression analysis was done adjusting for age group, race/ethnicity, language, income, and insurance status (Table 3). Compared with White patients, Black (aOR 0.25, 95% CI 0.11-0.56, P = .0007) and other race/ethnicity (aOR 0.30, 95% CI 0.11-0.77, P = .0081) individuals were significantly less likely to use diabetes technology. Although not statistically significant, Hispanic individuals (aOR 0.51, 95% CI 0.14-1.86, P = .31) and those who spoke a language other than English (Spanish aOR 0.57, 95% CI 0.13-2.58, P = .46; other languages aOR 0.40, 95% CI 0.09-1.84, P = .26) were about half as likely to use diabetes technology as White or English-speaking patients. Moreover, patients with public health insurance were about half as likely to use diabetes technology compared with patients with private health insurance (aOR, 0.54; 95% CI, 0.29-0.99, P = .05).
Table 3.
Multivariable Analysis of Sociodemographic Factors for Technology Usea.
| Sociodemographic factors | Adjusted odds ratio (95% CI) | |
|---|---|---|
| Race/ethnicity | White | Reference |
| Black | 0.25 (0.11-0.56) | |
| Hispanic | 0.51 (0.14-1.86) | |
| Other | 0.30 (0.11-0.77) | |
| Age | 18-25 years | Reference |
| 26-49 years | 2.00 (0.83-4.82) | |
| >50 years | 1.17 (0.42-3.25) | |
| Insurance | Private | Reference |
| Public | 0.54 (0.29-0.99) | |
| None | 0.35 (0.01-22.79) | |
| Annual household incomeb | >$75,000 | Reference |
| $50,000-$75,000 | 0.91 (0.47-1.78) | |
| <$50,000 | 1.07 (0.43-2.69) | |
| Languagec | English | Reference |
| Spanish | 0.57 (0.1-2.58) | |
| Other | 0.40 (0.09-1.84) | |
Multivariable analysis was adjusted for race/ethnicity, age, insurance, annual household income, and language. Firth’s bias correction applied.
One patient was missing annual household income data.
One patient was missing language data.
Glycemic Outcomes
Glycemic control varied with race/ethnicity and use of diabetes technology. White patients had the lowest mean HbA1c compared with all others; 8.1% ± 1.5% (65 ± 16 mmol/mol) in White, 10.1% ± 2.2% (87 ± 24 mmol/mol) in Black, 9.2% ± 2% (77 ± 22 mmol/mol) in Hispanic, and 8.9% ± 1.9% (74 ± 21 mmol/mol) in other race/ethnicity (P < .0001, Figure 1). Glycemic control was improved in patients using any form of diabetes technology when compared with those not using technology (8.1% ± 1.6% (65 ± 18 mmol/mol) vs 9.4% ± 2.1% (79 ± 23 mmol/mol), P < .0001). The use of CSII and CGM together was associated with the lowest HbA1c across all racial and ethnic groups (P < .001, Figure 1). Additionally, CGM-only users were found to have lower HbA1c compared with CSII-only users except in Black individuals, where CGM-only users had the highest HbA1c among those using any form of diabetes technology.
Discussion
We found that in an urban, safety-net setting more than half of White patients with T1D used diabetes technology, whereas less than one third of non-White patients were using diabetes technology. Similar to pediatric data,18,19 this study demonstrates racial and ethnic disparities in the use of diabetes technology even in a racially and ethnically diverse adult population with a high proportion of racial/ethnic minorities. After adjusting for age group, health insurance, language, and annual household income, non-White patients were about half as likely to use diabetes technology. This disparity was most profound in Black patients, where the odds of diabetes technology use were 75% lower compared with White patients.
In contrast to prior studies demonstrating an association between higher annual income and greater use of diabetes technology,13,19,25,26 this was not reflected in the Black and Hispanic patients in our study. Notably, at the highest household income levels (≥$75,000/y), Black and Hispanic individuals were significantly less likely than White individuals to use diabetes technology. It is striking that although nearly 20% of Black patients had an annual household income in the highest bracket, none in this income bracket used diabetes technology. Although socioeconomic status may contribute to disparities in treatment methods, additional factors appear to play a significant role and need to be further evaluated.
