Abstract
Endocannabinoids are key bioactive components of the endocannabinoid system, and the profound influence of endocannabinoids on the modulation of the immune system is being increasingly appreciated. The knowledge of endocannabinoid–immune cell crosstalk will pave the way to therapeutic implications of modulators of this pathway in autoimmune and chronic inflammatory disorders. Endocannabinoids seem to exert both anti‐inflammatory and pro‐inflammatory effects in specific contexts, based on specific receptor engagement and the downstream signalling pathways involved. In this review, we summarized the biosynthesis, signalling and degradation of two well‐studied endocannabinoids—anandamide and 2‐arachidonylglycerol in immune cells. Then, we discussed the effects of these two endocannabinoids on the functioning of major innate and adaptive immune cells, along with the choice of receptors employed in such interactions. Finally, we outline our current knowledge on the involvement of anandamide and 2‐arachidonylglycerol in context of inflammation, allergies, autoimmunity and metabolic disorders.
Keywords: 2‐AG, anandamide, autoimmunity, CB2, endocannabinoids, immune cells, inflammation, metabolic disorder
Endocannabinoids are key bioactive components of the endocannabinoid system, and the profound influence of endocannabinoids on the modulation of the immune system is being increasingly appreciated. Endocannabinoids seem to exert both anti‐inflammatory and pro‐inflammatory effects in specific contexts; thus, knowledge on endocannabinoid–immune cell crosstalk will pave the way to therapeutic implications of modulators of this pathway in autoimmune and chronic inflammatory disorders.

Abbreviations
- 2‐AG
2‐arachidonylglycerol
- AA
arachidonic acid
- ABHD12
α/β‐hydrolase domain‐containing protein‐12
- ABHD6
α/β‐hydrolase domain‐containing protein‐6
- AEA
N‐arachidonylethanolamine
- CB1
cannabinoid receptor 1
- CB2
cannabinoid receptor 2
- CNS
central nervous system
- COX‐2
cyclooxygenase‐2
- DAGL
diacylglycerol lipase
- DCs
dendritic cells
- DTH
delayed‐type hypersensitivity
- ECS
endocannabinoid system
- FAAH
fatty acid amide hydrolase
- FLS
fibroblast‐like synoviocytes
- GPR55
G protein‐coupled receptor 55
- GTP
guanosine triphosphate
- LOX
lipoxygenases
- LPS
lipopolysaccharides
- MAGL
monoacylglycerol lipase
- MDSCs
myeloid‐derived suppressor cells
- MPO
myeloperoxidase
- MS
multiple sclerosis
- MZ
marginal zone
- NAAA
N‐acylethanolamine‐selective acid amidase
- NAPE
N‐acyl‐phosphatidylethanolamine
- NAPE‐PLD
N‐acyl‐phosphatidylethanolamine phospholipase D
- OLDA
N‐Oleoyldopamine
- PAF
platelet‐activating factor
- PDC
plasmacytoid dendritic cell
- PEA
palmitoylethanolamide
- PGE‐2
prostaglandin‐E2
- PPARγ
peroxisome proliferator‐activated receptor gamma
- PTPN22
protein tyrosine phosphatase non‐receptor type 22
- RA
rheumatoid arthritis
- SLE
systemic lupus erythematosus
- SRBC
sheep red blood cells
- THC
tetrahydrocannabinol
- TLR
Toll‐like receptor
- TNF‐α
Tumour necrosis factor alpha
- TRPV1
transient receptor potential cation channel subfamily V member 1
AN OVERVIEW OF THE ENDOCANNABINOID SYSTEM
The endocannabinoid system (ECS) modulates critical physiological functions such as pain, satiety, fear, memory and inflammation. The major targets of ECS include the brain, spinal cord, adipocytes, hepatocytes, endothelial cells and the immune system. Bioactive lipid molecules termed endocannabinoids (or endogenous cannabinoids), enzymes responsible for their biosynthesis and degradation, and the endocannabinoid receptors driving their downstream signalling essentially constitute the ECS [1].
The knowledge of ECS commenced with the elucidation of the structure, function and metabolism of the phytocannabinoid Δ9‐tetrahydrocannabinol (THC), the major component of the marijuana plant [2]. THC specifically reduced cyclic‐AMP accumulation with a concomitant decrease in membrane adenylate cyclase activity in resting and prostacyclin‐treated neuroblastoma cells [3]. This enhanced in the presence of GTP but drastically dampened on treatment with a non‐hydrolysable GTP analogue or pertussis toxin [4, 5]. Eventually, cannabinoid receptors—cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2)—were discovered, which are G protein‐coupled receptors utilizing Gi/o proteins for downstream signalling [6, 7]. Subsequently, endogenous molecules with structural and functional similarity to THC were discovered and named ‘endocannabinoids’.
N‐arachidonylethanolamine (AEA) or anandamide (named after the Sanskrit word ‘Ananda’ meaning bliss) was the first endocannabinoid discovered from porcine brain extracts [8]. The second endocannabinoid molecule 2‐arachidonylglycerol (2‐AG) was first isolated from the canine gut [9]. 2‐AG is present at 170 times higher concentration than anandamide in the brain lysate [10] and displays higher binding affinities for both CB1 and CB2 compared with anandamide [11]. Other endocannabinoids were identified subsequently based on CB1/CB2 affinity, viz. noladin ether [12], virodhamine [13], N‐arachidonoyl dopamine [14] and OLDA [15]. For instance, virodhamine shows high affinity to CB2 and acts as partial antagonist for CB1 [13]. This review will focus on the current knowledge of immunomodulation by the two major and best‐characterized endocannabinoids—anandamide and 2‐AG.
BIOSYNTHESIS, DEGRADATION AND INDUCTION OF ENDOCANNABINOIDS IN IMMUNE CELLS
Endocannabinoids are synthesized from membrane precursors in response to stimuli, unlike neurotransmitters that are pre‐formed and stored in vesicles, although anandamide can also be stored inside cells [16, 17]. There are different pathways for anandamide biosynthesis, and N‐acyl‐phosphatidylethanolamine (NAPE) acts as the precursor for all of them [1]. The most studied pathway involves the action of phospholipase D (NAPE‐PLD) on NAPE to produce anandamide and phosphatidic acid [18, 19]. NAPE‐PLD is a member of the β‐lactamase family of hydrolases, which is stimulated by calcium and highly expressed in the brain, spleen, kidney, liver, lung and heart [20, 21]. The second pathway is operative in immune cells, wherein NAPE‐specific phospholipase C produces phospho‐anandamide from NAPE, followed by dephosphorylation by PTPN22 [22]. Palmitoylethanolamide (PEA) is an endocannabinoid‐like molecule with anti‐inflammatory properties, synthesized along with anandamide. It potentiates anandamide action by increasing receptor affinity or reducing degradation of anandamide, known as ‘entourage effect’ [23]. Biosynthesis of 2‐AG involves the membrane phospholipid 1‐stearoyl‐2‐arachidonoyl‐sn‐glycerol being hydrolysed by PLCβ followed by further cleavage of the product 1,2‐diacylglycerol by diacylglycerol lipase (DAGL) to yield 2‐AG [24, 25]. There exist two isoforms of DAGL, viz. DAGLα in the central nervous system (CNS) [26] and DAGLβ in immune cells [27](Figure 1).
FIGURE 1.
