Abstract
Background:
International consensus recommends a set of continuous glucose monitoring (CGM) metrics to assess quality of diabetes therapy. The impact of individual CGM sensors on these metrics has not been thoroughly studied yet. This post hoc analysis aimed at comparing time in specific glucose ranges, coefficient of variation (CV) of glucose concentrations, and glucose management indicator (GMI) between different CGM systems and different sensors of the same system.
Method:
A total of 20 subjects each wore two Dexcom G5 (G5) sensors and two FreeStyle Libre (FL) sensors for 14 days in parallel. Times in ranges, GMI, and CV were calculated for each 14-day sensor experiment, with up to four sensor experiments per subject. Pairwise differences between different sensors of the same CGM system as well as between sensors of different CGM system were calculated for these metrics.
Results:
Pairwise differences between sensors of the same model showed larger differences and larger variability for FL than for G5, with some subjects showing considerable differences between the two sensors. When pairwise differences between sensors of different CGM models were calculated, substantial differences were found in some subjects (75th percentiles of differences of time spent <70 mg/dL: 5.0%, time spent >180 mg/dL: 9.2%, and GMI: 0.42%).
Conclusion:
Relevant differences in CGM metrics between different models of CGM systems, and between different sensors of the same model, worn by the same study subjects were found. Such differences should be taken into consideration when these metrics are used in the treatment of diabetes.
Keywords: continuous glucose monitoring, glucose management indicator, glycemic variability, time in range, CGM metrics
Introduction
Optimization of diabetes therapy helps people with diabetes mellitus in reducing the risk of short-term and long-term complications.1 The quality of diabetes therapy can be estimated from different parameters, for example, the amount of glycated hemoglobin (HbA1c) and the number of severe hypoglycemic episodes. When continuous glucose monitoring (CGM) is used in diabetes therapy, glucose levels can be tracked around the clock, providing some advantages over self-monitoring of blood glucose (BG) or HbA1c monitoring. As Agiostratidou et al,2 for example, point out in their 2017 consensus publication on measures beyond HbA1c, HbA1c does not capture short-term variations in glucose concentrations or exposure to hypo- and hyperglycemia, and it does not reflect glucose variability.
Time within specific glucose ranges is increasingly often used as a marker for glycemic control in patients with diabetes, with international consensus statements encouraging wider use as a more meaningful marker for glycemic control than HbA1c.3 According to international consensus statements,3,4 use of the following glucose ranges is recommended for nonpregnant persons with diabetes: <54 mg/dL (time below range [TbR] level 2), 54 to <70 mg/dL (TbR level 1), 70 to 180 mg/dL (time in range [TiR]), >180 to 250 mg/dL (time above range [TaR] level 1), and >250 mg/dL (TaR level 2). Some years ago, the estimated A1c was introduced to estimate HbA1c from CGM values. This parameter was recently updated and renamed “glucose management indicator” (GMI),5 and it is recognized as a valuable parameter for the assessment of diabetes therapy quality.6 Glycemic variability is an aspect of glycemic control, because glucose concentrations should not only be acceptable on average, but glycemic excursions should also be minimized.7 The coefficient of variation (CV) has been proposed as a marker for glycemic variability, with a threshold of 36% for categorization of stable (CV <36%) and unstable (CV ≥36%) variability.3
These parameters and the recommendation for their use are based on clinical evidence gathered over long periods with different models of CGM systems. Considering that the level of analytical performance varies between the different models of CGM systems, this raises the question how large the impact of an individual model of CGM systems on these parameters is. This question is currently not answered in international consensus statements.
The aim of this post hoc analysis was to assess TiR, TbR, TaR, GMI, and CV as markers for glycemic control based on data from two different models of CGM systems.
Materials and Methods
Data from a clinical trial comparing the measurement accuracy of two different CGM systems, whose major outcomes were previously published,8,9 were used to assess different CGM metrics as reported by the two different CGM systems. Metrics of interest were time in different glycemic ranges (TiR, TbR, and TaR), GMI, and CV as a marker for glycemic variability. The original trial was registered in the German Clinical Trial Register (“Deutsches Register Klinischer Studien,” DRKS) with the registration number DRKS00011920, an approved Primary Register in the WHO International Clinical Trials Registry Platform.
