Summary
Cytomegalovirus (CMV) induction of large frequencies of highly functional memory T cells has attracted much interest in the utility of CMV‐based vaccine vectors, with exciting preclinical data obtained in models of infectious diseases and cancer. However, pathogenesis of human CMV (HCMV) remains a concern. Attenuated CMV‐based vectors, such as replication‐ or spread‐deficient viruses, potentially offer an alternative to fully replicating vectors. However, it is not well understood how CMV attenuation impacts vector immunogenicity, particularly when administered via relevant routes of immunization such as the skin. Herein, we used the murine cytomegalovirus (MCMV) model to investigate the impact of vector attenuation on T‐cell memory formation following subcutaneous administration. We found that the spread‐deficient virus (ΔgL‐MCMV) was impaired in its ability to induce memory CD8+ T cells reactive to some (M38, IE1) but not all (IE3) viral antigens. Impaired‐memory T‐cell development was associated with a preferential and pronounced loss of polyfunctional (IFN‐γ+ TNF‐α+) T cells and also reduced accumulation of TCF1+ T cells, and was not rescued by increasing the dose of replication‐defective MCMV. Finally, whilst vector attenuation reduced dendritic cell (DC) recruitment to skin‐draining lymph nodes, systematic depletion of multiple DC subsets during acute subcutaneous MCMV infection had a negligible impact on T‐cell memory formation, implying that attenuated responses induced by replication‐deficient vectors were likely not a consequence of impaired initial DC activation. Thus, overall, these data imply that the choice of antigen and/or cloning strategy of exogenous antigen in combination with the route of immunization may influence the ability of attenuated CMV vectors to induce robust functional T‐cell memory.
Keywords: cytomegalovirus, inflationary immune response, T cells, vaccine vectors
Cytomegalovirus (CMV) is a potentially exciting vaccine vector, but pathogenicity may preclude the use of replicating viruses. Here, we show that a spread‐deficient CMV vector is attenuated in the ability to induce T‐cell memory formation after subcutaneous immunization. This results in pronounced loss of polyfunctional T cells, possibly linked to reduced development of TCF1‐expressing T cells.
Abbreviations
- CD
cluster of differentiation
- cDC
classical dendritic cell
- CMV
cytomegalovirus
- DC
dendritic cell
- DT
diphtheria toxin
- DTR
diphtheria toxin receptor
- HCMV
human CMV
- HIV
human immunodeficiency virus
- IE1
immediate early 1
- IE3
immediate early 3
- IFN‐γ
interferon‐γ
- LC
Langerhans cell
- MCMV
murine CMV
- MPEC
memory precursor effector cell
- pDC
plasmacytoid dendritic cell
- RhCMV
rhesus CMV
- SIV
simian immunodeficiency virus
- SLEC
short‐lived effector cell
- TCM
central memory T cell
- TEM
effector memory T cell
- TNF‐α
tumour necrosis factor α
INTRODUCTION
The beta‐herpesvirus human cytomegalovirus (HCMV) is ubiquitous in the human population. Although primary infection is generally asymptomatic in immune‐competent individuals, HCMV is a significant cause of morbidity and mortality following congenital infection and primary infection or reactivation in immunocompromised individuals such as immune‐suppressed transplant recipients. Despite its pathogenic potential, HCMV has attracted considerable interest as a potential vaccine vector [1, 2]. HCMV induces unusually high frequencies of antigen‐specific CD4+ and CD8+ T cells [3] that maintain functionality and gradually increase in abundance over time during viral chronicity [4, 5], a process termed ‘memory inflation’ [6]. Studies using the rhesus CMV (RhCMV) model have demonstrated that this T‐cell response can be redirected towards exogenous antigen with exciting therapeutic potential. Using a CMV‐based vector expressing multiple antigens from simian immunodeficiency virus (SIV), induction of broad SIV‐specific T‐cell responses exhibiting MHC class II and HLA‐E restriction was observed [7, 8]. Importantly, these unusual T‐cell responses were accompanied by SIV clearance in approximately half of immunized macaques [7, 9], resulting in the consideration of the utility of HCMV‐based vaccines in a range of diseases including HIV, tuberculosis [10], Ebola [11] and cancer [12, 13].
Despite the potential of CMV‐based vaccines, the severity of medical problems associated with HCMV in vulnerable populations remains a major roadblock. Thus, the development of ‘safe’ non‐replicating CMV vectors was first explored using the murine CMV (MCMV) model. Whereas temperature‐sensitive MCMV failed to induce robust virus‐specific CD8+ T‐cell memory [14], OVA‐expressing virus lacking the gene M94 (MCMV‐ΔM94), which is essential for secondary envelopment and cell‐to‐cell spread of MCMV [15], induced OVA‐specific CD4+ and CD8+ T‐cell responses following systemic challenge [16]. Furthermore, ΔgL‐MCMV lacking the essential viral glycoprotein L (gL) required for viral cell spread induced MCMV‐specific CD8+ T‐cell memory inflation when administered systemically (intraperitoneally), albeit generally to a lesser degree than replicating virus.[17]
Although these studies have indicated excellent safety profiles and induction of effector memory T‐cell responses by these attenuated vectors, these investigations have relied upon systemic routes of infection, particularly intraperitoneal and intravenous injections. Microenvironmental and cellular differences among anatomic infection sites influence virus‐specific T‐cell responses [18]. Furthermore, the site of infection influences the cell types which viruses infect, transport antigen to draining lymph nodes and present antigen to T cells [19]. Indeed, dendritic cell subsets influence T‐cell priming in viral infections by secreting different cytokines leading to Th1 or Th2 dominant responses [20, 21]. and by preferentially priming of CD4+ or CD8+ T cells [22].
Injections into the skin are most applicable to vaccination in the clinical setting. Encouraging, preclinical data from the Rhesus macaque model using a replication‐competent CMV‐based vector were derived from subcutaneous immunization [10]. Importantly, replication‐defective human cytomegalovirus induces humoral and cellular immunity following intramuscular or intradermal injections [23]. Furthermore, conditionally replication‐defective HCMV‐based vectors are being developed [24]. However, currently, there is a lack of detailed understanding of how injection into the skin influences the immunogenicity of CMV‐based vectors, particularly in the context of attenuated vectors. Herein, we used in vivo mouse models of infection to perform a systematic investigation of how subcutaneous immunization with spread‐deficient virus (Δgl‐MCMV) impacts the expansion and function of virus‐specific T cells and the role that relevant DC subsets play in priming of CMV‐specific T cells in this context.
MATERIALS AND METHODS
Mice
BALB/c mice and C57BL/6 mice were purchased from the Charles River Laboratories.
