Abstract
Objectives
The primary objective is to determine the prevalence of SARS-CoV-2 antibodies persistence among HCWs and specifically among asymptomatic HCWs. A secondary objective is to determine the duration of persistent SARS-CoV-2 antibodies post infection and factors affecting this duration. The findings are expected to open the door for further research into the role of SARS-CoV-2 antibodies during the current COVID-19 pandemic.
Methodology
HCWs were divided into high, intermediate, and low risk based on their type and location of work. All participants filled a questionnaire. Blood samples were obtained for SARS-CoV-2 IgG/total antibodies. A documented SARS-CoV-2 PCR or Anti-SARS-CoV-2 IgG/total antibodies defined the primary outcome. The probability of persistence of antibody was calculated using the Kaplan–Meier estimator. Logistic and Cox regression were used where appropriate.
Results
A total of 1111 HCWs were included. The median age 37 years (IQR: 31–43). More than half (67.2%) were females. The primary outcome was seen in 373 (33.6%) participants with a median age of 36 years (IQR: 29–41). Only 37.2% of those with documented positive SARS-CoV-2 PCR had reactive serology, while only 16.2% of those with reactive serology had documented positive SARS-CoV-2 PCR. Male gender (OR 0.44, P < 0.001) and older age (OR 0.98, P < 0.019) were associated with a lower risk of acquiring SARS-CoV-2 infection. The probability of persistent SARS-CoV-2 antibodies at six months was 60.2% (95% CI: 49.5%–73.1%). Omanis had a higher probability of losing the antibody than others (HR 2.63, P = 0.021).
Conclusion
We report a high prevalence of anti-SARS-CoV-2 antibodies among HCWs in Oman, specifically among asymptomatic HCWs. Community was the most likely source of infection. Therefore, the society must adhere to the roles and regulations set to reduce the risk of transmission. We demonstrate a high percentage of seroconversion post initial infection, and the persistence of SARS-CoV-2 antibodies at six months in more than half of those previously infected. We demonstrated a new interesting finding of fast decline of SARS-CoV2 antibody levels over time among different nationalities and this requires further research.
Keywords: SARS-CoV-2, COVID-19, Serology, SARS-CoV-2 antibodies, Healthcare workers, Oman
Introduction
Hundreds of thousands of healthcare workers (HCWs) have been infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) since its outbreak in China in 2019 [1,2]. SARS-CoV-2 affects the respiratory system and many other body systems and has been labeled as coronavirus disease 2019 (COVID-19) [3]. Due to the rapid spread of the virus to most of the countries in the world, the World Health Organization (WHO) declared COVID-19 as pandemic on the 11th of March 2020 [4].
The majority of COVID-19 patients have no symptoms [5]. Therefore, symptoms-based testing using real-time polymerase chain reaction (RT-PCR) can miss many asymptomatic carriers [6]. Multiple studies over the last year demonstrated different SARS-CoV-2 antibodies directed against the different viral components [7,8]. The majority of patients exposed to SARS-CoV-2 will develop SARS-CoV-2 antibodies within one to two weeks post-infection [8]. These antibodies can be used to screen asymptomatic carriers, especially asymptomatic HCWs [9].
HCWs at front lines, providing care to millions of SARS-CoV-2 infected patients around the world [10] are at a high risk for infection [11]. Therefore, screening asymptomatic HCWs is essential in their allocation to provide care and prevent transmission of SARS-CoV-2 [6]. Several studies looked at the prevalence of SARS-CoV-2 antibodies among HCWs [12,13]. Garcia-Basteiro et al. reports a cumulative prevalence of SARS-CoV-2 antibodies of 11.2% in a large hospital in Spain [14]. The study of 28,792 HCWs in Denmark identified 2.81% prevalence for IgM and 2.67% for IgG and found a higher prevalence in front-liners working with COVID-19 patients than other HCWs [15]. Another study of 40,329 HCWs from New York City reported a prevalence of 13.7%, similar to the community prevalence of 14.0% in New York State [16,17]. A recent meta-analysis by Galanis et al. reported an estimated overall prevalence of SARS-CoV-2 antibodies among HCWs to be 8.7% [13]. The duration of SARS-CoV-2 antibodies persistence post-infection is not well known. Initial reports describe short-lived SARS-CoV-2 antibodies [18,19]. However, recent reports described the persistence of antibodies beyond 7–9 months [20,21].
