Abstract
Pelizaeus-Merzbacher disease (PMD) is a severe hypomyelinating disorder of the central nervous system (CNS) linked to mutations in the proteolipid protein-1 (PLP1) gene. Although there are multiple animal models of PMD, few of them fully mimic the human disease. Here, we report three spontaneous cases of male neonatal rhesus macaques with the clinical symptoms of hypomyelinating disease, including intention tremors, progressively worsening motor dysfunction, and nystagmus. These animals demonstrated a paucity of CNS myelination accompanied by reactive astrogliosis, and a lack of PLP1 expression throughout white matter. Genetic analysis revealed that these animals were related to one another and that their parents carried a rare, hemizygous missense variant in exon 5 of the PLP1 gene. These animals therefore represent the first reported non-human primate model of PMD, providing a novel and valuable opportunity for preclinical studies that aim to promote myelination in pediatric hypomyelinating diseases.
Keywords: Pelizaeus-Merzbacher Disease, hypomyelination, proteolipid protein, oligodendrocytes, non-human primate
1. Introduction
Myelin sheaths that wrap around the axons of neurons in vertebrates dramatically increase axon conduction velocities and are essential for normal nervous system function. In the central nervous systems (CNS) of most vertebrates, myelin is made by oligodendrocytes (OLs) that can supply segments of myelin for up to approximately 50 axons. In both the developing and adult CNS, oligodendrocytes arise from oligodendrocyte progenitor cells (OPCs) that mature into myelin-forming OLs to either generate new myelin or replace damaged myelin (Lubetzki et al., 2020). Conditions that interfere with OPC maturation into myelinating OLs or alter the structure of myelin prevent myelination or remyelination following demyelinating insults. Dysmyelination and remyelination failure result in cognitive, motor and sensory deficits.
Leukodystrophies are a group of genetic dysmyelinating disorders characterized by different combinations of hypomyelination and demyelination (Inoue, 2019). Hypomyelinating leukodystrophies are characterized by either a lack of myelination or abnormal myelination, while demyelinating leukodystrophies are characterized by progressive white matter degeneration. The most common hypomyelinating leukodystrophy is Pelizaeus-Merzbacher disease (PMD), which affects approximately 1 in 400,000 individuals (Osório et al., 2018). The vast majority of PMD patients are male. PMD patients present with hypotonia, nystagmus, respiratory distress, and stridor soon after birth with subsequent delays in motor and cognitive development and spastic quadriparesis (Singh and Samanta, 2020).
The majority of PMD cases are linked to mutations in the proteolipid protein-1 (PLP1) gene located on the long arm of the X chromosome. PLP1 deletions, missense mutations, point mutations, and increased PLP1 copy numbers have been linked to disease in PMD patients and in rodent models of PMD (Inoue, 2019). PLP1 encodes the PLP1 protein as well as a shorter version of the same protein, called DM20, which is encoded by a PLP1 splice variant (Nave et al., 1987). PLP1/DM20 is a tetraspan membrane protein expressed by OLs and localized to CNS myelin (Weimbs and Stoffel, 1992). Although the functions of PLP1 are not entirely clear, it has been implicated in the stabilization of compact myelin (Boison et al., 1995).
There is growing evidence supporting the use of cell-based therapies, including the use of OPCs and neural stem cells, to treat PMD and other leukodystrophies (Windrem et al., 2004; Windrem et al., 2008; Wang et al., 2011; Uchida et al., 2012; Gruenenfelder et al., 2020). However, the evaluation of the safety and efficacy of these approaches can be limited by the availability of appropriate animal models that broadly share the pathological features of the human disease (Rutherford and Hamilton, 2019). Small animal models with Plp1 mutations, including the jimpy (jp) mouse (Nave et al., 1986), the rumpshaker mouse (Griffiths et al., 1990), the myelin deficient rat (md) (Dentinger et al., 1982; Duncan et al., 1987), and the paralytic tremor (pt) rabbit (Tosic et al., 1994; Sypecka and Domanska-Janik, 2005), each exhibit some aspects of human PMD, but have limited predictive value for studies in humans due to significant differences in their anatomy and physiology, and because many of them are short-lived. The canine shaking pup (shp) model also has PLP1 mutations, lives longer, and has proven to be valuable for understanding aspects of human PMD (Griffiths et al., 1981a, b; Duncan et al., 1983; Mayer et al., 2015). However, as with the small animal models discussed above, canine models can have limited value for pre-clinical studies of human diseases for a number of reasons, including differences in their anatomy and physiology (Partridge and Rossmeisl Jr., 2020).
Non-human primate (NHP) models of human neurological disorders often have greater translational value compared to other animal models (Verdier et al., 2015). NHPs have a highly developed cerebral cortex that is anatomically similar to humans and they exhibit greater cognitive and complex motor skills. Among NHPs, rhesus macaques (Macaca mulatta) are the best characterized and most widely used NHPs in applied biomedical research. Furthermore, the recent development of macaque genomic resources (Rhesus Macaque Genome Sequencing and Analysis Consortium et al., 2007; Warren et al., 2020; Bimber et al., 2019) has spurred the discovery of novel NHP models of monogenic diseases. These include naturally occurring models of globoid cell leukodystrophy, also known as Krabbe disease (Baskin et al., 1998; Borda et al., 2008), and neuronal ceroid lipofuscinosis (McBride et al., 2018).