The following transgenic mouse strains were bred in‐house and used for diphtheria toxin (DT)‐induced DC depletions. For plasmacytoid dendritic cell (pDC) depletion, BDCA2‐DTR (014176‐C57BL/6‐Tg(CLEC4C‐HBEGF)956Cln/J) mice (Jax.org) were maintained as heterozygotes. pDC depletion lasts for 2–3 days [25]. For Langerhans cell (LC) depletion, Lang‐DTREGFP (B6·129S2‐Cd207tm3(DTR/GFP)Mal/J) mice (Jax.org) were maintained as homozygotes. Depletion was apparent 24h after DT injection and lasted for 6–7 days. Complete restoration of the LC pool has been reported after 6 weeks [26]. For depletion of cross‐presenting dendritic cells, XCR1‐DTR mice (Riken B6. Cg‐Xcr1<tm2(HBEGF/Venus)Ksho> (RBRC09485)) from Riken (Japan) were maintained as heterozygotes. CD8α+ DC depletion is apparent for 4 days, and pools are completely restored at day 8 [27]. For depletion of classical DCs (cDCs), zDC‐DTR (B6(Cg)‐Zbtb46tm1(HBEGF)Mnz/J) mice (Jax.org) were maintained as homozygotes. In bone marrow chimeras, CD11c+‐DCs were depleted at 12 hr post‐DT injection. Reconstitution starts at day 5, and pools are completely restored in the spleen at day 7 [28]. To generate chimeric mice, C57BL/6 mice were irradiated (2 × 550rad) and transfused with 2 × 106 zDC‐DTR or, in some experiments, XCR1‐DTR bone marrow cells 24 hr post‐irradiation. Mice were subsequently treated with antibiotic‐supplement water (Baytril, Bayer) for 4 weeks. DC depletion and MCMV infections were performed 6–8 weeks post‐irradiation. DT was injected intraperitoneally (i.p.) at 300 ng/mouse on day −1 prior to viral infection or, in XCR1‐DTR mice, at day −3/−2 and day −1. Where non‐chimeric heterozygote mice were used (BDCA2‐DTR), both transgenic and control mice without transgene were injected with DT. In the case of homozygote or chimeric mice (Lang‐DTREGFP, XCR1‐DTR, zDC‐DTR), control groups were injected with PBS.
Viral infections
Mice were injected subcutaneously (s.c.) in the sternum area with 2 × 105 PFU WT‐MCMV (BAC‐derived MCMV strain K181 (a kind gift from Alec Redwood, University of Western Australia)) in 50 µl sterile PBS or with replication‐deficient ΔgL‐MCMV/K181 [17] (a kind gift from Chris Snyder, Thomas Jefferson University) at 2 × 105 PFU, 8 × 105 PFU or 2 × 106 PFU in 50 µl sterile PBS. ΔgL‐MCMV was grown and titred using 3T3 cells that express glycoprotein L (gL). Mice were killed 24 hr or 16–18 or 43 weeks post‐infection, as indicated in the figure legends.
Leucocyte isolation and analysis
Leucocytes were isolated from spleen or axillary draining lymph nodes as described previously [29]. Peripheral blood was collected from the lateral tail vein into heparin‐coated tubes (Sarstedt) at time‐points stated in the legends. Depletion of Langerhans cells (LCs) was confirmed by a migration assay from ear skin using mouse 6Ckine, as described elsewhere [30]. In brief, ears were removed and dorsal skin sheet was isolated via peeling from the ear cartilage using tweezers. The dorsal skin sheets were placed ventral side down in RPMI 1640 (Gibco) containing 10% FCS for 2–4 hr at 37℃ to release non‐DCs. The skin sheets were then transferred into another 1ml medium containing 0·1 μg of recombinant mouse 6Ckine (BioLegend) to enhance DC migration. After 24 hr, skin sheets were again transferred into 1ml fresh medium containing 6Ckine and incubated for a further 24 hr. Supernatants from both time‐points were pooled and assessed for migrating DCs by flow cytometry.
For DC analysis, cells were stained with LIVE/DEAD Zombie Aqua dye (BioLegend) and incubated with FC block and subsequently stained with a combination of the following anti‐mouse antibodies (all from BioLegend, eBioscience or BD Biosciences): anti‐CD8α (53–6·7), anti‐CD11c (N418), anti‐CD11b (M1/70), anti‐IAIE (M5/114·15·2), anti‐Siglec H (551), anti‐CD103 (2E7), anti‐CD80 (16‐10A1), anti‐CD86 (GL‐1), anti‐CD205 (NLDC‐145), anti‐EpCAM1 (G8·8) and anti‐4‐1BBL (TKS‐1).
For detection and phenotypical analysis of MCMV‐specific CD8+ T cells, leucocytes were stained with LIVE/DEAD Zombie Aqua dye (BioLegend) and incubated with FC block (BioLegend), followed by tetramer incubation (25 μg/ml) for 15min at 37℃. Biotinylated monomers loaded with H‐2Kb‐restricted M38 (SSPPMFRV) and IE3 (RALEYKNL) peptides were generated by the NIH tetramer facility (Emory, USA), and were conjugated using PE‐labelled streptavidin (BioLegend) at a 4:1 molar ratio to generate tetramers [31]. Subsequently, a combination of the following anti‐mouse antibodies was used to stain for surface markers (all from BioLegend, eBioscience or BD Biosciences): anti‐CD3ε (145‐2C11), anti‐CD4 (RM4‐5), anti‐CD8α (53‐6·7), anti‐CD44 (IM7), anti‐CD62L (MEL‐14), anti‐CD11a (M17/4), anti‐CD69 (H1.2F3), anti‐CD103 (2E7), anti‐CD127 (A7R34) and anti‐KLRG1 (MAFA). For detection of TCF1 expression, leucocytes were then incubated for 45min with BD Cytofix/Cytoperm™ (BD) at 4℃ and then stained with an anti‐TCF1/TCF7 (C63D9) antibody from New England Biolabs in permeabilization buffer (eBioscience) for 30 min at RT.
For functional analysis, leucocytes were stimulated for 6 hr at 37℃ with peptide M38 (M38, IE3) at a final concentration of 2 μg/ml in the presence of brefeldin A (1 μg/ml) (BD Biosciences). Cells were then stained with LIVE/DEAD Zombie Aqua dye (BioLegend) and incubated with FC block. Cells were subsequently stained with anti‐CD8 (53–6·7), and cells were then fixed in 4% paraformaldehyde and permeabilized with saponin buffer (PBS, 2% FCS, 0·05% sodium azide and 0·5% saponin) in order to perform intracellular cytokine staining using anti‐TNFα (MP6‐XT22) and anti‐IFN‐γ (XMG1.2).
All data were acquired using Attune NxT Flow Cytometer from Thermo Fisher.
Statistics
Paired data were evaluated for statistical significance using the non‐parametric Mann–Whitney U‐test in Prism 8.2.1 for macOS (GraphPad). Where multiple comparisons were performed, a 1‐way ANOVA test was used for single time‐point multi‐group analysis and a 2‐way ANOVA was used for time course data.
Ethics
All mouse experiments were conducted according to U.K. Home Office guidelines under a Home Office project licence (PPL P7867DADD, granted to I. R. Humphreys) at the Home Office‐designated facility at Heath Park, Cardiff University.
RESULTS
MCMV replication is required for optimal T‐cell memory induction following subcutaneous infection
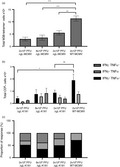
We first sought to investigate the immunogenicity of ΔgL‐MCMV following subcutaneous infection. C57BL/6 mice were administered 2 × 105 PFU ΔgL‐MCMV or WT‐MCMV (pARK25), and peripheral blood CD8+ T‐cell responses to the inflationary H2Kb‐restricted MHC class I‐restricted peptides M38 and IE3 were measured over time. ΔgL‐MCMV induced significantly lower frequencies of IFN‐γ‐secreting M38‐ and IE3‐specific CD8+ T cells in peripheral blood compared with WT‐MCMV during the acute phase of the response 7 days p.i (Figure 1a & b). Interestingly, during the memory phase (from 4 weeks p.i.), only M38‐specific IFN‐γ‐producing T cells remained significantly lower in mice infected with ΔgL‐MCMV as compared to WT‐MCMV (Figure 1a & b). Furthermore, when tissue responses were measured in the spleen at 19 weeks p.i., both peptide stimulation (Figure 1c, d & g) and tetramer staining (Figure 1e, f & i) of splenocytes demonstrated significantly reduced frequencies and numbers of M38‐specific but not IE3‐specific CD8+ T cells in ΔgL‐MCMV‐infected mice as compared to WT‐MCMV. Virus‐specific CD4+ T‐cell responses have similar low frequencies and numbers during latency in both WT‐MCMV‐ and ΔgL‐MCMV‐infected mice (data not shown).
