Abstract
Introduction:
Lipohypertrophy (LH) is caused by repetitively injecting insulin into the same location. This can lead to unpredictable insulin absorption and increased glucose variability (GV). A new medical device, ROTO Track, automatically guides the user to rotate abdominal insulin injections to avoid LH lesions. This study aimed to test whether the medical device could reduce the number of insulin injections in the same subcutaneous area as compared with non-aided standard insulin injection techniques.
Methods:
In this proof-of-concept cross-over study, baseline data about injection site in the abdominal region were collected blinded for 1 week with a nonguiding version of the device and compared to 1 and 12 weeks of device guidance in 35 people with type 1 diabetes. The device registered time and location of abdominal injections. The primary endpoint was a “rotation score.” Secondary endpoints included number and size of LH, GV, and hemoglobin A1c.
Results:
The rotation score improved significantly from a baseline mean of 40.2% to 49.9% after 1 week (confidence interval: 2.2-17.2%, P = .012) and improved further after 12 weeks to 52.2% (P < .001). After 12 weeks, LH was reduced both in median size from 9.2 (range: [0.9-29.4]) cm2 to 5.4 (range: [0.0- 26.8]) cm2 (P = .041) and mean count from 1.4 (range: [1-2]) to 1.1 (range: [0-2], P = .039) and the coefficient of variation of interstitial glucose was reduced from 38.6 to 35.1 (P = .009).
Conclusion:
This proof-of-concept study indicates that the device improves rotation of insulin injections, and reduces LH and GV.
Keywords: insulin injection technique, lipohypertrophy, proof of concept, type 1 diabetes
Introduction
Lipohypertrophy (LH), a common side effect of insulin therapy, is caused by the growth-stimulating properties of insulin injected repeatedly at the same location on the body.1 When insulin is injected into LH lesions, the absorption of insulin may be delayed and unpredictable. This can lead to unexpected hyperglycemia and hypoglycemia, and increased glucose variability (GV),1,2 which has been linked to the development of diabetes complications.3 The prevalence of LH is reported with wide variation and methods. Gentile et al reviewed LH prevalence to range from 3.6% to 64.0% in people with type 1 diabetes mellitus (T1DM).4 In a larger study, 13 289 patients from 42 countries answered an injection technique questionnaire and were examined by a nurse by visual inspection and palpation of injection sites. The prevalence of LH was reported to be around 30% both by patients and their healthcare providers.5
In most diabetes clinics people with T1DM are trained in insulin injection techniques at onset of diabetes. This training includes techniques to ensure satisfactory rotation of the injections, for example, manual registration of injection sites. Observational data have shown a significantly better glycemic control between patients who rotate injection sites correctly and those who do not.1 Grassi et al showed that through three months of injection technique training, hemoglobin A1c (HbA1c) improved by an average of 0.58% (=6.3 mmol/mol) in 259 patients.2 Another interventional study demonstrated that similar effects can be achieved by intensive nurse training in injection techniques followed by patient education and that the improved glycemic control was accompanied by lower insulin doses and healthcare cost savings.6
The high prevalence of LH, despite education in insulin injection techniques, indicates that real-life rotation of insulin injections is suboptimal for many patients. This may hamper patients’ other efforts to reach their glycemic goals. Thus, to improve injection techniques, we developed and tested a medical device that can automatically guide the person to rotate the abdominal insulin injections. The hypothesis is that the device can reduce the number of insulin injections in the same subcutaneous area as compared with non-aided standard insulin injection techniques in people with T1DM. This may potentially reduce LH and its consequences.
Methods
Design
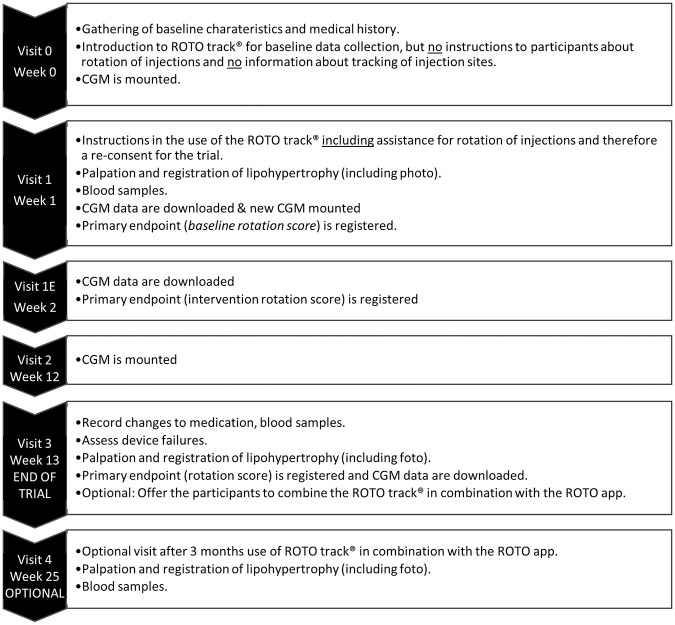
This is a 12-week cross-over single-center trial testing a new medical device that clips on to an insulin pen and assists with insulin injection rotation in the abdominal region. After a 1-week baseline data collection period, without intervention from the clip-on device and without the participants knowing of data collection, all participants were instructed in the use of the ROTO track (ROTO Health, Copenhagen, Denmark), to guide rotation of mealtime insulin injections on the abdomen for the next 12 weeks. After the end of 12 weeks, participants were offered to continue using the clip-on device together with an app for additional 12 weeks. The visit plan is illustrated in Figure 1. The study was approved by the Danish National Committee on Health Research Ethics with Journal No.: H-17041921, and by The Danish Data Monitoring Board with Journal No.: VD-2018-202. The trial was registered at ClinicalTrials.gov with identifier NCT03407677.