The total population of Oman is around 4.5 million [22]. As of 1st of April 2021, 160 thousand cases of positive COVID-19 have been reported, with a total of 1681 deaths and a case fatality rate of 1.1% [23]. The prevalence of COVID-19 among HCWs in Oman is unknown. Given the expected high risk of COVID-19 infection among HCWs and marked variation in the reported prevalence of COVID-19 among HCWs in different parts of the world, we decided to conduct this study. Therefore, the main objective of this study is to determine the prevalence SARS-CoV-2 antibodies specifically among asymptomatic HCWs in Oman. Determining this prevalence will add more information to the current available knowledge in relation to the sources of infection in Oman and in countries with similar culture and demographic structures. This information may help stratify the workforce for risk, establish better healthcare policies and procedures, and better mitigate transmission risks across different healthcare settings.
A secondary objective is to determine the duration of persistent SARS-CoV-2 antibodies post-infection and factors affecting this duration. The findings are expected to open the door for further research into the role of SARS-CoV-2 antibodies during the current COVID-19 pandemic.
Methodology
Study setting
This is a cross-sectional study conducted at the Armed Forces Hospital (AFH), a tertiary hospital located in the capital city of Muscat. Military personnel and their families are entailed for treatment at AFH. AFH was one of the tertiary hospitals in Muscat involved extensively in the management of COVID-19 patients. A dedicated emergency department (ED), intensive care unit (ICU), and admission wards were allocated for COVID-19 patients.
As exposure of HCWs to COVID-19 differs based on their specialty and place of work, AFH staff of different nationalities from all departments were included. These departments were classified based on contact with COVID-19 patients as; high-risk areas and include COVID-19 emergency, ICU, medical wards, and operating rooms; intermediate risk areas that have a non-COVID-19 emergency, ICU, admission wards, radiology, physiotherapy, endoscopy unit, and low risk areas that include laboratories, administration, and other back up services staff such as drivers, cleaners, and staff working at catering. The study was conducted between 9–24 of December 2020, which corresponds to the end of the first wave of SARS-CoV-2 in Oman.
This study was approved by the medical research committee at armed forces medical services (AFMS-MREC 028/20).
Participants
Participation in this study was voluntary. All HCWs, of different nationalities, working at the AFH were invited to participate in the study through an internal announcement. Subjects were asked to consent if they are willing to participate. All staff aged 20 years and above who have been working in the hospital since the January 2020 were included. Recently recruited (less than 3 months) medical and non-medical staff, as well as symptomatic HCWs at the time of conducting the study, were excluded. All the participants filled a questionnaire related to HCWs demography, past medical history, exposure to COVID-19 patients, and symptoms suggestive of COVID-19.
Staff risk was based on their place of work in high, intermediate and low risk areas, as mentioned earlier. All participants were not vaccinated for SARS-CoV-2. A 5 ml of peripheral venous blood sample was collected to test for SARS-CoV-2 antibodies from the participants. A minimum period of four weeks was deliberately calculated in participants with the onset of either COVID-19 symptoms or suspected exposure to allow for antibody response and detection.
A documentation of previous SARS-CoV-2 infection, using SARS-CoV-2 PCR testing of nasal and oropharyngeal swab, was obtained from the hospital information system.
SARS-CoV-2 PCR testing
Diagnosis of previous SARS-CoV-2 infection was based on symptom assessment, followed by a positive naso-pharyngeal SARS-CoV-2 PCR testing. GeneXpert SARS-CoV-2 real time PCR, [Xpert Express (Cepheid, Sunnyvale, California, USA)] was used to test for SARS-CoV-2. The test was recently released under emergency use by the FDA.
This test targets two SARS-CoV-2 virus genomic regions: the first region lies in the envelope (E) gene and used for screening. The second region, which confirms a SARS-CoV-2 infection, is in the N2 region of the nucleocapsid (N) gene. Both targets, as well as sample processing control, are multiplexed within the same PCR reaction. Amplification of the sample control is used to validate each PCR reaction. The assay requires a sample load of 300 μl, and has a detection limit of 250 copies/ml. The test is considered positive when it detects both targets, or when it detects N2 alone. The detection of E gene alone is considered presumptive positive. When compared with all methods of nucleic acid amplification tests (NAATs) standard of care, the Xpert test positive agreement was (99.5%; 95% CI, 97.5–99.9%) and negative agreement was (95.8%; 95% CI, 92.6–97.6%).
Antibody testing
Elecsys Anti-SARS-CoV-2 S test (Roche Diagnostics, Rotkreuz, Switzerland) was used to test for SARS-CoV-2 IgG/total antibodies. This immunoassay targets antibodies against the viral spikes protein, specifically its receptor-binding domain (RBD), a common target for virus-neutralizing antibodies and the focus of therapeutics and vaccine design. After the 5 ml of blood was obtained from each participant, it was placed into serum separator tubes and allowed to coagulate at room temperature for 60 min. It was then stored at 4 °C until centrifugation. The serum was then analyzed according to the manufacturer’s recommendations.