FIGURE 1.
Subcutaneous infection with ΔgL‑MCMV results in impaired‐memory T‐cell induction in C57BL/6 and BALB/c mice. (a,b) C57BL/6 mice were administered s.c. with 2 × 105 PFU of WT‐MCMV or ΔgL‑MCMV. Leucocytes were isolated from peripheral blood on weeks 1, 4, 8, 12 and 16. Frequencies and total numbers or functional (CD8+IFN‐γ+) T cells specific for M38 (a) or IE3 (b) were quantified using flow cytometry. Data shown as mean ± SEM (n = 4). (c–g) Leucocytes were isolated from spleens at week 19 post‐infection (p.i) and quantified using flow cytometry. Data shown as mean ± SEM (n = 6 mice per group). (c) Frequencies of functional (CD8+IFN‐γ+) T cells specific for M38 or IE3. (d) Total numbers of functional (CD8+IFN‐γ+) T cells specific for M38 or IE3. (e) Frequencies of tetramer‐binding CD8+ T cells specific for M38 or IE3. (f) Total numbers of tetramer‐binding CD8+ T cells specific for M38 or IE3. (g) Representative flow cytometry plots of frequencies of unstimulated and IFN‐γ‐producing CD8+ T cells specific for M38 or IE3 are shown. (h) BALB/c mice were infected/immunized s.c. with 2 × 105 PFU WT‐MCMV or ΔgL‑MCMV. Leucocytes were isolated from peripheral blood on weeks 1, 4, 8, 12 and 16 p.i. Frequencies of IE1‐specific functional T cells (CD8+IFN‐γ+) were quantified using flow cytometry. (i) Representative flow cytometry plots of frequencies of tetramer‐binding CD8+ T cells specific for M38 or IE3 are shown. Data shown as mean ± SEM (n = 3–4), representing for 2 experiments. *p 0·05, **p 0·01
We further investigated the epitope‐specific impact of MCMV attenuation on virus‐specific memory T‐cell formation by studying another inflationary CD8+ T‐cell population, namely IE1‐specific T cells in BALB/c mice [6]. BALB/c mice immunized with ΔgL‐MCMV or WT‐MCMV induced similar frequencies of IE1‐specific IFN‐γ‐secreting CD8+ T cells during the acute phase of infection. However, at week 4 p.i., these responses diverged, with reduced‐memory inflation in ΔgL‐MCMV‐infected mice throughout the time course of infection (Figure 1h). Thus, overall, these data suggest that ΔgL‐MCMV is impaired in its ability to induce memory T‐cell formation following subcutaneous infection, although the impact may vary depending on the antigen in question.
T‐cell memory induced by ΔgL‐MCMV is functionally and phenotypically distinct from T cells induced by replication‐competent MCMV
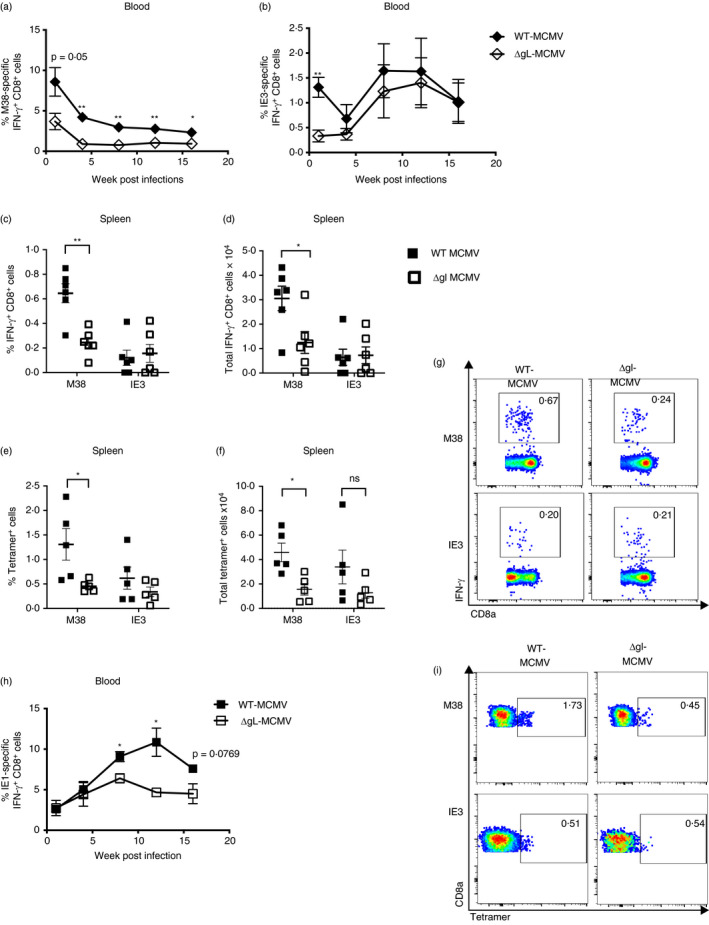
Next, we assessed whether MCMV replication impacted cytokine expression of inflationary CD8+ T‐cell responses, focusing on the robust replication‐dependent M38‐specific responses. After 18 weeks p.i., most M38‐specific T cells in spleens of C57BL/6 mice were polyfunctional (IFN‐γ+TNF‐α+) in WT‐MCMV infection (Figure 2a). Strikingly, we observed a preferential loss of polyfunctional T cells in spleens upon infection with ΔgL‐MCMV with no evidence of conversion to single IFN‐γ or TNF‐α producers. Indeed, we observed very few virus‐specific single TNF‐α+‐ or IFN‐γ+‐producing cells after either MCMV or ΔgL‐MCMV infection (Figure 2a & b). In addition, proportional compositions of the response were analysed and revealed that a lower proportion of M38‐specific polyfunctional cells were found in mice infected with ΔgL‐MCMV (Figure 2b & c). Thus, ΔgL‐MCMV infection via the subcutaneous route leads to a preferential impairment of MCMV‐specific polyfunctional memory T‐cell formation.
FIGURE 2.
