Abstract
Background:
Existing research shows that hypoglycemia fear (HF) is common in parents of children with established type 1 diabetes (T1D). We examined parental HF in the T1D recent-onset period and evaluated whether continuous glucose monitoring (CGM) adoption relates to improved outcomes of parental HF.
Methods:
In TACKLE-T1D, a prospective study of five- to nine-year olds with recent-onset T1D, parents completed the Hypoglycemia Fear Survey-Parents (HFS-P) at baseline (T1) and 6 (T2) and 12 (T3) months post-baseline. The HFS-P measures worry about hypoglycemia (HFS-Worry score) as well as hypoglycemia avoidance behaviors (HFS-Behavior score). We recorded CGM start dates for youth during the same time period through medical record review.
Results:
Between T1 and T2, 31 youth (32.3%) initiated CGM therapy, and between T2 and T3, an additional 17 youth (17.7%) began using CGM, leaving 48 youth who never initiated CGM therapy (50%) in the recent-onset period. Parents reported moderate HFS-Worry scores at T1 (32.9 ± 11.9), which increased between T1 and T2 (37.6 ± 11.4, P < .001) and plateaued between T2 and T3 (37.7 ± 12.4, P = .89). In contrast, parental HFS-Behavior scores decreased between T1 (33.1 ± 5.8) and T2 (32.2 ± 6.0, P = .005) and plateaued between T2 and T3 (32.2 ± 6.0, P = .95). Baseline HFS-Behavior and Worry scores were associated with increased adoption of CGM between T1-T2 and T2-T3, respectively. Parents of children initiating CGM therapy between T1 and T2 showed the largest decrease in HFS-Behavior (P = .03).
Conclusions:
Initiating CGM therapy within the first 12 months of T1D may help reduce parents’ use of hypoglycemia avoidance behaviors, but has little effect on parents’ hypoglycemia worry.
Keywords: children, continuous glucose monitors, fear of hypoglycemia, type 1 diabetes
Introduction
Parents of children with recent-onset type 1 diabetes (T1D) must learn to manage a complex disease around various activities in a child’s life that affect glycemic management. One common, but unpredictable, acute complication of insulin therapy is hypoglycemia. If left untreated, hypoglycemia can result in seizure, loss of consciousness, or death. Due to the potential risks associated with hypoglycemia, many individuals with T1D and their caregivers develop significant anxiety related to hypoglycemia, referred to as fear of hypoglycemia (HF).1-4 Moderate parental HF occurs at a high rate among parents of children and adolescents with T1D and directly relates to children’s blood glucose levels,5-7 and hemoglobin A1c (HbA1c).8 These associations may be due to parents modifying their T1D management behaviors in response to HF such that they allow their child’s blood glucose levels to run above their age-based glycemic target range. In this way, parental HF can be a significant barrier in a child’s diabetes management suggesting a need for solutions to alleviate parents’ fears.
Advances in diabetes technologies have made continuous glucose monitors (CGM) more accessible to people with T1D and their caregivers. The safety and effectiveness of these devices are well studied,9,10 and national and international clinical care guidelines recommend the use of these devices in pediatric populations.11 No published literature has yet examined the relationship between parental HF and the adoption of CGM technology among children with recent-onset T1D. Therefore, our objectives were to examine parental HF levels and their rate of CGM adoption over the recent-onset period and to investigate if the timing of CGM adoption affects parental HF. We hypothesized that higher parental HF at baseline would relate to higher rates of CGM adoption within the recent-onset period of T1D and that after CGM adoption, parental HF would be significantly lower among parents of families who adopted CGM vs parents of non-adopters, specifically in both the Behavior and Worry subscales.
Methods
Participants and Procedure
We recruited children aged five to nine years and their parents from two pediatric diabetes clinics in the United States to participate in a three-year longitudinal study examining psychosocial factors that may contribute to glycemic control in youth during the recent-onset period of T1D. More detailed methodology has been discussed elsewhere.12-14 In general, parents completed study questionnaires every six months. In this report, we utilized data collected at baseline (T1), 6 months post-baseline (T2), and 12 months post-baseline (T3). On average, we enrolled children at about 5 months post-diagnosis, thus T2 reflects families’ status at approximately 12 months post-diagnosis and T3 reflects families’ status at about 18 months post-T1D diagnosis. Parents received $50 at each time point to compensate them for completing study measures. CGM start dates from T1 through T3 were obtained from the child’s electronic medical record data. Youth who initiated CGM prior to study enrollment were excluded from these analyses.