Study Design
In this study, 20 subjects with type 1 diabetes on either multiple daily injections or insulin pump were enrolled. Participants wore two devices each of two different CGM systems: the Dexcom G5 (G5) system (Dexcom Inc., San Diego, CA, USA) and the first-generation FreeStyle Libre (FL) system (Abbott Diabetes Care, Alameda, CA, USA). G5 is a real-time CGM system, displaying current glucose values every five minutes, whereas FL is an intermittently scanned CGM system that displays current values upon placing the reader near the sensor. Additionally, FL continuously stores glucose data every 15 minutes. The study design is described in detail in the main outcome publication.8
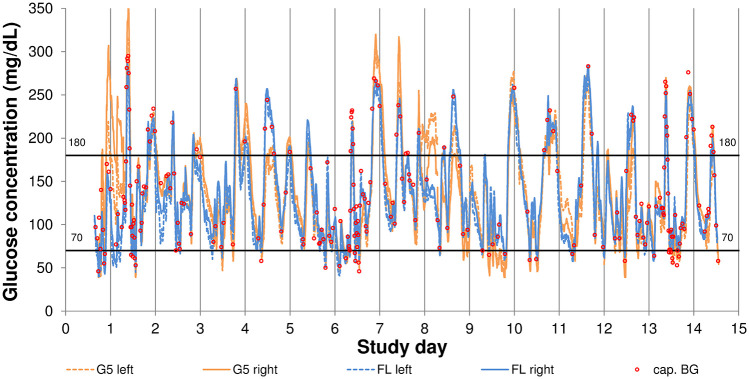
The devices were worn in parallel for 14 days each, and G5 sensors were replaced after seven days of use following manufacturers’ labeling. Following manufacturers’ labeling, G5 was worn on the abdomen and FL was worn on the arms. Application sites were labeled “left” and “right” to distinguish between the sensor experiments. The replacement G5 sensors applied after seven days were placed near the previous application site, but application sites did not overlap. A sensor experiment was defined as data from two successively worn G5 sensors at the same application site and as data from one individual FL sensor, respectively. An example graph is provided in Figure 1.
Figure 1.
Example of glucose concentration trace in one subject.
cap. BG, capillary blood glucose; FL, FreeStyle Libre; G5, Dexcom G5.
G5 was calibrated twice daily with BG values obtained with a FreeStyle Freedom Lite system (Abbott Diabetes Care). The BG monitoring system’s analytical performance was characterized before-hand in a study based on ISO 15197:2013. In that characterization, the system exhibited a bias according to Bland and Altman10 of −4.9% (95% limits of agreement: −13.1% to +3.3%) against a hexokinase-based laboratory analyzer, with 100% of results falling within ±15 mg/dL or ±15% of the comparison method result. Daily checks with Standard Reference Material 965b (National Institute of Standards and Technology, Gaithersburg, MD, USA) confirmed small bias (≤1.8%) and small imprecision (≤1.3%) of the laboratory analyzer.
All treatment decisions were based on capillary BG values.
Data Analysis
The continuously stored data from G5 and FL were downloaded and analyzed. Based on international consensus,3 data from participants were excluded from further analysis if they did not capture ≥80% of all possible glucose data points. The maximum possible number of data points was nmax,G5 = 4032 (14 days times 288 values per day) for G5, whereas it was nmax,FL = 1344 (14 days times 96 values per day) for FL.
Time within specific glucose ranges was calculated as the number of data points within the specific glucose range divided by the total number of data points for each sensor experiment (see above), separately, in agreement with a recent international consensus.3,4 The following times in ranges were calculated: <54 mg/dL (TbR<54), ≥54 to <70 mg/dL (TbR54-<70), <70 mg/dL (TbR<70), ≥70 to ≤180 mg/dL (TiR70-180), >180 mg/dL (TaR>180), >180 and ≤250 mg/dL (TaR>180-250), and >250 mg/dL (TaR>250).
