Abstract
Background:
Despite increasing use of technology in type 1 diabetes, persistent ethnic and socio-economic disparities have been reported. We analyzed how the use of insulin pump therapy and continuous glucose monitoring (CGM) evolved over the years in Germany depending on demographics and area deprivation.
Method:
We investigated the use of insulin pump and CGM between 2016 and 2019 in 37,798 patients with type 1 diabetes aged < 26 years from the German Prospective Follow-up Registry (DPV). Associations with federal state, area-deprivation quintile (German Index of Multiple Deprivation 2010 on district level), gender, and migration background were investigated over time using multiple logistic regression.
Results:
Between 2016 and 2019, the regional distribution of insulin pump use did not change substantially and the association with area deprivation remained non-linear and statistically non-significant. The effect of area deprivation on CGM use decreased continuously and disappeared in 2019 (OR [95%-CI] Q1 vs Q5: 1.85 [1.63-2.10] in 2016; 0.97 [0.88-1.08] in 2019). The effect of migration background on the use of either technology decreased over the years but remained significant in 2019. Girls had constantly higher odds of using an insulin pump than boys (OR: 1.25 [1.18-1.31] in 2019), whereas no gender difference was identified for CGM use.
Conclusions:
Although disparities decreased in Germany, access to diabetes technology still depends on migration background in 2019, and gender differences in pump use persist. As technological advances are made, further research is needed to understand the reasons for these persistent disparities.
Keywords: CGM, deprivation, insulin pump, migration, type 1 diabetes
Introduction
Despite considerable advances in the management of type 1 diabetes, achievement of optimal metabolic control remains challenging, especially for adolescents and young adults.1,2 Nevertheless, both efficacy and safety of diabetes technology improve continuously. Insulin pumps and continuous glucose monitoring systems (CGM), which are now widely used in many high-income countries,2-5 are associated with better glycemic control and have the potential to reduce the risk of acute complications.2,6 Furthermore, evidence on improved glycemic outcomes obtained with automated insulin delivery systems is accumulating.7-9 In 2018, the first hybrid closed-loop system has been approved for children aged 7 years or older in Europe and in the U.S.7
However, it remains illusory to believe that every child enjoys an equal access to these devices. Despite improved reimbursement for established diabetes technology, important disparities based on socio-economic factors4,10 or on migration background2,11-14 have been reported. Moreover, there is a concern that further advances in diabetes technology widen these disparities and increase the systematic disadvantage of children in more deprived situations.4,15 Nevertheless, considering the sharp increase in CGM and insulin pump use over the last years in Germany, it remains uncertain how exactly these disparities have evolved in more recent years.
We used real-world data from a representative registry to analyze how the use of these devices evolved in children, adolescents and young adults with type 1 diabetes between 2016 and 2019 in Germany. More particularly, we investigated whether the influence of area-based (federal states, district-based deprivation) and demographic factors (gender, migration background) on this technology use changed over time.
Methods
For this population-based study, we used data from the multicenter Diabetes Prospective Follow-up (DPV) Initiative based at the University of Ulm, Germany. Since 1995, all participating diabetes care centers, mainly located in Germany and Austria, prospectively document clinical and demographic data of patients with any type of diabetes into the standardized DPV database.16 Semi-annually, the collected data are transmitted in pseudonymous form to the University of Ulm, which aggregates the data for central analysis and quality assurance, after plausibility checks and corrections. The Ethics Committee of the Medical Faculty of the University of Ulm (vote number 202/09), as well as the local review boards of participating centers, approved both data collection and analysis of anonymized data from the DPV registry.
Patients with type 1 diabetes living in Germany, aged < 26 years, and with visits documented between 2016 and 2019 were eligible for this study. Further inclusion criteria were: documentation of insulin treatment, age ≥ 6 months at diagnosis, and diabetes duration ≥3 months. Individuals without available or assignable 5-digit postal code of residence (n = 393) were excluded from the analysis as this information was required to categorize participants into area deprivation quintiles.
Demographic and Socioeconomic Variables
According to prior publications from the DPV registry,4,10 migration background was defined as place of birth outside Germany for the patient or at least for one parent. Patients without information on migration background (n = 6,150) were assumed to have no history of migration. Additionally, patients with migration background were categorized into 2 groups depending on the patient’s place of birth: “first-generation immigrant” (patient born outside Germany) and “second-generation immigrant” (patient born in Germany with at least one parent born outside Germany). For 1.2% of the patients with migration background, this information was missing.
Area deprivation17 was assessed at district level using the German Index of Multiple Deprivation for the reference year 2010 (GIMD 2010).4,18,19 As described previously,10 the GIMD includes 7 deprivation domains differently weighted: income (25%), employment (25%), education (15%), municipal/district revenue (15%), social capital (10%), environment (5%), and security (5%). Districts were categorized into area deprivation quintiles from Q1 (lowest area deprivation quintile) to Q5 (highest area deprivation quintile). Patients were assigned to districts and consequently to GIMD quintiles using the 5-digit postal code of their residence.