Figure 1.
Visit schedule.
CGM, continuous glucose monitoring.
Population
Participants were identified in the diabetes outpatient clinic at Nordsjællands Hospital, Steno Diabetes Center Copenhagen, and other diabetes clinics at Zeeland, Denmark, through web-based advertisements (ie, Facebook) and a patient recruitment agency. Inclusion criteria included written informed consent, age >18 years, T1DM with a duration of 2 or more years, treatment with 3 or more daily injections of insulin aspart in a Flexpen (Novo Nordisk, Bagsværd, Denmark) in the abdominal region, and willingness to comply with trial protocol. Exclusion criteria included severely impaired eyesight and history of alcohol or drug abuse. Baseline characteristics can be seen in Table 1.
Table 1.
Descriptive Statistics of 35 Participants with Type 1 Diabetes Taking Part in the Study.
| Participants included at visit 1
(baseline) N = 35 |
|
|---|---|
| Male sex (count) | 21 (60%) |
| Age (years) | 55.4 (±18.0) |
| Duration of diabetes (years) | 21.2 (±14.8) |
| Height (m) | 1.75 (±0.07) |
| Weight (kg) | 83.0 (±15.3) |
| BMI (kg/m2) | 27.0 (±5.0) |
| Systolic blood pressure (mmHg) | 133 (±17) |
| Diastolic blood pressure (mmHg) | 76 (±9) |
| Insulin injection habits | |
| Self-reported LH (%, abdomen/thighs) |
(20.0/14.3) |
| Clinically observed LH (%, abdomen/thighs) |
(31.4/8.6) |
| Self-reported injects in LH (%) | 8.6 |
| Self-reported injection site rotation (%) | 97.1 |
| Rotation scheme used (%, left-right/two spots/1 cm between/pattern) |
(68.6/48.6/0.0/34.3) |
| Received injection-site rotation instruction (%) | 85.7 |
| Needle length used (%, 12 mm/8 mm/6 mm/5 mm/4 mm) |
(2.9/5.7/40.0/54.3/5.7) |
| Reuses needles (%) | 48.6 |
| Injection zones used, fast-acting insulin (%, abdomen, thighs, hips, arms) |
(100/8.6/2.9/2.9) |
| Injection zones used, long-acting insulin (%, abdomen, thighs, hips, arms) |
(20.0/71.4/17.1/0.0) |
| Late diabetic complications | |
| Normoalbuminuria (%) | 94.3 |
| Microalbuminuria (%) | 5.7 |
| Macroalbuminuria (%) | 0.0 |
| Retinopathy, none (%) | 54.3 |
| Retinopathy, mild (%) | 40.0 |
| Retinopathy, proliferative (%) | 5.7 |
| Autonomic neuropathy (%) | 5.7 |
| Peripheral neuropathy (%) | 28.6 |
| Diabetic foot ulcer (%) | 2.9 |
| History of severe hypoglycemia at any time before inclusion (%) | 17.1 |
| Symptomatic hypoglycemia events, one-month pretrial (count) | 6 (0-30) |
| Severe hypoglycemia events, one-year pretrial (count) | 0.4 (0-8) |
| History of diabetic ketoacidosis (%) | 29 |
| Diabetic ketoacidosis, one-year pretrial (count) | 0.0 (±0.0) |
Continuous variables are reported as means (±standard deviation).
BMI, body mass index; LH, lipohypertrophy.
The Clip-on Device
The clip-on device is an electronic injection log tracking injection sites in the abdominal region. The clip-on device attaches directly to the disposable insulin pen and activates whenever the insulin pen is picked up. Small LED lights on the device indicate where the next injection site is recommended according to the individual patient’s injection plan. By moving the pen and device to an “anchor point” in front of the navel, the device starts tracking where the insulin pen is positioned. The device contains a vibration motor to indicate when the device is in the correct area in the injection plan. The device can be individually programmed to avoid areas in a patient’s abdomen where insulin injections should be avoided, such as LH infiltrates, scar tissue, or a stoma. The device registers the location and the time automatically when the patient injects insulin. The registered data can be downloaded using a wireless connection to an app or a specialized laboratory program, where patients and healthcare professionals can see a visual presentation of the injection patterns as well as insulin injection diaries. For the study, all insulin injections were registered in a paper diary.