GMI was calculated as outlined by Bergenstal et al based on each individual sensor experiment’s mean glucose value.5
CV was also calculated for each sensor experiment separately as the standard deviation of that experiment’s glucose concentrations divided by their mean value.
Descriptive statistics were calculated for all of these parameters: minimum, 25th percentile, median, 75th percentile, and maximum. In addition, CV was categorized as indicating stable (CV <36%) or unstable (CV ≥36%) glycemic variability, in agreement with international consensus statements.3
Data from all sensor experiments were analyzed separately. In an additional analysis, the absolute values of paired differences between TbR/TiR/TaR/GMI/CV of the same type of CGM system (ie, “left” G5 vs “right” G5 in the same subject and “left” FL vs “right” FL in the same subject) as well as between the two types of CGM in the same subject (ie, “left” G5 vs “left” FL, “left” G5 vs “right” FL, “right” G5 vs “left” FL, and “right” G5 vs “right” FL) were calculated.
Although differences are provided in percent, they do relate to differences calculated in the unit of expression, which is percent for TiR, GMI, and CV, that is, percentage points, and they do not indicate relative changes.
Results
Comparison of CGM Metrics Separated by CGM System Model
For G5, 39 out of 40 possible sensor experiments were analyzed, whereas 34 out of 40 FL sensor experiments were analyzed. Detailed results are provided in Table 1.
Table 1.
Summary of CGM Metrics, Including Time in Various Glycemic Ranges, GMI, and CV (Also Categorized as Either Stable or Unstable), Based on Individual Sensor Experiments.
| CGM metric | G5 |
FL |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min. | 25th percentile | Median | 75th percentile | Max. | n | Min. | 25th percentile | Median | 75th percentile | Max. | n | |
| Mean CGM glucose (mg/dL) | 114.0 | 131.6 | 142.4 | 157.2 | 211.0 | 39 | 93.6 | 130.4 | 140.6 | 156.9 | 182.4 | 34 |
| SD of CGM glucose (mg/dL) | 32.8 | 47.7 | 55.8 | 64.7 | 76.2 | 39 | 38.6 | 48.5 | 56.9 | 64.3 | 76.6 | 34 |
| CV of CGM glucose | 27.2% | 35.7% | 37.9% | 41.2% | 43.8% | 39 | 29.2% | 35.5% | 39.1% | 42.1% | 53.7% | 34 |
| TbR<70 | 1.1% | 2.9% | 5.3% | 7.3% | 12.3% | 39 | 0.3% | 2.9% | 5.5% | 9.3% | 39.3% | 34 |
| TiR70-180 | 32.3% | 60.5% | 70.9% | 77.1% | 91.7% | 39 | 51.7% | 60.2% | 67.3% | 77.0% | 87.5% | 34 |
| TaR>180 | 3.4% | 15.3% | 22.1% | 31.8% | 65.5% | 39 | 5.8% | 15.1% | 22.4% | 32.1% | 47.7% | 34 |
| TbR<54 | 0.0% | 0.3% | 1.0% | 2.0% | 4.0% | 39 | 0.0% | 0.4% | 1.0% | 2.4% | 23.9% | 34 |
| TbR54-<70 | 0.9% | 2.5% | 4.0% | 5.1% | 8.4% | 39 | 0.3% | 2.6% | 5.0% | 6.5% | 15.4% | 34 |
| TaR>180-250 | 3.1% | 12.9% | 18.2% | 23.3% | 39.0% | 39 | 5.8% | 12.7% | 18.4% | 23.6% | 30.6% | 34 |
| TaR>250 | 0.1% | 2.1% | 4.7% | 9.0% | 28.9% | 39 | 0.0% | 1.9% | 4.5% | 8.6% | 18.4% | 34 |
| GMI | 6.04% | 6.46% | 6.72% | 7.07% | 8.36% | 39 | 5.55% | 6.43% | 6.67% | 7.06% | 7.67% | 34 |
| CV category | Stable: | 12 | Unstable: | 27 | Stable: | 10 | Unstable: | 24 | ||||
Abbreviations: CGM, continuous glucose monitoring; CV, coefficient of variation; FL, FreeStyle Libre; G5, Dexcom G5; GMI, glucose management indicator; SD, standard deviation; TaR, time above range; TbR, time below range; TiR, time in range.
