Abstract
Background
The advent of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines has been associated with a significant decline in coronavirus disease 2019 (COVID-19) hospitalizations and deaths. However, little is known about the benefits experienced by different population groups and/or using distinct vaccines.
Methods
The Spanish public registry was analyzed to examine associations between weekly vaccination scale-up and the incidence of COVID-19 hospitalizations by age, sex, and vaccine modality. The study period extended from January 2020 to June 2021.
Results
A total of 363 960 COVID-19 hospitalizations were recorded in Spain during the study period, with three peaks in March 2020, November 2020, and January 2021. The incidence of COVID-19 hospitalizations per 100 000 population increased exponentially with age, on average 71.5% for each decade older. Overall, individuals older than 60 years of age accounted for 65% of all COVID-19 hospitalizations. The speedy vaccination rollout since the end of 2020, with prioritization of the elderly groups, resulted in a rapid fall in COVID-19 hospitalizations starting in February 2021. The benefit was already noticed 3–4 weeks after the first dose, regardless of the vaccine modality.
Conclusions
COVID-19 hospitalizations increased exponentially with age in all three peaks of SARS-CoV-2 infection in Spain. Early mass vaccination of people over 60 years of age prevented a fourth wave of COVID-19 hospitalizations during the spring of 2021.
KEYWORDS: Severe COVID-19, Hospitalizations, Vaccine, Spain
1. Introduction
The recent global spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in humans is unprecedented in medicine(Morens and Fauci, 2020). By the end of June 2021, over 180 million cases had been reported worldwide, with nearly 4 million deaths. In Spain, these figures were nearly 4 million confirmed cases and over 80 000 deaths. In the absence of effective antiviral agents against SARS-CoV-2, the advent of vaccines has been eagerly awaited. The first vaccines achieved emergency approval in December 2020, after proving significant protection against the development of severe coronavirus disease 2019 (COVID-19) in clinical trials (Polack et al., 2020; Baden et al., 2021). Vaccine rollout began immediately, with prioritization of the most vulnerable populations, including the elderly and healthcare workers.
Several surges of COVID-19 have been recognized in different countries, largely depending on the mitigation interventions mandated at the country level. In Spain, three large waves have been well characterized so far, with peaks of COVID-19 hospitalizations and deaths in March 2020 (Soriano et al., 2020; Pollan et al., 2020; Moreno-Torres et al., 2021), November 2020 Soriano et al., 2021, and January 2021 (Soriano et al., 2021b).
The speedy vaccination rollout since the end of 2020 in Spain, with prioritization of the elderly groups and healthcare workers, resulted in a rapid fall in COVID-19 hospitalizations starting in February 2021. This study was performed to examine in detail the impact of vaccination on nationwide COVID-19 hospitalizations.
2. Methods
All COVID-19 hospitalizations in Spain from February 2020 to June 2021 were investigated using the national public registry, which is freely available on the Health Ministry website (CNE, 2021). The age, sex, and calendar week of hospitalization of all hospitalized COVID-19 patients during this 17-month period were analyzed. Likewise, all information available for the vaccines administered, including the calendar week and modality, as recorded on the Health Ministry website, were examined (MS, 2021).
For the analyses, age was grouped into consecutive decades. Calendar periods were grouped by weeks. There were four vaccine modalities, two using mRNA, marketed by Pfizer (Polack et al., 2020) and Moderna (Baden et al, 2021), one using a chimp adenovirus as vector, marketed by AstraZeneca (Voysey et al., 2021), and another that uses a replication-deficient human adenovirus vector from Janssen (Sadoff et al., 2021). All but the Janssen vaccine are given as two doses.
2.1. Statistical analyses
Crude data were recorded in Excel files as absolute numbers and percentages. Patients were grouped by age range in consecutive decades. The incidence of COVID-19 hospitalization for each age group was expressed as the number of hospitalizations per 100 000 population in that age range. Data on the national population and age and sex distribution were obtained from the Spanish Statistical database (SNIS, 2021). Calendar date COVID-19 hospitalizations were grouped by week, reported on the following Monday. Exponential regression was adjusted using the least-squares method.