Subcutaneous infection with ΔgL‐MCMV leads to a preferential loss of polyfunctional memory CD8+ T cells. C57BL/6 mice were infected s.c. with 2 × 105 PFU of WT‐MCMV or ΔgL‐MCMV. Leucocytes were isolated from spleens on week 19 pi and quantified using flow cytometry. Data shown as mean ± SEM (n = 6). (a) Frequencies and total numbers of polyfunctional and monofunctional T cells in spleens, gated on live CD8+ cells specific for M38. (b) Representative flow cytometry plots of polyfunctional and monofunctional T cells in spleens of mice infected with WT‐MCMV vs. ΔgL‑MCMV. (c) Proportion of M38‐specific functional response in the spleen. The sum of total numbers of polyfunctional and monofunctional T cells in spleens equals 100%. Proportions of poly‐ and monofunctional subsets have been calculated at proportions of 100%. *p ·05, **p ·01, ***p ·001 and ****p
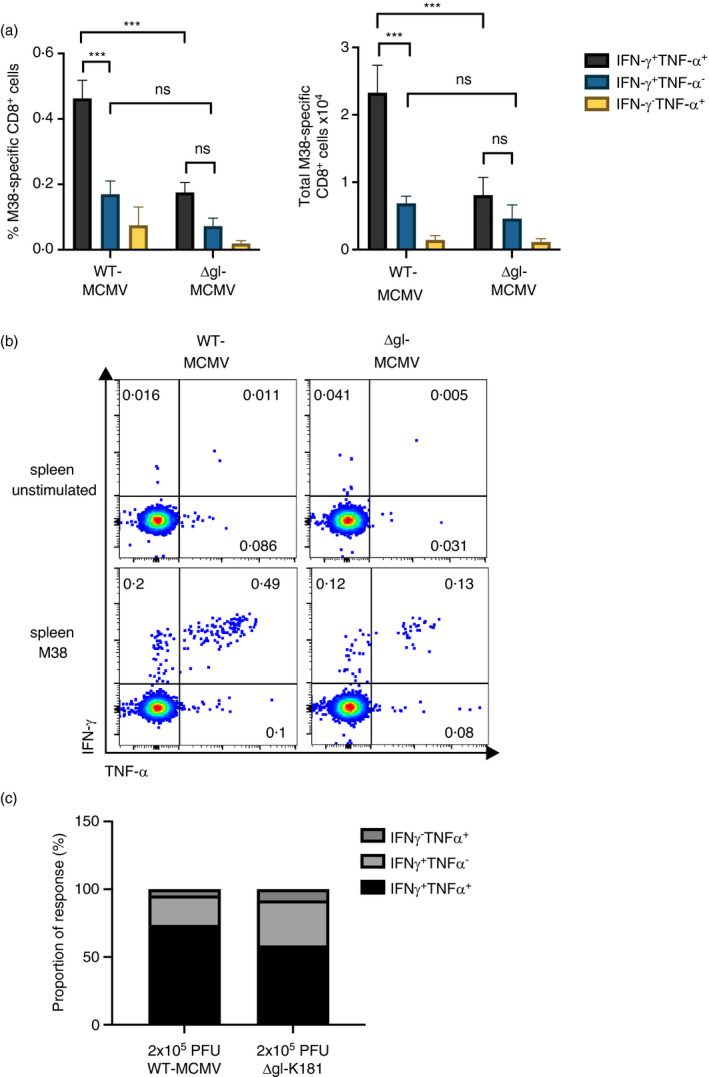
Next, phenotypic analysis of M38‐specific CD8+ T cells produced upon s.c. ΔgL‐MCMV infection was investigated. Proportions of splenic M38‐specific central memory T cells (TCM) and effector memory T cells (TEM) (assessed using CD62L and CD44 expression) were comparable after ΔgL‐MCMV and WT‐MCMV infection with, as expected, most cells exhibiting effector memory phenotype (Figure 3a). However, in accordance with reduced total numbers of M38‐specific CD8+ T‐cell responses, significant reduction in TCM, and TEM in ΔgL‐MCMV‐infected mice were observed (Figure 3b). Furthermore, when expression of KLRG1 and CD127 on CD44+ cells was measured, we noted a significant reduction in total numbers of both memory precursor effector cells (MPECs: CD127+KLRG1−) and short‐lived effector cells (SLECs: CD127−KLRG1+) in ΔgL‐MCMV‐infected mice (Figure 3c–e). Collectively, these data suggest that ΔgL‐MCMV induces fewer numbers of M38‐specific memory CD8+ T cells without preferentially impacting a particular cell subset.
FIGURE 3.
Virus replication is required for maximal formation of multiple memory CD8+ T‐cell subsets following subcutaneous infection. C57BL/6 mice were infected s.c. with 2 × 105 PFU of WT‐MCMV or ΔgL‑MCMV. Leucocytes were isolated from spleens at week 19 pi and quantified using flow cytometry. Data shown as mean ± SEM (n = 6). (a) Frequencies of M38 tetramer‐binding effector memory T cells (TEM: CD8+ CD44+ CD62L−) and central memory T cells (TCM: CD8+ CD44+ CD62L+). (b) Total numbers of M38 tetramer‐binding effector memory T cells (TEM: CD8+ CD44+ CD62L−) and central memory T cells (TCM: CD8+ CD44+ CD62L+). (c) Frequencies of MPECs (CD127+KLRG1+) and SLECs (CD127−KLRG1+). (d) Total numbers of MPECs (CD127+KLRG1+) and SLECs (CD127−KLRG1+). (e) Representative flow cytometry plots of MPECs (CD127+KLRG1+) and SLECs (CD127−KLRG1+) specific for M38 in mice infected with WT‐MCMV or ΔgL‑MCMV. (f) and (g) C57BL/6 mice were infected s.c. with 2 × 105 PFU of WT‐MCMV or ΔgL‑MCMV. Leucocytes were isolated from spleens at week 4 p.i. and quantified using flow cytometry. Total numbers of M38‐specific (f) and IE3‐specific (g) TCF+ T cells gated on CD8 are shown. *p 0·05, **p 0·01 and ***p 0·001
A recent publication [32] demonstrated that a small subset of T cells expressing transcription factor 1 (TCF1) is required to sustain the inflationary T‐cell pool in chronic MCMV infection. We therefore infected mice with WT‐MCMV or ΔgL‐MCMV and harvested spleens after 4 weeks to assess TCF1 expression of M38‐ and IE3‐specific T cells. There were significantly fewer M38‐specific (but not IE3‐specific) TCF1+ cells in mice infected with ΔgL‐MCMV as compared to WT‐MCMV (Figure 3f and g). These data indicate that ΔgL‐MCMV might be inferior in maintaining the M38‐specific inflationary T‐cell pool via impaired induction of TCF1.
Increasing dosing of ΔgL‐MCMV does not augment T‐cell memory formation
Impaired induction of functional T‐cell memory by ΔgL‐MCMV may simply reflect the requirement for more virus particles in the initial infection and thus, in a vaccination setting, could be overcome by using a larger dose of replication‐deficient MCMV. To assess this, mice were infected subcutaneously with 2x105 PFU, 8 × 105 PFU or 2 × 106 PFU ΔgL‐MCMV or, as control, 2 × 105 PFU WT‐MCMV. Increasing the dose of ΔgL‐MCMV as much as 10‐fold had only a negligible impact on M38‐specific CD8+ T‐cell memory accumulation 43 weeks later (Figure 4a). Functional analysis of T cells showed a trend towards fewer polyfunctional (IFN‐γ+TNF‐α+) T cells in all ΔgL‐MCMV doses as compared to WT‐MCMV (Figure 4b and c), with no discernible increase in frequencies of polyfunctional T cells after increasing the inoculum of ΔgL‐MCMV. Overall, these data suggest that impaired‐memory T‐cell formation following infection with replication‐deficient MCMV cannot be easily overcome by increasing the initial inoculum.
FIGURE 4.