TbR<70, time spent <70 mg/dL; TiR70-180, time spent between 70 and 180 mg/dL; TaR>180, time spent >180 mg/dL; TbR<54, time spent <54 mg/dL; TbR54-<70, time spent ≥54 and <70 mg/dL; TaR>180-250, time spent >180 and ≤250 mg/dL; and TaR>250, time spent >250 mg/dL.
Although the median TbR<70 was similar between G5 and FL (5.3% vs 5.5%, corresponding to ~80 minutes per day), the range of TbR values was considerably different. For example, the 75th percentile of TbR<70 was 7.3% (105 minutes per day) for G5 and 9.3% (133 minutes) for FL. Maximum TbR<70 was even more markedly different. G5 showed slightly higher TiR70-180 than FL as minimum, median, and maximum values for TiR70-180 were larger for G5 than for FL.
GMI values were comparable for at least 50% of subjects (indicated by the 25th and 75th percentiles), although G5 provided a slightly wider range of GMI values than FL.
Categorizing sensor experiments into “stable” and “unstable” glycemic variability based on CV showed that this categorization was similar between G5 and FL.
Paired Differences of CGM Metrics Within the Same CGM System Model
In total, pairwise comparison of the two sensor experiments captured by the same CGM system model was possible for 19 subjects for G5 and for 15 subjects for FL. Detailed results are shown in Table 2.
Table 2.
Summary of Paired Differences in CGM Metrics Between Sensors of the Same Model Within the Same Subject.
| CGM metric | G5 |
FL |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min. | 25th percentile | Median | 75th percentile | Max. | n | Min. | 25th percentile | Median | 75th percentile | Max. | n | |
| Mean CGM glucose (mg/dL) | 0.1 | 1.6 | 3.6 | 4.8 | 7.2 | 19 | 1.4 | 4.4 | 8.2 | 13.6 | 45.6 | 15 |
| SD of CGM glucose (mg/dL) | 0.0 | 0.8 | 1.7 | 4.0 | 10.5 | 19 | 0.1 | 1.5 | 2.1 | 5.8 | 19.3 | 15 |
| CV of CGM glucose | 0.1% | 0.4% | 0.8% | 1.8% | 6.4% | 19 | 0.2% | 1.0% | 1.8% | 2.5% | 3.7% | 15 |
| TbR<70 | 0.1% | 0.4% | 0.5% | 1.1% | 1.8% | 19 | 0.0% | 1.5% | 2.6% | 5.1% | 22.8% | 15 |
| TiR70-180 | 0.0% | 0.9% | 1.7% | 2.3% | 5.9% | 19 | 0.1% | 1.4% | 2.2% | 4.3% | 10.4% | 15 |
| TaR>180 | 0.1% | 0.8% | 1.6% | 2.3% | 4.6% | 19 | 0.6% | 2.1% | 4.0% | 5.0% | 15.5% | 15 |
| TbR<54 | 0.0% | 0.1% | 0.3% | 0.6% | 1.2% | 19 | 0.0% | 0.3% | 1.0% | 1.4% | 14.1% | 15 |
| TbR54-<70 | 0.0% | 0.2% | 0.3% | 0.6% | 1.2% | 19 | 0.2% | 1.0% | 2.0% | 3.3% | 8.7% | 15 |
| TaR>180-250 | 0.3% | 0.7% | 1.7% | 1.9% | 2.6% | 19 | 0.1% | 1.3% | 1.9% | 4.7% | 14.8% | 15 |
| TaR>250 | 0.1% | 0.3% | 0.7% | 2.2% | 4.0% | 19 | 0.1% | 0.8% | 1.5% | 2.0% | 6.9% | 15 |
| GMI | 0.00% | 0.04% | 0.09% | 0.11% | 0.17% | 19 | 0.03% | 0.11% | 0.20% | 0.33% | 1.09% | 15 |
| CV category identical? | Yes: | 15 | No: | 4 | Yes: | 15 | No: | 0 | ||||
Differences for time spent in various glycemic ranges, for glucose management indicator (GMI), and for coefficient of variation (CV) are shown as absolute changes (ie, percentage points). CV was categorized as either stable or unstable.