2.1.1. Estimates for COVID-19 hospitalizations in 2021 in the absence of vaccines
In order to determine the impact of the vaccines, the expected hospitalizations during 2021 were estimated, extrapolating data from the second wave, when hospital beds were always available (in contrast with the first wave) and the vaccines had still not been marketed. Estimates were made for the distinct age groups. As shown in the Supplementary Material (Figure S1 and Table S1), the proportions of each age group as part of the total from the second wave were very stable and reliable, and accordingly the period from the week reported on October 12 to the week reported on January 4 was chosen as the ‘reference window’. These proportions were considered as the established hospitalization incidence rates for each age group.
When the effect of the vaccines on COVID-19 hospitalizations began to be recognized, estimates for hospitalization incidence for each age group were assessed weekly, applying the established proportions. The age groups that had been less vaccinated were considered as controls, and curves of ‘hospitalizations in the absence of vaccines’ were estimated applying the established proportions for matched ages. In this way, the real COVID-19 hospitalization incidence was compared with estimated hospitalizations in the absence of vaccines.
The following three reference age groups were used: (1) For the weeks reported from 11-Jan-2021 to 08-Feb-2021, the reference age group was 50–59 years, since that age group had a very low vaccination coverage during that period (<2%). (2) For the weeks reported from 15-Feb-2021 to 12-Apr-2021, the reference age group was 70–79 years, since although the first vaccinations for this age group had been given in nursing homes, the rest of the population within this age range living in their own homes had to wait until the full completion of the 80–89 years age group vaccinations before receiving theirs. Less than 5% of this age group had received one dose during this period. (3) For the weeks reported from 19-Apr-2021 to 21-Jun-2021, the reference age group was the combination of 30–39 years and 40–49 years. These two age groups were combined to have greater statistical significance, because the hospitalization numbers were low. The vaccination coverage with one dose in these age groups reached 15% by mid-June. By then, the 80–89 years age group had reached 100% coverage, the 70–79 years group had reached 98%, the 60–69 years group had reached 92%, and the 50–59 years group had reached 76%. Therefore, vaccine coverage of 15% was an acceptable threshold to be considered as low impacted by vaccination.
2.1.2. Estimates for early vaccinations by age
From January 4 to March 29, 2021, the Spanish Health Ministry released information about the total population that had received at least one dose and those that had completed the two-dose vaccination schedule, as distributed by the vaccine vendor. From March 31, the percentages of vaccinated people were also reported by age group. In the study analyses, the missing age distribution of vaccination during the initial rollout was estimated using the following criteria:
-
●Mathematical conditions:
-
○The estimated curves for each age group should meet smoothly with the data first reported on March 31.
-
○Every week, the number of ‘people with at least one dose’ and ‘people with full vaccination’ in all age groups should match with the total number of vaccines administered in Spain.
-
○The number of ‘people with at least one dose’ and ‘people with full vaccination’ every week should be equal to or greater than the previous week for each age group.
-
○The number of ‘people with full vaccination’ in a certain week should not be larger than the number of ‘people with at least one dose’ from 3 weeks before for the Moderna/Pfizer vaccines.
-
○
-
●
The age groups 80+, 70–79, and 16–17 years were considered to have been vaccinated only with the Pfizer/Moderna vaccines.
-
●
The age groups 60–69, 50–59, 25–49, and 18–24 years were considered to have received AstraZeneca as well as Pfizer/Moderna after early March.
-
●
A small proportion of individuals in the age groups 60–69, 50–59, 25–49, and 18–24 years were considered to have received the first dose with AstraZeneca and the second with Pfizer/Moderna.
-
●
The age group 60–69 years only received the AstraZeneca vaccine starting in May. This restriction was removed 2 months later.
-
●
The age group 70–79 years initially received a large number of doses (roughly 100 000), which is explained by the easy access to the elderly living in skilled nursing homes. Thereafter, the pace of vaccination slowed down because the elderly living in their own homes had to be contacted individually by their general practitioners.
3. Results
The official population of Spain by mid-2020 was 47.5 million people, with 51% being female. Table 1 reports the age and sex distribution. Interestingly, persons older than 60 years of age represent 25.6% of the country's population. Up to June 21, 2021, a total of 363 960 COVID-19 hospitalizations had been recorded in Spain. Individuals older than 60 years of age accounted for 64.7% of all COVID-19 hospitalizations; those above 80 years accounted for 26.8%. Overall, more males than females were hospitalized with COVID-19, but below 40 years of age, females were more frequently hospitalized than males.