In this study, we have characterized the clinical and histopathologic features of three related infant rhesus macaques at the Oregon National Primate Research Center (ONPRC) who were clinically evaluated for a range of symptoms including respiratory difficulties, head bobbing, difficulties nursing, and other motor dysfunction. Histopathologic evaluation revealed that these animals had widespread CNS hypomyelination. Using both pedigree and genomic sequence analyses, we found that these animals were from a family that carried a rare PLP1 mutation, consistent with an inherited form of PMD. Animals from this family therefore represent a unique potential resource for studies aimed at developing novel therapies for PMD and other hypomyelinating or demyelinating diseases.
2. Materials and Methods
2.1. Animals
Rhesus macaques were housed in indoor or outdoor social groups prior to removal for clinical evaluation. All animals were housed with their dams. Animal care and procedures were conducted in accordance with all federal regulations and the guidelines established by the National Institutes of Health, the Guide, and the Institutional Animal Care and Use Committee at the ONPRC. The ONPRC is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
2.2. Clinical Evaluations
Individual animal health observations are performed daily by trained animal care personnel for all indoor- and outdoor-housed rhesus macaques at the ONPRC. Clinical signs were first documented during these daily observations while in outdoor housing for Cases 1 and 2, and after birth indoors for Case 3. Once behavioral abnormalities were identified, infants were referred with their dams to a dedicated hospital treatment area for triage and subsequent detailed clinical observation of neurologic signs. In-house complete clinical evaluations, as outlined in the Results below, were conducted by ONPRC veterinarians in affected neonates. Animals were humanely euthanized due to their conditions at 54, 10 and 2 days of age. Three control cases that all lacked any neurological deficits (51, 54, and 61 days of age) had previously been euthanized for other reasons.
2.3. Histopathology
Full necropsies were performed on all animals and representative tissues were preserved in 10% neutral buffered formalin. Tissues were routinely processed into paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin (H&E). A Luxol fast blue stain with a periodic acid Schiff counterstain (LFB PAS) was performed to assess myelination. Light microscopy was used to evaluate the tissues. Photomicroscopy was performed with a Leica DMC2900 camera and Leica LAS X microscope imaging software. Slides were scanned using a Leica Aperio AT2 slide scanner.
2.4. Immunohistochemistry
Tissue sections were deparaffinized in xylene and then rehydrated in graded alcohols. Slides were heated in citrate buffer (10 mmol, pH 6.0) for 5 min in a microwave for antigen retrieval. Sections were then pre-blocked blocking buffer, consisting of 5% normal goat serum in phosphate buffered saline (PBS). Primary antibodies were diluted in blocking buffer and sections were incubated overnight at 4°C. Slides were rinsed in blocking buffer three times, then incubated with the appropriate species-specific fluoro-conjugated secondary antibodies (Alexa546 or Alexa488, Molecular Probes Inc.) for 60 minutes. Primary antibodies used were: mouse anti-myelin basic protein (MBP; 1:500, Covance), mouse anti-neurofilament light chain (NFL; 1:200, Chemicon), mouse anti-glial fibrillary acidic protein (GFAP; 1:500, Sigma), rabbit anti-proteolipid protein (PLP; 1:250, Abcam), rabbit anti-Iba1 (1:300, Wako), and rabbit anti-Olig2 (1:250; Millipore, Sigma). Apoptosis was assessed using a TUNEL assay kit (In Situ Cell Death Detection Kit, Fluorescein, Millipore Sigma) with double labeling as above and according to the manufacturer’s instructions. Cell nuclei were visualized by staining with Hoeschst 33342 (1:5000, Molecular Probes). Following incubations with secondary antibodies, sections were washed in PBS and mounted with Prolong Gold mounting media (Invitrogen) then imaged using an Axioskop 40 fluorescence microscope (Zeiss).
Image quantification was achieved using ImageJ 1.53a software (National Institutes of Health). Adjacent sections from similar regions-of-interest (at least 4 sections per animal) were analyzed. The densities of PLP, MBP and NF immunostaining were quantified by setting the Otsu threshold to include all stained tissue. A positive percentage of the total area was measured as the density of positive staining. The numbers of Olig2 positive cells were analyzed using the analyze particles tool. GFAP, Olig2, Iba-1 and TUNEL positive cells were counted manually.
2.5. Genetic analysis
The Macaque Genotype and Phenotype Resource (mGAP; https://mgap.ohsu.edu; Bimber et al., 2019), which contains annotated genotype data for ONPRC rhesus macaques (mGAP release 1.9), was used to identify candidate functional genetic variants among the described cases. PLP1: n.682T>C; p.Cys228Arg was identified as a likely pathogenic variant, based on predicted protein impact, gene function, allele frequency and presence of the allele in the primary relatives of the affected cases. To validate the discovered allele, gDNA from Case 1 was amplified using PCR primers PLP1_F:AGAGATGGAAGAAGGGCTCTG and PLP1_R: GCACAGCCCATTTTCTTGAT. The product was treated with exonuclease I and shrimp alkaline phosphatase (New England Biolabs, Inc.), then sequenced on an ABI 3730XL DNA Analyzer (LifeTech, Inc.) by the ONPRC Molecular Biology Technology Core Facility. Additional genotyping of the affected macaques and relevant pedigree members was performed using the derived cleaved amplified polymorphic sequence (dCAPS; Neff et al., 2002) method. A partially mismatched primer (PLP1_dCAP_R:AACCCACTCACCTCAGCTGTTTTCC) generated a HpaII recognition site in the variant sequence to enable cleavage and allele discrimination.