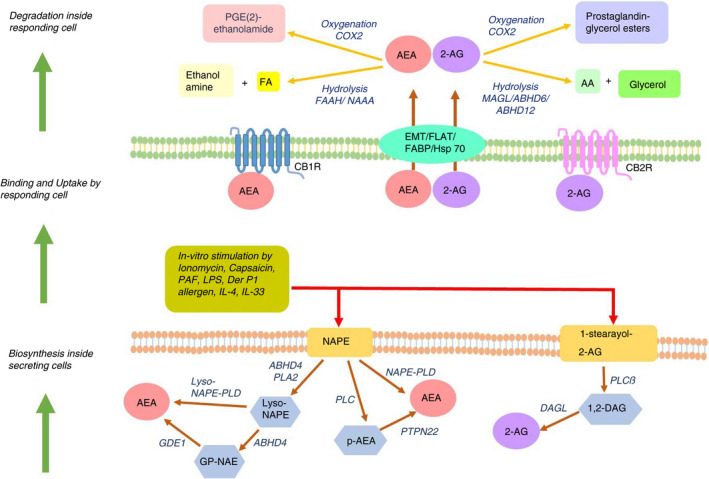
Biosynthesis, uptake and catabolism of the endocannabinoids—anandamide (AEA) and 2‐AG. Lower panel shows synthesis of endocannabinoids in immune cells in response to different stimuli and through different pathways for different endocannabinoids. Middle panel shows the binding and signalling of endocannabinoids thus released in the responding cells mostly through the cannabinoid receptors CB1R and CB2R, which are G protein‐coupled receptors. Top panel shows the hydrolysis or oxygenation of endocannabinoids in the cytoplasm of responding cells, thus terminating the direct effect of the endocannabinoids
Lipopolysaccharides (LPS), platelet‐activating factor (PAF), the TRPV1‐agonist capsaicin, Δ9‐THC and anandamide itself enhance anandamide synthesis in mouse macrophage cells RAW264·7 [28, 29]. Microglia polarized towards anti‐inflammatory M2 phenotype with IL‐4 and IL‐33 produces 2‐AG and anandamide, with elevated expression of DAGLα and CB2, while endocannabinoid signalling genes are downregulated in pro‐inflammatory M1 macrophages [30]. A functional ECS is present in dendritic cells (DCs), in terms of expression of CB1, CB2 and fatty acid amide hydrolase (FAAH). Stimulation of immature monocyte‐derived DCs with LPS or Derp1 allergen induces synthesis of 2‐AG, which is further enhanced in mature DCs [31]. In murine models of delayed‐type hypersensitivity (DTH) and strong antibody response, T cells and B cells produce significant levels of 2‐AG [32] (Figure 1).
Circulating concentrations of anandamide and 2‐AG have been reliably measured in human serum by high‐pressure liquid chromatography–tandem mass spectrometry [33, 34]. Although systemic endocannabinoid concentration is extremely dynamic and depends on multiple factors [35], physiological levels of serum anandamide range from 1 to 5 nM, while serum 2‐AG concentration varies broadly between 10 and 500 nM [34]. The physiological level of anandamide and 2‐AG is controlled by endocannabinoid‐degrading enzymes. Anandamide is primarily degraded by FAAH, a serine hydrolase active at alkaline pH [36]. N‐acylethanolamine‐selective acid amidase (NAAA) is another anandamide catabolic enzyme, a cysteine hydrolase working in acidic pH [37]. PEA serves as a specific substrate of NAAA, thereby interfering and reducing anandamide degradation [38]. Hydrolysis of 2‐AG into arachidonic acid (AA) is carried out by three serine hydrolases, viz. monoacylglycerol lipase (MAGL), α/β‐hydrolase domain‐containing protein‐6 (ABHD6) and α/β‐hydrolase domain‐containing protein‐12 (ABHD12). MAGL is the major 2‐AG‐hydrolysing enzyme in the CNS [39]—it contributes to 85% of hydrolytic activity in rodent brains, while ABHD6 and ABHD12 account for the rest [40, 41]. Anandamide and 2‐AG are also substrates for cyclooxygenase‐2 (COX‐2), lipoxygenases (LOX) and cytochrome P450 monooxygenases producing prostaglandin/prostaglandin esters, epoxy derivatives and hydroxyl derivatives respectively, which exerts varied physiological functions [42] (Figure 1).
ROLE OF ENDOCANNABINOIDS ON MAJOR IMMUNE CELLS
THC and cannabinoid receptors are long known to have immunomodulatory potential. CB1 is expressed at a modest level in human B cells, NK cells, monocytes, neutrophils, T cells and differentiated macrophages [43, 44], while being abundant in the CNS. CB2 is expressed predominantly in the immune cells, around 10–100 times higher than CB1 [43, 45]. Evidence for expression of the cannabinoid receptors on immune cells and reports on immunomodulatory effects of THC through these receptors (not reviewed here) led to studies on roles of endocannabinoids in the innate and adaptive immunity [46, 47]. Here, we curated the notable studies on immunomodulatory roles of anandamide and 2‐AG on the major immune cells (Figure 2).
FIGURE 2.
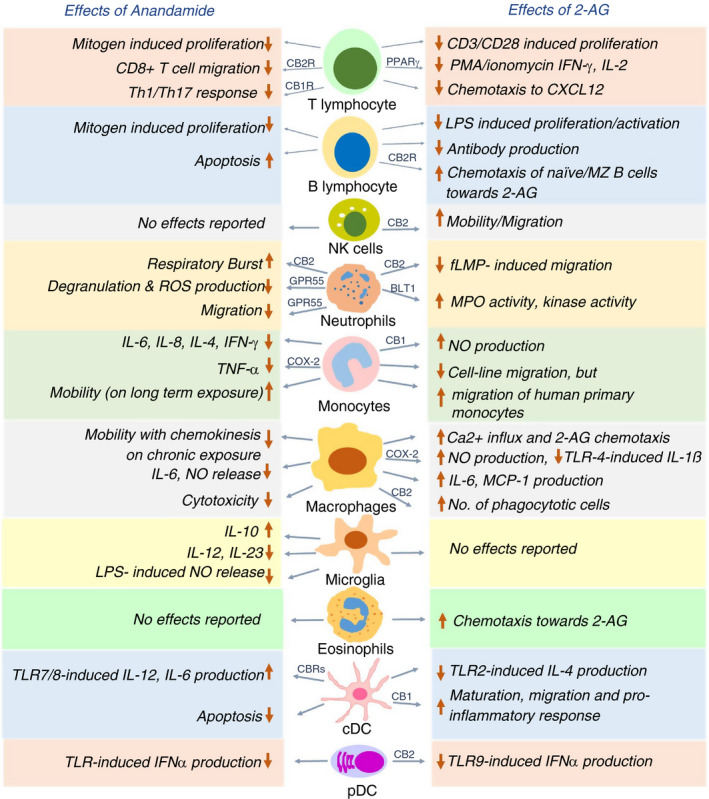
Effects of anandamide and 2‐AG on various immune cells in a nutshell. Both anandamide and 2‐AG exert immunomodulatory roles on major immune cells such as T cells, B cells, natural killer cells, neutrophils, monocytes, macrophages, microglia, eosinophils, classical dendritic cells (cDC) and plasmacytoid dendritic cells (pDCs) as schematically represented. The choice of receptors on each cell varies depending on the specific effect of the endocannabinoid. CB1R, CB2R, GPR55, BLT1 and COX‐2 are already reported receptors for some of the effects (as shown over the arrowhead), while the receptors involved in the other effects remain to be identified
T cells and NK cells
Anandamide inhibits mitogen‐induced proliferation of T cells at 10 μM dose [48] and hampers the chemokine SDF‐1‐induced migration of CD8+ T cells [49]. Anandamide directly suppresses cytokine release by CD4+ and CD8+ T cells via CB2 [50] and inhibits pro‐inflammatory T‐cell responses by blocking the production of Th1/Th17‐polarizing cytokines by keratinocytes through CB1‐mTOR axis [51]. 2‐AG interferes with nuclear translocation of transcription factors NFAT1 and NFAT2 in mouse splenocytes, thus reducing IL‐2 and IFN‐γ expression [52]. IL‐2 suppression by 2‐AG is CB1/CB2‐independent and involves a COX‐2 metabolite that signals via PPARγ [53]. On exposure to super‐antigens, human T cells upregulate expression of the otherwise negligible CB2 and increase phosphorylation and activation of ERK1/2 in response to 2‐AG. 2‐AG also abrogates T‐cell chemotaxis towards CXCL12 [54]. Thus, both the endocannabinoids exert immunosuppressive effect on T‐cell migration and function through different receptors. On the other hand, 2‐AG, but not anandamide, induces CB2‐dependent migration in NK cell line KHYG‐1 and primary human NK cells [55].