Table 1.
Population of Spain and the distribution by age and sex, and the cumulative number of COVID-19 hospitalizations during the study period (January 2020 to June 2021).
| Age (years) | Population, n | COVID-19 hospitalizations, n (n per 100 000 population) | ||||
|---|---|---|---|---|---|---|
| Total | Male | Female | Total | Male | Female | |
| 0–9 | 4 307 135 | 2 214 419 | 2 092 716 | 2307 (54) | 1251 (56) | 1056 (50) |
| 10–19 | 4 912 519 | 2 530 201 | 2 382 318 | 2695 (55) | 1292 (51) | 1403 (59) |
| 20–29 | 4 944 640 | 2 516 099 | 2 428 541 | 9256 (187) | 3981 (158) | 5275 (217) |
| 30–39 | 6 230 403 | 3 123 693 | 3 106 710 | 19 045 (306) | 9290 (297) | 9755 (314) |
| 40–49 | 7 891 737 | 3 992 962 | 3 898 775 | 37 937 (481) | 22 764 (570) | 15 173 (389) |
| 50–59 | 7 033 306 | 3 480 573 | 3 552 733 | 57 494 (817) | 34 933 (1004) | 22 561 (635) |
| 60–69 | 5 336 986 | 2 563 879 | 2 773 107 | 65 763 (1232) | 40 161 (1566) | 25 602 (923) |
| 70–79 | 3 960 045 | 1 794 301 | 2 165 744 | 72 177 (1823) | 41 791 (2329) | 30 386 (1403) |
| 80+ | 2 834 024 | 1 039 463 | 1 794 561 | 97 286 (3433) | 44 748 (4305) | 52 538 (2928) |
| Total | 47 450 795 | 23 255 590 | 24 195 205 | 363 960 (767) | 200 211 (861) | 163 749 (677) |
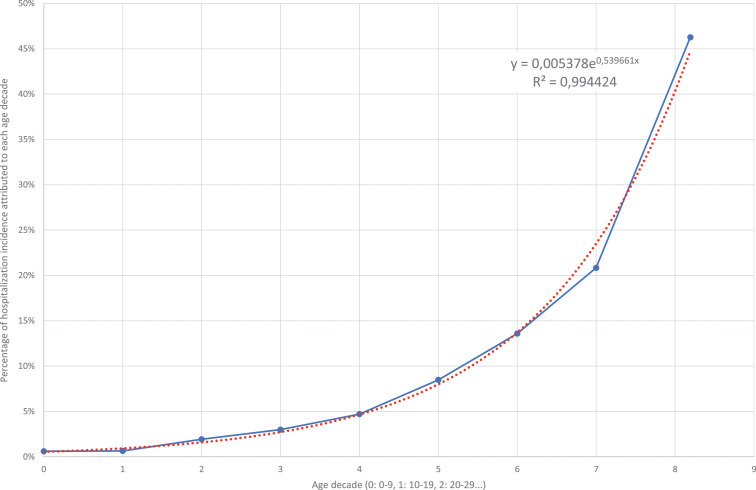
Figure 1 shows the COVID-19 hospitalization incidence for each age range. Regardless of sex, older age was by far the major determinant of COVID-19 hospitalization. The adjusted risk increased exponentially on average 71.5% for each decade older above 20 years old.
Figure 1.
Incidence of COVID-19 hospitalization by age in Spain. Real numbers (continuous line) versus estimated numbers in the exponential model (dotted line).