3. Results
3.1. Clinical and Neurological Evaluation of Cases
We identified three male infant rhesus macaques that each had similar clinical presentations consistent with hypomyelinating disease (Table 1). Case 1 demonstrated head bobbing. Although he had normal gripping behavior on the dam, nursing was not observed. On initial examination, the infant exhibited intermittent abnormal vocalizations accompanied by profound intention tremors which worsened with excitement. During subsequent neurologic examination, intermittent vertical and horizontal jerk nystagmus to the right was revealed despite apparently normal mentation. Direct and consensual pupillary light reflexes were intact but mildly decreased. The infant was approximately 30% below average weight for sex and age.
TABLE 1:
Symptoms and Signs of Rhesus Macaque Cases and Controls
| Animal | Age (days)* | Weight (kg) | Symptoms |
|---|---|---|---|
|
| |||
| Case 1 (male) | 54 | 0.490 | head bobbing, trouble nursing, abnormal vocalizations, intention tremors, nystagmus |
|
|
|
||
| Case 2 (male) | 10 | 0.465 | trouble nursing, low body temperature, respiratory problems, prematurity |
|
|
|||
| Case 3 (male) | 2 | 0.450 | head bobbing, trouble nursing, intention tremors, motor dysfunction |
| Control 1 (female) | 54 | 0.760 | no neurological symptoms |
| Control 2 (female) | 61 | 0.496 | chronic proliferative typhlocolitis, no neurological symptoms |
| Control 3 (male) | 51 | 0.620 | no neurological symptoms |
Age at time of euthanasia
Case 2 was hypothermic and weak with inadequate latching when nursing. During examination, the infant was tachypneic with markedly increased respiratory effort, bilateral pulmonary crackles on auscultation, and reduced oxygen saturation despite supplemental oxygen. Further examination revealed a premature appearance with significant webbing of digits.
Case 3 had difficulty nursing despite an intact suckle reflex, and on presentation appeared lethargic and dehydrated with diarrheic feces. After three days of stabilization, the infant displayed profound intention tremors and head bobbing with hindlimb ataxia and an impaired righting reflex. Increased bronchovesicular sounds were auscultated bilaterally. Neurologic signs did not improve and the hindlimb ataxia progressed to hypotonia over four additional days of observation and supportive care.
3.2. Gross pathological findings in the CNS
Due to the severity of disease in each of these cases, animals were euthanized. Examination of the brains of these animals revealed mild cerebral and cerebellar swelling characterized by widening of gyri and folia (Fig. 1A–B and data not shown). These findings, in conjunction with the behavioral findings described above, suggest that each of these animals had a congenital brain disorder.
Figure 1:
Gross appearance of the brain of case 1. (A) Dorsal view, showing mild swelling of the cerebrum and cerebellum with broad gyri (see black arrow for example) and shallow sulci (see black arrowhead for example). (B) Ventral view, showing broad folia in the cerebellum (white arrow). (C) A coronal section from the brain showed in A and B at the level of the thalamus, showing poorly developed white matter in the cerebrum (including the extreme capsule; black arrow) (D) An archival control (control 2) showing well-developed cerebral white matter (extreme capsule, black arrow). All three cases demonstrated similar abnormalities. Scale bar = 10 mm.
3.3. Histopathologic findings in the CNS
Histopathologic examination of the brains from these animals revealed that they each had a CNS dysmyelinating disorder. There was diffuse loss of the normal tinctorial differentiation of gray and white matter of the cerebrum and cerebellum. This is evident when comparing case 1 with an archival age-matched control (control 2, Table 1; compare Fig. 1C and 1D; age-matched controls were not available for cases 2 and 3).
Throughout the white matter of the cerebrum, cerebellum, and spinal cord, there was diffuse gliosis and subjectively increased numbers of apoptotic glial cells (compare Fig. 2A&B with Fig 2C&D). Rarely, round to irregular (8–12 μm) eosinophilic structures were present (interpreted as spheroids).
Figure 2:
H&E staining of white matter from the dorsal midbrain of case 1 compared to control 2. (A) showing hypercellularity and gliosis in case 1. (B) A higher power image of the same tissue section in A, showing increased numbers of cerebral glial cells with plump elongate reactive nuclei (arrows) and scattered glial apoptosis characterized by cell shrinkage, chromatin condensation, and nuclear fragmentation (arrowheads). (C) H&E staining of a similar area of white matter as in A from control 2. (D) A higher power image of the tissue from C. (A, C) Scale bar = 200 μm; (B, D) Scale bar = 50 μm.
Myelination was evaluated with an LFB PAS stain, which demonstrated reduced to absent LFB staining in all three cases compared with control 2, a 61-day old female infant rhesus macaque that was euthanized for non-neurologic reasons (chronic proliferative typhlocolitis) (Fig. 3A–D). Indeed, there was a complete lack of myelin throughout the white matter of all three cases (Fig. 3E&F and data not shown). These findings, in conjunction with the behavioral findings described above, suggest that each of these animals had a congenital hypomyelinating brain disorder.