Clinical Variables
Body Mass Index (BMI) was calculated as kg/m2. For children and adolescents less than 18 years of age, BMI values were transformed to standard deviation scores (SDS or z score) adjusting for age and gender, using national pediatric reference data20 by applying the LMS method.21 For young adults aged 18 years or older, we used BMI values. HbA1c was mathematically standardized to the reference range of the Diabetes Control and Complications Trial (DCCT) (4.05-6.05% [20.7-42.6 mmol/mol]) using the multiple of the mean method to adjust for differences between laboratories.22
Use of Diabetes Technology
Insulin pumps were increasingly used and refunded in Germany in the early 2000s.3 The statutory health insurance (covering approximately 90% of the pediatric patients) refunds insulin pump therapy in pediatric patients with type 1 diabetes on a case-by-case-basis. Indication criteria are numerous, in particular: insufficient glycemic control with intensified conventional insulin therapy, severe hypoglycemia, dawn phenomenon, preschool age, pregnancy, needle phobia, or participation in competitive sports.23 Application for reimbursement must contain an explanatory statement of the indication, a detailed documentation of the therapy and glycemic outcomes of the last 3 months, and certify that the patient will receive adequate pump education. Approval of the health insurance is first given for a probation period, and if glycemic values improves, final approval is given. Regional medical services of the health insurance funds often take part in decision-making, with more or less restrictive positions.
Real-time glucose monitoring (rtCGM) is refunded since September 2016 in Germany by the statutory health insurance for patients with insufficient glycemic control and/or severe hypoglycemia.23 Since July 2019, the second generation of intermittent scanning glucose monitoring (iscCGM) with alarm function can be reimbursed as well. Application process is similar to those for insulin pump therapy, but a probation period is not required.
For the 10% of children with private insurance, reimbursement of diabetes technology depends of contract specifications.
In the present analysis, use of insulin pump and use continuous glucose monitoring (CGM) were defined as any use of these technologies documented at least once per year. For many patients in our study population (between 31% and 46% depending on the year), the type of CGM (iscCGM or rtCGM) was not documented and we therefore decided to perform the analysis for all types of CGM without distinction.
Statistical Analysis
Data were aggregated per patient and year as median (age, diabetes duration, HbA1c, BMI SDS, and BMI) or maximum (pump use, CGM use). Age was categorized into 3 groups: 0.5- < 11 years, 11- < 16 years and 16- < 26 years. Diabetes duration was categorized into the following groups: 0.25- < 2 years, 2- < 5 years and ≥5 years.
Unadjusted patient characteristics were presented, as median with interquartile range (IQR) or proportion, for continuous or categorical variables, respectively. Wilcoxon tests (continuous variables) and X2 tests (categorical variables) were used to compare demographic and clinical characteristics between years, adjusting for multiple comparisons according to the Holm-Bonferroni stepdown procedure.
We represented the regional distribution of the use of pump or CGM in 2016 und 2019 in Germany, using tertile-based choropleth maps. For that purpose, we performed logistic regression models adjusting for gender, age group, diabetes duration group, and migration background, to estimate the use of pump of CGM for each of the 16 federal states of Germany. Then, the adjusted estimates were assigned to 3 categories: low, middle or high use.
In a second step, we performed logistic regression models to assess the association of the 3 independent variables (area deprivation, migration background, and gender) with pump or CGM use by year (interaction terms: area deprivation*year, migration background*year or gender*year), adjusting for area deprivation, migration background, gender, age group, diabetes duration group, and an interaction between migration background and area deprivation. To take the dependence of the data within regions into consideration, we used sandwich variance estimators. Results of regression analyses are presented as adjusted estimates of pump or CGM use with their respective 95% confidence intervals (95% CI) for each independent variable category, as well as odds ratios (OR) for the use of pump or CGM for female vs male, individuals without vs with migration background, and those with area deprivation quintile Q1 vs Q5. P-values for trend were calculated to test the overall logit-linear trend of the independent variables (area deprivation modelled as ordinal variables) in each year. Additionally, we tested if these associations were significantly different between years (trend-test for the total period).
A 2-side P-value <.05 was considered statistically significant. Statistical analysis was performed using SAS 9.4 software (SAS Institute, Cary, NC).
Results
The final study population of overall 37,798 youths with type 1 diabetes for the period 2016-2019 is described stratified by year in Table 1 (unadjusted results). From 2016 to 2019, the use of insulin pump increased from 51.7% to 57.6%, and the use of CGM from 17.9% to 70.3% (Table 1).
Table 1.