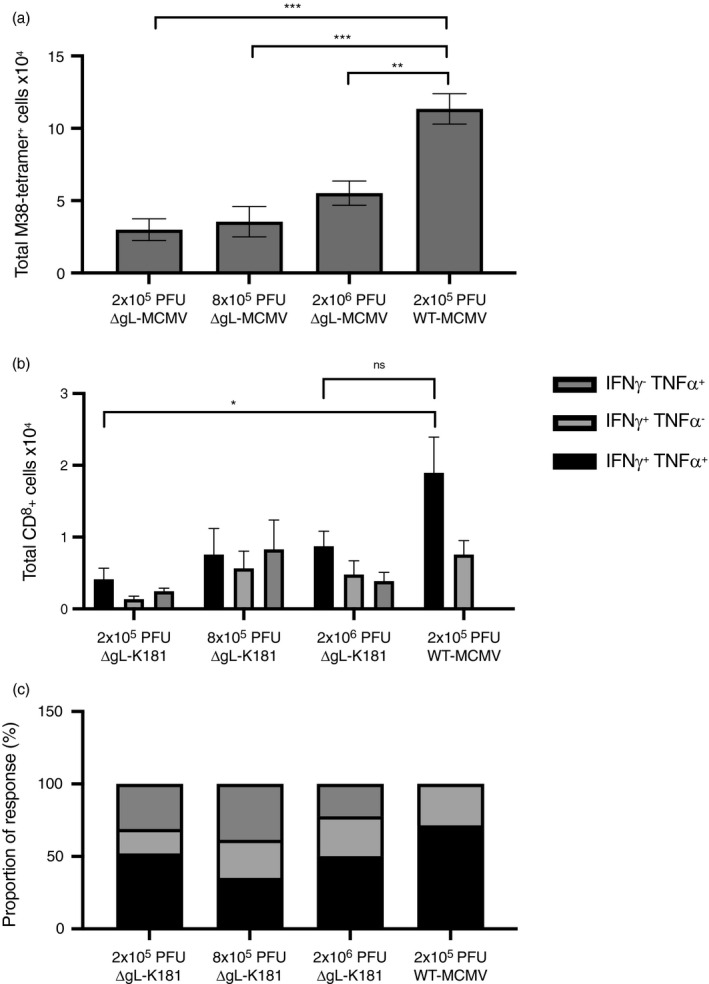
Increasing dosing of ΔgL‐MCMV does not augment memory T‐cell formation. C57BL/6 mice were infected s.c. with 2 × 105 PFU (n = 4), 8 × 105 PFU (n = 5) or 2 × 106 PFU (n = 5) of ΔgL‐MCMV or 2 × 105 PFU WT‐MCMV (n = 6). Leucocytes were isolated from spleens 43 wk p.i. and quantified using flow cytometry. (a) Total numbers of tetramer‐binding CD8+ T cells specific for M38. (b) Total numbers of polyfunctional (IFN‐γ+TNF‐α+) and monofunctional (IFN‐γ+TNF‐α− or IFN‐γ−TNF‐α+) T cells in spleens, gated on live CD8+ cells specific for M38. (c) Proportion of M38‐specific functional response. The sum of total numbers of polyfunctional and monofunctional T cells in spleens equals 100%. Proportions of poly‐ and monofunctional subsets have been calculated at proportions of 100%. Data shown as mean SEM. *p 0·05, **p ·01 and ***p ·001
Initial T‐cell priming by dendritic cells has minimal impact on inflationary T‐cell memory formation after subcutaneous MCMV infection
We have previously demonstrated that manipulation of cytokine signalling during initial MCMV infection and T‐cell priming can influence memory T‐cell inflation [33, 34] and can be manipulated to enhance immunogenicity of replication‐deficient MCMV [25]. Given that reduced‐memory T‐cell formation following subcutaneous ΔgL‐MCMV infection was accompanied by impaired responses during the acute phase of infection, we hypothesized that reduced‐memory T‐cell formation in these mice may reflect defective T‐cell priming. Thus, we measured cDC responses in skin‐draining axillary lymph nodes that we confirmed had drained from the injection site used, using Evans blue injection. Following ΔgL‐MCMV infection, we observed reduced classical DC (cDC, CD11c+MHCIIhi) accumulation in draining lymph nodes 24 hr after ΔgL‐MCMV infection, as compared to WT‐MCMV (Figure 5a), suggesting a reduction in global DC responses may contribute to reduced‐memory T‐cell formation induced by ΔgL‐MCMV.
FIGURE 5.
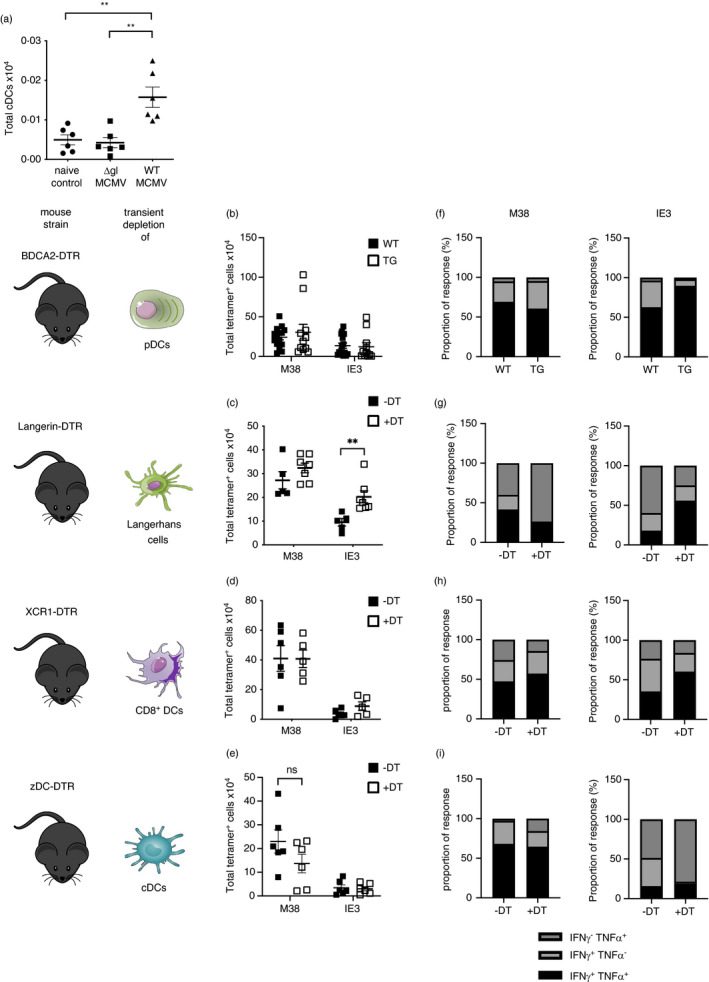
Depleting DC subsets during initial subcutaneous ΔgL‐MCMV infection does not dramatically impact memory T‐cell formation. (a) Total numbers of cDCs (CD11c+ MHCII+) isolated from axillary lymph nodes 24 hr post‐s.c. infection were quantified using flow cytometry. (b–e) total numbers of tetramer‐binding CD8+ T cells specific for M38 or IE3 in spleens quantified by flow cytometry. (f–i): Proportion of M38‐ and IE3‐specific functional response. The sum of total numbers of M38‐ or IE3‐specific polyfunctional (IFN‐γ+TNF‐α+) and monofunctional (IFN‐γ+TNF‐α− or IFN‐γ−TNF‐α+) T cells in spleens =100%. Proportions of poly‐ and monofunctional subsets have been calculated as proportions of 100%. (b) BDCA2‐DTR transgenic (TG) and non‐transgenic (WT) mice were injected with 300 ng diphtheria toxin (DT) 24 hr before s.c. infection with 2 × 105 PFU WT‐MCMV. Leucocytes were isolated from spleens at 19/18 wk pi. Data shown as mean ± SEM (n = 14 WT and 12 TG) representative for 2 experiments. (c) XCR1‐DTR transgenic (TG) and non‐transgenic (WT) mice were injected with 300 ng diphtheria toxin (DT) 72 and 24 hr before s.c. infection with 2 × 105 PFU WT‐MCMV. Leucocytes were isolated from spleens at 18 wk pi. Data shown as mean ± SEM (n = 6 WT and 5 TG). (d) LANG‐DTREGFP mice were injected with 300 ng diphtheria toxin (+DT) or not (−DT) 24 hr before s.c. infection with 2 × 105 PFU WT‐MCMV. Leucocytes were isolated from spleens at 18 weeks pi. Data shown as mean ± SEM (n = 10 +DT and 7 −DT). (d) zDC‐DTR bone marrow chimeras were injected with 300 ng diphtheria toxin (+DT) or not (−DT) 24 hr before s.c. infection with 2 × 105 PFU WT‐MCMV. Leucocytes were isolated from spleens 19 wk pi. Data shown as mean ± SEM (n = 5 +DT and 6 −DT). *p ·05 and **p ·01
We thus hypothesized that understanding what early DC responses contribute to the immunogenicity of replicating MCMV may afford insight into mechanisms that could be exploited to enhance the immunogenicity of replication‐defective vectors during immunization. Thus, we systematically depleted DCs using established transgenic mice that enable diptheria toxin‐mediated transient depletion of either pDCs, cross‐presenting (XCR1+) DCs, classical DCs and Langerhans cells. Published methodology demonstrated that DC depletion lasts for at least 3–7 days [25, 26, 27, 28] and is thus removing DCs during the T‐cell priming in acute infection, which we confirmed in‐house (Figure S1). As depletion of classical DCs in zDC‐DTR mice is fatal [28], bone marrow chimeras of WT C57BL/6 mice were generated prior to cDC depletion. The same approach was used with XCR1‐DTR mice for the depletion of cross‐presenting DCs to ensure comparability when assessing the two overlapping DC populations.