Figure 3:
LFB PAS staining (blue) of sections from the dorsal midbrain of control 2 (A, E) and from case 1 (B, F), case 2 (C), and case 3 (D). Cortical sections demonstrate substantial myelin in the white matter of the control (A) but a lack of myelin in all three cases (B-D). Similarly, higher power observation of white matter demonstrates staining consistent with dense myelin in the control (E) but an absence of myelin in case 1 (F). Similar staining was observed in cases 2 and 3. Scale bars = (C) 1 mm; (F) 50 μm.
3.4. Gross and histopathologic findings outside the CNS
In addition to the abnormalities observed in the CNS, cases 1 and 2 both had evidence of bronchopneumonia, bilateral mottling and multifocal consolidation of the lung lobes (data not shown). All three cases had either gross or microscopic evidence of pneumonia accounting for the respiratory signs identified on clinical examination. Cases 1 and 2 had marked neutrophilic bronchopneumonia, while case 3 exhibited moderate pleocellular interstitial pneumonia. Bacterial cultures of the lungs from case 1 yielded Orchrobactrum anthropi (2+), while cultures from case 2 identified Staphylococcus aureus (2+) and S. epidermidis (1+) (data not shown). Bacterial culture was not performed on the lungs of case 3. These findings are consistent with the respiratory difficulties observed during clinical observations.
All three cases had marked thymic atrophy. Microscopic findings in case 1 included rhinitis, tracheitis, enteric cryptosporidiosis, as well as lymphoid depletion of the thymus and spleen. Cases 2 and 3 had evidence of thymic lymphoid depletion. Lastly, all three cases had mild renal tubular epithelial degeneration; this was considered a consequence of dehydration-induced hypoperfusion.
3.5. Both oligodendrocyte lineage cell proliferation and apoptosis are elevated in animals with hypomyelination
To identify which cells were demonstrating apoptotic morphologies in Fig. 2, we examined the numbers of TUNEL+ cells in sections from case 1 and the three control cases that were of a similar to age to case 1. We compared total TUNEL labeling as well as TUNEL co-labeling with GFAP (to label astrocytes; Fig. 4A&B), Iba-1 (to label microglia; Fig. 4C&D), and Olig2 (to label oligodendrocyte lineage cells; Fig. 4E&F). Although there were more TUNEL+ cells in sections from case 1 compared to any of the three controls (Fig. 4G), we were unable to identify TUNEL+/GFAP+ cells (TUNEL+ nuclei clearly associated with astrocytes and not other cells in microscopic fields; Fig. 4A&B). We did observe a small number of Iba-1+ cells that were also TUNEL+ (Fig. 4C&D) but found no differences between case 1 and the controls (Fig. 4H). However, we observed an increase in TUNEL+/Olig2+ cells (Fig. 4E, F and I) in case 1 compared to controls, indicating that at least some of the apoptosis occurring in these animals involves the oligodendrocyte lineage.
Figure 4:
TUNEL labeling of dorsal midbrain cortical white matter from control 2 (A, C, E) and case 1 (B, D, F). Sections were double labeled for TUNEL (green), GFAP (red, A&B), Iba-1 (red, C&D), and Olig2 (red, E&F). Arrowheads in A and B show TUNEL+ cells that are not clearly associated with GFAP+ cells. Arrows in C-F show nuclei associated with microglia (C&D) and Olig2+ cells (E&F). Sections were stained with DAPI to label nuclei (blue). (G) Positive cell numbers per square millimeter of TUNEL-labeled nuclei in the three controls compared to case 1. (H) Positive numbers of Iba-1+/TUNEL+ cells in the three controls compared to case 1. (I) Positive numbers of Olig2+/TUNEL+ cells in the three controls compared to case 1. (J&K) TUNEL labeling of the dentate gyrus of (J) control 2 and (K) case 1. Sections were also stained with DAPI (blue) to label cell nuclei. Note the absence of TUNEL labeling in both panels. Scale bar = 100 μm.
Hypomyelinating and dysmyelinating conditions are often accompanied by neuronal apoptosis in different areas of the brain including the hippocampus (see, for example, Sima et al., 2009). We therefore examined TUNEL labeling in the hippocampus of case 1 compared to control 2. However, in contrast to the white matter in the cortex and cerebellum, we did not observe TUNEL+ cells in the hippocampus at this stage of disease (Fig. 4J&K).
Given the absence of LFB staining in the three cases, we decided to assess the numbers of Olig2+ cells in sections from case 1 compared to the three controls of similar ages to determine if cells in the oligodendrocyte lineage were absent. Adjacent sections were stained with an anti-Olig2 antibody and counterstained with DAPI to label cell nuclei (Fig. 5A&B). When counting the total numbers of Olig2+ cells, we found that there was actually an increase in Olig2+ cells in case 1 compared to the controls (Fig. 5C). This is consistent with findings in perinatal brain injury where OPCs increase in number with a concurrent increase in OPC apoptosis (for example, Segovia et al., 2008) and with the jimpy mouse model of PMD which demonstrate increased OPC proliferation in conjunction with OPC apoptosis (Wu et al., 2000).