B cells
Immunization of mice with either SRBC (sheep red blood cells) or cholera toxin and analysis of the migration of sorted B cells revealed that naïve B cells exhibit higher chemotaxis towards high 2‐AG concentrations of 1 and 10 μM, in comparison with the B220+ class‐switched B cells, which is CB2‐dependent [56]. However, polyclonally activated murine B cells release 2‐AG to a greater extent than naïve B cells, and the released 2‐AG, in turn, reduces proliferation and antibody production in polyclonally activated or antigen‐stimulated B cells [32]. Marginal zone (MZ) B cells display CB2‐mediated chemotaxis towards 2‐AG, and the retention of MZ B cells in the marginal zone compartment requires the presence of CB2 [57]. Thus, 2‐AG serves dual role of chemo‐attracting naïve B cells and MZ B cells and inhibiting function of activated B cells, with the latter being better 2‐AG producers.
Neutrophils
Methanandamide, a stable synthetic analogue of anandamide [58], has been shown to stimulate respiratory burst in human neutrophils through CB2, while anandamide does not show any effect due to its rapid hydrolysis. 2‐AG suppresses fMLP‐induced migration of human neutrophils by CB2‐dependent modulation of RhoA activity [59]. Anandamide also inhibits neutrophil migration, utilizing a receptor distinct from CB1 and CB2 [60]. The identity of this receptor was discovered to be GPR55, often considered the ‘third’ cannabinoid receptor and highly expressed in neutrophils. Activated GPR55 enhances the migration of human neutrophils towards 2‐AG, while simultaneously inhibiting their degranulation and release of reactive oxygen species [61]. Human neutrophils get activated in terms of enhanced myeloperoxidase (MPO) release, increased kinase activity and calcium mobilization on 2‐AG pretreatment. It was eventually revealed that 2‐AG hydrolysis fuels the synthesis of leukotriene LTB4, which leads to the stimulation of neutrophils [62]. Accordingly, supernatants from 2‐AG‐treated neutrophils exhibit potent antibacterial and antiviral effects, which is completely abrogated on the blockade of LTB4 synthesis or antagonizing its cognate receptor BLT1 [63]. To summarize, 2‐AG and anandamide exert mostly stimulatory effect on certain neutrophil functions while largely suppressing their migration.
Macrophages and monocytes
Anandamide regulates macrophage and monocyte function in both dose‐dependent and temporal manner, while the effect of 2‐AG relies on by its downstream products, which stimulate nitric oxide (NO) production and suppress pro‐inflammatory function of macrophages. On assessing the effect of anandamide on cytokine production by stimulated human peripheral blood mononuclear cells (PBMCs), it was found that inhibition of IL‐6 and IL‐8 occurs only at nanomolar concentrations (3–30 nM), while inhibition of IL‐4‐, TNF‐α‐, IFN‐γ‐ and p75 TNF‐α‐soluble receptors require micromolar concentrations (3–30 μM) of anandamide. The release of AA by unstimulated and fMLP‐stimulated human monocytes is enhanced at 30 μM anandamide dose, with no effect on IL‐10 release [64]. Interestingly, acute anandamide exposure renders macrophages round and immobile, while long‐term anandamide exposure mobilizes macrophages and enhances its endothelial adherence, favouring transmembrane migration [65]. Anandamide suppresses NO and IL‐6 release by LPS‐activated J774 macrophages in a dose‐dependent fashion, whereas 2‐AG suppresses IL‐6 but increases iNOS‐mediated NO production. This puzzling effect of 2‐AG is probably an outcome of 2‐AG being hydrolysed and further oxygenated by COX‐2 to yield AA and prostaglandin‐E2 (PGE‐2), both of which promote iNOS. Notably, PGE(2)‐ethanolamide (oxygenated product of anandamide) does not have any effect on IL‐6 or NO production [66]. Rather, PGE(2)‐ethanolamide represses IL‐12p40 promoter in LPS/IFN‐γ‐stimulated microglia. Consistently, anandamide itself blocks IL‐12 and IL‐23 production and enhances IL‐10 production by activated microglia [67], through activation of JNK and ERK1/2, and NF‐κB inhibition [68]. Anandamide causes neuroprotection by inhibiting NO release in LPS‐activated microglia, signalling through cannabinoid receptors and upregulating MKP‐1 expression, thereby repressing the MAP kinase pathway [69]. PGE(2)‐ethanolamide also hinders TNF‐α production by LPS‐stimulated human monocytes, utilizing the EP2 receptor and downstream cAMP pathway [70].
Primary human monocytes show enhanced production of NO with increasing doses of 2‐AG through CB1. Interestingly, 2‐AG treatment of human primary monocytes renders them round and immobile, which appear immunosuppressive in terms of reduced cytokine production and adhesion [71].
Human promyelocytic leukaemic HL‐60 cells differentiate into macrophage‐like cells on exposure to vitamin D3 [72]. A lot of studies were done on HL‐60 cells to understand the effect of 2‐AG on human macrophages. The influx of Ca2+ influx increases transiently in HL‐60 cells on activation of 2‐AG‐CB2‐phospholipase C‐axis[73]; migration towards 2‐AG is enhanced in a dose‐dependent manner (10 nM–10 μM range) [72]; and IL‐6 and MCP‐1 production is induced, which is further enhanced when treated with both 2‐AG and LPS [74]. Furthermore, 2‐AG increases the phagocytic ability of HL‐60 cells based on β2 integrins, and signalling through CB2‐Gi/o pathway [75]. A recent study revealed that elevated 2‐AG inhibits TLR4‐induced pro‐inflammatory response in macrophages, mediated by oxygenation of 2‐AG into prostaglandin‐glycerol ester PGD2‐G [76].
Dendritic cells
Exploratory work with murine bone marrow‐derived DCs (BMDCs) revealed that anandamide induces their apoptosis by engaging both the cannabinoid receptors [77]. Anandamide inhibits TLR7/8‐induced IL‐12 and IL‐6 production by human myeloid DCs (mDCs) and IFN‐α production by human plasmacytoid DCs (pDCs) [78]. In a mouse model for DTH, 2‐AG suppresses TLR2 agonist‐mediated IL‐4 production by mDCs and enhances chemotaxis of CD8+ CD11c+ DCs, causing stronger Th1‐skewed DTH response—both these effects are CB2‐dependent [79].
In a murine model of pancreatic ductal adenocarcinoma (PDAC), 2‐AG exerts CB1‐mediated anti‐tumour effects by suppressing the proliferation of cancer cells and by favouring maturation of dendritic cells to produce pro‐inflammatory mediators through p‐STAT6 upregulation [80]. Genetic ablation of DAGLβ in BMDCs reduces the cellular level of 2‐AG along with a direct reduction in LPS‐stimulated TNF‐α production. However, maturation of Daglb −/− DCs and their T‐cell priming activity remains unhindered [81].
On assessing the effect on pDCs, it has been recently shown that 2‐AG strongly suppresses TLR9‐mediated type I interferon production by human primary pDCs. The effect is solely mediated by CB2, and abrogation of this 2‐AG inhibition is found to be crucial in SLE pathogenesis [82].
ENDOCANNABINOIDS AND IMMUNE PATHOLOGIES
Majority of evidences support an anti‐inflammatory role of anandamide, although 2‐AG exerts both pro‐ and anti‐inflammatory roles depending on cell type. Endocannabinoids might be beneficial in the suppression of inflammation, and their dysregulation may underlie certain instances of chronic inflammation. We next summarize our current understanding of the crosstalk of endocannabinoids with the immune system in three instances of chronic inflammatory diseases, viz. allergic disorders, autoimmune diseases and systemic inflammation associated with metabolic syndrome.