While 80% of the cohort was English-speaking, less than one third of Hispanic patients spoke English. Despite this difference, once diabetes technology use accounted for race/ethnicity, age, insurance, and annual household income, language was imprecise as a predictor for diabetes technology use. Although spoken language does not appear to be a strong predictor of diabetes technology use in our study, a finding that may be due to small sample size, it has been previously found that language barriers can decrease access to health care, affect adherence to treatment regimens, and create difficulty in communication between patients and healthcare providers.27
As a result of healthcare reform in Massachusetts in 2006, eligibility for access to state subsidized health insurance through Medicaid was expanded to those with a family income below 300% of the federal poverty limit. Subsequently, Massachusetts has a low rate of uninsured individuals with less than 3% of the population uninsured.28 Despite overall high rates of insurance coverage in our cohort, those with public health insurance were about half as likely to use diabetes technology as privately insured individuals with T1D. Although a majority of public health insurance programs in the state include benefits for the use of diabetes technology under the use of durable medical equipment, both public and private insurers have significant requirements regarding self-management behaviors to meet medical necessity.29,30 Self-management behaviors have been found to require significant resources, time, and effort of individuals. These requirements may systematically disadvantage those of lower socioeconomic status and racial and ethnic minority patients who may be restricted by economic and social conditions at home or at work, are more likely to be publicly insured, and receive care at urban safety-net institutions.31 However, data have shown that even in those who do not perform routine self-management behaviors such as self-monitoring of blood glucose, improvements in glycemic control, and increase in self-monitoring behaviors are seen with initiation of diabetes technology.32 To this end, removing restrictive eligibility and insurance coverage criteria may enable increased access to diabetes technology, which can serve as a tool to increase self-monitoring behaviors, engage in self-care, and improve health outcomes.
As the vast majority of patients in our study had adequate health insurance to cover the use of diabetes technology and our findings did not support language spoken or higher socioeconomic status as major predictors of the use of diabetes technology; the patient and provider level factors contributing to these findings, including the roles of patient preference and of implicit bias and structural racism, should be further explored. Patient’s behavior and knowledge, provider behavior, patient–provider interactions, and organizational and institutional factors may contribute to racial and ethnic disparities.33 Importantly, evidence suggests that implicit bias contributes to health care disparities.34,35 Implicit bias influences clinician–patient communication and it is suggested that biases are likely to influence diagnosis and treatment decisions as well as negatively influence perceptions regarding shared clinical decision making.34,36 Institutional eligibility and allocation of diabetes technologies may be impacted by patient interaction, framing of medical technologies to patients, and how clinics structure decision-making processes.37 Given our findings, these processes, including clinician decision making regarding the use of diabetes technology, the role of communication regarding diabetes technology with patients, and the barriers patients face after prescription of diabetes technology should be evaluated.
As expected, individuals using diabetes technology had lower HbA1c than those not using diabetes technology. Consistent with recent literature, CGM use in our population, irrespective of CSII use, was overall associated with improved glycemic control.38 Black individuals had the highest HbA1c across all racial and ethnic groups. While there are some data demonstrating biological differences in glycation of hemoglobin between Black and White individuals, the clinical impact may be minimal and does not explain these results.39 Across all racial and ethnic groups, HbA1c was lowest in those who used both CSII and CGM together. Given extensive self-management requirements for CSII eligibility, it is possible that the improved HbA1c outcomes from patients with CSII and CGM may be due in part to selecting patients who exhibit more rigorous self-management behaviors and are therefore felt to be more likely to successfully use diabetes technology.
Our findings should be interpreted in light of several limitations. This study was completed at a single center and the cohort was small. The small sample size may have limited our ability to find statistically significant findings. For example, small sample size may have accounted for the lack of statistically significantly different rates of diabetes technology use among individuals in the lowest income bracket (<$50,000). We used retrospective data available from the electronic medical records, therefore it is subject to issues of missing data, including in race and ethnicity, as reported by patients and limitations in the ability to accurately capture certain data such as diabetes duration. Similarly, we were unable to collect data on factors that have previously been reported as relevant in the pediatric literature such as education level.40 In addition to small sample size, an important limitation is the inability to determine whether patients had previously used and discontinued or had been offered the use of diabetes technology but had declined this option.
Despite the limitations, this is the first study to our knowledge to examine variation in diabetes technology use in an adult, urban, safety-net population and contributes to filling a gap in knowledge regarding management and outcomes of adult patients with T1D cared for in urban racially/ethnically diverse safety-net institutions. Additionally, this study contributes to the literature demonstrating racial and ethnic disparities in access to diabetes technology in tT1D across age groups.
Conclusion
The odds of Black adults with T1D using diabetes technology were less than half that of White patients in our study, consistent with prior pediatric data. Importantly, and in contrast to prior research, sociodemographic factors such as annual household income and language spoken do not appear to be the major drivers of these disparities and raises the question of implicit bias, structural racism, or other unmeasured factors as primary contributors to observed disparities in diabetes technology use. As emerging technologies become more widely available and demonstrate glycemic benefit and improved quality of life,4 it is imperative to understand disparities in application of diabetes technologies to facilitate their equitable allocation. Further research is needed to understand patient, provider, and organizational barriers and facilitators to use of diabetes technology in patients with T1D in an effort to develop interventions aimed to increase prescription of diabetes technology, improve treatment outcomes, and mitigate healthcare disparities.