Test specificity and sensitivity
Clinical specificity of 99.98%, with a 95% lower confidence limit of 99.91%, determined in 5991 pre-pandemic patient samples. Clinical sensitivity of 98.8%, with a 95% lower confidence limit of 98.91%; determined in 1423 samples with a sampling date of 14 days or later after diagnosis with PCR. Serum IgG titers were considered “positive” if detected at dilutions of 1:0000 or greater and “weakly positive” if detected at dilutions of 1:000.
Statistical analysis
Descriptive statistics were summarized as medians and interquartile ranges for continuous variables and as frequencies and percentages for categorical variables. A documented positive SARS-CoV-2 PCR or reactive Anti-SARS-CoV-2 IgG/total antibodies defined the primary outcome. The association of different baseline predictors with the primary outcome was assessed using univariable logistic regression. The assessed baseline variables included gender, age, ethnicity (Omanis vs. others), profession (doctors or nurses vs. others), obesity (defined as BMI of 30 kg/m2 or higher), working in risk area (high risk as a reference), and unprotected contact with COVID-19 patient. Variables with a P-value of <0.05 were then entered into a multivariable logistic regression.
Kaplan–Meier estimator was used to calculate the probability of “persistence of SARS-CoV-2 antibodies”. The impact of baseline variables on the probability of the persistence of antibodies was assessed using univariable Cox regression. These variables included gender (male vs. female), age (<50 years), obesity, and ethnicity (Omanis vs. others). All statistical analyses were performed using the R program (R version 3.5.1, R Core Team, 2018).
Results
A total of 1111 HCWs tested for SARS-CoV-2 antibodies were included in the current study. The median age was 37 years (IQR: 31–43). Seven hundred and forty-seven participants (67.2%) were female. There were 630 (56.7%) Omani participants. Table 1 shows the demographic and clinical characteristics of the whole cohort (n = 1111) and those with the primary outcome.
Table 1.
Baseline demographic and clinical characteristics of the cohort and univariable analyses (n = 1111).
| Characteristic | Whole cohort (n = 1111) | Participants with primary outcome (n = 373) |
|---|---|---|
| Age in years (median IQR) | 37 (31–43) | 36 (29–41) |
| Females | 747 (67.2%) | 293 (78.6%) |
| BMI (median IQR) | 25.8 (23.4–28.3) | 25.9 (23.2–28.3) |
| Omanis | 630 (56.7%) | 227 (60.9%) |
| Physicians and nurses | 587 (52.8%) | 189 (50.7%) |
| Working in COVID-19 areas | 96 (8.6%) | 25 (6.7%) |
| History of unprotected contact with a confirmed case | 174 (15.7%) | 91 (24.4%) |
| Wearing a mask when in public: | ||
| - Always | 1069 (96.2%) | 358 (95.7%) |
| - Sometimes | 41 (3.7%) | 14 (3.8%) |
| - Never | 1 (0.1%) | 1 (0.3%) |
| Work risk area: | ||
| - High risk | 231 (20.8%) | 61 (16.4%) |
| - Intermediate risk | 476 (42.8%) | 163 (43.7%) |
| - Low risk | 404 (36.4%) | 149 (39.9%) |
| Clinical characteristics: | ||
| - Diabetes mellitus | 45 (4.1%) | 16 (4.3%) |
| - Hypertension | 47 (4.2%) | 17 (4.6%) |
| - Smoking | 10 (0.9%) | 3 (0.8%) |
The total number of participants with a positive primary outcome was 373 (33.6%) participants with a median age of 36 years (IQR: 29–41). Less than half (41.6%) of these participants had a previous infection confirmed by PCR (113 seronegative and 42 seropositive). Only 37.2% of those with documented positive SARS-CoV-2 PCR had reactive serology, although the test clinical sensitivity is 98.8%, while only 16.2% of those with reactive serology had a confirmed positive SARS-CoV-2 PCR. Reactive SARS-CoV-2 antibody serology without previous history of COVID-19 suggested symptoms was found in 58.45% (n = 218) participants. The majority of participants with a positive primary outcome worked in intermediate (43.7%) and low-risk (39.9%) areas. Only 16.4% of participants with a positive primary outcome were working in a high-risk area.
The most common symptoms among those with primary outcome were headache 139 (37.3%) followed by joint pain 99 (36.5%) and fever 132 (35.4%). The rest of the symptoms were described in Table 2 .
Table 2.