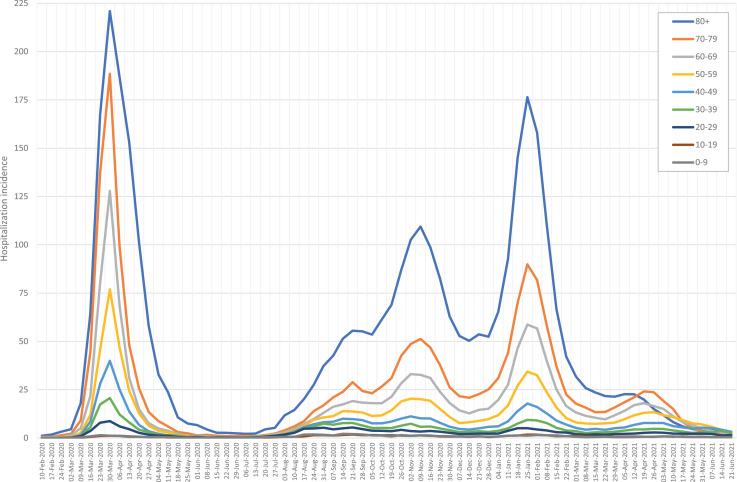
Figure 2 shows the weekly change in new COVID-19 hospitalizations during the study period, grouped by age range. Three different waves of COVID-19 hospitalizations were observed. The first with a peak in March 2020, after the nationwide lockdown with mandatory home confinement starting on March 14. The second wave occurred in late October 2020, following the summer holidays and return to work, schools, and universities. The third wave was seen in January 2021, after Christmas gatherings. In each of the waves, over 200 COVID-19 hospitalizations per 100 000 people were observed.
Figure 2.
Incidence of COVID-19 hospitalizations (per 100 000 population) by age group and calendar week.
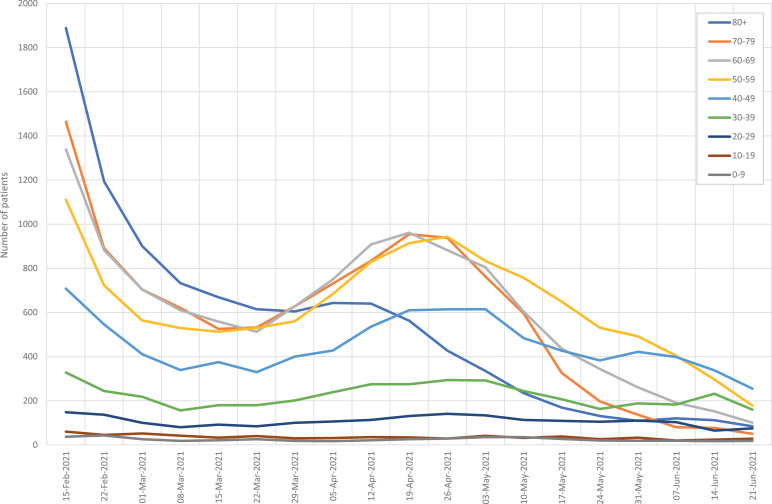
Starting in February 2021 there was a rapid decline in COVID-19 hospitalizations with a small rebound in April, after the Easter holidays (Figure 3 ). Of note, there was no evidence of a fourth wave of COVID-19. However, COVID-19 hospitalizations in this mild spring surge mostly occurred in people younger than 80 years of age.
Figure 3.
Impact of COVID-19 hospitalizations in Spain by calendar week and age starting in February 2021.
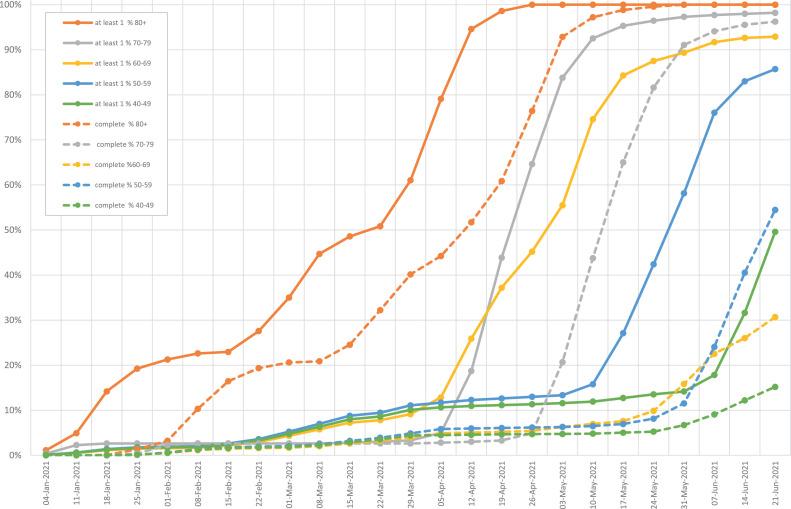
SARS-CoV-2 vaccination in Spain began at the end of 2020. Two main groups were prioritized: the elderly living in nursing homes and healthcare workers. Figure 4 records the scaling up of vaccination by age group. The Pfizer and Moderna vaccines were the only available vaccines at that time. The recommended schedule was two doses given 3–4 weeks apart. Starting in March 2021, the AstraZeneca vaccine was also prescribed, given in two doses 10–12 weeks apart. The Janssen vaccine was only introduced in May 2021.