Inflammation and allergy
Both anandamide and 2‐AG suppress neutrophil recruitment and TNF‐α production in LPS‐induced bronchopulmonary inflammation in mice [83, 84]. Endocannabinoids are increased in response to trinitrobenzene sulphonic acid (TNBS)‐induced colitis in rodents. Elevated endocannabinoids appears anti‐inflammatory as inhibiting their degradation reduces the severity of inflammation, while CB1 and CB2 deletion aggravates colitis. Anandamide level also correlated with disease severity in biopsies from patients with untreated ulcerative colitis [85, 86]. Capsaicin‐ and anandamide‐induced activation of TRPV1 in gut mononuclear phagocytes enhances anandamide biosynthesis, and CB2–anandamide interaction expands the population of regulatory CX3CR1hi macrophages. This pathway increases anti‐inflammatory Tr1 cells through IL‐27, thus providing a mechanism of ECS‐mediated gut immunotolerance [87]. In a murine model of cutaneous contact dermatitis, lack of cannabinoid receptors enhances hypersensitivity, while deficiency of endocannabinoid‐hydrolysing FAAH increases anandamide level and dampens hypersensitivity [88]. Methylated BSA‐stimulated Th17‐mediated DTH gets diminished in mice exposed to anandamide, presumably through favoured IL‐10 and reduced IL‐17 and IFN‐γ production [89]. A similar reduction in Th1‐ and Th17‐related cytokines, and suppressed proliferation of lymphocytes are seen on 2‐AG exposure to mice with DTH [32]. 2‐AG suppresses acute neuroinflammation by reducing CNS‐infiltrating immune cells and inducing immunosuppressive MDSCs (myeloid‐derived suppressor cells) [90]. Likewise, enhanced anandamide levels due to systemic/central inhibition of FAAH attenuates neuroinflammation by inhibiting TLR4‐induced pro‐inflammatory cytokines in the frontal cortex, partially utilizing TRVP1 [91]. Endocannabinoids also suppress anti‐tumour immunity by reducing Th1 response and inducing MDSCs [92, 93].
On the other hand, a pro‐inflammatory role of endocannabinoids, especially 2‐AG, has been reported in the context of allergic inflammation. This is mostly because 2‐AG acts as a chemotactic agent for human eosinophils through CB2, probably by enhancing endothelial/leucocyte adhesion and transmigration [94, 95, 96]. A series of reports indicate that in murine models of contact dermatitis and allergic bronchitis, the 2‐AG level is highly increased in the inflamed tissue, correlating strongly with disease severity and immune cell infiltration, which is effectively counteracted on CB2 blockade [97, 98, 99, 100].
Autoimmune disorders
Endocannabinoids plays vital role in protecting against neuroinflammation in multiple sclerosis (MS) by engaging both CB1 and CB2, for indirect release of immunomodulatory molecules by nerves and direct effect on immune cells respectively [101]. The pathogenic cytokines of MS, that is IL‐12p70 and IL‐23, are effectively downregulated in the spinal cord and microglia by anandamide, in a virus‐induced animal MS model [102]. In vivo administration of 2‐AG to EAE (experimental autoimmune encephalitis) mice delays disease onset, severity and mortality, with a concomitant increase in anti‐inflammatory macrophages [103]. Anandamide and 2‐AG are also increased in cerebrospinal fluid and plasma of human MS patients with active disease and lesions [104, 105, 106]. Evaluating endocannabinoid levels showed that anandamide level is elevated in B cell, T cells and NK cells of MS patients, and declines to normal levels following 1 year of treatment with IFN‐β [107]. Comparative analysis further revealed that monocytes from MS patients upregulate CB2 and downregulate FAAH, perhaps to increase the local anandamide level as a feedback mechanism to combat inflammation. Anandamide, however, only significantly suppresses TLR7/8‐stimulated, but not TLR4 or TLR5‐stimulated, cytokine release in MS monocytes, owing to high expression of TLR4 and TLR5 in MS monocytes compared with healthy monocytes [108]. Likewise, the inhibitory activity of anandamide on pDC function is compromised in MS patients, due to abundant FAAH expression and high anandamide‐degrading capacity in these cells [78]. Understanding the local endocannabinoid tone in the microenvironment of the immune cells can be crucial when designing drugs to treat disorders.
Anandamide and 2‐AG are detected at appreciable levels in the synovial fluid from patients with rheumatoid arthritis (RA) and osteoarthritis (OA) while being absent in healthy volunteers. Synovial biopsies from these patients display functional cannabinoid receptors and FAAH [109]. It was subsequently reported that low dose of glucocorticoid drives synovial fibroblasts to produce endocannabinoids [110]. Anandamide inhibits IL‐6, IL‐8, and MMP‐3 induction in primary synoviocytes and synovial fibroblasts from RA patients by TRPA1/TRPV1 channel desensitization [111], thus exerting anti‐inflammatory effects. Indeed, synthetic cannabinoids such as ajulemic acid and the CB2 agonist HU‐320 hamper metalloproteinase and pro‐inflammatory cytokine production by FLS (fibroblast‐like synoviocytes), thereby reducing the severity of collagen‐induced arthritis (CIA) [112, 113]. Moreover, a loss‐of‐function dinucleotide polymorphism in the CB2 gene has been linked with increased susceptibility of RA [114, 115].
A missense mutation in anandamide biosynthetic enzyme PTPN22 and a gain‐of‐function polymorphism in the 2‐AG‐hydrolysing enzyme ABHD6 have been highly associated with systemic lupus erythematosus (SLE)—these observations uncover a link between the ECS and lupus pathogenesis [116, 117, 118]. It was recently shown that enhanced expression of ABHD6 in a subset of SLE patients increases their 2‐AG‐hydrolysing ability, the latter being important in strong suppression of the pathogenic type I interferon response characterizing SLE [82].
Inflammation associated with metabolic syndrome
Chronic low‐grade inflammation characterizes the visceral adipose tissue (VAT) in obesity [119]. Obese VAT shows altered expression of FAAH and CB1 under pro‐inflammatory conditions in vitro [120]. High‐fat diet‐induced obesity in mice is also associated with enhanced plasma levels of endocannabinoids, presumably due to increased synthesis in VAT, evident from a strong correlation of endocannabinoid levels with endocannabinoid‐synthesizing enzyme expression in VAT [121, 122]. Blockade of CB1 receptor reduces the expression of pro‐inflammatory molecules MIP‐1β and IL‐7 in cultured adipocytes obtained from human adipose tissue explants [123]. A small‐molecule inhibitor of CB1, rimonabant, has been used to reduce weight in humans, although currently discontinued due to psychiatric side‐effects [124]. Chronic stimulation of CB1 in high‐fat diet‐fed mice promotes glucose intolerance and supports enhanced macrophage infiltration in muscles [125]. Interestingly, endocannabinoids favour type 2 diabetes by activating Nlrp3‐inflammasome, which increases IL‐1β in the infiltrating M1 macrophages of pancreatic islets, leading to inflammatory damage of the pancreatic β cells [126, 127].
CONCLUDING REMARKS
Research done over the past few decades has thoroughly proved the presence of extensive crosstalk between the ECS and the various facets of the immune system. The interaction is indeed complex, and the effects of endocannabinoids on the immune system can vary highly in a spatiotemporal and context‐dependent manner. The levels of endocannabinoids can be significantly altered in the context of disease pathologies. The endocannabinoid tone affects and is also in turn affected by immune homeostasis and immune perturbations. The concentration of endocannabinoids in in vitro studies may not always reflect the physiological endocannabinoid levels in systemic and local environment; thus, heavily relying on these studies can further complicate the understanding of their immunomodulation. These studies have to be complemented with detailed exploration of physiological endocannabinoid levels in biological tissues, which is known to be dependent on multiple factors as reviewed elsewhere [35]. Filling up this knowledge gap can enable utilizing endocannabinoid levels as potential ‘biomarkers’ for disease course.
The ubiquitous nature of the ECS makes it appear difficult to target specific diseases without causing undesired side‐effects. In this regard, one approach has been developing peripheral CB1 agonists, which cannot cross blood–brain barrier, thereby precluding undesirable effects on the CNS involving psychotropic manifestations. It is also beneficial to target CB2 due to its predominant expression in the immune cells. Quite encouragingly, a handful of peripheral CB1 and CB2 agonists such as drugs for example JBT‐101, APD371 and NEO1940 are now in their phase II/phase III trials for use in inflammatory disorders and cancer [128]. Other likely drug target candidates such as GPR55, MAGL, ABHD6 and TRPV1 are only recently starting to be recognized. To sum up, the complex and multifactorial nature of ECS pathway opens up great opportunities for identifying therapeutic targets in multiple immunological aberrations, which can be harnessed optimally only with increased research and extremely detailed understanding of the ECS pathway.
CONFLICT OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
OR and DG conceptualized and wrote the manuscript. OR prepared the figures.