Abbreviations: CGM, continuous glucose monitoring; FL, FreeStyle Libre; G5, Dexcom G5; SD, standard deviation; TaR, time above range; TbR, time below range; TiR, time in range.
TbR<70, time spent <70 mg/dL; TiR70-180, time spent between 70 and 180 mg/dL; TaR>180, time spent >180 mg/dL; TbR<54, time spent <54 mg/dL; TbR54-<70, time spent ≥54 and <70 mg/dL; TaR>180-250, time spent >180 and ≤250 mg/dL; and TaR>250, time spent >250 mg/dL.
For FL, the median paired difference in the different TbR, TiR, and TaR measures was 1.0% to 4.0% (14 to 57 minutes per day), whereas for G5, it was considerably smaller. The 75th percentile for differences in these measures ranged from 0.6% to 2.3% for G5, and from 1.4% to 5.1% for FL. Variability in differences in TiR, TbR, and TaR measures was markedly more pronounced in FL than in G5. An example graph for the impact of the model of CGM system on TbR<70 is shown in Figure 2.
Figure 2.
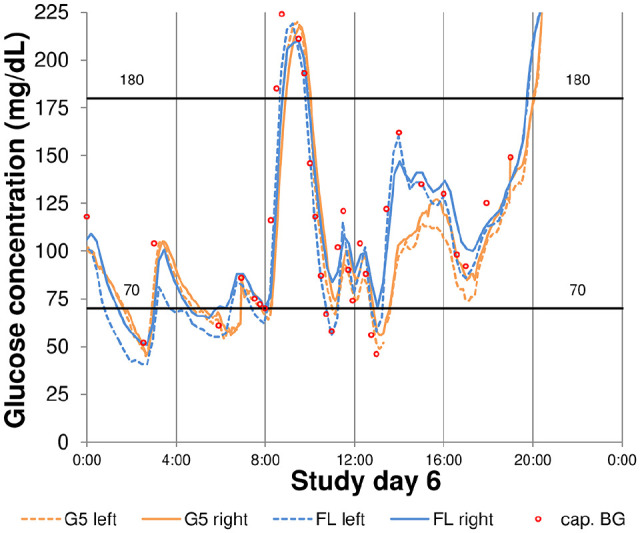
Example for relevant differences in time below 70 mg/dL between different models of CGM systems and sensors.
cap. BG, capillary blood glucose; FL, FreeStyle Libre; G5, Dexcom G5.
Paired differences for GMI also showed larger variability for FL than for G5. The 75th percentile for differences in GMI was 0.11% for G5 and 0.33% for FL (absolute differences).
When CV values were categorized into “stable” and “unstable” glycemic variability, however, the categorization was independent from the individual sensor for FL, whereas in four subjects, G5 values indicated different categories. Paired differences in CV as measured by the G5 sensors in these four subjects ranged from 1.3% to 6.4% (absolute differences).
Paired Differences of CGM Metrics Between Different CGM System Models
For a total of 66 pairings, paired differences were calculated. Detailed results are shown in Table 3.
Table 3.
Summary of Paired Differences in CGM Metrics Between All Possible Combinations of Dexcom G5 Sensor Experiments and FreeStyle Libre Sensor Experiments Within the Same Subject.