Characteristics of the Study Population, Stratified by Year.
| 2016 (n = 25,442) | 2017 (n = 25,807) | 2018 (n = 26,218) | 2019 (n = 26,628) | P-valuesb | |
|---|---|---|---|---|---|
| Girls, n (%) | 12,060 (47.4) | 12,233 (47.4) | 12,349 (47.1) | 12,568 (47.2) | n.s. (1.00) |
| Age, years (median, IQR) | 13.7 (10.2-16.4) | 13.7 (10.2-16.5) | 13.7 (10.2-16.5) | 13.7 (10.3-16.5) | n.s. (1.00) |
| Diabetes duration, years (median, IQR) | 4.6 (2.1-8.1) | 4.6 (2.1-8.1) | 4.7 (2.1-8.1) | 4.7 (2.1-8.1) | n.s. (1.00) |
| Migration backgrounda, n (%) | 5,750 (22.6) | 6,065 (23.5) | 6,266 (23.9) | 6,471 (24.3) | .0002 |
| - First generation | 1,018 (4.0) | 1,161 (4.5) | 1,258 (4.8) | 1,358 (5.1) | <.0001 |
| - Second generation | 4,630 (18.2) | 4,826 (18.7) | 4,903 (18.7) | 5,059 (19.0) | n.s. (.75) |
| BMI SDS (median, IQR) for patients aged <18 years | 0.33 (−0.23–0.92) | 0.35 (−0.22–0.94) | 0.35 (−0.22–0.94) | 0.39 (−0.19–0.99) | <.0001 |
| BMI (median, IQR) for patients aged ≥18 years | 23.7 (21.6-26.3) | 23.7 (21.6-26.6) | 23.7 (21.6-26.2) | 23.8 (21.6-26.4) | n.s. (1.00) |
| HbA1c, % (median, IQR) | 7.58 (6.87-8.38) | 7.65 (6.97-8.48) | 7.52 (6.83-8.33) | 7.52 (6.83-8.31) | <.0001 |
| Use of insulin pump, n (%) | 13,154 (51.7) | 13,910 (53.9) | 14,708 (56.1) | 15,338 (57.6) | <.0001 |
| Use of CGM, n (%) | 4,554 (17.9) | 10,839 (42.0) | 15,573 (59.4) | 18,719 (70.3) | <.0001 |
Unadjusted data.
BMI SDS, standard deviation score of Body Mass Index (kg/m2); IQR, interquartile range.
Migration background is defined as birth of the patient himself outside Germany (first-generation immigrant) or at least one of his parents (second-generation immigrant). Documentation of the place of birth was missing for 0.8-1.8% of the patients with migration history.
Comparison between years using Wilcoxon test for continuous variables and X2 test for variables with binomial distribution, adjusted for multiple comparisons according to the Holm-Bonferroni stepdown procedure. P < .05 (two-sided) was considered statistically significant.
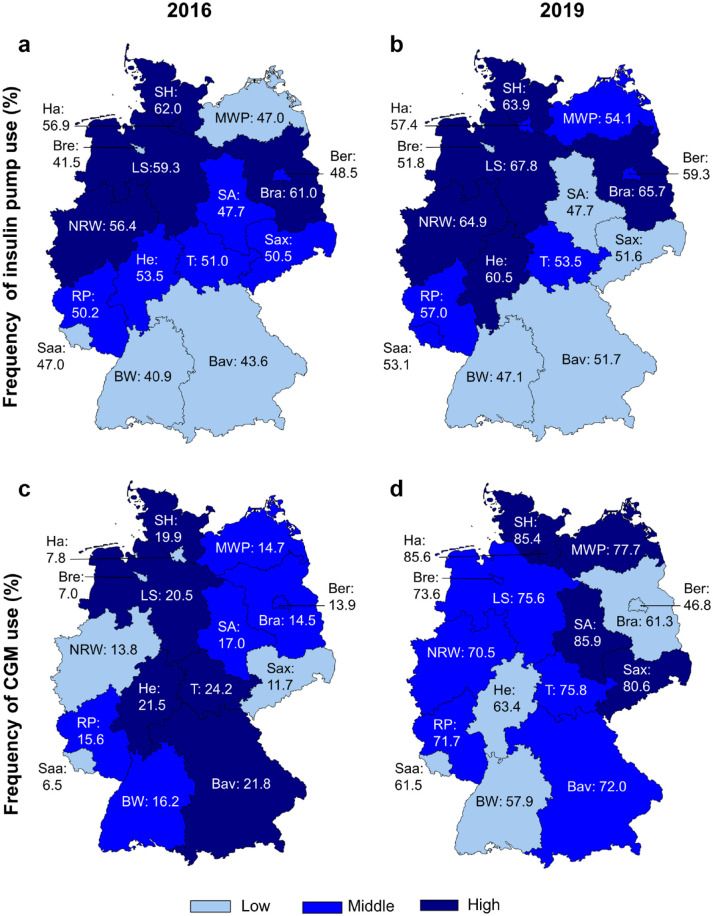
Evolution by Federal State (Figure 1)
Figure 1.