Measures
Demographics
Parent’s reported all demographic information at T1 including child age, child sex, child race/ethnicity, duration of T1D, caregiver relation to child, caregiver age, caregiver marital status, and family income.
Hypoglycemia Fear Survey-Parents
Parents completed the Hypoglycemia Fear Survey-Parents (HFS-P), a validated 25-item survey.15 Parents answered items on a five-point Likert scale (1 = Never, 2 = Almost Never, 3 = Sometimes, 4 = Almost Always, and 5 = Always); higher scores suggest greater HF. The HFS-P has two subscales: HFS-Worry and HFS-Behavior. The HFS-Worry subscale includes 15 items where higher worry scores suggest parents experience increased worry about their child having a low blood glucose event. The HFS-Behavior subscale includes 10 items where higher behavior scores indicate parents engage in treatment behaviors to keep their child’s glucose level above the target range. In this study, we used parents HFS-Worry and HFS-Behavior scores. To interpret HFS scores, we used previously published literature for comparison.3,4
HbA1c
We collected children’s HbA1c by finger-stick blood sample and capillary tube collection during study visits at T1, T2, and T3. All study samples were analyzed at a central laboratory using automated high-performance liquid chromatography (reference range 4.0%-6.0%, Tosoh 2.2, Tosoh Corporation, San Francisco, CA), which has been described previously.12-14 We report these levels to help characterize our sample.
Analyses
Descriptive statistics were used to examine our sample characteristics, the main outcome variables, and study hypotheses. We used two-tailed correlations to examine if baseline HFS-Worry or HFS-Behavior scores related to CGM adoption. We used a series of independent samples t-tests to examine differences in HFS-Worry and HFS-Behavior scores for parents of children who started CGM vs parents of children who did not start CGM.
This study was approved by the Colorado Multiple Institutional Review Board and the Pediatric Institutional Review Board at The Children’s Mercy Hospital & Clinics. Investigators obtained written informed consent prior to all study procedures.
Results
One hundred and twenty-eight parent-child dyads enrolled in the longitudinal study. Of these, 96 had data regarding CGM use through the first 12 months of follow-up and completed the HFS-P at T3, resulting in our final sample (75% included in the present analysis). Children were an average of 7.5 ± 1.3 years old and 4.7 ± 3.3 months post-diagnosis at baseline (T1). Average HbA1c at T1 was 7.6 ± 1.4% (60 ± 15 mmol/mol), 8.1 ± 1.2% (65 ± 13 mmol/mol) at T2, and 8.3 ± 1.2% (67 ± 13 mmol/mol) at T3. About half of children were female and most were non-Hispanic White. Mothers made up the majority of the parent respondent group. There were no significant differences in child age, sex, or race on parental HFS scores or CGM adoption (Table 1).
Table 1.
Demographic and Clinical Characteristics of Child-Parent Dyads Enrolled in TACKLE-T1D.
| Minimum | Maximum | Mean ± SD | |
|---|---|---|---|
| Child age, years | 5 | 9 | 7.5 ± 1.4 |
| Age at diagnosis, years | 4 | 9 | 7.0 ± 1.4 |
| Diabetes duration (months) | 0 | 12 | 4.7 ± 3.3 |
| Parent age, years | 24 | 54 | 36.8 ± 6.4 |
| n (%) | |||
| Child gender (% F) | 60 (52.2%) | ||
| Demographics (% NHW) | 98 (85.2%) | ||
| Caregiver type (% mothers) | 101 (87.8%) | ||
| Families with other T1D children | 17 (14.8%) | ||
Abbreviations: SD, standard deviation; T1D, type 1 diabetes.
Fear of Hypoglycemia in Parents
Average total HFS-P score at T1 was 66.0 ± 13.7, which indicates a moderate level of fear. Between T1 and T2, parents’ HFS-P total scores increased significantly (69.8 ± 14.5, P = .005) and then plateaued between T2 and T3 (69.8 ± 15.3, P = .95). At T1, the mean HFS-Behavior score was 33.1 ± 5.8. Between T1 and T2, parents reported a significant decrease in HFS-Behavior scores (ΔT1–T2 = −0.9, P = .05). However, there was no difference between parents’ HFS-Behavior scores between T2 and T3 (ΔT2–T3 = 0.1, P = .20). In contrast, parents’ average HFS-Worry score was 32.9 ± 11.7 at T1 and increased significantly from T1 to T2 (ΔT1–T2= 5.1, P ≤ .001). After T2, we observed no change in parents’ HFS-Worry scores (ΔT2–T3 = −0.1, P = .72).