Primary Endpoint
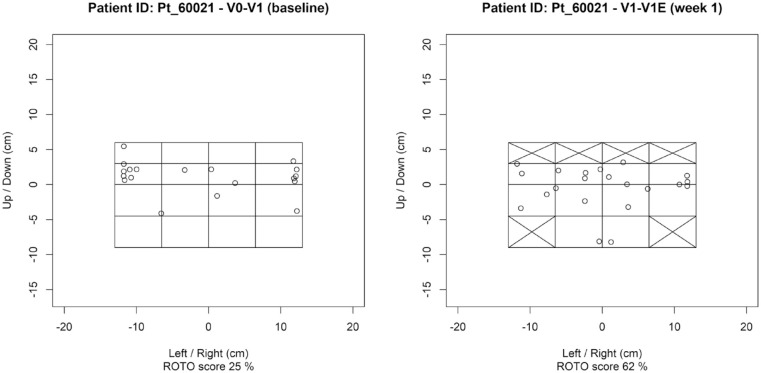
The primary endpoint was change in injection site rotation from the baseline period (V0-V1) to the first week of intervention (V1-V1E) measured as a rotation score (Supplemental Figure 1). The rotation score was calculated as follows: The abdominal skin area was divided into a 4 × 4 grid (16 fields, Figure 2) and we assumed that the clip-on device would be able to “identify” these fields and guide insulin injections to one of the specific fields based on a preset rotation pattern, thereby improving the rotations between fields. If a participant had LH infiltrates or scar tissue in an area, the implicated fields were removed from the participant’s injection plan. The rotation score is a measure of rotation of injection sites based on both the order of injections and the fields of skin area used. A rotation score of 100% implies “perfect rotation,” such that each defined area of skin was used for exactly one injection, before reusing the first available fields again given by the injection plan. A rotation score of 0% implies no rotation, that is, every injection was done in the same area of skin. The rotation score is adjusted such that a 75% rotation score implies that approximately 75% of the available fields were used, but if not used in the order given by the injection plan, the score will be lower than 75%.
Figure 2.
Injection-site plots of baseline and first week of intervention for a participant illustrating injection patterns on the abdominal grid.
Secondary Endpoints
To assess the validity of the rotation score, as a measure of injection-site rotation, we examined the percentage of available injection fields used, as well as the median number of injections taken before reusing an injection field. The percentage of available injection fields used in the abdominal injection zone is an indication of the total area used to inject insulin. Each injection field is counted as used if one or more injections were registered in the field—this means that the metric is best suited to compare periods of equal length.
Other secondary endpoints were the above-mentioned rotation metrics from baseline (V0-V1) to week 12 (V1-V3) and delta HbA1c from V0 to V3 and various glycemic indices collected by continuous glucose monitoring (CGM): Glycemic variability as expressed by the coefficient of variation (CV), mean blood glucose (MBG), and time in range (TIR), time below range (TBR), and time above range (TAR). These were calculated as the fraction of time in the following ranges: TIR: 70-180 mg/dL, TBR level 1: 54-69 mg/dL, TBR level 2: ≥53 mg/dL, TAR level 1: 181- 250 mg/dL, TAR level 2: ≥251 mg/dL.7
Visit Schedule and Data Collection
All participants were seen at V0-V3 as illustrated in Figure 1, and optional at V4. Results from the optional participation with data from V3-V4 are publicly available in Ref.8 and in Supplemental Table 1. The following data were collected throughout the visits:
Lipohypertrophic areas were captured through palpation of the skin and documented by photos of drawings on a wound care grid (Comfeel 3052 from Coloplast, Denmark). The infiltrates were counted and the area of affected subcutaneous tissue was measured by ImageJ from NIH (MD, USA). Daily insulin dose requirements and hypoglycemic events with or without symptoms (defined as a blood glucose at 70 mg/dL or below) were recorded in patient diaries. Insulin treatment satisfaction, injection technique, and quality of life were addressed by validated questionnaires (ITSQ9 and EQ-5D10) at V0 and V3. The changes in insulin treatment satisfaction over the 12-week intervention were measured on a seven-point Likert scale. The usability of the clip-on device was addressed in a questionnaire at V3, using a five-point Likert scale. HbA1c measurements were done at the same laboratory at Nordsjællands Hospital for all participants and measured using standard methods. CGM data were collected with Ipro (Medtronic, Minneapolis, MN, USA) during 3 × 6 days of observation (V0-V1, V1-V1E, and V2-V3). If the sensor failed to collect at least three full days of data, the sample was discarded.