Use of diabetes technology by federal state in 2016 and 2019. Legend: Tertile-based choropleth map representing the regional distribution of the use of insulin pump in 2016 (a), in 2019 (b), as well as of CGM in 2016 (c) and 2019 (d), using estimates from logistic regression models, adjusting for gender, age group, diabetes duration, and migration background, for each of the 16 federal states of Germany.
Bav, Bavaria; Ber, Berlin; Bra, Brandenburg; Bre, Bremen; BW, Baden-Württemberg; Ha, Hamburg; He, Hesse; LS, Lower Saxony; MWP, Mecklenburg-Western Pomerania; NRW, North Rhine-Westphalia; RP, Rhineland-Palatinate; SA, Saxony-Anhalt; Saa, Saarland;
Sax, Saxony; SH, Schleswig-Holstein; T, Thuringia.
Between 2016 and 2019, the regional distribution of the use of insulin pumps did not change substantially, with the lowest use in Baden-Württemberg (from 2016 to 2019: 40.9%-47.1%) and Bavaria (43.6%-51.7%), and the highest use in Schleswig-Holstein (62.0%-63.9%), Brandenburg (61.0%-65.7%), and Lower-Saxony (59.3%-67.8%). The relatively strongest increase, from 41.5% to 51.8%, was observed in Bremen, and the weakest in Saxony-Anhalt, were the use of insulin pump remained nearly stable around 47.7%.
In the same time period, the regional distribution of the use of CGM changed substantially. In 2016, the lowest use was reported in Saarland, Bremen, and Hamburg (6.5%, 7.0%, and 7.8% respectively) and the highest in Thuringia (24.2%). By contrast in 2019, the lowest use was observed in Berlin (46.8%) and the highest use in Schleswig-Holstein, Hamburg, and Saxony-Anhalt (85.4, 85.6, and 85.9% respectively). The increase in CGM use was weakest in Berlin (+33 percentage points) and strongest in Hamburg (+78 percentage points), as well as in Saxony-Anhalt and Saxony (both: +69 percentage points).
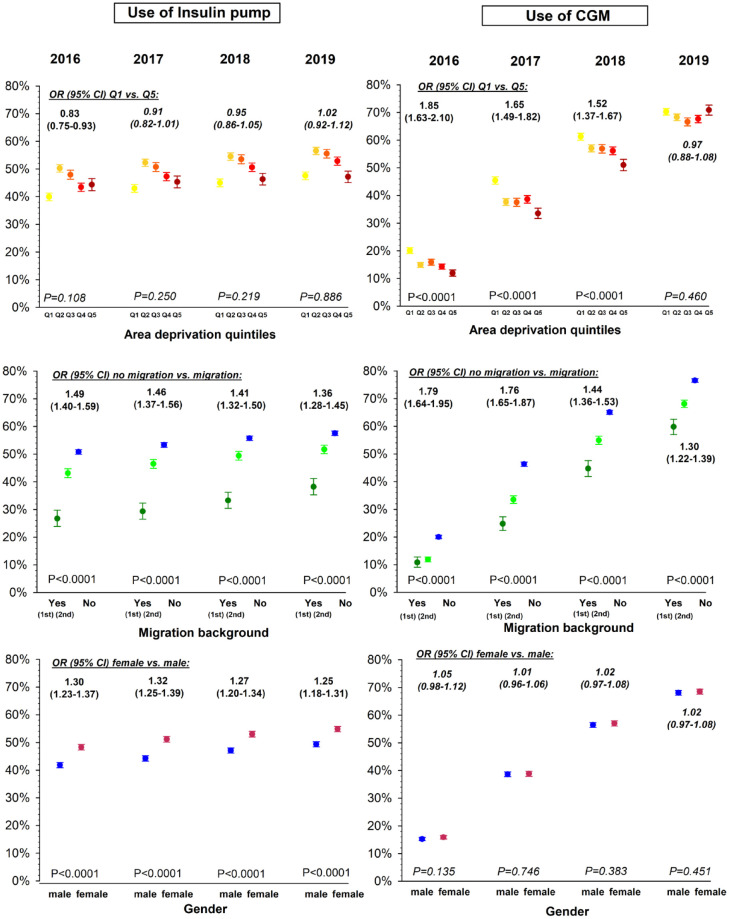
Evolution Depending on Area Deprivation (Figure 2)
Figure 2.
Use of diabetes technology by year and by area deprivation, migration background, and gender. Legend: Use of insulin pump and CGM in percentage by year and area deprivation, migration background, or gender interaction are represented using estimates with 95% CI from logistic regression models, adjusting for area deprivation, migration background, gender, age group, diabetes duration, and migration background - area deprivation interaction. Q1 is the least deprived quintile (yellow) and Q5 is the most deprived quintile (dark red). Migration background is defined as birth of the patient himself outside Germany (1st: first generation, dark green) or at least one of his parents (2nd: second generation, light green). P-values for trend are given for the effect of gender, migration background, or area deprivation (modelled as an ordinal term) by year. Non-significant P-values are indicated in italics.