CGM Use and Parent HFS
Between T1 and T2, 31 (32.3%) youth began CGM therapy. Between T2 and T3, an additional 17 (17.7%) children began CGM therapy. Table 2 reports parents’ HFS scores across time points for parents of children starting CGM therapy vs parents of children not starting CGM therapy. We saw a significant association between CGM starts between baseline (T1) and T2 and parents’ T1 HFS-Behavior scores (r = 0.25, P = .01). There was no association between parents’ T1 HFS-Behavior scores and CGM starts between T2 and T3. We saw no association between parents’ T1 HFS-Worry scores and CGM starts between T1 and T2, but there was a significant association with CGM starts between T2 and T3 and parents’ T1 HFS-Worry scores (r = 0.22, P < .04).
Table 2.
Longitudinal Measures of Parental Fear of Hypoglycemia and Relation to Adoption of Diabetes Technology (mean ± SD).
| CGM T1-T2 (n = 31) | CGM T2-T3 (n = 17) | No CGM (n = 48) | |
|---|---|---|---|
| HF Scores T1 | |||
| Total | 66.4 ± 12.8 | 72.1 ± 15.8 | 62.8 ± 13.4 |
| Behavior | 34.3 ± 5.6 | 33.5 ± 6.0 | 31.4 ± 5.6 |
| Worry | 32.2 ± 10.9 | 38.5 ± 13.2 | 31.4 ± 12.1 |
| HF Scores T2 | |||
| Total | 68.7 ± 14.6 | 77.8 ± 15.6 | 67.7 ± 13.5 |
| Behavior | 32.0 ± 5.9 | 33.6 ± 6.2 | 31.3 ± 6.0 |
| Worry | 36.7 ± 11.1 | 44.2 ± 13.0 | 36.5 ± 10.9 |
| HF Scores T3 | |||
| Total | 70.4 ± 15.2 | 74.2 ± 15.7 | 67.1 ± 15.3 |
| Behavior | 33.0 ± 6.3 | 32.0 ± 5.7 | 31.0 ± 5.9 |
| Worry | 37.5 ± 11.9 | 42.2 ± 12.1 | 36.1 ± 13.0 |
Abbreviations: CGM, continuous glucose monitoring; HF, fear of hypoglycemia; SD, standard deviation.
Parents’ T1 HFS-Behavior score was significantly higher among parents of children starting CGM at some point between T1 and T2 compared to parents of children who did not start CGM (P = .02). Moreover, after controlling for T1D duration, parents of children starting a CGM between T1 and T2 reported a significantly greater decline in their HFS-Behavior scores between T1 and T2 than parents of children who did not adopt CGM (P = .03) (Figure 1). Compared to parents of children who did not start CGM, those starting CGM between T2 and T3 had a higher T1 HFS-Worry score (P = .04). We did not see a difference in T1 HFS-Worry scores for parents of children who started CGM between T1 and T2 vs parents of children who did not start CGM during this period.
Figure 1.
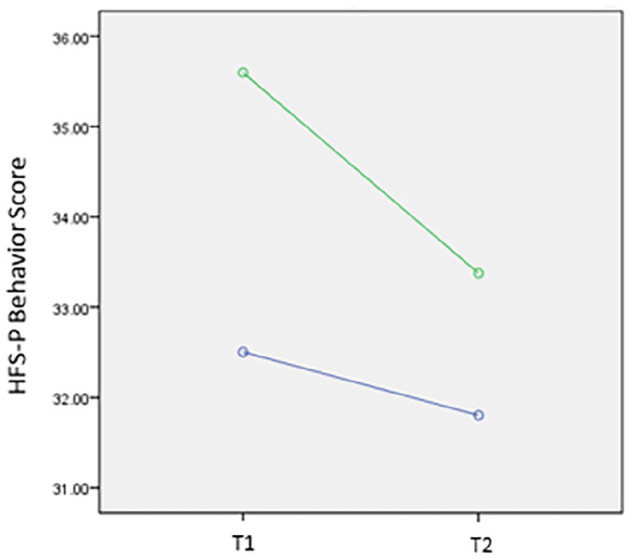
Change in HFS-P Behavior score for parents whose child started on CGM technology between baseline (T1) and six months (T2) compared to those who did not start on a CGM adjusted for diabetes duration. Green line shows change in HFS-P Behavior score for parents whose child started a CGM between T1 and T2. The blue line shows change in HFS-Behavior in parents whose child did not start CGM. CGM, continuous glucose monitoring; HFS-P, Hypoglycemia Fear Survey-Parents.