Rahaman O, Ganguly D. Endocannabinoids in immune regulation and immunopathologies. Immunology. 2021;164:242–252. 10.1111/imm.13378
Funding information
This work was supported by funding from Swarnajayanti Fellowship to DG and Senior Research Fellowship from University Grants Commission, India, to OR
REFERENCES
- 1.Lu HC, Mackie K. An introduction to the endogenous cannabinoid system. Biol Psychiatry. 2016;79:516–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–7. [Google Scholar]
- 3.Howlett AC. Inhibition of neuroblastoma adenylate cyclase by cannabinoid and nantradol compounds. Life Sci. 1984;35:1803–10. [DOI] [PubMed] [Google Scholar]
- 4.Howlett AC. Cannabinoid inhibition of adenylate cyclase. Biochemistry of the response in neuroblastoma cell membranes. Mol Pharmacol. 1985;27:429–36. [PubMed] [Google Scholar]
- 5.Howlett AC, Qualy JM, Khachatrian LL. Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol Pharmacol. 1986;29:307–13. [PubMed] [Google Scholar]
- 6.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–4. [DOI] [PubMed] [Google Scholar]
- 7.Munro S, Thomas KL, Abu‐Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–5. [DOI] [PubMed] [Google Scholar]
- 8.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–9. [DOI] [PubMed] [Google Scholar]
- 9.Mechoulam R, Ben‐Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2‐monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. [DOI] [PubMed] [Google Scholar]
- 10.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long‐term potentiation. Nature. 1997;388:773–8. [DOI] [PubMed] [Google Scholar]
- 11.Sugiura T, Waku K. 2‐Arachidonoylglycerol and the cannabinoid receptors. Chem Phys Lipids. 2000;108(1–2):89–106. [DOI] [PubMed] [Google Scholar]
- 12.Hanus L, Abu‐Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, et al. 2‐arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci USA. 2001;98:3662–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porter AC, Sauer JM, Knierman MD, Becker GW, Berna MJ, Bao J, et al. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J Pharmacol Exp Ther. 2002;301:1020–4. [DOI] [PubMed] [Google Scholar]
- 14.Huang SM, Bisogno T, Trevisani M, Al‐Hayani A, De Petrocellis L, Fezza F, et al. An endogenous capsaicin‐like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA. 2002;99:8400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pertwee RG. The therapeutic potential of drugs that target cannabinoid receptors or modulate the tissue levels or actions of endocannabinoids. AAPS J. 2005;7:E625–E654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Marzo V, Deutsch DG. Biochemistry of the endogenous ligands of cannabinoid receptors. Neurobiol Dis. 1998;5(6):386–404. 10.1006/nbdi.1998.0214 [DOI] [PubMed] [Google Scholar]
- 17.Oddi S, Fezza F, Pasquariello N, De Simone C, Rapino C, Dainese E, et al. Evidence for the intracellular accumulation of anandamide in adiposomes. Cell Mol Life Sci. 2008;65:840–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmid PC, Reddy PV, Natarajan V, Schmid HH. Metabolism of N‐acylethanolamine phospholipids by a mammalian phosphodiesterase of the phospholipase D type. J Biol Chem. 1983;258:9302–6. [PubMed] [Google Scholar]
- 19.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–91. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–305. [DOI] [PubMed] [Google Scholar]
- 21.Ueda N, Liu Q, Yamanaka K. Marked activation of the N‐acylphosphatidylethanolamine‐hydrolyzing phosphodiesterase by divalent cations. Biochim Biophys Acta. 2001;1532(1–2):121–7. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Wang L, Harvey‐White J, Osei‐Hyiaman D, Razdan R, Gong Q, et al. A biosynthetic pathway for anandamide. Proc Natl Acad Sci USA. 2006;103:13345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambert DM, Di Marzo V. The palmitoylethanolamide and oleamide enigmas: are these two fatty acid amides cannabimimetic? Curr Med Chem. 1999;6:757–73. [PubMed] [Google Scholar]
- 24.Jung KM, Astarita G, Zhu C, Wallace M, Mackie K, Piomelli D. A key role for diacylglycerol lipase‐alpha in metabotropic glutamate receptor‐dependent endocannabinoid mobilization. Mol Pharmacol. 2007;72:612–21. [DOI] [PubMed] [Google Scholar]
- 25.Shonesy BC, Winder DG, Patel S, Colbran RJ. The initiation of synaptic 2‐AG mobilization requires both an increased supply of diacylglycerol precursor and increased postsynaptic calcium. Neuropharmacology. 2015;91:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain T, Wager‐Miller J, Mackie K, Straiker A. Diacylglycerol lipase alpha (DAGLalpha) and DAGLbeta cooperatively regulate the production of 2‐arachidonoyl glycerol in autaptic hippocampal neurons. Mol Pharmacol. 2013;84:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu KL, Tsuboi K, Adibekian A, Pugh H, Masuda K, Cravatt BF. DAGLbeta inhibition perturbs a lipid network involved in macrophage inflammatory responses. Nat Chem Biol. 2012;8:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pestonjamasp VK, Burstein SH. Anandamide synthesis is induced by arachidonate mobilizing agonists in cells of the immune system. Biochim Biophys Acta. 1998;1394(2–3):249–60. [DOI] [PubMed] [Google Scholar]
- 29.Di Marzo V, Lastres‐Becker I, Bisogno T, De Petrocellis L, Milone A, Davis JB, et al. Hypolocomotor effects in rats of capsaicin and two long chain capsaicin homologues. Eur J Pharmacol. 2001;420(2–3):123–31. [DOI] [PubMed] [Google Scholar]
- 30.Mecha M, Feliu A, Carrillo‐Salinas FJ, Rueda‐Zubiaurre A, Ortega‐Gutierrez S, de Sola RG , et al. Endocannabinoids drive the acquisition of an alternative phenotype in microglia. Brain Behav Immun. 2015;49:233–45. [DOI] [PubMed] [Google Scholar]
- 31.Matias I, Pochard P, Orlando P, Salzet M, Pestel J, Di Marzo V. Presence and regulation of the endocannabinoid system in human dendritic cells. Eur J Biochem. 2002;269:3771–8. [DOI] [PubMed] [Google Scholar]
- 32.Sido JM, Nagarkatti PS, Nagarkatti M. Production of endocannabinoids by activated T cells and B cells modulates inflammation associated with delayed‐type hypersensitivity. Eur J Immunol. 2016;46:1472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lam PM, Marczylo TH, Konje JC. Simultaneous measurement of three N‐acylethanolamides in human bio‐matrices using ultra performance liquid chromatography‐tandem mass spectrometry. Anal Bioanal Chem. 2010;398:2089–97. [DOI] [PubMed] [Google Scholar]
- 34.Hillard CJ, Weinlander KM, Stuhr KL. Contributions of endocannabinoid signaling to psychiatric disorders in humans: genetic and biochemical evidence. Neuroscience. 2012;204:207–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hillard CJ. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology. 2018;43:155–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty‐acid amides. Nature. 1996;384:83–7. [DOI] [PubMed] [Google Scholar]
- 37.Ueda N, Yamanaka K, Terasawa Y, Yamamoto S. An acid amidase hydrolyzing anandamide as an endogenous ligand for cannabinoid receptors. FEBS Lett. 1999;454:267–70. [DOI] [PubMed] [Google Scholar]
- 38.Ueda N, Yamanaka K, Yamamoto S. Purification and characterization of an acid amidase selective for N‐palmitoylethanolamine, a putative endogenous anti‐inflammatory substance. J Biol Chem. 2001;276:35552–7. [DOI] [PubMed] [Google Scholar]