The effect of area deprivation on the use of insulin pumps followed each year a similar non-linear pattern. Between 2016 und 2018, pump use remained lowest in the lowest area deprivation quintile Q1 (39.9% in 2016 and 45.0% in 2018) whereas in 2019, the lowest use was both in Q1 (47.6%) and Q5 (47.2%), compared to other quintiles (Q2: 56.6%, Q3: 55.5%, Q4: 52.9%). Nevertheless, a decrease of insulin pump use with higher deprivation, between area deprivation quintile Q2 and Q5, could be observed throughout the observation period.
Between 2016 and 2018, patients living in districts of the lowest deprivation quintile Q1 had significant higher odds of using a CGM compared to those living in districts of the highest deprivation quintile Q5, but over the years, the effect of area deprivation decreased continuously (OR [95%-CI] Q1 vs Q5: 1.85 [1.63-2.10] in 2016 to 1.52 [1.37-1.67] in 2018, P-value for interaction area deprivation*year <.001), and eventually disappeared in 2019 (0.97 [0.88-1.08], P-value for trend = 0.460).
Evolution Depending on Migration Background (Figure 2)
Between 2016 and 2019, the odds of using an insulin pump was constantly and significantly higher in patients without migration background compared to those with a history of migration (OR [95%-CI]: from 1.49 [1.40-1.59] in 2016 to 1.36 [1.28-1.45] in 2019, all P < .001). A trend towards a weaker effect on migration background on pump use could be observed, but did not reach statistical significance (P-value for interaction migration*year = .169).
Similarly, the odds of using a CGM was significantly higher in patients without migration background over the whole study period. Nevertheless, the effect on former immigration on the use of CGM decreased significantly over the years (OR [95%-CI]: from 1.79 [1.64-1.95] in 2016 to 1.30 [1.22-1.39] in 2019, P-value for interaction migration*year < .001).
Taking into consideration the generation of immigration, second-generation immigrants had a significant higher use of insulin pump and CGM than first-generation immigrants (except for CGM in 2016), but still had a lower use than patients without migration background (all P < .001).
Evolution Depending on Gender (Figure 2)
During the whole observation period, the odds of using an insulin pump was higher in girls than in boys (OR [95%-CI]: 1.25 [1.18-1.31] in 2019, P < .001). The effect of gender on the use of insulin pump did not change significantly over the years (P-value for interaction gender*year = 0.415). By contrast, the odds of using a CGM remains similar in girls and boys, throughout the observed years.
Discussion
In this population-based study, we analyzed the evolution of the use of insulin pump and CGM in Germany between 2016 and 2019, focusing on their regional distribution, as well as on the influence of regional socio-economic and demographic factors.
The regional distribution of the use of insulin pumps did not change substantially since an initial analysis for the years 2012-2013,24 which reported the lowest use of insulin pump in Southern Germany and the highest use in Northern-Western Germany. The pattern of the association with area deprivation partly reflects these regional disparities: the lowest use was found nearly every year in districts of the least deprived quintile Q1 which predominate in Southern Germany, that is, Bavaria (where 57% of the districts are Q1 vs <1% of the districts Q5) and Baden-Württemberg (44% of the districts Q1 vs <1% of the districts Q5). Regional disparities, like the lower use of insulin pumps in Southern Germany, result from complex interactions between several factors. Among other things, local preferences (patients and/or physicians) as well as less or more restrictive positions of the regional medical services of the health insurance funds can play a role. Nevertheless, apart from the lower use in Q1, insulin pumps tend to be less frequently used with higher deprivation (from quintile Q2 to Q5), following the same pattern described in previous publications.4,10 Lower health literacy skills and more particularly lower parental perceived self-efficacy associated with lower socioeconomic status, may lead to a lower use of diabetes technology in more deprived regions.12,25,26 Besides, the necessity to apply for reimbursement and the uncertainty of approval by health insurance may discourage some families in most deprived socioeconomic situations.10,12 Overall, the persistent non-linear association between pump use and area deprivation indicates that several covariates with partly opposite effects are interacting.