Figure 4.
Scale-up of SARS-CoV-2 vaccination in Spain.
The administration of at least one dose of SARS-CoV-2 vaccine was recorded for 55% of the population in Spain up to June 21, 2021. The complete vaccination schedule with two doses (for all but Janssen) was recorded for 34% of the population. The distribution of vaccines administered was as follows: Pfizer 67%, Moderna 9%, AstraZeneca 20%, and Janssen 4%. No differential impact of the distinct vaccines on COVID-19 hospitalization rates were identified, considering age ranges or sex (data not shown).
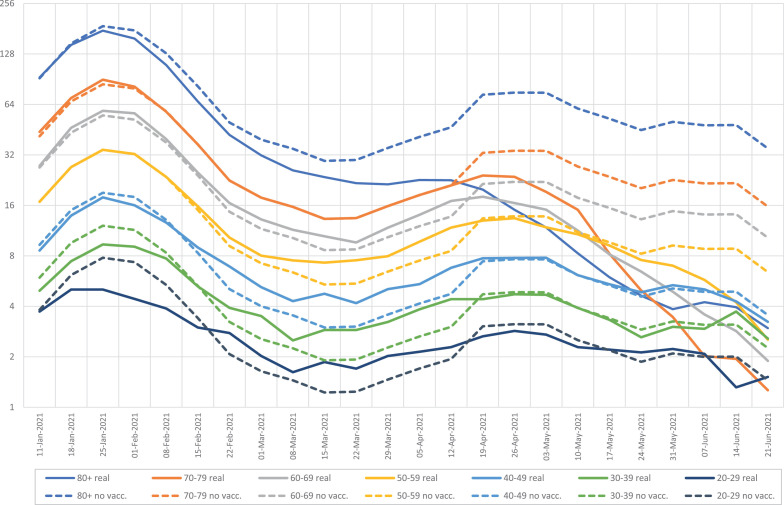
Figure 5 shows the COVID-19 hospitalizations by age group since the introduction of the vaccines, considering real numbers and estimates if no vaccines had existed, derived from data obtained during the ‘reference window’ (fall 2020) described above, when no vaccines were available. This figure shows the benefit of the vaccines, with declines in hospitalizations, largely reflecting their benefit on the oldest population.
Figure 5.
COVID-19 hospitalizations by age group and calendar week starting in January 2021. Real numbers (continuous line) versus estimated numbers without vaccination (dotted line); logarithmic scale.
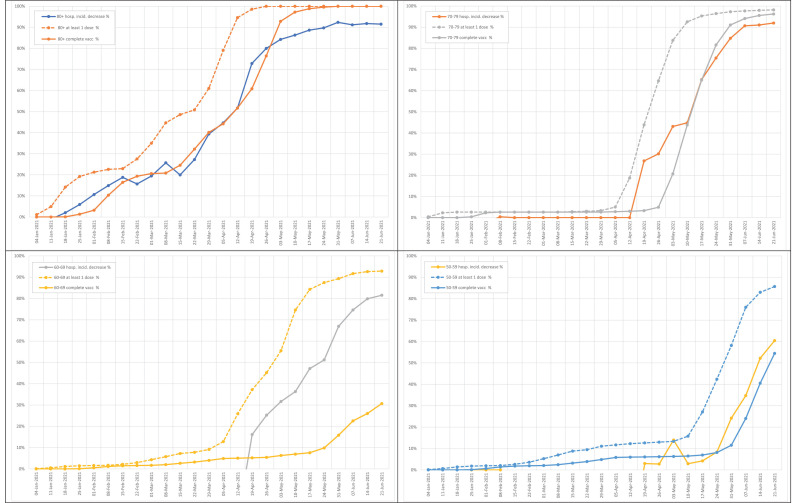
The strong inverse relationship between vaccination rollout and COVID-19 hospitalizations was initially noticed in the oldest groups of people who were vaccinated earlier. When declines in COVID-19 hospitalizations in distinct age groups were examined alongside vaccinations in the same age group, a significant correlation was found, with a 3–4-week delay on average after the first dose (Figure 6 ). Indeed, the second dose only added a slight benefit on hospitalizations.