- 39.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA. 2002;99:10819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2‐arachidonoylglycerol. Chem Biol. 2007;14:1347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savinainen JR, Saario SM, Laitinen JT. The serine hydrolases MAGL, ABHD6 and ABHD12 as guardians of 2‐arachidonoylglycerol signalling through cannabinoid receptors. Acta Physiol (Oxf). 2012;204:267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piscitelli F, Di Marzo V. "Redundancy" of endocannabinoid inactivation: new challenges and opportunities for pain control. ACS Chem Neurosci. 2012;3:356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. [DOI] [PubMed] [Google Scholar]
- 44.Han KH, Lim S, Ryu J, Lee CW, Kim Y, Kang JH, et al. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc Res. 2009;84:378–86. [DOI] [PubMed] [Google Scholar]
- 45.Nong L, Newton C, Friedman H, Klein TW. CB1 and CB2 receptor mRNA expression in human peripheral blood mononuclear cells (PBMC) from various donor types. Adv Exp Med Biol. 2001;493:229–33. [DOI] [PubMed] [Google Scholar]
- 46.Chiurchiu V. Endocannabinoids and immunity. Cannabis Cannabinoid Res. 2016;1:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiurchiu V, Battistini L, Maccarrone M. Endocannabinoid signalling in innate and adaptive immunity. Immunology. 2015;144:352–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwarz H, Blanco FJ, Lotz M. Anadamide, an endogenous cannabinoid receptor agonist inhibits lymphocyte proliferation and induces apoptosis. J Neuroimmunol. 1994;55:107–15. [DOI] [PubMed] [Google Scholar]
- 49.Joseph J, Niggemann B, Zaenker KS, Entschladen F. Anandamide is an endogenous inhibitor for the migration of tumor cells and T lymphocytes. Cancer Immunol Immunother. 2004;53:723–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cencioni MT, Chiurchiu V, Catanzaro G, Borsellino G, Bernardi G, Battistini L, et al. Anandamide suppresses proliferation and cytokine release from primary human T‐lymphocytes mainly via CB2 receptors. PLoS One. 2010;5:e8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiurchiu V, Rapino C, Talamonti E, Leuti A, Lanuti M, Gueniche A, et al. Anandamide suppresses proinflammatory T cell responses in vitro through type‐1 cannabinoid receptor‐mediated mTOR inhibition in human keratinocytes. J Immunol. 2016;197:3545–53. [DOI] [PubMed] [Google Scholar]
- 52.Ouyang Y, Hwang SG, Han SH, Kaminski NE. Suppression of interleukin‐2 by the putative endogenous cannabinoid 2‐arachidonyl‐glycerol is mediated through down‐regulation of the nuclear factor of activated T cells. Mol Pharmacol. 1998;53:676–83. [DOI] [PubMed] [Google Scholar]
- 53.Rockwell CE, Snider NT, Thompson JT, Vanden Heuvel JP, Kaminski NE. Interleukin‐2 suppression by 2‐arachidonyl glycerol is mediated through peroxisome proliferator‐activated receptor gamma independently of cannabinoid receptors 1 and 2. Mol Pharmacol. 2006;70:101–11. [DOI] [PubMed] [Google Scholar]
- 54.Coopman K, Smith LD, Wright KL, Ward SG. Temporal variation in CB2R levels following T lymphocyte activation: evidence that cannabinoids modulate CXCL12‐induced chemotaxis. Int Immunopharmacol. 2007;7:360–71. [DOI] [PubMed] [Google Scholar]
- 55.Kishimoto S, Muramatsu M, Gokoh M, Oka S, Waku K, Sugiura T. Endogenous cannabinoid receptor ligand induces the migration of human natural killer cells. J Biochem. 2005;137:217–23. [DOI] [PubMed] [Google Scholar]
- 56.Tanikawa T, Kurohane K, Imai Y. Induction of preferential chemotaxis of unstimulated B‐lymphocytes by 2‐arachidonoylglycerol in immunized mice. Microbiol Immunol. 2007;51:1013–9. [DOI] [PubMed] [Google Scholar]
- 57.Muppidi JR, Arnon TI, Bronevetsky Y, Veerapen N, Tanaka M, Besra GS, et al. Cannabinoid receptor 2 positions and retains marginal zone B cells within the splenic marginal zone. J Exp Med. 2011;208:1941–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abadji V, Lin S, Taha G, Griffin G, Stevenson LA, Pertwee RG, et al. (R)‐methanandamide: a chiral novel anandamide possessing higher potency and metabolic stability. J Med Chem. 1994;37:1889–93. [DOI] [PubMed] [Google Scholar]
- 59.Kurihara R, Tohyama Y, Matsusaka S, Naruse H, Kinoshita E, Tsujioka T, et al. Effects of peripheral cannabinoid receptor ligands on motility and polarization in neutrophil‐like HL60 cells and human neutrophils. J Biol Chem. 2006;281:12908–18. [DOI] [PubMed] [Google Scholar]
- 60.McHugh D, Tanner C, Mechoulam R, Pertwee RG, Ross RA. Inhibition of human neutrophil chemotaxis by endogenous cannabinoids and phytocannabinoids: evidence for a site distinct from CB1 and CB2. Mol Pharmacol. 2008;73:441–50. [DOI] [PubMed] [Google Scholar]
- 61.Balenga NA, Aflaki E, Kargl J, Platzer W, Schroder R, Blattermann S, et al. GPR55 regulates cannabinoid 2 receptor‐mediated responses in human neutrophils. Cell Res. 2011;21:1452–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chouinard F, Lefebvre JS, Navarro P, Bouchard L, Ferland C, Lalancette‐Hebert M, et al. The endocannabinoid 2‐arachidonoyl‐glycerol activates human neutrophils: critical role of its hydrolysis and de novo leukotriene B4 biosynthesis. J Immunol. 2011;186:3188–96. [DOI] [PubMed] [Google Scholar]
- 63.Chouinard F, Turcotte C, Guan X, Larose MC, Poirier S, Bouchard L, et al. 2‐Arachidonoyl‐glycerol‐ and arachidonic acid‐stimulated neutrophils release antimicrobial effectors against E. coli, S. aureus, HSV‐1, and RSV. J Leukoc Biol. 2013;93:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berdyshev EV, Boichot E, Germain N, Allain N, Anger JP, Lagente V. Influence of fatty acid ethanolamides and delta9‐tetrahydrocannabinol on cytokine and arachidonate release by mononuclear cells. Eur J Pharmacol. 1997;330(2–3):231–40. [DOI] [PubMed] [Google Scholar]
- 65.Stefano GB, Salzet M, Rialas CM, Mattocks D, Fimiani C, Bilfinger TV. Macrophage behavior associated with acute and chronic exposure to HIV GP120, morphine and anandamide: endothelial implications. Int J Cardiol. 1998;64(Suppl 1):S3–13. [DOI] [PubMed] [Google Scholar]
- 66.Chang YH, Lee ST, Lin WW. Effects of cannabinoids on LPS‐stimulated inflammatory mediator release from macrophages: involvement of eicosanoids. J Cell Biochem. 2001;81:715–23. [DOI] [PubMed] [Google Scholar]
- 67.Correa F, Docagne F, Clemente D, Mestre L, Becker C, Guaza C. Anandamide inhibits IL‐12p40 production by acting on the promoter repressor element GA‐12: possible involvement of the COX‐2 metabolite prostamide E(2). Biochem J. 2008;409:761–70. [DOI] [PubMed] [Google Scholar]
- 68.Correa F, Hernangomez M, Mestre L, Loria F, Spagnolo A, Docagne F, et al. Anandamide enhances IL‐10 production in activated microglia by targeting CB(2) receptors: roles of ERK1/2, JNK, and NF‐kappaB. Glia. 2010;58:135–47. [DOI] [PubMed] [Google Scholar]
- 69.Eljaschewitsch E, Witting A, Mawrin C, Lee T, Schmidt PM, Wolf S, et al. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP‐1 in microglial cells. Neuron. 2006;49:67–79. [DOI] [PubMed] [Google Scholar]
- 70.Brown KL, Davidson J, Rotondo D. Characterisation of the prostaglandin E2‐ethanolamide suppression of tumour necrosis factor‐alpha production in human monocytic cells. Biochim Biophys Acta. 2013;1831:1098–107. [DOI] [PubMed] [Google Scholar]