Supplemental Material
Supplemental material, sj-pdf-1-dst-10.1177_1932296821995810 for Racial Disparities in Diabetes Technology Use and Outcomes in Type 1 Diabetes in a Safety-Net Hospital by Kathryn L Fantasia, Kamonkiat Wirunsawanya, Christopher Lee and Ivania Rizo in Journal of Diabetes Science and Technology
Acknowledgments
We thank Drs. Devin Steenkamp and Sun Lee for their excellent recommendations and expertise in reviewing our manuscript. Similarly, we thank the Boston University CTSI for their assistance with review of statistical methods, they are supported by the following grant: 1UL1TR001430.
Footnotes
Abbreviations: T1D, Type 1 diabetes mellitus; CSII, continuous subcutaneous insulin infusion; CGM, Continuous glucose monitor; HbA1c, hemoglobin A1c; ANOVA, analysis of variance.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research included in this manuscript was not supported/funded. The authors report the following funding for work unrelated to this manuscript: KLF was supported by the following grant: NIH T32DK007201-42.
ORCID iDs: Kathryn L Fantasia  https://orcid.org/0000-0002-2949-9568
https://orcid.org/0000-0002-2949-9568
Ivania Rizo  https://orcid.org/0000-0002-3933-9461
https://orcid.org/0000-0002-3933-9461
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Bergenstal R, Tamborlane W, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363:311-320. [DOI] [PubMed] [Google Scholar]
- 2.Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections. JAMA. 2017;317:371. [DOI] [PubMed] [Google Scholar]
- 3.Polonsky WH, Hessler D, Ruedy KJ, Beck RW.The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: further findings from the DIAMOND randomized clinical trial. Diabetes Care. 2017;40:736-741. [DOI] [PubMed] [Google Scholar]
- 4.Hommel E, Olsen B, Battelino T, et al. Impact of continuous glucose monitoring on quality of life, treatment satisfaction, and use of medical care resources: analyses from the SWITCH study. Acta Diabetol. 2014;51:845-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections the gold randomized clinical trial. JAMA. 2017;317:379-387. [DOI] [PubMed] [Google Scholar]
- 6.Thabit H, Prabhu JN, Mubita W, et al. Use of factory-calibrated real-time continuous glucose monitoring improves time in target and HbA1c in a multiethnic cohort of adolescents and young adults with type 1 diabetes: the MILLENNIALS study. Diabetes Care. 2020;43:2537-2543. [DOI] [PubMed] [Google Scholar]
- 7.Jendle J, Pöhlmann J, De Portu S, Smith-Palmer J, Roze S.Cost-effectiveness analysis of the MiniMed 670G hybrid closed-loop system versus continuous subcutaneous insulin infusion for treatment of type 1 diabetes. Diabetes Technol Ther. 2019;21:110-118. [DOI] [PubMed] [Google Scholar]
- 8.Roze S, Isitt J, Smith-Palmer J, Javanbakht M, Lynch P. Long-term cost-effectiveness of dexcom G6 real-time continuous glucose monitoring versus selfmonitoring of blood glucose in patients with type 1 diabetes in the U.K. Diabetes Care. 2020;43:2411-2417. [DOI] [PubMed] [Google Scholar]
- 9.Roze S, Saunders R, Brandt AS, de Portu S, Papo NL, Jendle J.Health-economic analysis of real-time continuous glucose monitoring in people with type 1 diabetes. Diabet Med. 2015;32:618-626. [DOI] [PubMed] [Google Scholar]
- 10.Grunberger G, Handelsman Y, Bloomgarden ZT, et al. American association of clinical endocrinologists and American college of endocrinology 2018 position statement on integration of insulin pumps and continuous glucose monitoring in patients with diabetes mellitus. Endocr Pract. 2018;24:302-308. [DOI] [PubMed] [Google Scholar]
- 11.Peters AL, Ahmann AJ, Battelino T, et al. Diabetes technology-continuous subcutaneous insulin infusion therapy and continuous glucose monitoring in adults: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:3922-3937. [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association. Diabetes technology: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S77-S88. [DOI] [PubMed] [Google Scholar]
- 13.Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther. 2019;21:66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lado JJ, Lipman TH.Racial and ethnic disparities in the incidence, treatment, and outcomes of youth with type 1 diabetes. Endocrinol Metab Clin. 2016;45:453-461. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal S, Hilliard M, Butler A, Butler A.Disparities in care delivery and outcomes in young adults with diabetes. Curr Diab Rep. 2018;18:65. [DOI] [PubMed] [Google Scholar]