Figure 5:
Immunostaining for Olig2 (red) in dorsal midbrain white matter of (A) control 2 and (B) case 1. Sections were stained with DAPI (blue) to label cell nuclei. (C) Quantification of Olig2 staining from four adjacent sections in controls compared with case 1. Scale bar = 100 μm.
3.6. Animals with hypomyelination demonstrate astrogliosis, microglial activation and a lack of PLP expression
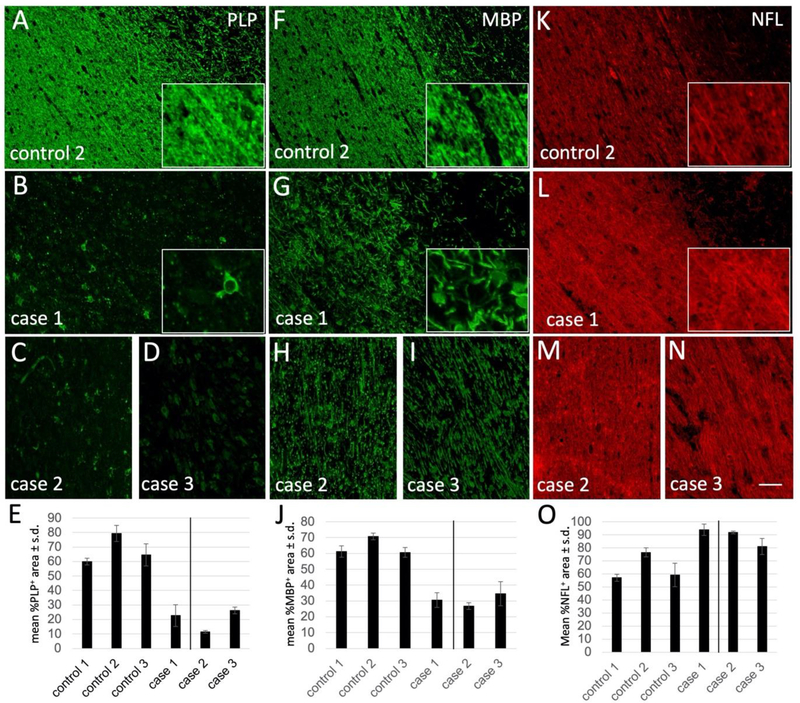
We further examined the white matter of these animals by immunohistochemistry using myelin, astrocyte and microglial markers. Compared to controls, all three cases demonstrated a nearly complete lack of immunostaining for the myelin protein PLP throughout the corpus callosum (compare Fig. 6A with 6B–D; quantified in Fig. 6E) and other white matter tracts (data not shown). In addition, there was a substantial reduction in the expression of another myelin protein, MBP, with highly disorganized staining and a lack of clear myelinated structures in each of the cases (compare Fig. 6F with 6G–I; quantified in Fig. 6J), and evidence of myelin debris in some areas (e.g. Fig. 6G, inset). In contrast, staining with NFL as a marker of axons was relatively normal in each case compared to the control (Fig. 6K–O).
Figure 6:
Immunostaining for PLP (A-D), MBP (F-I), and NFL (K-N), in control 2 (A, F, K), case 1 (B, G, L), case 2 (C, H, M), and case 3 (D, I, N). Insets are high-power images from the center of each microscopic field. Note that PLP staining is nearly absent from cases 1, 2, and 3, while MBP is significantly reduced and disorganized. Scale bar = 50 μm. (E, J, O) Quantification of fluorescence signal for (E) PLP, (J) MBP, and (O) NFL in controls compared to each of the cases. Note that case 2 and 3, to the right of the line, are different ages compared to the controls. Scale bar = 100 μm.
We also examined tissues for evidence of both astrogliosis and microglial activation. Consistent with the histopathology findings above (Fig. 2), immunostaining for GFAP demonstrated that compared to the controls, animals with hypomyelination had extensive astrogliosis throughout their white matter (compare Fig. 7A with 7B–D; quantified in Fig. 7E) as well as increased numbers of Iba-1+ cells with morphologies consistent with activated microglia (compare Fig. 7F Fig. 7G–I; quantified in Fig. 7J).
Figure 7:
Immunostaining for GFAP (A-D) and Iba-1 (F-I) in control 2 (A, F), case 1 (B, G), case 2 (C, H), and case 3 (D, I). Note the increased GFAP and Iba-1 staining in cases 1, 2, and 3. Scale bar = 50 μm. (E&J) Quantification of cells expressing (E) GFAP and (J) Iba-1 in controls compared to each of the cases. Note that case 2 and 3, to the right of the line, are different ages compared to the controls. Scale bar = 100 μm.