Figure 6.
Decline in the incidence of COVID-19 hospitalizations alongside vaccine scaling up for distinct age groups.
4. Discussion
Since the end of 2019, SARS-CoV-2 infection has spread worldwide transforming human lives and societies. The first wave of COVID-19 erupted abruptly in Europe in March 2020, with Spain being one of the epicenters (Dur-e-Ahmad and Imran, 2020) Liu et al., 2021). The high mortality rates among the elderly in skilled nursing homes and the collapse of hospitals occurred within a few weeks (Soriano and Barreiro, 2020). Home confinement and lockdown of most activities were implemented over 6 weeks. In the present analysis of nationwide COVID-19 hospitalizations, the surge in March 2020 should be interpreted as an underestimation of the real number of severe cases, since access to diagnostic tests was poor and a large proportion of older people did not die in hospitals but in their skilled nursing homes (Candel et al., 2021). The relaxation of social distancing measures during the summer was followed by a new surge in October 2020, alongside a partial return to work and the reopening of schools and universities. Finally, a third wave of COVID-19 hospitalizations occurred after the Christmas period, most likely reflecting family gatherings (Guallar et al., 2020; Thompson et al., 2021).
It is noteworthy that older age was the major determinant of hospitalization. Above 20 years old, for each decade older there was an exponential increased risk of hospitalization of 71.5%, more clearly manifested during the second and third waves. On average, individuals over 80 years of age represented 27% of hospital admissions. In contrast, persons younger than 40 years of age and children represented only 9% of hospitalizations. Of note, the incidence of hospitalization in the younger population was higher for females than males; although among those older than 40 years old, the hospitalization rate was higher for males. This finding is most likely due to the fact that almost all infected pregnant women were recommended to stay in hospital for close monitoring until resolution (Cruz et al., 2021).
The introduction of vaccines at the end of 2020 had a significant impact on COVID-19 hospitalizations. The effect of the prioritization of vaccination of the elderly in skilled nursing homes and of healthcare workers started to be noticed in February 2021. Subsequently, the steady vaccination of the oldest persons living in their own homes and essential workers consolidated the decline in COVID-19 hospitalizations. Accordingly, new COVID-19 hospitalizations diminished and these mostly occurred in younger patients who had not been vaccinated.
From the last week of March 2021, people over 80 years of age were no longer the most frequently hospitalized. This was also the case for those above 70 years old starting in mid-May. From early June 2021, COVID-19 hospitalization numbers were low, with the residual population younger than 50 years old and not yet vaccinated being the most represented.
It is noteworthy that overall protection from COVID-19 hospitalizations increased to nearly 91% for those who were vaccinated, in line with data from pre-registration vaccine trials regarding severe COVID-19 (Polack et al., 2020; Baden et al., 2021; MS, 2021). Furthermore, the clinical protection from COVID-19 hospitalization was seen after the first vaccine dose in all age groups, including the oldest ones in whom immune responses could hypothetically be impaired. The study results are in line with preliminary evidence from registrational clinical trials (Connors et al., 2021) and challenge the recognition of a low humoral response after the first dose, suggesting that protection derived from cellular immune responses might be more important than previously thought (Stankov et al., 2021). On the other hand, the study results support recent evidence suggesting that people who have already had a SARS-CoV-2 infection may safely skip the second vaccine dose (Wang et al., 2021). This information is important because it could help to stretch scarce vaccine supplies in some countries.
No difference in vaccine protection could be attributed to the distinct vaccines, although the numbers for the Janssen vaccine were low and the follow-up was too short to draw definitive conclusions. The Pfizer vaccine was the most frequently prescribed COVID-19 vaccine by far up to June 2021 in Spain.