- 71.Stefano GB, Bilfinger TV, Rialas CM, Deutsch DG. 2‐arachidonyl‐glycerol stimulates nitric oxide release from human immune and vascular tissues and invertebrate immunocytes by cannabinoid receptor 1. Pharmacol Res. 2000;42:317–22. [DOI] [PubMed] [Google Scholar]
- 72.Kishimoto S, Gokoh M, Oka S, Muramatsu M, Kajiwara T, Waku K, et al. 2‐arachidonoylglycerol induces the migration of HL‐60 cells differentiated into macrophage‐like cells and human peripheral blood monocytes through the cannabinoid CB2 receptor‐dependent mechanism. J Biol Chem. 2003;278:24469–75. [DOI] [PubMed] [Google Scholar]
- 73.Sugiura T, Kondo S, Kishimoto S, Miyashita T, Nakane S, Kodaka T, et al. Evidence that 2‐arachidonoylglycerol but not N‐palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. Comparison of the agonistic activities of various cannabinoid receptor ligands in HL‐60 cells. J Biol Chem. 2000;275:605–12. [DOI] [PubMed] [Google Scholar]
- 74.Kishimoto S, Kobayashi Y, Oka S, Gokoh M, Waku K, Sugiura T. 2‐Arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces accelerated production of chemokines in HL‐60 cells. J Biochem. 2004;135:517–24. [DOI] [PubMed] [Google Scholar]
- 75.Gokoh M, Kishimoto S, Oka S, Sugiura T. 2‐Arachidonoylglycerol enhances the phagocytosis of opsonized zymosan by HL‐60 cells differentiated into macrophage‐like cells. Biol Pharm Bull. 2007;30:1199–205. [DOI] [PubMed] [Google Scholar]
- 76.Alhouayek M, Masquelier J, Cani PD, Lambert DM, Muccioli GG. Implication of the anti‐inflammatory bioactive lipid prostaglandin D2‐glycerol ester in the control of macrophage activation and inflammation by ABHD6. Proc Natl Acad Sci USA. 2013;110:17558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Do Y, McKallip RJ, Nagarkatti M, Nagarkatti PS. Activation through cannabinoid receptors 1 and 2 on dendritic cells triggers NF‐kappaB‐dependent apoptosis: novel role for endogenous and exogenous cannabinoids in immunoregulation. J Immunol. 2004;173:2373–82. [DOI] [PubMed] [Google Scholar]
- 78.Chiurchiu V, Cencioni MT, Bisicchia E, De Bardi M, Gasperini C, Borsellino G, et al. Distinct modulation of human myeloid and plasmacytoid dendritic cells by anandamide in multiple sclerosis. Ann Neurol. 2013;73:626–36. [DOI] [PubMed] [Google Scholar]
- 79.Maestroni GJ. The endogenous cannabinoid 2‐arachidonoyl glycerol as in vivo chemoattractant for dendritic cells and adjuvant for Th1 response to a soluble protein. FASEB J. 2004;18:1914–6. [DOI] [PubMed] [Google Scholar]
- 80.Qiu C, Yang L, Wang B, Cui L, Li C, Zhuo Y, et al. The role of 2‐arachidonoylglycerol in the regulation of the tumor‐immune microenvironment in murine models of pancreatic cancer. Biomed Pharmacother. 2019;115:108952. [DOI] [PubMed] [Google Scholar]
- 81.Shin M, Buckner A, Prince J, Bullock TNJ, Hsu KL. Diacylglycerol lipase‐beta is required for TNF‐alpha response but Not CD8(+) T cell priming capacity of dendritic cells. Cell Chem Biol. 2019;26:1036–41.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rahaman O, Bhattacharya R, Liu CSC, Raychaudhuri D, Ghosh AR, Bandopadhyay P, et al. Cutting edge: dysregulated endocannabinoid‐rheostat for plasmacytoid dendritic cell activation in a systemic lupus endophenotype. J Immunol. 2019;202:1674–9. [DOI] [PubMed] [Google Scholar]
- 83.Berdyshev E, Boichot E, Corbel M, Germain N, Lagente V. Effects of cannabinoid receptor ligands on LPS‐induced pulmonary inflammation in mice. Life Sci. 1998;63(8):PL125–9. [DOI] [PubMed] [Google Scholar]
- 84.Bottemanne P, Paquot A, Ameraoui H, Alhouayek M, Muccioli GG. The alpha/beta‐hydrolase domain 6 inhibitor WWL70 decreases endotoxin‐induced lung inflammation in mice, potential contribution of 2‐arachidonoylglycerol, and lysoglycerophospholipids. FASEB J. 2019;33:7635–46. [DOI] [PubMed] [Google Scholar]
- 85.D'Argenio G, Valenti M, Scaglione G, Cosenza V, Sorrentini I, Di Marzo V. Up‐regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J. 2006;20:568–70. [DOI] [PubMed] [Google Scholar]
- 86.Engel MA, Kellermann CA, Burnat G, Hahn EG, Rau T, Konturek PC. Mice lacking cannabinoid CB1‐, CB2‐receptors or both receptors show increased susceptibility to trinitrobenzene sulfonic acid (TNBS)‐induced colitis. J Physiol Pharmacol. 2010;61:89–97. [PubMed] [Google Scholar]
- 87.Acharya N, Penukonda S, Shcheglova T, Hagymasi AT, Basu S, Srivastava PK. Endocannabinoid system acts as a regulator of immune homeostasis in the gut. Proc Natl Acad Sci USA. 2017;114:5005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Karsak M, Gaffal E, Date R, Wang‐Eckhardt L, Rehnelt J, Petrosino S, et al. Attenuation of allergic contact dermatitis through the endocannabinoid system. Science. 2007;316:1494–7. [DOI] [PubMed] [Google Scholar]
- 89.Jackson AR, Nagarkatti P, Nagarkatti M. Anandamide attenuates Th‐17 cell‐mediated delayed‐type hypersensitivity response by triggering IL‐10 production and consequent microRNA induction. PLoS One. 2014;9:e93954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mecha M, Feliu A, Machin I, Cordero C, Carrillo‐Salinas F, Mestre L, et al. 2‐AG limits Theiler's virus induced acute neuroinflammation by modulating microglia and promoting MDSCs. Glia. 2018;66:1447–63. [DOI] [PubMed] [Google Scholar]
- 91.Henry RJ, Kerr DM, Flannery LE, Killilea M, Hughes EM, Corcoran L, et al. Pharmacological inhibition of FAAH modulates TLR‐induced neuroinflammation, but not sickness behaviour: an effect partially mediated by central TRPV1. Brain Behav Immun. 2017;62:318–31. [DOI] [PubMed] [Google Scholar]
- 92.McKallip RJ, Nagarkatti M, Nagarkatti PS. Delta‐9‐tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. J Immunol. 2005;174:3281–9. [DOI] [PubMed] [Google Scholar]
- 93.Hegde VL, Nagarkatti M, Nagarkatti PS. Cannabinoid receptor activation leads to massive mobilization of myeloid‐derived suppressor cells with potent immunosuppressive properties. Eur J Immunol. 2010;40:3358–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oka S, Ikeda S, Kishimoto S, Gokoh M, Yanagimoto S, Waku K, et al. 2‐arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces the migration of EoL‐1 human eosinophilic leukemia cells and human peripheral blood eosinophils. J Leukoc Biol. 2004;76:1002–9. [DOI] [PubMed] [Google Scholar]
- 95.Kishimoto S, Oka S, Gokoh M, Sugiura T. Chemotaxis of human peripheral blood eosinophils to 2‐arachidonoylglycerol: comparison with other eosinophil chemoattractants. Int Arch Allergy Immunol. 2006;140(Suppl 1):3–7. [DOI] [PubMed] [Google Scholar]
- 96.Gasperi V, Evangelista D, Chiurchiu V, Florenzano F, Savini I, Oddi S, et al. 2‐Arachidonoylglycerol modulates human endothelial cell/leukocyte interactions by controlling selectin expression through CB1 and CB2 receptors. Int J Biochem Cell Biol. 2014;51:79–88. [DOI] [PubMed] [Google Scholar]
- 97.Ueda Y, Miyagawa N, Matsui T, Kaya T, Iwamura H. Involvement of cannabinoid CB(2) receptor‐mediated response and efficacy of cannabinoid CB(2) receptor inverse agonist, JTE‐907, in cutaneous inflammation in mice. Eur J Pharmacol. 2005;520(1–3):164–71. [DOI] [PubMed] [Google Scholar]
- 98.Oka S, Yanagimoto S, Ikeda S, Gokoh M, Kishimoto S, Waku K, et al. Evidence for the involvement of the cannabinoid CB2 receptor and its endogenous ligand 2‐arachidonoylglycerol in 12‐O‐tetradecanoylphorbol‐13‐acetate‐induced acute inflammation in mouse ear. J Biol Chem. 2005;280:18488–97. [DOI] [PubMed] [Google Scholar]