3.7. Animals with hypomyelination have mutations in the PLP1 gene
The clinical and pathologic findings discussed above are consistent with a hypomyelinating disease linked to the loss of PLP protein. We therefore searched the macaque Genotype and Phenotype database (mGAP) to determine if these animals carried mutations in the PLP1 gene. The mGAP database contains annotated information for over 41 million rhesus macaque genetic variants identified in more than 2,000 rhesus macaques (Bimber et al., 2019). While genomic sequence data were not available for the affected cases, the database did contain information for the sibling of case 1 and the mother/dam of cases 2 and 3, as identified by pedigree analysis (Fig. 8A), demonstrating that the three cases are related to one another. An n.682T>C allele of PLP1 was identified as a candidate causal variant (Fig. 8B), based on the function of the PLP1 gene, the predicted damaging nature of the variant, and the rarity of the allele (minor allele frequency = 0.001). The missense variant was identified by the PolyPhen2 (Adzhubei et al., 2019) algorithm as ‘probably damaging’ and had a Combined Annotation Dependent Depletion (CADD; Rentzsch et al., 2019) score of 20.5. Of the 485 individuals genotyped at that locus at the time of discovery, the sibling of case 1 was the only observed carrier.
Figure 8.
Genotype analysis of rhesus macaques with hypomyelinating disease. (A) Genotype and affected status of chrX:97,544,288 T>C among the disease pedigree members. Genotype analysis identified an inheritance pattern consistent with an X-linked recessive trait. Affected individuals are represented in solid black, while unaffected wildtype and carriers are shown in solid white and split fill, respectively. Males are squares and females are circles. Red arrow indicates the identity of the initial carrier identified in mGAP. (B) An n.682T>C; p.Cys228Arg variant is located within exon 5 of the PLP1 gene. (C) Representative Sanger sequence traces are shown for wildtype, heterozygous carrier and a male hemizygote.
Sanger resequencing and PCR based genotyping confirmed that the affected males were hemizygous for the n.682T>C allele, and identified three additional female carriers (Fig. 8C). The expanded pedigree analysis determined that the variant is likely a de novo mutation, as both the dam and sire of carrier 2 carried only wildtype alleles. The allele was not reported in the sequenced data deposited by six other primate research centers, and we did not identify any non-affected male hemizygotes or homozygous females.
4. Discussion
There are no definitive treatments for hypomyelinating leukodystrophies, including PMD. Ongoing studies are exploring the possibility of replacing mutant OPCs or, in the case of patients with particular PLP1 mutations, altering the way PLP1 is expressed (Singh and Samanta, 2020). A recent study demonstrated that suppression of Plp1 expression in jimpy mice, for example, demonstrated increased myelination, improved motor function, and increased lifespan (Elitt et al., 2020). However, it is unclear if this approach and others will be efficacious in humans. As discussed above, a challenge with such studies is that many small-animal models suffer from anatomical and physiological differences from humans that limit their value in predicting outcomes in human patients.
Here, we have characterized the first spontaneous cases of PMD described in rhesus macaques. The three macaque cases, all related, presented with symptoms of hypomyelinating disease and lacked CNS myelin. These cases also presented with pneumonia, likely related to respiratory difficulties that are often reported in human patients (Ueda et al., 2018) and/or difficulties with feeding. As in PMD patients, the white matter from these cases lacked PLP expression and demonstrated low and disorganized MBP expression accompanied by reactive astrogliosis and activated microglia. All together, these findings indicate that this rhesus macaque form of PMD (RM-PMD) shares the symptoms, signs and pathophysiology of at least some forms of PMD in humans. RM-PMD may, therefore, be an excellent model of PMD and other hypomyelinating diseases that could be highly predictive of clinical outcomes in response to novel therapies. Indeed, with supportive care, animals with RM-PMD could survive for several months to facilitate testing novel therapies.
The RM-PMD cases were linked to a missense variant in exon 5 of the PLP1 gene. Patients with similar missense mutations in exon 5 of the human PLP1 gene (e.g. c.689C>T; p.Thr230Ile and c.634T>C; p.Trp212Arg) developed symptoms at birth that were similar to what we observed in RM-PMD including nystagmus, muscular hypotonia, and spasticity and weakness (Grossi et al., 2011). PLP1 missense mutations can result in so-called connatal PMD characterized by glial cell apoptosis, including OLs, and axonal injury that lead to a more severe phenotype, similar to that we observed in the RM-PMD cases described here, than observed with patients (and animals) with null mutations, duplications, or other mutations. Thus, the RM-PMD model could be of value in understanding the evolution and progression of connatal PMD.
The fact that mutant PLP1 carriers remain in the ONPRC rhesus macaque colony presents the unique possibility to develop RM-PMD into an NHP model of PMD. Because this is a spontaneous disease, developing this model will require either targeted breeding groups or in vitro fertilization approaches, which may limit the availability of animals for preclinical studies. However, breeding of carriers should allow sufficient numbers of animals to be born over time, as well as the ability to send animals to other institutions to develop new colonies. Alternatively, using gene editing approaches, it would be possible to generate new carriers with the PLP1 mutation we characterized here or other mutations. The severity of RM-PMD also requires intensive clinical care for long-term studies, limiting the types of facilities that can utilize the model. Nonetheless, these animals have the potential to provide novel insights into the functions of the PLP protein and the pathogenesis of PMD and other hypomyelinating diseases. Furthermore, in addition to serving as a platform for testing cell-based and pharmacologic therapies as discussed above, RM-PMD offers the chance of developing gene therapy-based strategies to prevent or reverse PMD in the NHP nervous system. Although RM-PMD is ideally suited for studies of connatal PMD, it could also serve as model for testing strategies to promote myelination in other dysmyelinating diseases, including other forms of PMD, offering an unprecedented opportunity for a wide range of pre-clinical studies aimed at preventing or reversing disease.