Statistical Analyses
All statistical analyses were performed using R (2019): A language and environment for statistical computing. A Jupyter Notebook containing all data preprocessing and statistical analyses is made available online.8 A two-tailed P-value of ≤.05 was considered statistically significant. Where the population sigma was known or could reasonably be estimated, a paired z-test was used; otherwise a paired t-test was used to test the statistical significance of the observed data.
For sample size assessment, the first 10 participants to complete V0-V1E was used as a pilot to validate the initial assumptions about the required sample size. The mean rotation score in the pilot period was 38.6% with a standard deviation (SD) of 21.2%. From the pilot SD a required sample size of 34 participants was determined to be necessary to achieve a power of 0.8 to detect a difference of 15% in rotation score.
Results
Population
In total, 35 persons with T1DM were included. 60% were male, the mean age was 55 years, and mean diabetes debut was 21 years. Of the initially included population, 20% reported having LH in the abdominal injection zone, but after the initial clinical examination 31% of the population was observed to have LH. Ninety-seven percent reported rotation of their injection sites and 34% described their injection rotation as forming a pattern. The majority reported alternating between the left and the right side (69%) or just a few spots (49%). After week 1, 4 participants were lost to follow-up, and thus 31 participants were evaluated for all endpoints after week 1. For the primary endpoint all 35 was evaluated.
Data Completeness
In some cases, the clip-on device did not collect location information, either due to excessive background movement (eg, by using the clip-on device in a car, plane, or on a boat) or due to incorrect handling of the clip-on device. After 13 weeks of use, the clip-on device collected more location information, which could indicate a learning curve for the participants. During the baseline period (V0-V1), this affected 15.1% (±14.4% points) of the injections. During the intervention (V1-V3), it affected 9.7% (±8.3% points) of the injections. The injections without location information were excluded before calculating injection-site rotation metrics but were included when calculating the accuracy of the clip-on device time log. The median time not using the clip-on device during the 12 weeks of intervention, as measured by whole days without device log entries, was 1 day (range [1-14 days]). The most frequent barrier for not using the device was a need for standing up to provide the full abdominal area for injection of insulin (participants were asked to take their insulin standing). This was not always possible for participants due to travelling, and so on.
CGM data were excluded for six participants for the V0-V1 vs V1-V1E comparison and six participants for the V0-V1 vs V2-V3 comparison, because the CGM failed to record at least three full days of data for either or both two periods.
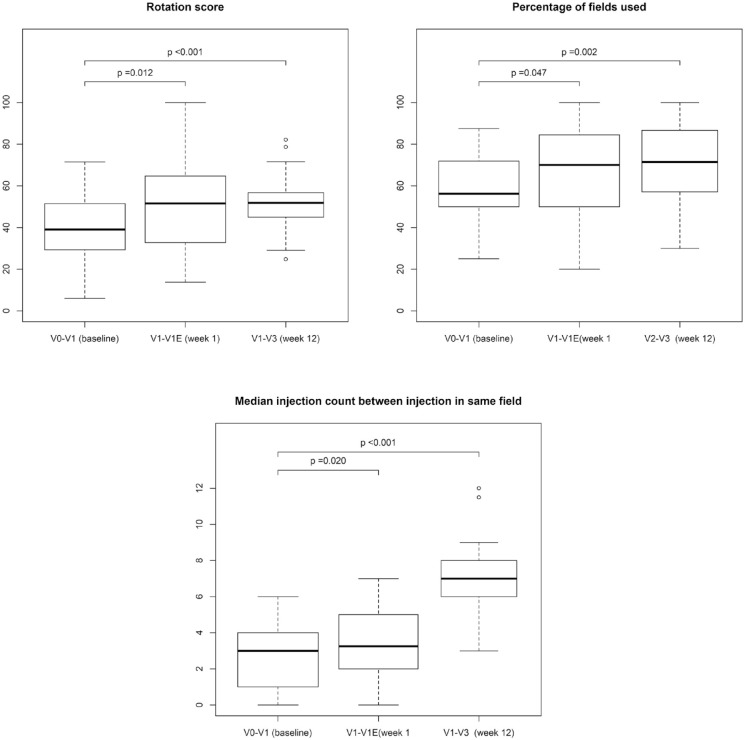
Rotation Score
Figure 3 shows the 3 injection-site rotation metrics at baseline, after first week of the intervention and the entire 12 weeks of the intervention along with the P-values for the primary and secondary endpoints. The mean rotation score was significantly higher after using the clip-on device for 1 week (+9.7% points, confidence interval [CI]: 2.2%-17.2%, P = .012), see Table 2, and this improvement was sustained during the entire 12 weeks (+12.6% points, CI: 5.2%-19.9%, P < .001). The lowest observed rotation at baseline was 6% and the highest observed score was 72%. This increased after one week’s intervention, with the lowest score being 14% and the highest being 100%. After 12 weeks, 23 of the 31 participants (74%) had improved their rotation score.