| CGM metric | All |
|||||
|---|---|---|---|---|---|---|
| Min. | 25th percentile | Median | 75th percentile | Max. | n | |
| Mean CGM glucose (mg/dL) | 0.1 | 5.5 | 9.0 | 17.4 | 47.1 | 66 |
| SD of CGM glucose (mg/dL) | 0.1 | 2.9 | 4.6 | 7.9 | 15.9 | 66 |
| CV of CGM glucose | 0.0% | 1.0% | 2.8% | 4.1% | 9.9% | 66 |
| TbR<70 | 0.2% | 1.1% | 2.4% | 5.0% | 32.5% | 66 |
| TiR70-180 | 0.1% | 2.2% | 4.8% | 7.8% | 21.5% | 66 |
| TaR>180 | 0.3% | 3.1% | 5.6% | 9.2% | 17.3% | 66 |
| TbR<54 | 0.0% | 0.3% | 1.0% | 1.9% | 22.2% | 66 |
| TbR54-<70 | 0.0% | 0.6% | 1.4% | 2.8% | 10.2% | 66 |
| TaR>180-250 | 0.3% | 2.1% | 3.5% | 6.1% | 14.8% | 66 |
| TaR>250 | 0.0% | 0.8% | 2.0% | 3.9% | 8.2% | 66 |
| GMI | 0.00% | 0.13% | 0.21% | 0.42% | 1.13% | 66 |
| CV category identical? | Yes: | 52 | No: | 14 | ||
Differences for time spent in various glycemic ranges, for glucose management indicator (GMI), and for coefficient of variation (CV) are shown as absolute changes (ie, percentage points). CV was categorized as either stable or unstable.
Abbreviations: CGM, continuous glucose monitoring; SD, standard deviation; TaR, time above range; TbR, time below range; TiR, time in range.
TbR<70, time spent <70 mg/dL; TiR70-180, time spent between 70 and 180 mg/dL; TaR>180, time spent >180 mg/dL; TbR<54, time spent <54 mg/dL; TbR54-<70, time spent ≥54 and <70 mg/dL; TaR>180-250, time spent >180 and ≤250 mg/dL; and TaR>250, time spent >250 mg/dL.
Although there was at least one subject in which two sensor experiments from different CGM system models yielded near-identical results, median TbR<70 and TaR>180 differed by 2.4% and 5.6%, respectively, corresponding to 35 and 81 minutes per day, respectively. The maximum paired difference (ie, the worst-case scenario of this pairwise comparison) in TiR, TbR, and TaR differed by 17.3% to 32.5%, corresponding to more than 4 hours and more than 7.5 hours per day, respectively. GMI differed by up to 1.13% (absolute differences), with a 75th percentile of 0.42%.
In nearly 80% of paired comparisons, CV categorization yielded the same result. For those comparisons where CV categorization yielded different results, the paired differences in CV ranged from 1.9% to 7.8% (absolute differences).
Discussion
CGM provides the opportunity of assessing quality of diabetes therapy through multiple different metrics. However, factors that influence the reliability of such assessments should be kept in mind. In a study comparing metrics based on SMBG and on CGM data, Avari et al found significant differences in TiR.11 While their finding is influenced by glucose sensing modalities and calculation procedures, their work raises the question whether relevant differences also exist between different models of CGM system. This post hoc analysis of CGM data indicates that the specific model of CGM system is one of these influencing factors. Michalak et al12 came to a similar conclusion analyzing data from children with type 1 diabetes who used two different models of CGM system. In addition, this post hoc analysis indicates that there might even be relevant sensor-to-sensor differences when using the same model of CGM system.
In this study, G5 showed smaller differences in CGM metrics TbR/TiR/TaR, GMI, and CV between sensors of the same model worn in parallel by the same subject than FL. This might be influenced by G5 being manually calibrated every 12 hours, so that twice daily, glucose levels as shown by G5 were forcefully matched. This was not possible for FL, which is factory-calibrated. Interestingly, the categorization of glucose control as “stable” or “unstable” based on CV was different for some subjects depending on which G5 sensor’s data were used, but this was not the case for FL. Considering that the international consensus on glycemic targets3 states that patients with type 1 and type 2 diabetes should spend less than 4% of the time at glucose concentrations below 70 mg/dL, median differences in that metric of only 2.4%, as found for the comparison between different models of CGM systems, can lead to relevant adjustments of diabetes therapy, solely based on the model of CGM system used. Generally, HbA1c changes of 0.5% are considered clinically relevant,13 although, sometimes, smaller changes may lead to therapy adjustments.14 In this context, the 75th percentile for differences in GMI between different models of CGM systems of 0.42% (absolute differences) indicates that in a relevant number of cases, therapy adjustments might have been performed, again, solely based on the model of CGM system used.