Table 2:
Comparison of Rhesus Macaque PMD With Other Models of Connatal PMD
| Model | Pathological Features | Symptoms | Citations | |
|---|---|---|---|---|
| Human connatal PMD | dysmyelination, astrogliosis, microglial activation, glial cell apoptosis | hypotonia, tremors, nystagmus, respiratory distress, stridor, | Singh & Samanta, 2020 | |
| Rhesus Macaque PMD | dysmyelination, astrogliosis, microglial activation, glial cell apoptosis | hypotonia, tremors, nystagmus, respiratory distress, stridor, feeding difficulties | ||
| Shaking pup (dog) | dysmyelination, astrogliosis, microglial activation, glial cell apoptosis | tremors, difficulties with walking, balance and coordination, feeding | Griffiths et al., 1981; Mayer et al., 2015 | |
| Paralytic tremor (rabbit) | dysmyelination, gliosis | tremors, spastic limb paresis | Tosic et al., 1994; Sypecka & Domanska-Janik, 2005 | |
| Myelin-deficient rat | dysmyelination, gliosis, glial apoptosis | tremors, seizures | Dentinger et al., 1982; Jackson & Duncan 1988 | |
| Jimpy mouse | dysmyelination, gliosis, glial apoptosis | tremors, seizures | Nave et al., 1986; Thompson et al., 1999 | |
| (jp, jp-msd, ip-4J) |
Highlights.
Neonatal rhesus macaques (RM) with PLP1 mutations develop motor and other symptoms
PLP1 mutant animals resemble human patients with Pelizaeus-Merzbacher disease (PMD)
RM-PMD is characterized by hypomyelination, astrogliosis, and microglial activation
Oligodendrocyte progenitor cell proliferation and apoptosis are elevated in RMPMD white matter
RM-PMD is a novel non-human primate model of PMD
Acknowledgements
We thank Wendy Price and Sireen Alkhatib for their help with histological analyses. This work was supported by NIH R24 OD021324 (to BF), R24 NS104161 (to LSS), 1S10OD025002-10 supporting the Integrated Pathology Core at the ONPRC, and P51 OD011092 supporting the ONPRC.
Footnotes
The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adzhubei I, Jordan DM, Sunyaev SR, 2013. Predicting functional effect of human missense mutations using PolyPhen-2. Curr. Protoc. Hum. Genet. Chapter 7:Unit 7.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin GB, Ratterree M, Davison BB, et al. , 1998. Genetic galactocerebrosidase deficiency (globoid cell leukodystrophy, Krabbe disease) in rhesus monkeys (Macaca mulatta). Lab. Anim. Sci. 48, 476–482. [PubMed] [Google Scholar]
- Bimber BN, Yan MY, Peterson SM, et al. , 2019. mGAP, the macaque genotype and phenotype resource, a framework for accessing and interpreting macaque variant data, and identifying new models of human disease. BMC Genomics 20, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, Büssow H, D’Urso D, et al. , 1995. Adhesive properties of proteolipid protein are responsible for the compaction of CNS myelin sheaths. J. Neurosci. 15, 5502–5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borda JT, Alvarez X, Mohan M, et al. , 2008. Clinical and immunopathologic alterations in rhesus macaques affected with globoid cell leukodystrophy. Am. J. Pathol. 172, 98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentinger MP, Barron KD, Csiza CK, 1982. Ultrastructure of the central nervous system in a myelin deficient rat. J Neurocytol. 1982 Aug;11(4):671–91. [DOI] [PubMed] [Google Scholar]
- Duncan ID, Griffiths IR, Munz M, 1983. ‘Shaking pups’: a disorder of central myelination in the spaniel dog. III. Quantitative aspects of glia and myelin in the spinal cord and optic nerve. Neuropathol. Appl. Neurobiol. 9, 355–368. [DOI] [PubMed] [Google Scholar]
- Duncan ID, Hammang JP, Trapp BD, 1987. Abnormal compact myelin in the myelin-deficient rat: absence of proteolipid protein correlates with a defect in the intraperiod line. Proc. Natl. Acad. Sci. USA 84, 6287–6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elitt MS, Barbar L, Shick HE, et al. , 2020. Suppression of proteolipid protein rescues Pelizaeus-Merzbacher disease. Nature 585, 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths IR, Duncan ID, McCulloch M, et al. , 1981. Shaking pups: a disorder of central myelination in the Spaniel dog. Part 1. Clinical, genetic and light-microscopical observations. J. Neurol. Sci. 50, 423–433. [DOI] [PubMed] [Google Scholar]
- Griffiths IR, Duncan ID, McCulloch M, 1981. Shaking pups: a disorder of central myelination in the spaniel dog. II. Ultrastructural observations on the white matter of the cervical spinal cord. J. Neurocytol. 10, 847–858. [DOI] [PubMed] [Google Scholar]
- Griffiths IR, Scott I, McCulloch MC, et al. , 1990. Rumpshaker mouse: a new X-linked mutation affecting myelination: evidence for a defect in PLP expression. J. Neurocytol. 19, 273–283. [DOI] [PubMed] [Google Scholar]
- Grossi S, Regis S, Biancheri R, et al. , 2011. Molecular genetic analysis of the PLP1 gene in 38 families with PLP1-related disorders: identification and functional characterization of 11 novel PLP1mutations. Orphanet J. Rare Dis 6:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenenfelder FI, McLaughlin M, Griffiths IR, et al. , 2020. Neural stem cells restore myelin in a demyelinating model of Pelizaeus-Merzbacher disease. Brain 143, 1383–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, 2019. Pelizaeus-Merzbacher Disease: Molecular and Cellular Pathologies and Associated Phenotypes. Adv. Exp. Med. Biol. 1190, 201–216. [DOI] [PubMed] [Google Scholar]