Discussion
This study is the first reported in the literature to use a prospective longitudinal design to report on parents’ HF in the recent-onset period. Specifically, this design enabled us to see that parents’ HFS-Worry scores started out at moderate levels, increased slightly between baseline (T1) and T2, and then plateaued and remained high-moderate between T2 and T3. In contrast, we saw parents’ HFS-Behavior scores decrease slightly between T1 and T2 and then remain at a consistent level between T2 and T3. Baseline HFS-Behavior and Worry scores were associated with increased CGM adoption between T1-T2 and T2-T3, respectively. Further, compared to those not using CGM, those who adopt CGM technology within the first 18 months post-diagnosis have greater decrease in HFS-Behavior score.
Youth with recent-onset T1D are vulnerable to extreme glycemic variability as families learn T1D self-care and learn to balance exogenous insulin injections with their child’s carbohydrate intake and residual endogenous insulin release.16 The combination of exogenous insulin injections and endogenous insulin release may increase the likelihood that a child will experience a hypoglycemic event. Families initially may take extra measures to protect their child from a hypoglycemia event, such as feeding their child large snacks or over-treating blood glucose levels. It is possible our data captured families’ potential over-reliance on hypoglycemic avoidance behaviors early in T1D as evidence by higher HFS-Behavior scores at T1 than at later time points. Moreover, we would anticipate that parents and children would gain more knowledge and experience in managing T1D, which may translate into less reliance on hypoglycemic avoidance behaviors and parents’ slightly lower HFS-Behavior scores at T2 and T3. In contrast, we believe parents’ HFS-Worry scores increased between T1 and T2 and then remained moderately high between T2 and T3 related to their increased time and experience managing T1D. From the literature, we know that many parents of similarly aged youth with established T1D report moderately high HFS-Worry scores.4,8,17,18 In this way, it is possible our data provide a glimpse at how parental HFS-Worry levels may start out in youth with recent-onset T1D and evolve as children and parents move out of the recent-onset period.
We were keenly interested to see how CGM adoption in these families of youth with recent-onset T1D may relate to parents’ HF and if CGM adoption might relate to lower parental HF. First, our data provide a snapshot of the degree of which our sample started to engage with CGM early after onset of T1D. There was a relatively large number of families who were quick to adopt CGM in the first year of their child’s diagnosis, followed by a smaller number of families who adopted CGM between approximately 12 and 18 months post-diagnosis; though, one-third of families never started CGM during this analysis period and we excluded families who adopted CGM prior to baseline. However, the correlations we observed between parents’ T1 HFS-Behavior scores and the number of CGM starts between T1 and T2 as well as the association between parents’ T1 HFS-Worry scores and the number of CGM starts between T2 and T3 suggest that parents’ HF may be one factor that relates to whether families start CGM in the recent-onset period. In follow-up comparisons we found that T1 HFS-Total scores were significantly lower for parents of children who never started CGM vs parents of children who started CGM between T1 and T2 and between T2 and T3, potentially reinforcing the possibility that some parents may adopt CGM because they are experiencing higher levels of HF in the recent-onset period. Our results showed that while starting CGM may relate to a decline in HFS-Behavior scores, but it may not impact parents’ HFS-Worry scores. There is an emotional quality to anxiety which may not be managed just by starting a new device. Parents may require support and counseling to ultimately reduce their HF. It is notable that existing research shows that starting CGM soon after diagnosis of T1D may help children to achieve better HbA1c in this early period.12 It is possible that kids using CGM achieve lower HbA1c in the recent-onset period because their parents may be relying less on hypoglycemia avoidance behaviors that lead to high child blood glucose levels.
This study is the first that evaluates parental HF and its relationship to the timing of the adoption of technology. We assert that a notable strength of the study is its prospective longitudinal design, which enabled us to track parent HF levels across the recent-onset period and to see how they may relate to the timing of CGM initiation. Yet, our study’s sample population is a limitation. We only had 48 children start a CGM at any time between T1 and T3. It is beyond the scope of our study to identify if families did not start CGM because of distrust/disinterest in the device, lack of awareness, insurance problems, or clinic/physician practice variations. It is possible that our study is underpowered to see differences in parent HF, particularly for parents of children who started CGM between T2 and T3 when only 17 children began CGM therapy. Additionally, our sample was predominately made up of non-Hispanic White children and their parents as well as families of higher income levels. Although these demographics are representative of the populations at both of the participating pediatric academic centers, future research should evaluate parental HF and CGM adoption across children and families from a variety of racial/ethnic and income backgrounds.