Some authors have argued that massive SARS-CoV-2 vaccination in the midst of a pandemic might be the wrong intervention, favoring the selection of vaccine-escape mutants. We did not find any evidence in favor of such a threat. Although vaccine breakthrough infections due to new variants have been well demonstrated, they have often been clinically mild and have generally not required hospitalization (Hacisuleyman et al., 2021). In Spain, up to June 30, the more transmissible SARS-CoV-2 variants alpha to delta have consecutively replaced the original SARS-CoV-2 strain (Alcoba-Florez et al., 2021; Campoy et al., 2021). However, vaccine protection remains clinically evident based on the low rate of COVID-19 hospitalizations. The study data are in agreement with most studies that have examined the protective efficacy of current vaccines over the new variants. All have uniformly highlighted that changes in neutralization activity do not seem to translate into complete vaccine failure with symptomatic illness (Wang et al., 2021; Kustin et al., 2021).
Several caveats and potential limitations of this study should be acknowledged, especially with respect to the conclusion that the lack of a fourth wave of COVID-19 hospitalizations in Spain was largely the result of the prior mass vaccine rollout in the most vulnerable populations by Easter. There are at least three other possible reasons that could explain this benefit: (1) the growing and already large subset of people infected and with immunity by then; (2) the acquisition of knowledge on social distancing and other protective measures that were uniformly implemented; and (3) the evolving and changing criteria for hospitalization of infected persons, with prioritization of disease management at home when possible. Each of these points is addressed separately below.
First, the population already infected and with natural immunity by Easter was low. Since there was an unexpected surge of cases (fifth wave) in July 2021 driven by the highly transmissible SARS-CoV-2 delta variant (Seppäla et al., 2021), it is clear that herd immunity has not been achieved in Spain. Moreover, it was possible to estimate the proportion of protection during the fourth wave that resulted from vaccination. By far the vast majority of hospitalizations in elderly people during the fourth and the largest fifth wave mostly occurred in the remaining subset of non-vaccinated individuals.
Second, a hard lockdown was only enforced during the first wave in March–April 2020. Social distancing measures of variable intensity were implemented intermittently during subsequent months, but there was no further enforced home confinement. Recommendations included wearing face masks indoors, restrictions on gatherings, curfews, etc. Therefore, the low plateau of new diagnoses of severe cases (those requiring hospitalization) after Easter (corresponding to the fourth wave) largely resulted from the beneficial impact of massive vaccination coverage for the most vulnerable oldest population.
Third, the criteria for admission to hospital wards during the pandemic have evolved as a result of improved knowledge, allowing people with no acute severe signs/symptoms and who have no predictors of a poorer outcome (older age, obesity, male sex, etc.) to avoid hospitalization. However, hospital room capacity has increased since the first wave, when many severely ill patients could not be hospitalized because the health system became overwhelmed. Overall, our interpretation is that hospitalization criteria did not greatly affect the study results.
In summary, nationwide records of COVID-19 hospitalizations in Spain indicate that the mass vaccination of people over 60 years of age prevented a fourth wave of COVID-19 hospitalization after Easter time. The protective effect on COVID-19 hospitalizations was noticed shortly after the first dose of the current marketed vaccines.
Declarations
Funding sources: UNIR TRAPES and SEVERITYGEN projects.
Ethical approval: According to Spanish law, this study did not require any specific ethical approval, as it used anonymous information that was retrieved from a public database.
Conflict of interest: None for all authors.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.09.022.
Appendix. Supplementary materials
References
- Alcoba-Florez J, Lorenzo-Salazar JM, Gil-Campesino H, et al. Monitoring the rise of the SARS-CoV-2 lineage B.1.1.7 in Tenerife (Spain) since mid-December 2020. J Infect. 2021;82:e1–e3. doi: 10.1016/j.jinf.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden L, El Sahly H, Essink B, et al. COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campoy P, Buenestado-Serrano S, Pérez-Lago L, et al. First importations of SARS-CoV-2 P.1 and P.2 variants from Brazil to Spain and early community transmission. Enferm Infecc Microbiol Clin. Last checked: August 31st 2021 (in press). [DOI] [PMC free article] [PubMed]
- Candel FJ, Barreiro P, San Román J, et al. The demography and characteristics of SARS-CoV-2 seropositive residents and staff of nursing homes for older adults in the Community of Madrid: the SeroSOS study. Age Ageing. 2021;50:1038–1047. doi: 10.1093/ageing/afab096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centro Nacional de Epidemiología. https://www.cnecovid.isciii.es/covid19/. Data file: https://cnecovid.isciii.es/covid19/resources/casos_hosp_uci_def_sexo_edad_provres.csv August 31st 2021.