COVID-19-compatible symptoms.
| Symptoms | Whole cohort (n = 1111) | Participants with primary outcome (n = 373) |
|---|---|---|
| Fever | 205 (18.5%) | 132 (35.4%) |
| Cough | 168 (15.1%) | 93 (24.9%) |
| Breathing difficulty | 56 (5%) | 42 (11.3%) |
| Sore throat | 206 (18.5%) | 101 (27.1%) |
| Joint pain | 143 (12.9%) | 99 (36.5%) |
| Diarrhea | 70 (6.3%) | 42 (11.3%) |
| Runny nose | 151 (13.6%) | 79 (21.2%) |
| Anosmia | 132 (11.9%) | 111 (29.8%) |
| Ageusia | 121 (10.9%) | 104 (27.9%) |
| Headache | 257 (23.1%) | 139 (37.3%) |
| Muscle ache | 183 (16.5%) | 117 (31.4%) |
| Nausea | 60 (5.4%) | 40 (10.7%) |
Predictors of the primary outcome
Univariate analysis was used to examine the effect of certain baseline characteristics, type of work, and place of work on the clinical outcome (Table 3 ). Age of 50 years or less, being of Omani nationality, female gender, lack of unprotected contact with a confirmed COVID-19 case, and working in low or intermediate risk areas were found to be significantly associated with the primary outcome. In the multivariable model (Table 3), only female gender (OR 2.27, P < 0.0001) and age (OR 0.98, P 0.019) remained statistically significant.
Table 3.
Univariate and multivariate analysis of positive primary outcome (33.6%) in different groups.
| Variable | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| Percentage | P-value | OR | P-value | |
| Age >50 years vs. 50 years or less | 2.6% vs. 31% | P = 0.005 | 0.98 | P = 0.019 |
| Female vs. male | 26.4% vs. 7.2% | P < 0.0001 | 2.27 | P < 0.0001 |
| Omanis vs. others | 20.4% vs.13.1% | P = 0.047 | 1.08 | P = 0.580 |
| Physicians or nurses vs. other professions | 17% vs. 16.6% | P = 0.304 | ||
| Obesity (BMI > 30 kg/m2) vs. not obese | 5.3% vs. 28.3% | P = 0.274 | ||
| Working in risk area: | ||||
| • High risk areaa | 5.5% | |||
| • Intermediate risk areas | 14.7% | P = 0.036 | 1.22 | P = 0.287 |
| • Low-risk areas | 13.4% | P = 0.008 | P = 0.054 | |
| Unprotected contact with COVID-19 patients vs. no contact | 8.2% vs. 25.4% | P < 0.0001 | 1.13 | P = 0.724 |
High-risk area was taken as reference.
Persistence of SARS-CoV-2 antibodies
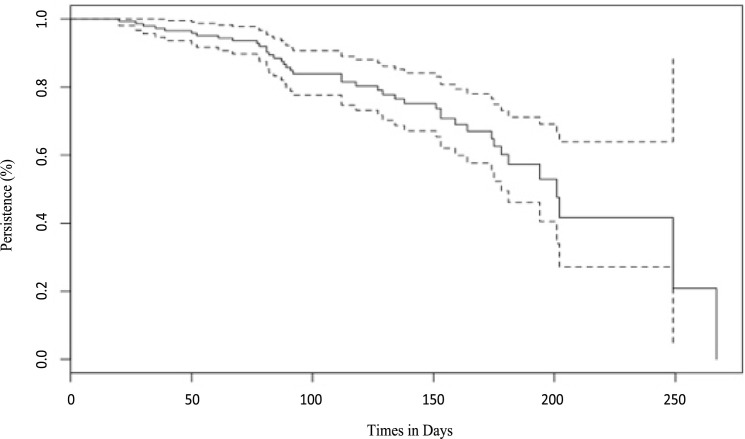
Among the 113 participants diagnosed with COVID-19 based on symptoms and positive SARS-CoV-2 PCR, 42 (37.2%) participants tested positive for SARS-CoV-2 antibodies. The median duration from testing for SARS-CoV-2 PCR to testing for SARS-CoV2 antibodies serology for all participants was 105 days (IQR: 74–159). This duration was 112 days (IQR: 78–159) for those with positive serology and 104 days (IQR: 74–159) for those with negative serology. Kaplan–Meier plot showed very high percentage of seroconversion post initial infection (Fig. 1 ). The median time from infection to losing the antibody was 201 days (95% CI: 178 – Not reached). The probability of persistent SARS-CoV-2 antibodies at six months was 60% (95% CI: 50–73) (Fig. 1).
Fig. 1.
Kaplan–Meier plot for persistence of SARS-CoV-2 antibodies.
In the univariable Cox regression, Omani nationality was the only statistically significant predictor of losing the positive serology (HR 2.63, P = 0.021). Female gender (HR 1.49, P = 0.549), age <50 years (HR 2.67, P = 0.332) and obesity (HR 0.94, P = 0.892) were not statistically significant.