We observed no impairment of memory T‐cell formation after subcutaneous MCMV infection after depletion of pDCs (Figure 5b), Langerhans cells (Figure 5c) or, in contrast to data derived from mice with genetic defects in cross‐presenting DCs (and thus throughout the time course of infection [35]), XCR1+ DCs (Figure 5d). Following depletion of classical DCs (including cross‐presenting DCs) using zDC mice, we noted a trend in impaired M38‐ but not IE3‐specific CD8+ T‐cell memory development after robust DC depletion (Figure S1), although differences were not statistically significant (Figure 5e). We also assessed the influence of early DC responses on memory T‐cell functionality. We observed that after depletion of pDCs, Langerhans cells and XCR1+ DCs (Figure 5f–h), memory CD8+ T‐cell polyfunctionality was unaltered (Figure 5i). Interestingly, in the case of Langerhans cell depletion, we observed an increased accumulation of IE3‐specific T cells after depletion (Figure 5c), consistent with known suppressive functions of Langerhans cells [36]. Thus, these data argue against a major influence for early DC activation in the immunogenicity of replicating MCMV. Overall, our data imply that strategies beyond increasing dosing and targeting DC activation should be considered when attempting to improve the immunogenicity of subcutaneous vaccination with replication‐deficient CMV vectors.
DISCUSSION
In this study, we demonstrated that subcutaneous infection with spread‐deficient MCMV induces significantly inferior T‐cell memory responses to inflationary epitopes when administered subcutaneously. Previous detailed investigations of the impact of MCMV replication on virus‐induced T‐cell responses focused primarily on a systemic infection via the peritoneum where ΔgL‐MCMV induced memory T‐cell responses, albeit at lower frequencies as compared to replicating virus. The same study noted that footpad infection of ΔgL‐MCMV failed to induce notable memory responses [17]. We now show that subcutaneous infection with ΔgL‐MCMV induces inferior polyfunctional memory T‐cell responses as compared to replicating MCMV, which corresponds to a reduction in viral‐specific memory T‐cell subsets.
Why ΔgL‐MCMV induces inferior memory T‐cell responses following subcutaneous infection is unclear. Studies using replication‐deficient Δm94‐MCMV have reported that latent load of spread‐deficient virus is comparable to that of replicating virus [16], suggesting that impaired‐memory induction is not a consequence of reduced latent viral load that leads to CMV reactivation and antigen presentation required for maintenance of long‐term T‐cell memory [37, 38]. In accordance with this, we observed no differences in latent viral load in spleens or draining lymph nodes 4 weeks post‐infection (data not shown). Furthermore, merely increasing the dose of ΔgL‐MCMV did not improve induction of memory formation, suggesting that the initial burst of replication and the virions produced also did not greatly influence memory T‐cell formation. Cell‐to‐cell spread is a key feature of HCMV replication and viral infection of DCs [39]. Whether this route of cell spread, lacking in ΔgL‐MCMV infection, induces immune activation relevant for induction of memory T‐cell formation is currently unclear.
We noted that subcutaneous infection with ΔgL‐MCMV induced the accumulation of fewer classical DCs into draining lymph nodes during acute infection, and this correlated with reduced T‐cell responses during this phase, at least in the C57BL/6 model. This contrasts with data obtained in the intraperitoneal infection model where non‐replicating MCMV actually enhanced initial DC accumulation by triggering less type I IFN that drives DC apoptosis in this model. Consequently, the authors observed elevated T‐cell responses during the acute phase of non‐replicating MCMV infection [40]. Similarly, in BALB/c mice, Dalod and colleagues demonstrated that type I IFN ablated splenic cDC responses, with a subsequent delay of acute CD8+ T‐cell responses [41]. Differences between these observations and our study may reflect differential cell tropism of the virus, or altered induction by MCMV of type I IFN following systemic versus subcutaneous infection. Irrespectively, these differences highlight the importance of the consideration of route of infection when considering early immunological events during CMV‐based vector immunization.
Despite the impact of MCMV replication on DC accumulation in lymph nodes, systematic depletion of different DC subsets during the first few days of infection revealed no obvious role for pDC, Langerhans cells or XCR1+ DCs in facilitating inflationary memory T‐cell formation. The latter observation is in accordance with data derived from Batf −/− mice that lack cross‐presenting DCs, demonstrating that the absence of these cells throughout the infection time course had a minimal impact on memory inflation despite impacting T‐cell priming [35]. Transient depletion of all cDCs (including cross‐presenting cells) showed a trend in reduced M38‐ but not IE3‐specific CD8+ memory T cells. However, these data argue against a dominant early role for DCs in determining memory T‐cell formation although they do not preclude a role for DCs in T‐cell priming once they recover from DT depletion ~1 week post‐infection in the models used. It is also important to note that one limitation of our study is that we did not perform systematic depletion of DC subsets after ΔgL‐MCMV infection. However, in fitting with the conclusion that initial DC responses during acute infection have a minimal impact on memory T‐cell development, we failed to boost M38‐ and IE3‐specific memory CD8+ T‐cell responses induced by subcutaneous ΔgL‐MCMV infection co‐administered with activating ligands of DC‐expressed endosomal TLR7 or TLR9 (data not shown). This contrasts the improved memory T‐cell formation when using the alarmin IL‐33 [34], that is released upon cell damage after viral infections [42], as an adjuvant. Similarly, blocking the function of the immune‐suppressive cytokine IL‐10 during initial MCMV infection increases memory T‐cell formation, thus demonstrating that early immunological events can be manipulated to increase CMV‐induced memory T‐cell formation. However, in line with the established role for non‐haematopoietic cells in sustaining memory T‐cell inflation [43, 44], these data imply that DCs may not represent useful targets for strategies that aim to improve immunogenicity of replication‐deficient CMV vectors.