Our results on CGM use reflect more dynamic changes in the regional distribution during the study period. Over the years, the effect of area deprivation decreased and eventually disappeared in 2019. In accordance with this finding, we observed in the study period a strong increase in the use of CGM in the most deprived areas. In Saxony-Anhalt, where the highest use in Germany was reported in 2019, 94% of the districts are classified in the highest area deprivation quintile Q5. In Mecklenburg-Western Pomerania, Saxony, and Thuringia, where districts of quintile Q5 are also predominant, the use of CGM increased also strongly between 2016 and 2019. In Germany, rtCGM are reimbursed by statutory health insurance since 2016, and iscCGM with alarm-function since 2019. At first, and in the same manner as for the insulin pump, the necessity to apply individually for reimbursement as well as the apparent higher complexity of using diabetes technology in everyday life may have constituted an obstacle for some families in more deprived regions. However, these barriers seem to have diminished gradually over time. One plausible explanation is that approval for reimbursement of CGM has become easier to obtain over the years. In particular, reimbursement in 2019 of the second generation of iscCGM systems, which are particularly popular in youth (marketing and delivery directly to the consumer, easy use, no calibration required), may have contribute to increase the use of these devices especially in the most deprived regions.
This study revealed that migration background affects the access to diabetes technology in Germany independently of area deprivation. Lower use of insulin pump in patients from ethnic minority groups has often been described, not only in Germany,3,12 but also in Austria, England, Wales27 or New-Zealand.28 In the U.S., persistent and strong racial disparities in diabetes technology use, independent of socio-economic status, have been described until recently.2,13,14,27,29 Overall, complex discriminatory reasons15,30-32 cannot be excluded. Besides, language barriers certainly limit access of many migrant families to diabetes technology in Germany.12,30 Sufficient German language skills are required not only to apply for reimbursement, but also for pump education, which is predominantly given in an inpatient setting, and for pump management in everyday life. Children from parents who migrated into Germany (second-generation immigrants) used diabetes technology more often than first-generation immigrants, however their technology uptake was lower compared to patients without a migration background. Indeed, second-generation immigrants may experience less language and cultural barriers. Nevertheless, even if the children are born in Germany, one or both parents born outside of Germany may still have difficulties with the language and health care system in Germany. This may limit the access to technology especially for younger children, for whom the parents are still playing the main role in the therapy.
Regarding access to CGM, a previous report found no significant difference depending on migration background in Germany,3 but the results were not adjusted, contrary to those of the present study. Barriers to CGM use in patients with migration background may be similar to those described above for pump use. Nevertheless, our findings indicate that the effect of a history of migration on the access to CGM decreased over the years in Germany. However, while the effect of area deprivation on access to CGM disappeared in 2019, the effect of a migration history remained significant.
In accordance with numerous reports,3,27,28,33 we found a higher use of insulin pump in female versus male patients, consistent over the study period. In Germany, this gender difference has only been observed in children aged 10 and older, and was more pronounced in those aged above 15 years.3 Despite higher psychological barriers to technology use and higher concern of wearing a pump in public,34 many indications, like poorer metabolic control,35 variable insulin requirement during the menstrual cycle, or possibility of pregnancy,23 contribute to a higher use of insulin pump in female adolescents and young adults compared to male of the same age. By contrast, as reported in other studies,3,33,34 CGM use did not depend on gender, whatever the year.
One major strength of the present study is the large size of the study population with more than 37,000 patients with type 1 diabetes, from a national prospective diabetes registry capturing more than 85% pediatric patients with type 1 diabetes in Germany.10 Moreover, we used robust statistical methodology to investigate how the effect of demographic and socio-economic factors changed over the years, adjusting for several confounder, including the interaction between migration background and area deprivation. Thus, our study confirms that a history of migration and socio-economic factors like area deprivation affect diabetes treatment of pediatric patients independently.4,11 One limitation of our study is the lack of information on health insurance type in the DPV-Registry, due to data protection reasons. However, nearly all children and adolescents are covered by health insurance in Germany (about 90% statutory and 10% private health insurance) and differences between insurance types have only minimal consequences on the access to diabetes technology in the pediatric population. In addition, since education level and household income are incompletely documented in DPV, information on individual socio-economic status was not available. However, indices of area deprivation have been frequently used in epidemiological research, either as surrogate for individual socio-economic status4 or much more to take an “area effect” into consideration, with its multiple dimensions.10,17-19,36
Conclusions
Over the last years in Germany, the effect of area deprivation on the use of CGM disappeared, and the effect of migration decreased continuously. By contrast, the effect of area deprivation and migration on the use of insulin pump did not change significantly. The decrease of ethnic and socio-economic disparities in CGM use contrasts with the situation observed in other countries with similar rates of CGM-use, like the U.S.,2,4,13,29 and is therefore encouraging. Nevertheless, disparities based on migration background, independent of area deprivation, still impede providing every child an equal access to diabetes technology. As safety and efficacy of hybrid closed-loop and closed-loop systems will further increase,7-9 our findings raise the concern that inequitable access to diabetes technology will continue to systematically disadvantage children living in more deprived regions and/or with a history of migration.15 Certainly, efforts are required to consider solutions to overcome the language barriers. Moreover, further research is needed to deepen our understanding of the reasons for these persistent disparities.