- 99.Oka S, Wakui J, Ikeda S, Yanagimoto S, Kishimoto S, Gokoh M, et al. Involvement of the cannabinoid CB2 receptor and its endogenous ligand 2‐arachidonoylglycerol in oxazolone‐induced contact dermatitis in mice. J Immunol. 2006;177:8796–805. [DOI] [PubMed] [Google Scholar]
- 100.Mimura T, Oka S, Koshimoto H, Ueda Y, Watanabe Y, Sugiura T. Involvement of the endogenous cannabinoid 2 ligand 2‐arachidonyl glycerol in allergic inflammation. Int Arch Allergy Immunol. 2012;159:149–56. [DOI] [PubMed] [Google Scholar]
- 101.Baker D, Jackson SJ, Pryce G. Cannabinoid control of neuroinflammation related to multiple sclerosis. Br J Pharmacol. 2007;152:649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Correa F, Hernangomez‐Herrero M, Mestre L, Loria F, Docagne F, Guaza C. The endocannabinoid anandamide downregulates IL‐23 and IL‐12 subunits in a viral model of multiple sclerosis: evidence for a cross‐talk between IL‐12p70/IL‐23 axis and IL‐10 in microglial cells. Brain Behav Immun. 2011;25:736–49. [DOI] [PubMed] [Google Scholar]
- 103.Lourbopoulos A, Grigoriadis N, Lagoudaki R, Touloumi O, Polyzoidou E, Mavromatis I, et al. Administration of 2‐arachidonoylglycerol ameliorates both acute and chronic experimental autoimmune encephalomyelitis. Brain Res. 2011;1390:126–41. [DOI] [PubMed] [Google Scholar]
- 104.Centonze D, Bari M, Rossi S, Prosperetti C, Furlan R, Fezza F, et al. The endocannabinoid system is dysregulated in multiple sclerosis and in experimental autoimmune encephalomyelitis. Brain. 2007;130(Pt 10):2543–53. [DOI] [PubMed] [Google Scholar]
- 105.Di Filippo M, Pini LA, Pelliccioli GP, Calabresi P, Sarchielli P. Abnormalities in the cerebrospinal fluid levels of endocannabinoids in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2008;79:1224–9. [DOI] [PubMed] [Google Scholar]
- 106.Jean‐Gilles L, Feng S, Tench CR, Chapman V, Kendall DA, Barrett DA, et al. Plasma endocannabinoid levels in multiple sclerosis. J Neurol Sci. 2009;287(1–2):212–5. [DOI] [PubMed] [Google Scholar]
- 107.Sanchez Lopez AJ, Roman‐Vega L, Ramil Tojeiro E, Giuffrida A, Garcia‐Merino A. Regulation of cannabinoid receptor gene expression and endocannabinoid levels in lymphocyte subsets by interferon‐beta: a longitudinal study in multiple sclerosis patients. Clin Exp Immunol. 2015;179:119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chiurchiu V, Leuti A, Cencioni MT, Albanese M, De Bardi M, Bisogno T, et al. Modulation of monocytes by bioactive lipid anandamide in multiple sclerosis involves distinct Toll‐like receptors. Pharmacol Res. 2016;113(Pt A):313–9. [DOI] [PubMed] [Google Scholar]
- 109.Richardson D, Pearson RG, Kurian N, Latif ML, Garle MJ, Barrett DA, et al. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res Ther. 2008;10:R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lowin T, Zhu W, Dettmer‐Wilde K, Straub RH. Cortisol‐mediated adhesion of synovial fibroblasts is dependent on the degradation of anandamide and activation of the endocannabinoid system. Arthritis Rheum. 2012;64:3867–76. [DOI] [PubMed] [Google Scholar]
- 111.Lowin T, Apitz M, Anders S, Straub RH. Anti‐inflammatory effects of N‐acylethanolamines in rheumatoid arthritis synovial cells are mediated by TRPV1 and TRPA1 in a COX‐2 dependent manner. Arthritis Res Ther. 2015;17:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Johnson DR, Stebulis JA, Rossetti RG, Burstein SH, Zurier RB. Suppression of fibroblast metalloproteinases by ajulemic acid, a nonpsychoactive cannabinoid acid. J Cell Biochem. 2007;100:184–90. [DOI] [PubMed] [Google Scholar]
- 113.Sumariwalla PF, Gallily R, Tchilibon S, Fride E, Mechoulam R, Feldmann M. A novel synthetic, nonpsychoactive cannabinoid acid (HU‐320) with antiinflammatory properties in murine collagen‐induced arthritis. Arthritis Rheum. 2004;50:985–98. [DOI] [PubMed] [Google Scholar]
- 114.Fukuda S, Kohsaka H, Takayasu A, Yokoyama W, Miyabe C, Miyabe Y, et al. Cannabinoid receptor 2 as a potential therapeutic target in rheumatoid arthritis. BMC Musculoskelet Disord. 2014;15:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ismail M, Khawaja G. Study of cannabinoid receptor 2 Q63R gene polymorphism in Lebanese patients with rheumatoid arthritis. Clin Rheumatol. 2018;37:2933–8. [DOI] [PubMed] [Google Scholar]
- 116.Kyogoku C, Langefeld CD, Ortmann WA, Lee A, Selby S, Carlton VE, et al. Genetic association of the R620W polymorphism of protein tyrosine phosphatase PTPN22 with human SLE. Am J Hum Genet. 2004;75:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee YH, Rho YH, Choi SJ, Ji JD, Song GG, Nath SK, et al. The PTPN22 C1858T functional polymorphism and autoimmune diseases–a meta‐analysis. Rheumatology (Oxford). 2007;46:49–56. [DOI] [PubMed] [Google Scholar]
- 118.Oparina NY, Delgado‐Vega AM, Martinez‐Bueno M, Magro‐Checa C, Fernandez C, Castro RO, et al. PXK locus in systemic lupus erythematosus: fine mapping and functional analysis reveals novel susceptibility gene ABHD6. Ann Rheum Dis. 2015;74:e14. [DOI] [PubMed] [Google Scholar]
- 119.Ghosh AR, Bhattacharya R, Bhattacharya S, Nargis T, Rahaman O, Duttagupta P, et al. Adipose recruitment and activation of plasmacytoid dendritic cells fuel metaflammation. Diabetes. 2016;65:3440–52. [DOI] [PubMed] [Google Scholar]
- 120.Kempf K, Hector J, Strate T, Schwarzloh B, Rose B, Herder C, et al. Immune‐mediated activation of the endocannabinoid system in visceral adipose tissue in obesity. Horm Metab Res. 2007;39:596–600. [DOI] [PubMed] [Google Scholar]
- 121.Engeli S, Bohnke J, Feldpausch M, Gorzelniak K, Janke J, Batkai S, et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54:2838–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kuipers EN, Kantae V, Maarse BCE, van den Berg SM , van Eenige R , Nahon KJ, et al. High fat diet increases circulating endocannabinoids accompanied by increased synthesis enzymes in adipose tissue. Front Physiol. 2018;9:1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ge Q, Maury E, Rycken L, Gerard J, Noel L, Detry R, et al. Endocannabinoids regulate adipokine production and the immune balance of omental adipose tissue in human obesity. Int J Obes (Lond). 2013;37:874–80. [DOI] [PubMed] [Google Scholar]
- 124.Sam AH, Salem V, Ghatei MA. Rimonabant: from RIO to Ban. J Obes. 2011;2011: 432607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Geurts L, Muccioli GG, Delzenne NM, Cani PD. Chronic endocannabinoid system stimulation induces muscle macrophage and lipid accumulation in type 2 diabetic mice independently of metabolic endotoxaemia. PLoS One. 2013;8:e55963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jourdan T, Godlewski G, Cinar R, Bertola A, Szanda G, Liu J, et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat Med. 2013;19:1132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jourdan T, Szanda G, Cinar R, Godlewski G, Holovac DJ, Park JK, et al. developmental role of macrophage cannabinoid‐1 receptor signaling in type 2 diabetes. Diabetes. 2017;66:994–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stasiulewicz A, Znajdek K, Grudzien M, Pawinski T, Sulkowska AJI. A guide to targeting the endocannabinoid system in drug design. Int J Mol Sci. 2020;21:2778. [DOI] [PMC free article] [PubMed] [Google Scholar]