Figure 3.
Injection-site rotation metrics of baseline compared with first week of intervention (primary endpoint) and 12 weeks/last week of intervention (secondary endpoint).
Table 2.
Rotation Score (Primary Endpoint) and Other Measures of Insulin Injection Rotation (Secondary Endpoints) in 35 Participants with Type 1 Diabetes.
| Metric | Baseline |
Intervention week 1 |
|||
|---|---|---|---|---|---|
| n | Mean (±SD) | Mean (±SD) | Median change [CI]a | P-valueb | |
| Rotation score (%) | 35 | 40.2 (16.1) | 49.9 (20.1) | 9.7 [2.2; 17.2] | .012 |
| Percentage of fields used (%) | 35 | 59.1(15.2) | 66.3 (22.3) | 7.2 [0.1; 14.3] | .047 |
| Field reuse count (fields) | 34 | 2.8 (1.6) | 3.8 (2.1) | 1.0 [0.2; 1.8] | .020 |
CI, confidence interval; SD, standard deviation.
Baseline and one-week results are reported.
95% confidence interval on change from baseline.
Paired z test, compared with baseline.
Percentage of Injection Fields Used
The mean percentage of available fields used was 58.7% at baseline and 66.7% during the first week of intervention with the clip-on device, which was an increase of +8.0% points, CI: 0.3%-15.8%, P = .042. During the last week of intervention this increased to 69.9%, an increase of 11.3% points, CI: 4.0%-18.6%, P = .002, see Figure 3 and Table 3.
Table 3.
Rotation Score and Other Measures of Insulin Injection Rotation (Secondary Endpoints) in 31 Participants with Type 1 Diabetes.
| Metric | Baseline |
Intervention week 1 |
Intervention week 12 |
|||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (±SD) | Mean (±SD) | Mean change [CI]a | P-valueb | Mean (± SD) | Mean change [CI]a | P-valueb | |
| Rotation score (%) | 31 | 39.6% (±16.4) | 49.2% (±20.2) | 9.6% [1.2%; 18.0%] | .025 | 52.2% (±13.0) | 12.6% [5.2%; 19.9%] | <.001 |
| Percentage of fields used | 31 | 58.7% (±15.7) | 66.7% (±22.4) | 8.0% [0.3%; 15.8%] | .042 | 69.9% (±21.4) | 11.3% [4.0%; 18.6%] | .002 |
| Field reuse count (fields) | 30 | 2.7 (±1.6) | 3.7 (±1.9) | 1.0 [0.1; 1.9] | .034 | 6.9 (±2.1) | 4.2 [3.3; 5.1] | <.001 |
CI, confidence interval; SD, standard deviation.
Baseline, 1-week, and 12-week results are reported.
95% confidence interval on change from baseline.
Paired z test, compared with baseline.
Time Between Reuse of Injection Fields
The median number of injections between reuse of an injection field increased from baseline to the first week of the intervention (+1 injection, CI: 0-2, P = .034. After 12 weeks’ intervention this increase was more pronounced (+4 injections, CI: 3-5, P < .001), see Figure 3 and Table 3.
Injection-Site Plots
Figure 2 shows an example of injection plots at baseline and at week 1 for the same participant. Baseline represents the participant’s own rotation of injections and one week represents the participant’s response to the injection plan provided by the clip-on device. As seen by the plot, several abdominal areas were removed from the injection plan, provided by the device, to avoid LH areas.
Hemoglobin A1c
HbA1c did not change after 12 weeks’ intervention (–0.6 mmol/mol, CI: –2.6 to 1.3, P = .510/–0.1%, CI: –0.2 to 0.1, P = .510), see Table 4.
Table 4.
HbA1c and CGM Data in 28 Participants with Type 1 Diabetes.