- Connors M, Graham B, Lane HC, Fauci A. SARS-CoV-2 vaccines: much accomplished, much to learn. Ann Intern Med. 2021;174:687–690. doi: 10.7326/M21-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz S, de la Cruz ML, Carmona P, et al. Pregnancy outcomes and SARS-CoV-2 infection: The Spanish Obstetric Emergency Group Study. Viruses. 2021;13:853. doi: 10.3390/v13050853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dur-e-Ahmad M, Imran M. Transmission dynamics model of coronavirus COVID-19 for the outbreak in most affected countries of the world. Int J Interactive Multimedia Artific Intel. 2020;6:7–10. [Google Scholar]
- Guallar MP, Meiriño R, Donat-Vargas C, Corral O, Jouvé N, Soriano V. Inoculum at the time of SARS-CoV-2 exposure and risk of disease severity. Int J Infect Dis. 2020;97:290–292. doi: 10.1016/j.ijid.2020.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacisuleyman E, Hale C, Saito Y, et al. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med. 2021;384:2212–2218. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustin T, Harel N, Finkel U, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. Last checked August 31st 2021 (in press) August 31st 2021. [DOI] [PMC free article] [PubMed]
- Liu X, Fong S, Dey N, Gonzalez-Crespo R, Herrera-Viedma E. A new SEAIRD pandemic prediction model with clinical and epidemiological data analysis on COVID-19 outbreak. Appl Intellig. 2021;51:4162–4198. doi: 10.1007/s10489-020-01938-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministerio de Sanidad. Vaccination strategy for COVID-19 in Spain. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/vacunaCovid19.htm. Data files: Open data file format: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Informe_Comunicacion_yyyymmdd.ods (where: yyyyy = year, mm = month, dd = day).
- Moreno-Torres V, de la Fuente S, Mills P, et al. Major determinants of death in patients hospitalized with COVID-19 during the first epidemic wave in Madrid, Spain. Medicine (Baltimore). 2021;100:e25634. doi: 10.1097/MD.0000000000025634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens D, Fauci A. Emerging pandemic diseases: how we got to COVID-19. Cell. 2020;183:837–846. doi: 10.1016/j.cell.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F, Thomas S, Kitchin N, et al. C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1-2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppälä E, Veneti L, Starrfelt J, et al. Vaccine effectiveness against infection with the Delta (B.1.617.2) variant, Norway, April to August 2021. Euro Surveill. (in press). [DOI] [PMC free article] [PubMed]
- Soriano V, Barreiro P. Why such excess of mortality for COVID-19 in Spain? Ther Adv Infect Dis. 2020;7 doi: 10.1177/2049936120932755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano V, Ganado-Pinilla P, Sanchez-Santos M, et al. Main differences between the first and second waves of COVID-19 in Madrid, Spain. Int J Infect Dis. 2021;105:374–376. doi: 10.1016/j.ijid.2021.02.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano V, de Mendoza C, Gómez-Gallego F, Corral O, Barreiro P. Third wave of COVID-19 in Madrid, Spain. Int J Infect Dis. 2021;107:212–214. doi: 10.1016/j.ijid.2021.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanish National Institute of Statistics. https://www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736176951&menu=ultiDatos&idp=1254735572981. Last checked August 31st 2021.
- Stankov M, Cossmann A, Bonifacius A, et al. Humoral and cellular immune responses against SARS-CoV-2 variants and human coronaviruses after single BNT162b2 vaccination. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab555. Jun 16:ciab555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H, Mousa A, Dighe A, et al. SARS-CoV-2 setting-specific transmission rates: a systematic review and meta-analysis. Clin Infect Dis. 2021;73:e754–e764. doi: 10.1093/cid/ciab100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M, Clemens S, Madhi S, et al. Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Muecksch F, Schaefer-Babajew D, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595:426–431. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.