- 16.Valenzuela JM, Seid M, Waitzfelder B, et al. Prevalence of and disparities in barriers to care experienced by youth with type 1 diabetes. J Pediatr. 2014;164:1369-1375.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willi SM, Miller KM, DiMeglio LA, et al. Racial–ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135:424-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Connor MR, Carlin K, Coker T, Zierler B, Pihoker C.Disparities in insulin pump therapy persist in youth with type 1 diabetes despite rising overall pump use rates. J Pediatr Nurs. 2019;44:16-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin MH, Connor CG, Ruedy KJ, et al. Race, socioeconomic status, and treatment center are associated with insulin pump therapy in youth in the first year following diagnosis of type 1 diabetes. Diabetes Technol Ther. 2013;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canedo JR, Miller ST, Schlundt D, Fadden MK, Sanderson M.Racial/ethnic disparities in diabetes quality of care: the role of healthcare access and socioeconomic status. J Racial Ethn Heal Disparities. 2018;5:7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med. 2017;376:1419-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewin M, Altman S, eds. America’s Health Care Safety Net: Intact but Endangered. Washington, DC: National Academies Press; 2000. [PubMed] [Google Scholar]
- 23.Gaskin DJ, Hadley J.Population characteristics of markets of safety-net and non-safety-net hospitals. J Urban Heal. 1999;76:351-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berkowitz SA, Traore CY, Singer DE, Atlas SJ.Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: Results from a primary care network. Health Serv Res. 2015;50:398-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borschuk AP, Everhart RS.Health disparities among youth with type 1 diabetes: a systematic review of the current literature. Fam Syst Heal. 2015;33:297-313. [DOI] [PubMed] [Google Scholar]
- 26.McKergow E, Parkin L, Barson DJ, Sharples KJ, Wheeler BJ.Demographic and regional disparities in insulin pump utilization in a setting of universal funding: a New Zealand nationwide study. Acta Diabetol. 2017;54:63-71. [DOI] [PubMed] [Google Scholar]
- 27.Nam S, Chesla C, Stotts NA, Kroon L, Janson SL.Barriers to diabetes management: patient and provider factors. Diabetes Res Clin Pract. 2011;93:1-9. [DOI] [PubMed] [Google Scholar]
- 28.Long SK, Skopec L, Shelto A, Nordahl K, Walsh KK.Massachusetts health reform at ten years: great progress, but coverage gaps remain. Health Aff. 2016;35:1633-1637. [DOI] [PubMed] [Google Scholar]
- 29.MassHealth. Guidelines for Medical Necessity Determination for Ambulatory Infusion Pumps (Insulin Pumps); 2011. [Google Scholar]
- 30.Anderson JE, Gavin JR, Kruger DF.Current eligibility requirements for CGM coverage are harmful, costly, and unjustified. Diabetes Technol Ther. 2020;22:169-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinder S, Greenhalgh T.“this does my head in”. Ethnographic study of self-management by people with diabetes. BMC Health Serv Res. 2012;12:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halbron M, Bourron O, Andreelli F, et al. Insulin pump combined with flash glucose monitoring: a therapeutic option to improve glycemic control in severely nonadherent patients with type 1 diabetes. Diabetes Technol Ther. 2019;21:409-412. [DOI] [PubMed] [Google Scholar]
- 33.Tripp-Reimer T, Choi E, Kelley LS, Enslein JC.Cultural barriers to care: inverting the problem. Diabetes Spectr. 2001;14:13-22. [Google Scholar]
- 34.Fitzgerald C, Hurst S.Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics. 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health. 2015;105:e60-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peek ME, Odoms-Young A, Quinn MT, Gorawara-Bhat R, Wilson SC, Chin MH.Race and shared decision-making: perspectives of African-Americans with diabetes. Soc Sci Med. 2010;71:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puckett C, Wong JC, Daley TC, Cossen K.How organizations shape medical technology allocation: insulin pumps and pediatric patients with type 1 diabetes. Soc Sci Med. 2020;249:112825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Šoupal J, Petruželkova L, Grunberger G, et al. Glycemic outcomes in adults with T1D are impacted more by continuous glucose monitoring than by insulin delivery method: 3 years of follow-up from the COMISAIR study. Diabetes Care. 2020;43:37-43. [DOI] [PubMed] [Google Scholar]
- 39.Herman WH, Cohen RM.Racial and ethnic differences in the relationship between HbA1c and blood glucose: implications for the diagnosis of diabetes. J Clin Endocrinol Metab. 2012;97:1067-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blackman S, Raghinaru D, Adi S, et al. Insulin pump use in young children in the T1D exchange clinic registry is associated with lower hemoglobin A1c levels than injection therapy. Pediatr Diabetes. 2014;15:564-572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-dst-10.1177_1932296821995810 for Racial Disparities in Diabetes Technology Use and Outcomes in Type 1 Diabetes in a Safety-Net Hospital by Kathryn L Fantasia, Kamonkiat Wirunsawanya, Christopher Lee and Ivania Rizo in Journal of Diabetes Science and Technology