| Metric | Baseline |
Intervention week 1 |
Intervention week 12 |
|||||
|---|---|---|---|---|---|---|---|---|
| N | Mean (±SD) | Mean (±SD) | Mean change [CI]a | P-valueb | Mean (±SD) | Mean change [CI]a | P-valueb | |
| HbA1c (mmol/mol) | 28 | 57 (8) | NA | 57 (9) | −0.6 [–2.6; 1.3] | .510 | ||
| (DCCT %) | 28 | 7.4 (0.7) | NA | 7.3 (0.8) | −0.1 [–0.2; 0.1] | .510 | ||
| CGM (MBG, mg/dL) | 20 | 175 (29) | 168 (24) | −6.2 [–15.6; 3.1] | .180 | 180 (26) | 5.9 [–3.3; 15] | .195 |
| CGM (CV, %) | 20 | 38.8 (8.9) | 36.7 (9.6) | −2.1 [–5.7; 1.6] | .249 | 35.9 (7.6) | −2.9 [–5.3; –0.4] | .024 |
| CGM (TIR, %) | 20 | 53.9 (16.9) | 57.3 (16.5) | 3.4 [–2.2; 9.1] | .220 | 50.1 (13.6) | −3.8 [–10.0; 2.4] | .214 |
| CGM (TBR level 1, %) | 20 | 2.2 (2.8) | 2.6 (3.0) | 0.4 [–0.9; 1.6] | .556 | 2.2 (2.9) | 0.0 [–0.9; 0.9] | .986 |
| CGM (TBR level 2, %) | 20 | 1.7 (4.6) | 1.8 (5.5) | 0.0 [–0.6; 0.6] | .914 | 1.0 (2.8) | −0.7 [–1.7; 0.3] | .154 |
| CGM (TAR level 1, %) | 20 | 25.9 (10.4) | 26.8 (10.0) | 0.9 [–1.7; 3.5] | .476 | 31.4 (9.8) | 5.6 [1.8; 9.3] | .006 |
| CGM (TAR level 2, %) | 20 | 16.3 (11.4) | 11.6 (8.1) | −4.7 [–10.0; 0.5] | .076 | 15.2 (9.5) | −1.0 [–5.8; 3.8] | .646 |
SD, standard deviation.
Baseline, 1-week, and 12-week results are reported.
95% confidence interval, paired z test, compared with baseline.
Paired z test, compared with baseline.
HbA1c results are only available for baseline and 12 weeks. CGM data only reported for participants, where sensor data were available for baseline, week 1, and 12 of the intervention.
CGM, continuous glucose monitoring; CV, coefficient of variation; DCCT, Diabetes Control and Complications Trial units, HbA1c, hemoglobin A1c; MBG, mean blood glucose; TAR, time above range; TBR, time below range; TIR, time in range.
CGM Data
CGM data show that CV decreased by 2.9% points, CI: –5.3% to –0.4%, P = .024 from baseline (V0-V1) to end of trial (V2-V3). MBC levels and TIR were unchanged. TBR level 1 did not change significantly (0.0% points, CI: –0.9% to 0.9%, P = .986). TAR level 1 increased significantly (5.6% points, CI: 1.8%-9.3%, P = .006). See Table 4. A subgroup analysis to separate the potential effect on CV of injecting basal insulin into areas affected by LH is shown in Supplemental Table 2.
Number and Size of Insulin Infiltrates
Table 5 contains the daily insulin dose requirement, number and size of insulin infiltrates, and hypoglycemic events. The number of clinically observed infiltrates was significantly reduced from baseline to after 12 weeks intervention (–0.3, CI: –0.6 to 0.0, P = .039). The mean area of the infiltrates was significantly reduced from 11.9 to 8.2 cm2 (–3.7 cm2, CI: –7.2 to –0.2, P = .041), representing a 31% reduction of the mean infiltrate area.
Table 5.
Total Daily Insulin Requirements, Infiltrates, and Hypoglycemic Events (Secondary Endpoints) for 31 Participants with Type 1 Diabetes.
| Metric | Baseline |
Intervention week 1 |
Intervention week 12 |
|||||
|---|---|---|---|---|---|---|---|---|
| n | Median [range] | Median [range] | Median change [CI]a | P-valueb | Median [range] | Median change [CI]a | P-valueb | |
| Total daily dose (IU) | 22 | 39 [19; 206] | 38 [20; 227] | −1 [–1.1; 3.5] | .286 | 35 [22; 228] | −4 [–7.8; 4.2] | .543 |
| Infiltrates, count | 12 | 1 [1; 2] | NA | 1 [0; 2] | 0 [–0.6; –0.0] | .039 | ||
| Infiltrates, area (cm2) | 15 | 9.2 [0.9; 29.4] | NA | 5.4 [0.0; 26.8] | −3.8 [–7.2; –0.2] | .041 | ||
| Hypoglycemic events, one-week comparison | 28 | 0 [0; 4] | 0.5 [0; 5] | 0.5 [–0.5; 0.9] | .613 | 0 [0; 7] | 0 [–0.9; 0.4] | .922 |
| Hypoglycemic events, pretrial vs trial | 28 | 2 [0; 30] | NA | 4 [0; 44] | 2 [–2.5; 5.7] | .218 | ||
95% CI, paired z test, compared with baseline.
Paired z test, compared with baseline.
CI, confidence interval.
Total daily insulin requirement is reported only for participants, who filled and returned their insulin diaries. Infiltrates area and count are reported only for participants, who had at least one infiltrate at trial start. Hypoglycemic events are reported only for participants, who filled and returned their hypoglycemic event diary.
Hypoglycemic Events
There were no significant changes in hypoglycemic events, neither after the first or after 12 weeks of intervention.
Insulin Treatment Satisfaction, Injection Technique, and Quality of Life
There were no changes in treatment satisfaction, satisfaction with blood GV, or in insulin treatment pain from baseline to week 12, data not shown (Table 6).