We noted an epitope‐specific impact of virus replication on memory T‐cell induction after subcutaneous infection with ΔgL‐MCMV, with an impairment of IE1‐specific responses in BALB/c mice, and M38 but not IE3‐specific responses in C57BL/6 mice. Given that IE1 and IE3 are expressed during the same phase of infection, kinetics of gene expression are unlikely to explain these discrepancies, although we cannot rule out that differences in IE3 and IE1 protein turnover and processing may exist in the APCs of relevance in this model. Differential dependency of MCMV‐specific CD8+ T‐cell memory on CD4+ T‐cell help has been described in C57BL/6 mice [45]. However, given that IE3‐specific responses are preferentially impacted by CD4+ T‐cell deficiency, the observation that IE3‐specific inflationary responses were unaffected by replication‐deficient MCMV argues against this hypothesis. Moreover, both IE3‐ and M38‐specific T‐cell responses during the acute phase of infection were impacted by replication deficiency, although we noted that M38‐specific T cells did not follow the usual kinetics of inflationary T‐cell responses that we (and others) routinely observe after systemic MCMV infection [33, 46, 47]. Notably, however, we did observe a significant reduction in TCF1+ T cells reactive for M38 but not IE3, suggesting that inflationary T cells of different specificities have differential requirements for virus replication in the induction of TCF1 and that this occurs in the first month of infection. Regulation of TCF1 expression in memory CD8+ T cells is incompletely understood. However, given that IL‐12 and type I IFNs induce TCF1 expression by MCMV‐specific CD8+ T cells [32], one could speculate that increased sensitivity of IE3‐specific T cells to these cytokines (e.g. due to higher expression of receptors) could result in reduced sensitivity to the reduced early cytokine induction that is observed following replication‐deficient MCMV infections [40]. Understanding why antigen‐specific memory T‐cell responses are differentially affected by virus replication following infection via the skin may inform the design of CMV vectors in the future.
One way to improve memory induction by a replicating vector could be simply to increase the infectious dose and initial antigen exposure, triggering a greater primary T‐cell response and therefore possibly increasing the formation of a memory T‐cell pool. Indeed, careful dose titration experiments using replicating MCMV clearly show a positive correlation between virus dose and amplitude of virus‐specific memory induction [48]. In our study, however, we observed that increasing the dose of ΔgL‐MCMV had little impact on memory T‐cell formation and polyfunctionality, certainly at the doses used. Whether the amount of antigen is already maximal, or whether any beneficial effects of increasing antigen load are counteracted by an elevated type I IFN responses and subsequent DC apoptosis is unclear. Irrespectively, collectively our data suggest that alternate approaches beyond dose elevation and DC manipulation may be needed to maximize immunogenicity of CMV‐based vaccine vectors for safe and efficacious utility in the future.
CONFLICT OF INTEREST
The authors have no competing interests.
AUTHOR CONTRIBUTIONS
SD, SGB, MM, PS, LC and MC performed the experiments. SD, SGB and IH designed the experiments. SD and IH wrote the paper.
Supporting information
Figure S1
ACKNOWLEDGEMENT
We thank Paula Longhi for advice regarding dendritic cell depletion models and Richard Stanton for critical reading of the manuscript.
Dimonte S, Gimeno‐Brias S, Marsden M, Chapman L, Sabberwal P, Clement M, et al. Optimal CD8+ T‐cell memory formation following subcutaneous cytomegalovirus infection requires virus replication but not early dendritic cell responses. Immunology. 2021;164:279–291. 10.1111/imm.13368
Funding information
This research was funded by a Wellcome Trust Senior Research Fellowship (Ref. 207503/Z/17/Z), a Wellcome Trust Collaborative Award in Science (Ref. 209213/Z/17/Z to I.R.H as co‐applicant), an MRC Confidence in Concept Award (to I.R.H), a Systems Immunity University Research Institute PhD Studentship (to P.S.) and a Cardiff University School of Medicine PhD Studentship (to S. G‐B.).
Contributor Information
Sandra Dimonte, Email: DimonteS@cf.ac.uk.
Ian R Humphreys, Email: humphreysir@cardiff.ac.uk.
REFERENCES
- 1.Humphreys IR, Sebastian S. Novel viral vectors in infectious diseases. Immunology. 2018;153:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Früh K, Picker L. CD8+ T cell programming by cytomegalovirus vectors: applications in prophylactic and therapeutic vaccination. Curr Opin Immunol. 2017;47:52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, et al. Broadly targeted human cytomegalovirus‐specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson SE, Sedikides GX, Mason GM, Okecha G, Wills MR. Human cytomegalovirus (HCMV)‐specific CD4+ T cells are polyfunctional and can respond to HCMV‐infected dendritic cells in vitro. J Virol. 2017;91:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lachmann R, Bajwa M, Vita S, Smith H, Cheek E, Akbar A, et al. Polyfunctional T cells accumulate in large human cytomegalovirus‐specific T cell responses. J Virol. 2012;86:1001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, et al. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol. 2003;170:2022–9. [DOI] [PubMed] [Google Scholar]
- 7.Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, Scholz I, et al. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science. 2013;340:1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen SG, Wu HL, Burwitz BJ, Hughes CM, Hammond KB, Ventura AB, et al. Broadly targeted CD8+ T cell responses restricted by major histocompatibility complex E. Science. 2016;351:714–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen SG, Piatak M, Ventura AB, Hughes CM, Gilbride RM, Ford JC, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502:100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen SG, Zak DE, Xu G, Ford JC, Marshall EE, Malouli D, et al. Prevention of tuberculosis in rhesus macaques by a cytomegalovirus‐based vaccine. Nat Med. 2018;24:130–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuda Y, Parkins CJ, Caposio P, Feldmann F, Botto S, Ball S, et al. A cytomegalovirus‐based vaccine provides long‐lasting protection against lethal Ebola virus challenge after a single dose. Vaccine. 2015;33:2261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beyranvand Nejad E, Ratts RB, Panagioti E, Meyer C, Oduro JD, Cicin‐Sain L, et al. Demarcated thresholds of tumor‐specific CD8 T cells elicited by MCMV‐based vaccine vectors provide robust correlates of protection. J Immunother Cancer. 2019;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu Z, Grenier JM, Khanna KM. Reviving virus based cancer vaccines by using cytomegalovirus vectors expressing modified tumor antigens. Oncoimmunology. 2016;5:e1056974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beswick M, Pachnio A, Al‐Ali A, Sweet C, Moss PA. An attenuated temperature‐sensitive strain of cytomegalovirus (tsm5) establishes immunity without development of CD8(+) T cell memory inflation. J Med Virol. 2013;85:1968–74. [DOI] [PubMed] [Google Scholar]