HbA1c is primarily used to assess quality of long-term glycemic control. Additionally, since HbA1c was shown to correlate with clinical outcomes in the landmark Diabetes Control and Complications Trial,15 it has been established as surrogate parameter for a range of other endpoints. Its increasing use as outcome parameter in past clinical studies resulted in large amounts of clinical evidence. However, different mechanisms that are known to affect HbA1c, for example, lifespan of erythrocytes, or blood disorders, have to be kept in mind,16,17 and there seems to be considerable variation among HbA1c assays.18 For TiR, as well as for GMI, such large amounts of data have to be gathered in the future, and influencing factors have to be studied. Transferring the clinical evidence gathered for HbA1c to TiR and/or GMI might not be adequate or possible, for example, because of the inexact relationship between HbA1c and TiR as well as HbA1c and GMI,19 and the influencing factors that compound all of these parameters. Whereas there are standardized methods and procedures to measure HbA1c, such standardization is still missing for CGM systems. While some CGM systems are calibrated with self-monitored BG values in capillary blood, other systems seem to exhibit smaller measurement differences when compared to venous BG.20 Reference methods that directly measure in the interstitial fluid have yet to be established, so that traceability to higher order methods and materials remains unclear. In this study, G5 was manually calibrated per manufacturer’s labeling using a FreeStyle Freedom Lite system. Selecting a BG monitoring system manufactured by the same company as FL did likely lead to a reduction in systematic differences in glucose concentrations (“bias”) between G5 and FL. If a BG monitoring system manufactured by another company was used, or a BG monitoring system with more/less bias against the hexokinase method, the differences in CGM metrics between G5 and FL might have been more pronounced. In light of these influences of CGM performance on perceived quality of therapy, there is a clear need for standardization of the output of CGM systems. Efforts are currently made, for example, by the IFCC Working Group on CGM as well as the Clinical and Laboratory Standards Institute (CLSI), who called for an update of its POCT05 guideline.21
These issues have to be handled adequately, so that physicians can make maximum use of CGM systems and the metrics derived from CGM datasets. Additionally, user handling of CGM systems has to be taken into consideration. For example, manually calibrated CGM systems, like G5 in the presented study, should be calibrated according to manufacturer’s instructions, that is, during times of sufficiently stable glucose concentrations, at least twice daily, and with high-quality BG values. The FL system used in this study had to be scanned at least once every eight hours, otherwise data gaps could occur, because only the last eight hours’ worth of continuous data were stored. If FL users liked to sleep longer than eight hours, this would result in systematic gaps in CGM data in the early night, which could affect reliability of derived CGM metrics.
Some limitations apply to this study. The total duration of 14 days, while sufficient in the context of the international consensus statement on the use of CGM, was comparably short. In addition, few individual FL sensors did exhibit considerable deviations from the BG monitoring system’s results. Users who perform confirmatory BG measurements might have prematurely replaced these sensors in their daily life. Another potential influence is that the G5 system used in the study was manually calibrated. Although subjects were trained in the use of the system, and 6 of the 14 study days were performed in-clinic with supervised calibrations,8 the possibility of erroneous calibrations cannot be fully excluded. A larger number of subjects, that is, more than the 20 subjects in this study, would allow for better generalization of this study’s findings.
In summary, this post hoc analysis revealed relevant differences in CGM metrics between different models of CGM systems, and between different sensors of the same model, worn by the same study subjects. Such differences should be taken into consideration when these metrics are used in the treatment of diabetes. Furthermore, efforts should be made to standardize measurement methods and traceability in CGM systems, so that such differences are minimized as best as possible.
Acknowledgments
The authors would like to thank the participants of the study, as well as IDT study staff, for their contribution to the study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: GF is general manager of the Institut für Diabetes-Technologie Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm (IDT), Ulm, Germany, which carries out clinical studies on the evaluation of BG meters and medical devices for diabetes therapy on its own initiative and on behalf of various companies. GF/IDT have received speakers’ honoraria or consulting fees from Abbott, Ascensia, Dexcom, i-SENS, LifeScan, Menarini Diagnostics, Metronom Health, Novo Nordisk, PharmaSense, Roche, Sanofi, Sensile, and Ypsomed. SP, UK, DW, ML, EZ, NJ, and CH are employees of IDT or were employees at the time the study was conducted.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors thank Roche Diabetes Care Deutschland GmbH for the financial support of this investigator-initiated trial.