Discussion
Multiple studies from different countries looking at the prevalence of SARS-CoV-2 antibodies among HCWs have been reported [[14], [15], [16], [17]]. There is marked variation in the reported prevalence of SARS-CoV-2 antibodies among HCWs. Our study is the first study from Oman to report the seroprevalence and durability of SARS-CoV-2 antibodies among HCWs. The current study reports that 33.6% of participating HCWs were previously diagnosed with COVID-19 or tested positive for its IgG antibodies. Liu et al. reported zero prevalence among HCWs care for COVID-19 patients at Wuhan, China, during the initial outbreak [24]. However, a high prevalence of 32.6% similar to our study was reported from New York City [25]. These variations in reported prevalence can be explained by various factors such as the prevalence of COVID-19 in different countries, the provision of Personal Protective Equipment (PPE), adherence of HCWs to different protective measures, sample size, sampling methodology, and sensitivity of antibody tests used. In addition, the time between the endemicity of SARS-CoV-2 and therefore the study conduction might affect the prevalence of COVID-19 among HCWs. As time goes on, more people, including HCWs, are exposed and could become infected with the virus, increasing its prevalence.
The demographic characteristics of the HCWs in this study showed that the mean age of HCWs was 37 years, and the majority of them had no comorbidities. In addition, most of the HCWs in this study (96.2%) complied with wearing face masks when in public places. However, these factors do not eliminate other responsible viral transmission factors like large gathering and transmission from asymptomatic carriers, described extensively [26,27].
The majority of the infected HCW in this study worked in low to intermediate risk areas, and half of the infected HCWs are not physicians or nurses but other categories of supportive staff. Strict measures taken by the AFH, including but not limited to the designation of specific areas for COVID-19 patients and rigorous application of PPE, helped reduce SARS-CoV-2 infection among HCWs in high-risk areas. Therefore, the most likely source of infection in most infected hospital staff may well be the community instead of the hospital. More than half of infected HCWs were Omanis who tend to return to their families and the larger community after working hours hence the higher risk of exposure to infected people than non-Omanis who tend to have smaller families and minimal interaction with the community.
The presence of comorbidities like diabetes, obesity, smoking, and immunosuppressive status as risk factors for acquiring SARS-CoV-2 infection [28] could not be thoroughly evaluated because the number of HCWs with these comorbidities in this study is very small. The foremost common symptoms among those with primary outcome were joints pain, headache, and fever. Similar symptoms were reported by other non-infected HCWs. These symptoms are common in other viral upper respiratory tract infections. However, specific symptoms described in SARS-CoV-2 infection such as loss of taste and loss of smell were more common among HCWs with primary outcome compared to those who were not infected with SARS-CoV-2 [29,30].
Our results showed that age above 50 years and male gender were associated with a lower risk of acquiring SARS-CoV-2 infection in our cohort. This finding of the high prevalence of SARS-CoV-2 infection among a younger age group is comparable to the result reported by Khamis et al., looking at the epidemiology of COVID-19 infection in Oman where the bulk of infected people were below the age of 50 years [31]. A possible explanation might be that the middle-aged group tends to stay home and apply social distancing and other protective measures compared to the younger age group [32]. In a study by AlMaskari et al., looking at the characteristics of HCWs infected with COVID-19 in Oman, the mean age of infected HCWs was 36 years [33], which is similar to the finding in the current study.
Our findings of lower risk of acquiring SARS-CoV-2 among the male gender differ from the report described by Khamis et al. [31] but similar to AlMaskari et al., who described a low prevalence of COVID-19 among male HCWs [33]. There is a wide variation in the reported prevalence of COVID-19 among different genders. This difference may be explained to be due to other unknown mechanisms of COVID-19 transmission or adherence to various protective measures applied by one gender more than the other in communities.
The humoral immune system produces different types of antibodies directed against different components of SARS-CoV-2. These are antibodies against SARS-CoV-2 nucleocapsid (N) proteins, S1 subunit of the SARS-CoV-2 spike, and receptor-binding domain (RBD) [34]. These antibodies can be of IgA, IgM, and IgG subtypes. So far, the magnitude of protection associated with different antibodies is not fully known [35]. This study demonstrated that the majority of infected participants (97.7%) had SARS-CoV-2 IgG antibodies by day 30 post-SARS-CoV-2 PCR testing. Our findings are consistent with previous studies showing that the majority of patients infected with SARS-CoV-2 develop antibodies within the first 21 days post confirmed infection. Long, QX et al. demonstrated that 100% of the 285 patients infected with SARS-CoV-2 developed IgG antibodies within 19 days of symptoms onset [36]. Another observational study by Wajnberg et al. of 624 PCR confirmed cases of COVID-19 found that 99% of the patients developed SARS-CoV-2 antibodies within 50 days of onset of symptoms [8].