- 15.Maninger S, Bosse JB, Lemnitzer F, Pogoda M, Mohr CA, von Einem J, et al. M94 Is essential for the secondary envelopment of murine cytomegalovirus ▿. J Virol. 2011;85:9254–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohr CA, Arapovic J, Muhlbach H, Panzer M, Weyn A, Dolken L, et al. A spread‐deficient cytomegalovirus for assessment of first‐target cells in vaccination. J Virol. 2010;84:7730–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder CM, Cho KS, Bonnett EL, Allan JE, Hill AB. Sustained CD8+ T cell memory inflation after infection with a single‐cycle Cytomegalovirus. PLoS Pathog. 2011;7:e1002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fulton JR, Smith J, Cunningham C, Cuff CF. Influence of the route of infection on development of T‐cell receptor β‐chain repertoires of reovirus‐specific cytotoxic T lymphocytes. J Virol. 2004;78:1582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rouse BT, Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat Rev Immunol. 2010;10:514–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, et al. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA. 1999;96:1036–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maldonado‐López R, Moser M. Dendritic cell subsets and the regulation of Th1/Th2 responses. Semin Immunol. 2001;13:275–82. [DOI] [PubMed] [Google Scholar]
- 22.Joffre OP, Segura E, Savina A, Amigorena S. Cross‐presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–69. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Freed DC, Li L, Tang A, Li F, Murray EM, et al. A replication‐defective human cytomegalovirus vaccine elicits humoral immune responses analogous to those with natural infection. J Virol. 2019;93(23):e00747‐19. 10.1128/JVI.00747-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D, Freed DC, He X, Li F, Tang A, Cox KS, et al. A replication‐defective human cytomegalovirus vaccine for prevention of congenital infection. Sci Transl Med. 2016;8:362ra145. [DOI] [PubMed] [Google Scholar]
- 25.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33:955–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kissenpfennig A, Henri S, Dubois B, Laplace‐Builhé C, Perrin P, Romani N, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–54. [DOI] [PubMed] [Google Scholar]
- 27.Yamazaki C, Sugiyama M, Ohta T, Hemmi H, Hamada E, Sasaki I, et al. Critical roles of a dendritic cell subset expressing a chemokine receptor, XCR1. J Immunol. 2013;190:6071–82. [DOI] [PubMed] [Google Scholar]
- 28.Meredith MM, Liu K, Darrasse‐Jeze G, Kamphorst AO, Schreiber HA, Guermonprez P, et al. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J Exp Med. 2012;209:1153–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stacey MA, Marsden M, Wang ECY, Wilkinson GWG, Humphreys IR. IL‐10 restricts activation‐induced death of NK cells during acute murine cytomegalovirus infection. J Immunol. 2011;187:2944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henri S, Vremec D, Kamath A, Waithman J, Williams S, Benoist C, et al. The dendritic cell populations of mouse lymph nodes. J Immunol. 2001;167:741–8. [DOI] [PubMed] [Google Scholar]
- 31.Price DA, Brenchley JM, Ruff LE, Betts MR, Hill BJ, Roederer M, et al. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J Exp Med. 2005;202:1349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welten SPM, Yermanos A, Baumann NS, et al. Tcf1 + Cells Are Required to Maintain the Inflationary T Cell Pool Upon MCMV Infection [Internet]. Nat Commun. 2020;11:2295. [cited 2020 May 21]. Available from: https://pubmed.ncbi.nlm.nih.gov/32385253/?from_single_result=Tcf1%2B+cells+are+required+to+maintain+the+inflationary+T+cell+pool+upon+MCMV+infection&expanded_search_query=Tcf1%2B+cells+are+required+to+maintain+the+inflationary+T+cell+pool+upon+MCMV+infection [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones M, Ladell K, Wynn KK, Stacey MA, Quigley MF, Gostick E, et al. IL‐10 restricts memory T cell inflation during cytomegalovirus infection. J Immunol. 2010;185:3583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLaren JE, Clement M, Marsden M, Miners KL, Llewellyn‐Lacey S, Grant EJ, et al. IL‐33 augments virus‐specific memory T cell inflation and potentiates the efficacy of an attenuated cytomegalovirus‐based vaccine. J Immunol. 2019;202:943–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torti N, Walton SM, Murphy KM, Oxenius A. Batf3 transcription factor‐dependent DC subsets in murine CMV infection: differential impact on T‐cell priming and memory inflation. Eur J Immunol. 2011;41:2612–8. [DOI] [PubMed] [Google Scholar]
- 36.West HC, Bennett CL. Redefining the role of langerhans cells as immune regulators within the skin. Front Immunol [Internet]. 2018;8:1941. [cited 2020 Sep 12]. Available from: https://www.frontiersin.org/articles/10.3389/fimmu.2017.01941/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snyder CM, Cho KS, Bonnett EL, van Dommelen S, Shellam GR, Hill AB. Memory inflation during chronic viral infection is maintained by continuous production of short‐lived, functional T cells. Immunity. 2008;29:650–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sierro S, Rothkopf R, Klenerman P. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur J Immunol. 2005;35:1113–23. [DOI] [PubMed] [Google Scholar]
- 39.Murrell I, Bedford C, Ladell K, Miners KL, Price DA, Tomasec P, et al. The pentameric complex drives immunologically covert cell–cell transmission of wild‐type human cytomegalovirus. Proc Natl Acad Sci. 2017;114:6104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loo CP, Snyder CM, Hill AB. Blocking virus replication during acute murine cytomegalovirus infection paradoxically prolongs antigen presentation and increases the CD8+ T cell response by preventing type I IFN‐dependent depletion of dendritic cells. J Immunol. 2017;198:383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robbins SH, Bessou G, Cornillon A, Zucchini N, Rupp B, Ruzsics Z, et al. Natural killer cells promote early CD8 T cell responses against cytomegalovirus. PLoS Pathog. 2007;3:e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cayrol C, Girard J‐P. IL‐33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol. 2014;31:31–7. [DOI] [PubMed] [Google Scholar]
- 43.Seckert CK, Schader SI, Ebert S, Thomas D, Freitag K, Renzaho A, et al. Antigen‐presenting cells of haematopoietic origin prime cytomegalovirus‐specific CD8 T‐cells but are not sufficient for driving memory inflation during viral latency. J Gen Virol. 2011;92:1994–2005. [DOI] [PubMed] [Google Scholar]
- 44.Torti N, Walton SM, Brocker T, Rülicke T, Oxenius A. Non‐hematopoietic cells in lymph nodes drive memory CD8 T cell inflation during murine cytomegalovirus infection. PLoS Pathog. 2011;7:e1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snyder CM, Loewendorf A, Bonnett EL, Croft M, Benedict CA, Hill AB. CD4+ T cell help has an epitope‐dependent impact on CD8+ T cell memory inflation during murine cytomegalovirus infection. J Immunol. 2009;183:3932–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Humphreys IR, Loewendorf A, de Trez C, Schneider K, Benedict CA, Munks MW, et al. OX40 costimulation promotes persistence of cytomegalovirus‐specific CD8 T Cells: A CD4‐dependent mechanism. J Immunol. 2007;179:2195–202. [DOI] [PubMed] [Google Scholar]
- 47.Humphreys IR, Lee S‐W, Jones M, Loewendorf A, Gostick E, Price DA, et al. Biphasic role of 4–1BB in the regulation of mouse cytomegalovirus‐specific CD8(+) T cells. Eur J Immunol. 2010;40:2762–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Redeker A, Remmerswaal EBM, van der Gracht ETI, Welten SPM, Höllt T, Koning F, et al. The contribution of cytomegalovirus infection to immune senescence is set by the infectious dose. Front Immunol. 2017;8:1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