Acknowledgments
We thank all participating centers in the DPV initiative, especially the centers contributing data to this investigation and their patients. A list of contributing centers is available at http://www.d-p-v.eu
Footnotes
Author Contribution: M.A. and R.W.H. designed the study. M.A. analyzed the study data, created the figures, and wrote the manuscript. J.R., W.M., S.v.S., J.H., T.K., G.F., S.S., E.L., and R.W.H. contributed to the discussion, reviewed, and approved the manuscript.
Abbreviations: BMI, body mass index; CI, confidence interval; DPV, German diabetes prospective follow-up registry; IQR, interquartile range; LMS method, method using lambda-mu-sigma parameters;
SDS: standard deviation score.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S.v.S. reports beeing consultant for Abbott, Lilly, Medtronic and NovoNordisk and received lecture fees from Abbott, Berlin-Chemie, Dexcom, Hexal, Infectopharm, Lilly, Medtronic and NovoNordisk. The other authors declare no competing financial interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The DPV registry and this analysis are supported by the German Center for Diabetes Research (DZD, grant number: 82DZD14A02). Further financial support for the DPV registry was provided by the German Diabetes Association (DDG), the German Robert Koch Institute (RKI).
Ethical approval: The Ethics Committee of the Medical Faculty of the University of Ulm (vote number 202/09), as well as the local review boards of participating centers, approved both data collection and analysis of anonymized data from the DPV database.
ORCID iDs: Marie Auzanneau  https://orcid.org/0000-0002-5906-6579
https://orcid.org/0000-0002-5906-6579
Guido Freckmann  https://orcid.org/0000-0002-0406-9529
https://orcid.org/0000-0002-0406-9529
References
- 1.Charalampopoulos D, Hermann JM, Svensson J, et al. Exploring variation in glycemic control across and within eight high-income countries: a cross-sectional analysis of 64,666 children and adolescents with type 1 diabetes. Diabetes Care. 2018;41(6):1180-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Boom L, Karges B, Auzanneau M, et al. Temporal trends and contemporary use of insulin pump therapy and glucose monitoring among children, adolescents, and adults with type 1 diabetes between 1995 and 2017. Diabetes Care. 2019;42(11):2050-2056. [DOI] [PubMed] [Google Scholar]
- 4.Addala A, Auzanneau M, Miller K, et al. A decade of disparities in diabetes technology use and HbA1c in pediatric type 1 diabetes: a transatlantic comparison. Diabetes Care. 2021;44(1):133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeSalvo DJ, Miller KM, Hermann JM, et al. Continuous glucose monitoring and glycemic control among youth with type 1 diabetes: International comparison from the T1D Exchange and DPV Initiative. Pediatr Diabetes. 2018;19(7):1271-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karges B, Schwandt A, Heidtmann B, et al. Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. JAMA. 2017;318(14):1358-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dovc K, Battelino T.Closed-loop insulin delivery systems in children and adolescents with type 1 diabetes. Null. 2020;17(2):157-166. [DOI] [PubMed] [Google Scholar]
- 8.Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tauschmann M, Thabit H, Bally L, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet. 2018;392(10155):1321-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auzanneau M, Lanzinger S, Bohn B, et al. Area deprivation and regional disparities in treatment and outcome quality of 29,284 pediatric patients with type 1 diabetes in Germany: a cross-sectional multicenter DPV analysis. Diabetes Care. 2018;41(12):2517-2525. [DOI] [PubMed] [Google Scholar]
- 11.Willi SM, Miller KM, DiMeglio LA, et al. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135(3):424-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheuing N, Wiegand S, Bachle C, et al. Impact of maternal country of birth on type-1-diabetes therapy and outcome in 27,643 children and adolescents from the DPV registry. PLoS One. 2015;10(8):e0135178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai CW, Lipman TH, Willi SM, Hawkes CP.Racial and ethnic disparities in rates of continuous glucose monitor initiation and continued use in children with type 1 diabetes. Diabetes Care. 2021;44(1):255-257. [DOI] [PubMed] [Google Scholar]
- 14.Lipman TH, Willi SM, Lai CW, Smith JA, Patil O, Hawkes CP.Insulin pump use in children with type 1 diabetes: over a decade of disparities. J Pediatr Nurs. 2020;55:110-115. [DOI] [PubMed] [Google Scholar]
- 15.Acerini C.The rise of technology in diabetes care. Not all that is new is necessarily better. Pediatr Diabetes. 2016;17(3):168-173. [DOI] [PubMed] [Google Scholar]