- Lubetzki C, Sol-Foulon N, Desmazières A, 2020. Nodes of Ranvier during development and repair in the CNS. Nat. Rev. Neurol 16, 426–439. [DOI] [PubMed] [Google Scholar]
- Mayer JA, Griffiths IR, Goldman JE, et al. , 2015. Modeling the natural history of Pelizaeus-Merzbacher disease. Neurobiol. Dis 75, 115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride JL, Neuringer M, Ferguson B, et al. , 2018. Discovery of a CLN7 model of Batten disease in non-human primates. Neurobiol. Dis 119, 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA, Lai C, Bloom FE, et al. , 1986. Jimpy mutant mouse: a 74-base deletion in the mRNA for myelin proteolipid protein and evidence for a primary defect in RNA splicing. Proc. Natl. Acad. Sci. USA 83, 9264–9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA, Lai C, Bloom FE, et al. , 1986. Splice site selection in the proteolipid protein (PLP) gene transcript and primary structure of the DM-20 protein of central nervous system myelin. Proc. Natl. Acad. Sci. USA 84, 5665–5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff MM, Turk E, Kalishman M, 2002. Web-based primer design for single nucleotide polymorphism analysis. Trends Genet. 18, 613–615. [DOI] [PubMed] [Google Scholar]
- Osório MJ, Goldman SA, 2018. Neurogenetics of Pelizaeus-Merzbacher disease. Handb. Clin. Neurol 148, 701–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge B, Rossmeisl JH Jr., 2020. Companion animal models of neurological disease. J. Neurosci. Methods 331, 108484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzsch P, Witten D, Cooper GM, et al. , 2019. CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 8, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhesus Macaque Genome Sequencing and Analysis Consortium, Gibbs RA, Rogers J, et al. , 2007. Evolutionary and biomedical insights from the rhesus macaque genome. Science 316, 222–234. [DOI] [PubMed] [Google Scholar]
- Rutherford HA, Hamilton N, 2019. Animal models of leukodystrophy: a new perspective for the development of therapies. FEBS J. 286, 4176–4191. [DOI] [PubMed] [Google Scholar]
- Segovia KN, McClure M, Moravec M, et al. 2008. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol. 63, 520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sima AA, Pierson CR, Woltjer RL, et al. , 2009. Neuronal loss in Pelizaeus-Merzbacher disease differs in various mutations of the proteolipid protein 1. Acta Neuropathol. 118, 531–5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Samanta D, 2020. Pelizaeus-Merzbacher Disease. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan–. [PubMed] [Google Scholar]
- Sypecka J, Domańska-Janik K, 2005. Rabbit paralytic tremor phenotype--a plp1 gene mutation as a model of human Pelizaeus-Merzbacher disease. Acta Neurobiol. Exp. (Wars) 65, 221–229. [PubMed] [Google Scholar]
- Tosic M, Dolivo M, Domańska-Janik K, et al. , 1994. Paralytic tremor (pt): a new allele of the proteolipid protein gene in rabbits. J. Neurochem. 63, 2210–2216. [DOI] [PubMed] [Google Scholar]
- Uchida N, Chen K, Dohse M, et al. , 2012. Human neural stem cells induce functional myelination in mice with severe dysmyelination. Sci. Transl. Med 4, 155ra136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Shimbo H, Yada Y, et al. , 2018. Pelizaeus-Merzbacher disease can be a differential diagnosis in males presenting with severe neonatal respiratory distress and hypotonia. Hum. Genome Var. 5, 18013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdier JM, Acquatella I, Lautier C, et al. , 2015. Lessons from the analysis of nonhuman primates for understanding human aging and neurodegenerative diseases. Front. Neurosci 9, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Piao JH, Larsen EC, et al. , 2011. Migration and remyelination by oligodendrocyte progenitor cells transplanted adjacent to focal areas of spinal cord inflammation. J. Neurosci. Res 89, 1737–1746. [DOI] [PubMed] [Google Scholar]
- Warren WC, Harris RA, Haukness M, et al. 2020. Sequence diversity analyses of an improved rhesus macaque genome enhance its biomedical utility. Science 370, eabc6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem MS, Nunes MC, Rashbaum WK, et al. , 2004. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat. Med 10, 93–97. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Schanz SJ, Guo M, et al. , 2008. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell 2, 553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Miller RH, Ransohoff RM, Robinson S, Bu J, Nishiyama A 2000. Elevated levels of the chemokine GRO-1 correlate with elevated oligodendrocyte progenitor proliferation in the jimpy mutant. J Neurosci. 20, 2609–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]