Conclusions
Overall, this study found that starting technology 6 to 12 months following diagnosis of T1D related to reduced parental HF. Randomized prospective studies should examine timing of CGM initiation following diagnosis of T1D on parental HF. Additional research should define cutoff scores for elevated parental HF to determine scores in which parents may benefit from the adoption of technology.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors declare that there is no conflict of interest related to the present work. MAC has received financial compensation from Glooko for employment and from Eli Lilly and Medtronic for consulting.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (R01DK100779).
ORCID iD: Erin M. Youngkin  https://orcid.org/0000-0002-1775-5106
https://orcid.org/0000-0002-1775-5106
References
- 1.Gonder-Frederick L, Nyer M, Shepard JA, Vajda K, Clarke W. Assessing fear of hypoglycemia in children with type 1 diabetes and their parents. Diabetes Manag Lond Engl. 2011;1(6):627-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Driscoll KA, Raymond J, Naranjo D, Patton SR. Fear of hypoglycemia in children and adolescents and their parents with type 1 diabetes. Curr Diab Rep. 2016;16(8):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patton SR, Noser AE, Clements MA, Dolan LM, Powers SW. Reexamining the hypoglycemia fear survey for parents of young children in a sample of children using insulin pumps. Diabetes Technol Ther. 2017;19(2):103-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patton SR, Dolan LM, Henry R, Powers SW. Fear of hypoglycemia in parents of young children with type 1 diabetes mellitus. J Clin Psychol Med Settings. 2008;15(3):252-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrero DG, Guare JC, Vandagriff JL, Fineberg NS. Fear of hypoglycemia in the parents of children and adolescents with diabetes: maladaptive or healthy response? Diabetes Educ. 1997;23(3):281-286. [DOI] [PubMed] [Google Scholar]
- 6.Shepard JA, Vajda K, Nyer M, Clarke W, Gonder-Frederick L. Understanding the construct of fear of hypoglycemia in pediatric type 1 diabetes. J Pediatr Psychol. 2014;39(10):1115-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freckleton E, Sharpe L, Mullan B. The relationship between maternal fear of hypoglycaemia and adherence in children with type-1 diabetes. Int J Behav Med. 2014;21(5):804-810. [DOI] [PubMed] [Google Scholar]
- 8.Haugstvedt A, Wentzel-Larsen T, Graue M, Søvik O, Rokne B. Fear of hypoglycaemia in mothers and fathers of children with Type 1 diabetes is associated with poor glycaemic control and parental emotional distress: a population-based study. Diabet Med. 2010;27(1):72-78. [DOI] [PubMed] [Google Scholar]
- 9.Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316(13):1407-1408. [DOI] [PubMed] [Google Scholar]
- 10.Plotnick LP, Clark LM, Brancati FL, Erlinger T. Safety and effectiveness of insulin pump therapy in children and adolescents with type 1 diabetes. Diabetes Care. 2003;26(4):1142-1146. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association. Introduction: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S1-S2. [DOI] [PubMed] [Google Scholar]
- 12.Patton SR, Noser AE, Youngkin EM, Majidi S, Clements MA. Early initiation of diabetes devices relates to improved glycemic control in children with recent-onset type 1 diabetes mellitus. Diabetes Technol Ther. 2019;21(7):379-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noser AE, Majidi S, Finch J, Clements MA, Youngkin EM, Patton SR. Authoritarian parenting style predicts poorer glycemic control in children with new-onset type 1 diabetes. Pediatr Diabetes. 2018;19(7):1315-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noser AE, Dai H, Marker AM, et al. Parental depression and diabetes-specific distress after the onset of type 1 diabetes in children. Health Psychol. 2019;38(2):103-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care. 1987;10(5):617-621. [DOI] [PubMed] [Google Scholar]
- 16.Abdul-Rasoul M, Habib H, Al-Khouly M. ‘The honeymoon phase’ in children with type 1 diabetes mellitus: frequency, duration, and influential factors. Pediatr Diabetes. 2006;7(2):101-107. [DOI] [PubMed] [Google Scholar]
- 17.Patton SR, Dolan LM, Henry R, Powers SW. Parental fear of hypoglycemia: young children treated with continuous subcutaneous insulin infusion. Pediatr Diabetes. 2007;8(6):362-368. [DOI] [PubMed] [Google Scholar]
- 18.Clarke WL, Gonder-Frederick LA, Snyder AL, Cox DJ. Maternal fear of hypoglycemia in their children with insulin dependent diabetes mellitus. J Pediatr Endocrinol Metab. 2011;11(suppl 1):189-194. [DOI] [PubMed] [Google Scholar]