One interesting finding of this study is the persistence of SARS-CoV-2 antibodies (IgG against S1 subunit of the SARS-CoV-2 spike and receptor-binding domain) at six months in 60% of HCWs who were diagnosed with COVID-19 based on symptoms and positive SARS-CoV-2 PCR. This finding is different from a report by Kuehn et al. that demonstrated a marked decline in SARS-CoV-2 antibodies in the majority of patients two months after the initial testing for SARS-CoV-2 antibodies, with almost one-third of patients testing negative for SARS-CoV-2 antibodies [37]. However, a recent study by Marot et al. showed early detection of IgG antibodies that remained stable up to the three months post-infection [19]. A Danish study by Hansen et al. showed persistent antibodies due to COVID-19 at six months [22]. In the same study, the estimated persistence of antibodies for more than seven months from prior COVID-19 infection was 77.7%. Another recent study by He et al. looked at the seroprevalence and durability of SARS-CoV-2 antibodies based on a longitudinal cross-sectional, multistage study from the 13 districts in Wuhan city in China involving 9500 individuals from 3600 families showed that around 40% of infected people developed SARS-CoV-2 antibodies that can be detected for up to 9 months [21].
People older than 65 years of age were found to have shorter durability of persistent SARS-CoV-2 antibodies when compared to younger patients with no difference between the two genders [20]. In comparison, the current study showed no difference between those younger or older than 50 years of age in relation to loss of SARS-CoV-2 antibodies. In addition, there was no difference between the two genders.
We found that the tendency to lose SARS-CoV-2 antibodies in Omani nationality was faster than in other nationalities. This finding requires further studies to elucidate genetic differences in antibody response to infections. Previous studies of post-vaccination immune response clearly demonstrated different responses in different ethnic groups [38,39].
Findings from the studies mentioned above showed a decline in the level of SARS-CoV-2 antibodies as time passes on and thus unlikely to achieve the estimated level of (67%) population immunity required to achieve herd immunity [5]. In addition, measures are taken by the entire world to prevent viral transmission will decrease the probability of reaching herd immunity. Further research is required to answer questions related to the level of protection provided by SARS-CoV-2 antibodies, especially with the increasing reports of recent SARS- CoV-2 strains and SARS-CoV-2 re-infection [40].
Our study has a few limitations. The first limitation is that the setup established at our hospital since the beginning of COVID-19 pandemic might not be representative of the wider healthcare system in Oman. This is due to the fact that the AFH setup included specific areas such as ED, ICU and certain wards were dedicated to COVID-19 patients, while this setup was not established in other health care facilities in Oman; therefore the risk of HCWs exposure to SARS-CoV-2 in the AFH might not be comparable in other healthcare facilities. The second limitation is the voluntary participation in the study, which may lead to selection bias. Participants who refused to participate might be anti-SARS-CoV-2 positive, and this might reduce the reported prevalence of anti-SARS-CoV-2. Another limitation is the exclusion of symptomatic HCWs at the time of the study, which might also decrease the reported prevalence of anti-SARS-CoV-2. Lastly, we relied on self-reported answers to a questionnaire questions related to symptoms and contact with COVID-19 patients, especially outside the hospital over the last one year.
Conclusion
The high seroprevalence of anti-SARS-CoV-2 antibodies among HCWs in Oman demonstrated in this study is reported for the first time in the region and is one of the highest reported seroprevalences among HCWs. Compared to the previously published studies, there is no relationship in the risk of SARS-CoV-2 acquisition when compared to HCW’s job type or location of work, suggesting a community source of infection rather than a hospital-acquired infection. This finding indicates that the transmission rate within the society is high. Therefore, strict rules must be enforced within the society such as adherence to social distancing, restriction of travel to high prevalence areas, wearing face mask and identification and isolation of suspected cases in order to prevent further speared of the infection and limit the burden on the health care system. As the study confirms previous findings of asymptomatic transmission, it confirms the findings of a few studies that showed a high percentage of persistence of SARS-CoV-2 antibodies at six months post-infection in more than half of those who were infected. This finding may confirm long-term protection against SARS-CoV-2 infection and therefore may suggest the need to study the vaccination protocols in areas with limited supply. Our study demonstrated a new and interesting finding of a noticeable difference in the rate of decline in SARS-CoV2 antibodies over time between different nationalities, which can be related to genetic differences in antibody response to SARS-CoV2, which could be considered as an initial lead to further studies related to SARS-CoV2 transmission, recovery, and vaccination protocols in the future.
Author contributions
All authors participated in this research and contributed to the final version of the manuscript. Khalid Al Naamani and Issa Al Jahdhami participated in concept design, manuscript writing, and supervising the study. Wafa AlTamtami participated in concept design, laboratory analysis, and supervising the study. Kawther AlAmri participated in concept design and supervising the study. Murtadha Al-Khabori and Heba Omer performed the statistical analysis Siham Al Sinani and Elias A. Said participated in manuscript writing, editing, and final revision. Hamad Al-Bahluli, Saada Al-Ryiami, Saleh Al-Hakmani, Najat Al-Naamani, Ruqaiya Al-Jahwari, and Musheera Al-Hinai participated in participants recruitment, blood sample, and data collection. All authors have read and agreed on the submitted manuscript.