- 16.Bohn B, Karges B, Vogel C, et al. 20 years of pediatric benchmarking in Germany and Austria: age-dependent analysis of longitudinal follow-up in 63,967 children and adolescents with type 1 diabetes. PLoS One. 2016;11(8):e0160971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noble M, Wright G, Smith G, Dibben C.Measuring multiple deprivation at the small-area level. Environ Plan. 2006;38:169-185. [Google Scholar]
- 18.Maier W, Scheidt-Nave C, Holle R, et al. Area level deprivation is an independent determinant of prevalent type 2 diabetes and obesity at the national level in Germany. Results from the National Telephone Health Interview Surveys ‘German Health Update’ GEDA 2009 and 2010. PLoS One. 2014;9(2):e89661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maier W.Indices of multiple deprivation for the analysis of regional health disparities in Germany: experiences from epidemiology and healthcare research. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2017;60(12):1403-1412. [DOI] [PubMed] [Google Scholar]
- 20.Rosario AS, Kurth BM, Stolzenberg H, Ellert U, Neuhauser H.Body mass index percentiles for children and adolescents in Germany based on a nationally representative sample (KiGGS 2003-2006). Eur J Clin Nutr. 2010;64(4):341-349. [DOI] [PubMed] [Google Scholar]
- 21.Cole TJ, Green PJ.Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11(10):1305-1319. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbauer J, Dost A, Karges B, et al. Improved metabolic control in children and adolescents with type 1 diabetes: a trend analysis using prospective multicenter data from Germany and Austria. Diabetes Care. 2012;35(1):80-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neu A, Burger-Busing J, Danne T, et al. Diagnosis, therapy and follow-up of diabetes mellitus in children and adolescents. Exp Clin Endocrinol Diabetes. 2019;127(Suppl 1):S39-S72. [DOI] [PubMed] [Google Scholar]
- 24.Bohn B, Rosenbauer J, Icks A, et al. Regional disparities in diabetes care for pediatric patients with type 1 diabetes. A cross-sectional DPV multicenter analysis of 24,928 German children and adolescents. Exp Clin Endocrinol Diabetes. 2016;124(2):111-119. [DOI] [PubMed] [Google Scholar]
- 25.Pulgaron ER, Sanders LM, Patino-Fernandez AM, et al. Glycemic control in young children with diabetes: the role of parental health literacy. Patient Educ Couns. 2014;94(1):67-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Buhr E, Tannen A. Parental health literacy and health knowledge, behaviours and outcomes in children: a cross-sectional survey. BMC Public Health. 2020;20(1):1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherr JL, Hermann JM, Campbell F, et al. Use of insulin pump therapy in children and adolescents with type 1 diabetes and its impact on metabolic control: comparison of results from three large, transatlantic paediatric registries. Diabetologia. 2016;59(1):87-91. [DOI] [PubMed] [Google Scholar]
- 28.McKergow E, Parkin L, Barson DJ, Sharples KJ, Wheeler BJ.Demographic and regional disparities in insulin pump utilization in a setting of universal funding: a New Zealand nationwide study. Acta Diabetol. 2017;54(1):63-71. [DOI] [PubMed] [Google Scholar]
- 29.Lipman TH, Hawkes CP.Racial and socioeconomic disparities in pediatric type 1 diabetes: time for a paradigm shift in approach. Diabetes Care. 2021;44(1):14-16. [DOI] [PubMed] [Google Scholar]
- 30.Icks A, Razum O, Rosenbauer J, et al. Lower frequency of insulin pump treatment in children and adolescents of Turkish background with type 1 diabetes: analysis of 21,497 patients in Germany. Diabetes Technol Ther. 2012;14(12):1105-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burgess DJ, Fu SS, van Ryn M.Why do providers contribute to disparities and what can be done about it? J Gen Intern Med. 2004;19(11):1154-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valenzuela JM, La Greca AM, Hsin O, Taylor C, Delamater AM.Prescribed regimen intensity in diverse youth with type 1 diabetes: role of family and provider perceptions. Pediatr Diabetes. 2011;12(8):696-703. [DOI] [PubMed] [Google Scholar]
- 33.Shah VN, Wu M, Polsky S, et al. Gender differences in diabetes self-care in adults with type 1 diabetes: findings from the T1D exchange clinic registry. J Diabetes Complications. 2018;32(10):961-965. [DOI] [PubMed] [Google Scholar]
- 34.Tanenbaum ML, Hanes SJ, Miller KM, Naranjo D, Bensen R, Hood KK.Diabetes device use in adults with type 1 diabetes: barriers to uptake and potential intervention targets. Diabetes Care. 2017;40(2):181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samuelsson U, Anderzen J, Gudbjornsdottir S, Steineck I, Akesson K, Hanberger L.Teenage girls with type 1 diabetes have poorer metabolic control than boys and face more complications in early adulthood. J Diabetes Complications. 2016;30(5):917-922. [DOI] [PubMed] [Google Scholar]
- 36.Lindner LME, Rathmann W, Rosenbauer J. Inequalities in glycaemic control, hypoglycaemia and diabetic ketoacidosis according to socio-economic status and area-level deprivation in Type 1 diabetes mellitus: a systematic review. Diabet Med. 2018;35:12-32. [DOI] [PubMed] [Google Scholar]