Table 6.
Insulin Treatment Satisfaction Questionnaire9 Data for 28 Participants with Type 1 Diabetes (Secondary Endpoints).
| Question | Baseline |
Intervention week 12 |
||
|---|---|---|---|---|
| Mean (±SD) | Mean (±SD) | Mean change [CI]a | P-valueb | |
| Insulin treatment, blood glucose stability (1 = very satisfied, 7 = not at all satisfied) |
3.1 (1.3) | 2.9 (1.3) | −0.2 [–0.7; 0.4] | .517 |
| Insulin treatment, pain with current insulin
therapy (1 = no pain or discomfort, 7 = terrible pain or discomfort) |
1.5 (0.8) | 1.5 (0.6) | 0.0 [–0.4; 0.4] | >.999 |
| Insulin treatment, overall treatment
satisfaction (slider, 1 = very satisfied, 7 = not at all satisfied) |
2.2 (1.3) | 2.1 (1.3) | −0.1 [–0.8; 0.7] | .871 |
CI, confidence interval; SD, standard deviation.
95% confidence interval, paired z test, compared with baseline.
Paired z test, compared with baseline.
Reponses are coded as values (1 = very satisfied, 7 = not at all satisfied) to calculate mean response of participants.
Usability of the Clip-On Device
Figure 4 shows a bar plot of the individual responses to the clip-on device usability questionnaire; the answers to the individual questions are available in Supplemental Table 3.
Figure 4.
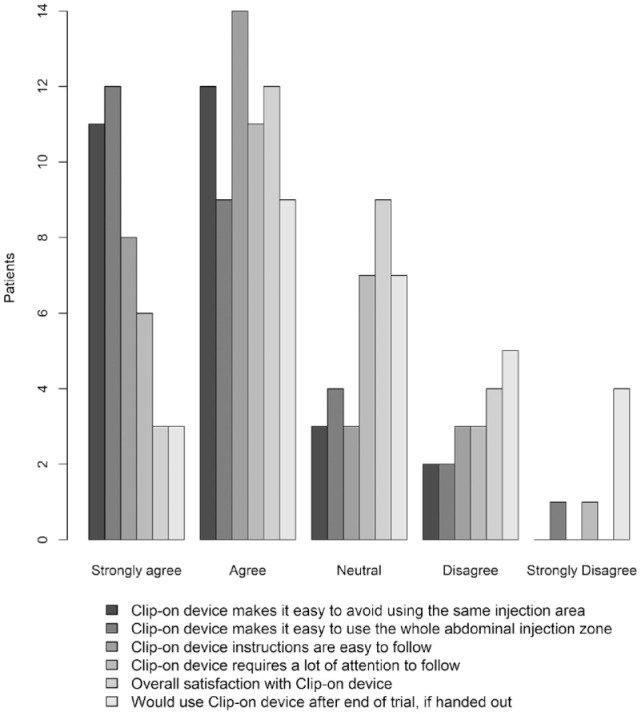
Patient self-reported satisfaction with clip-on device.
Fifty-four percent of participants responded that they were “satisfied” or “very satisfied” with the clip-on device and 43% responded that it was “likely” or “very likely” that they would use the clip-on device if handed out after trial.
Adverse Effects
In total, 10 adverse effects (AEs) were reported. Three AEs were hypoglycemic episodes, however, without the participant needing assistance, and could be explained by exercise or not enough food intake, and thus a causal relationship to the clip-on-device was highly unlikely. Seven mild AEs with unlikely causal relationship to the clip-on device were reported such as tonsillectomy and mycotic eczema.
Discussion
This is the first study of an automatic insulin injection log device, which can track both when and where a person with diabetes injects insulin in the abdominal region in real-life conditions. Combining this knowledge with an in-device algorithm designed to minimize the risk of injection in the same area, the device was able to guide 23 out of 31 users to improve the rotation of insulin injections in the abdominal area. Of the 31 participants, 30 stated at baseline that they already used some kind of injection plan. Thus, the significant improvement in rotation score, when using the clip-on-device, might be even higher in patients that does not already use an injection plan. Injection-site rotation has been studied before, but these studies have relied on a binary (yes/no) categorization of correct injection site rotation and thus do not provide accurate measures for the distribution of injections on the skin. The studies relied on patient reporting and/or nurse assessment during patient follow-up—with significant differences between the two assessments.1,2 One study asked patients to categorize the total injection area used as the size of a post card, a playing card, a credit card, or a postage stamp, but also assessed rotation within the area using the binary yes/no categorization.5
The present proof-of-concept study shows that the clip-on device can help people with T1DM to improve the rotation of insulin injections and furthermore reduce the number and size of LH lesions. This might reduce unpredictable insulin absorption and consequently unexpected hypoglycemia and hyperglycemia. We observed a reduction in blood GV (measured as the CV) but was not able to show a reduction in HbA1c and number of hypoglycemic episodes, possibly because the study was not powered for this. GV might be a new risk factor for diabetes complications including cardiovascular disease.3 A reduction of LH lesions and avoidance of existing LH areas for insulin injection could help improve glucose control, which potentially could reduce the risk of diabetes complications.