Ethical approval
This study was approved by the Armed Forces Medical Services Research Ethics committees (AFMS-MREC: 028/2020).
Funding
No funding sources.
Conflict of interest
None declared.
Acknowledgments
We would like to express our sincerest thanks to all staff and administration of the Armed Forces Hospital. We greatly appreciate HCWs who dedicated themselves to patient care and who volunteered in the process of blood sampling. Finally, we would like to thank Roche Diagnostics, for the kind donation of antibody test kits.
References
- 1.Marsden B.D., Cox S., James T., Warren F., Peck L.J., Ritter T.G. 2020. Antibody status and incidence of SARS-CoV-2 infection in health care workers; pp. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stock A.D., Bader E.R., Cezayirli P., Inocencio J., Chalmers S.A., Yassari R. COVID-19 infection among healthcare workers: serological findings supporting routine testing. Front Med. 2020;7:1–7. doi: 10.3389/fmed.2020.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kakodkar P., Kaka N., Baig M. A comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 2019 (COVID-19) Cureus. 2020;2019 doi: 10.7759/cureus.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwok K.O., Lai F., Wei W.I., Wong S.Y.S., TJ Herd immunity — estimating the level required to halt the COVID-19 epidemics in affected countries. J Infect. 2020;80:e32–e33. doi: 10.1016/j.jinf.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivett L., Sridhar S., Sparkes D., Routledge M., Jones N.K., Forrest S. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. eLife. 2020;9:1–20. doi: 10.7554/eLife.58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lumley S.F., O’Donnell D., Stoesser N.E., Matthews P.C., Howarth A., Hatch S.B. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384:533–540. doi: 10.1056/nejmoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wajnberg Ania, Mansour Mayce, Leven Emily, Bouvier Nicole M., Patel Gopi, Firpo-Betancourt Adolfo. Humoral response and PCR positivity in patients with COVID-19 in the New York City region, USA: an observational study. Lancet Microbe. 2021;1:e283–e289. doi: 10.1016/S2666-5247(20)30120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko J.-H., Joo E.-J., Park S.-J., Baek J.Y., Kim W.D., Jee J. Neutralizing antibody production in asymptomatic and mild COVID-19 patients, in comparison with pneumonic COVID-19 patients. J Clin Med. 2020;9:2268. doi: 10.3390/jcm9072268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams J.G., Walls R.M. Supporting the health care workforce during the COVID-19 global epidemic. JAMA. 2020;323:1439–1440. doi: 10.1001/jama.2020.3972. [DOI] [PubMed] [Google Scholar]
- 11.Shah A.S.V., Wood R., Gribben C., Caldwell D., Bishop J., Weir A. Risk of hospital admission with coronavirus disease 2019 in healthcare workers and their households: nationwide linkage cohort study. BMJ. 2020;371 doi: 10.1136/bmj.m3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu J., Yang N., Wei Y., Yue H., Zhang F., Zhao J. Clinical characteristics of 54 medical staff with COVID-19: a retrospective study in a single center in Wuhan, China. J Med Virol. 2020;92:807–813. doi: 10.1002/jmv.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galanis P., Vraka I., Fragkou D., Bilali A., KD Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J Hosp Infect. 2021;108:120–134. doi: 10.1016/j.jhin.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Basteiro A.L., Moncunill G., Tortajada M., Vidal M., Guinovart C., Jiménez A. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun. 2020;11 doi: 10.1038/s41467-020-17318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iversen K., Bundgaard H., Hasselbalch R.B., Kristensen J.H., Nielsen P.B., Pries-Heje M. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis. 2020;2 doi: 10.1016/S1473-3099(20)30589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moscola J., Sembajwe G., Jarrett M., Farber B., Chang T., McGinn T. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City area. J Am Med Assoc. 2020;324:893–895. doi: 10.1001/jama.2020.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandrasekaran B., Fernandes S. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus. The COVID-19 resource centre is hosted on Elsevier Connect, the company’s public news and information website. Diabetes Metab Syndr. 2020;14(4):337–339. [Google Scholar]