ORCID iDs: Stefan Pleus  https://orcid.org/0000-0003-4629-7754
https://orcid.org/0000-0003-4629-7754
Delia Waldenmaier  https://orcid.org/0000-0003-3280-2369
https://orcid.org/0000-0003-3280-2369
Guido Freckmann  https://orcid.org/0000-0002-0406-9529
https://orcid.org/0000-0002-0406-9529
References
- 1.Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. [DOI] [PubMed] [Google Scholar]
- 2.Agiostratidou G, Anhalt H, Ball D, et al. Standardizing clinically meaningful outcome measures beyond HbA1c for type 1 diabetes: a consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care. 2017;40(12):1622-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergenstal RM, Beck RW, Close KL, et al. Glucose management indicator (GMI): A new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018;41(11):2275-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Standards of medical care in diabetes—2019. Diabetes Care. 2019;24(suppl 1):S1-S193. [PubMed] [Google Scholar]
- 7.Hirsch IB. Glycemic variability and diabetes complications: does it matter? Of course it does! Diabetes Care. 2015;38(8):1610-1614. [DOI] [PubMed] [Google Scholar]
- 8.Freckmann G, Link M, Pleus S, Westhoff A, Kamecke U, Haug C. Measurement performance of two continuous tissue glucose monitoring systems intended for replacement of blood glucose monitoring. Diabetes Technol Ther. 2018;20(8):541-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freckmann G, Link M, Westhoff A, Kamecke U, Pleus S, Haug C. Prediction quality of glucose trend indicators in two continuous tissue glucose monitoring systems. Diabetes Technol Ther. 2018;20(8):550-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327(8476):307-310. [PubMed] [Google Scholar]
- 11.Avari P, Uduku C, George D, Herrero P, Reddy M, Oliver N. Differences for percentage times in glycemic range between continuous glucose monitoring and capillary blood glucose monitoring in adults with type 1 diabetes: analysis of the REPLACE-BG dataset. Diabetes Technol Ther. 2020;22(3):222-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michalak A, Pagacz K, Mlynarski W, Szadkowska A, Fendler W. Discrepancies between methods of continuous glucose monitoring in key metrics of glucose control in children with type 1 diabetes. Pediatr Diabetes. 2019;20(5):604-612. [DOI] [PubMed] [Google Scholar]
- 13.Little RR, Rohlfing CL. The long and winding road to optimal HbA1c measurement. Clin Chim Acta. 2013;418:63-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenters-Westra E, Schindhelm RK, Bilo HJ, Groenier KH, Slingerland RJ. Differences in interpretation of haemoglobin A1c values among diabetes care professionals. Neth J Med. 2014;72(9):462-466. [PubMed] [Google Scholar]
- 15.Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328(23):1676-1685. [DOI] [PubMed] [Google Scholar]
- 16.Heinemann L, Freckmann G. Quality of HbA1c measurement in the practice: the German perspective. J Diabetes Sci Technol. 2015;9(3):687-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malka R, Nathan DM, Higgins JM. Mechanistic modeling of hemoglobin glycation and red blood cell kinetics enables personalized diabetes monitoring. Sci Transl Med. 2016;8(359):359ra130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.EurA1c Trial Group. EurA1c: the European HbA1c trial to investigate the performance of HbA1c assays in 2166 laboratories across 17 countries and 24 manufacturers by use of the IFCC model for quality targets. Clin Chem. 2018;64(8):1183-1192. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch IB, Welsh JB, Calhoun P, Puhr S, Walker TC, Price DA. Associations between HbA1c and continuous glucose monitoring-derived glycaemic variables. Diabet Med. 2019;36(12):1637-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freckmann G, Link M, Kamecke U, Haug C, Baumgartner B, Weitgasser R. Performance and usability of three systems for continuous glucose monitoring in direct comparison. J Diabetes Sci Technol. 2019;13(5):890-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. POCT05-A. Performance Metrics for Continuous Interstitial Glucose Monitoring. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. [Google Scholar]