The ultimate goal of an insulin pen tracking device is to make insulin injections as easy as possible for people with diabetes and at the same time increase the quality of the injection. Especially people with newly diagnosed T1DM or type 2 diabetes initiating insulin therapy could benefit from a tracking device to fully learn how to increase rotation of injections. Moreover, people with diabetes who struggle with LH could be helped by the device to avoid injections in areas with LH. Furthermore, in clinical trials, additional precision and increased compliance can be obtained with this device.
The satisfaction with the clip-on device was predominantly good, although dissatisfaction was also the case for some participants. Further development of the device, by increasing precision of the device and the user interface, is ongoing. Likewise, the possibility to use the device for other regions of the body is explored.
Our study has some limitations. First, our pilot study was not a randomized controlled trial of the efficacy of the click-on device compared with usual care. However, using a baseline week in which the participants did not know that the click-on device was tracking their insulin injection locations partly compensated for the lack of a randomized design. Second, the precision of the tracking device was not verified in the study. Finally, guidelines now recommend 10 full days of CGM evaluation in clinical trials instead of the 6 planned in this trial.11
Conclusion
This proof-of-concept study indicates that the new clip-on device for improving insulin injection technique is safe, improves rotation, reduces LH, and reduces GV. Future studies are needed to validate the results from this study in other and larger populations.
Supplemental Material
Supplemental material, Supplementary_Table_nd_figure_PDF for A New Medical Device for Improved Rotation of Insulin Injections in Type 1 Diabetes Mellitus: A Proof-of-Concept Study by Carina Kirstine Klarskov, Yasmin Hassan Hamid, Rasmus Tjalk-Bøggild, Lise Tarnow and Peter Lommer Kristensen in Journal of Diabetes Science and Technology
Acknowledgments
Research laboratory technician Susanne Månsson, Charlotte Pietraszek, and research nurse Lizette Helbo Nislev are thanked for excellent handling of participants, data, and devices.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RT-B is a board member of Nordic Healthcare Technology and an employee of Nordic Healthcare Technology. None of the other authors have any conflicts of interests.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Innovation Fund Denmark (grant numbers 8063-00049B) and Nordic Healthcare Technology.
ORCID iD: Carina Kirstine Klarskov  https://orcid.org/0000-0001-7702-8595
https://orcid.org/0000-0001-7702-8595
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Blanco M, Hernández MT, Strauss KW, Amayada M.Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013;39(5):445-453. [DOI] [PubMed] [Google Scholar]
- 2.Grassi G, Scuntero P, Trepiccioni R, Marubbi F, Strauss K.Optimizing insulin injection technique and its effect on blood glucose control. J Clin Transl Endocrinol. 2014;1(4):145-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsch IB.Glycemic variability and diabetes complications: does it matter? Of course it does! Diabetes Care. 2015;38(8):1610-1614. [DOI] [PubMed] [Google Scholar]
- 4.Gentile S, Guarino G, Giancaterini A, Guida P, Strollo FAMD-OSDI Italian Injection Technique Study Group. A suitable palpation technique allows to identify skin lipohypertrophic lesions in insulin-treated people with diabetes. Springerplus. 2016;5:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frid AH, Hirsch LJ, Menchior AR, Morel DR, Strauss KW.Worldwide injection technique questionnaire study: population parameters and injection practices. Mayo Clin Proc. 2016;91(9):1212-1223. [DOI] [PubMed] [Google Scholar]
- 6.Smith M, Clapham L, Strauss K.UK lipohypertrophy interventional study. Diabetes Res Clin Pract. 2017;126:248-253. [DOI] [PubMed] [Google Scholar]
- 7.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klarskov CK, Hamid Y, Tjalk-Bøggild RT, Tarnow L, Kristensen PL. Data and analyses for “The impact of ROTO track® in helping patients with diabetes rotate their insulin injections better (ROTOone)”. figshare. 2020. https://figshare.com/articles/_/11925726.
- 9.Andersen RT, Skovlov SE, Marrero D, et al. Development and validation of the insulin treatment satisfaction questionnaire. Clin Ther. 2004;26(4):565-578. [DOI] [PubMed] [Google Scholar]
- 10.Janssen MF, Pickard AS, Golicki D, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res. 2013;22(7):1717-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Table_nd_figure_PDF for A New Medical Device for Improved Rotation of Insulin Injections in Type 1 Diabetes Mellitus: A Proof-of-Concept Study by Carina Kirstine Klarskov, Yasmin Hassan Hamid, Rasmus Tjalk-Bøggild, Lise Tarnow and Peter Lommer Kristensen in Journal of Diabetes Science and Technology