- 18.Seow J., Graham C., Merrick B., Acors S., Pickering S., Steel K.J.A. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5:1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marot S., Malet I., Leducq V., Zafilaza K., Sterlin D., Planas D. Rapid decline of neutralizing antibodies against SARS-CoV-2 among infected healthcare workers. Nat Commun. 2021;12:1–7. doi: 10.1038/s41467-021-21111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen C.H., Michlmayr D., Gubbels S.M., Mølbak K., Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet (Lond, Engl) 2021;397 doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He Z., Ren L., Yang J., Guo L., Feng L., Ma C. Seroprevalence and humoral immune durability of anti-SARS-CoV-2 antibodies in Wuhan, China: a longitudinal, population-level, cross-sectional study. Lancet. 2021;397:1075–1084. doi: 10.1016/S0140-6736(21)00238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Affairs UN department of E and S . 2019. Overal total population-world population prospects. [Google Scholar]
- 23.MOH . 2021. COVID-19 cases in Oman.https://covid19.moh.gov.om/#/home [Google Scholar]
- 24.Liu M., Cheng S.Z., Xu K.W., Yang Y., Zhu Q.T., Zhang H. Use of personal protective equipment against coronavirus disease 2019 by healthcare professionals in Wuhan, China: cross sectional study. BMJ. 2020;369:6–11. doi: 10.1136/bmj.m2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansour M., Leven E., Muellers K., Stone K., Mendu D.R., Wajnberg A. Prevalence of SARS-CoV-2 antibodies among healthcare workers at a tertiary academic hospital in New York City. J Gen Intern Med. 2020;35:2485–2486. doi: 10.1007/s11606-020-05926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S., Meyler P., Mozel M., Tauh T., Merchant R. Asymptomatic carriage and transmission of SARS-CoV-2: what do we know? Can J Anesth. 2020;67:1424–1430. doi: 10.1007/s12630-020-01729-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gudbjartsson D.F., Helgason A., Jonsson H., Magnusson O.T., Melsted P., Norddahl G.L. Spread of SARS-CoV-2 in the icelandic population. N Engl J Med. 2020;382:2302–2315. doi: 10.1056/nejmoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dennison D., Khabori M.Al, Mamari S.Al, Aurelio A., Hinai H.Al, Maamari K.Al. Circulating activated neutrophils in COVID-19: an independent predictor for mechanical ventilation and death. Int J Infect Dis. 2021;106:155–159. doi: 10.1016/j.ijid.2021.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gulati Aishwarya, Pomeranz Corbin, Zahra Qamar M., Thomas Stephanie, Frisch Daniel, George Gautam. A comprehensive review of manifestations of novel coronaviruses in the context of deadly COVID-19 global pandemic. Am J Med Sci. 2020;360:5–34. doi: 10.1016/j.amjms.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khamis F., Al Rashidi B., Al-Zakwani I., Al Wahaibi A.H., Al Awaidy S.T. Epidemiology of COVID-19 infection in Oman: analysis of the first 1304 cases. Oman Med J. 2020;35:1–4. doi: 10.5001/omj.2020.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aldarhami A., Bazaid A.S., Althomali O.W., BN Public perceptions and commitment to social distancing “Staying-at-Home” during COVID-19 pandemic: a national survey in Saudi Arabia. Int J Gen Med. 2020;13:677–686. doi: 10.2147/IJGM.S269716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al Maskari Z., Al Blushi A., Khamis F., Al Tai A., Al Salmi I., Al Harthi H. Characteristics of healthcare workers infected with COVID-19: a cross-sectional observational study. Int J Infect Dis. 2021;102:32–36. doi: 10.1016/j.ijid.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siracusano G., Pastori C., Lopalco L. Humoral immune responses in COVID-19 patients: a window on the state of the art. Front Immunol. 2020;11:1–9. doi: 10.3389/fimmu.2020.01049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Post N., Eddy D., Huntley C., van Schalkwyk M.C.I., Shrotri M., Leeman D. Antibody response to SARS-CoV-2 infection in humans: a systematic review. PLoS One. 2020;15:1–27. doi: 10.1371/journal.pone.0244126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 37.Kuehn B.M. Health workers’ antibody levels wane after SARS-CoV-2 infection. J Am Med Assoc. 2021;325 doi: 10.1001/jama.2020.25457. [DOI] [PubMed] [Google Scholar]
- 38.Haralambieva I.H., Salk H.M., Lambert N.D., Ovsyannikova I.G., Kennedy R.B., Warner N.D. Associations between race, sex and immune response variations to rubella vaccination in two independent cohorts. Vaccine. 2014;32:1946–1953. doi: 10.1016/j.vaccine.2014.01.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.To K.K.-W., Hung I.F.-N., Ip J.D., Chu A.W.-H., Chan W.-M., Tam A.R. Coronavirus disease 2019 (COVID-19) re-infection by a phylogenetically distinct severe acute respiratory syndrome coronavirus 2 strain confirmed by whole genome sequencing. Clin Infect Dis. 2020;2019:1–6. doi: 10.1093/cid/ciaa1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall V.J., Foulkes S., Charlett A., Atti A., Monk E.J., Simmons R. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN) Lancet. 2021;397(10283):1459–1469. doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]