Abstract
Purpose
In acute respiratory distress syndrome (ARDS), dead space fraction has been independently associated with mortality. We hypothesized that early measurement of the difference between arterial and end-tidal CO2 (arterial-ET difference), a surrogate for dead space fraction, would predict mortality in mechanically ventilated patients with ARDS.
Methods
We performed two separate exploratory analyses. We first used publicly available databases from the ALTA, EDEN, and OMEGA ARDS Network trials (N = 124) as a derivation cohort to test our hypothesis. We then performed a separate retrospective analysis of patients with ARDS using University of Chicago patients (N = 302) as a validation cohort.
Results
The ARDS Network derivation cohort demonstrated arterial-ET difference, vasopressor requirement, age, and APACHE III to be associated with mortality by univariable analysis. By multivariable analysis, only the arterial-ET difference remained significant (P = 0.047). In a separate analysis, the modified Enghoff equation ((PaCO2–PETCO2)/PaCO2) was used in place of the arterial-ET difference and did not alter the results. The University of Chicago cohort found arterial-ET difference, age, ventilator mode, vasopressor requirement, and APACHE II to be associated with mortality in a univariate analysis. By multivariable analysis, the arterial-ET difference continued to be predictive of mortality (P = 0.031). In the validation cohort, substitution of the arterial-ET difference for the modified Enghoff equation showed similar results.
Conclusion
Arterial to end-tidal CO2 (ETCO2) difference is an independent predictor of mortality in patients with ARDS.
Keywords: ARDS, Mortality, Blood gas analysis, End-tidal CO2
Introduction
Acute Respiratory Distress Syndrome (ARDS) is an acute inflammatory process which leads to protein-rich, non-hydrostatic pulmonary edema with reduced lung compliance, refractory hypoxemia, and impaired ability to eliminate carbon dioxide [1]. This inefficiency in CO2 elimination is caused by increased physiologic dead space as microvascular injury reduces perfusion of ventilated alveoli. Despite advances in the understanding of the pathophysiology in ARDS and identification of improved strategies that prevent further ventilator-induced lung injury (VILI) [2], mortality in ARDS remains high [1].
Given the high morbidity and mortality of ARDS, it is important to identify reliable prognostic indicators, not only to predict outcomes in individual patients, but also to stratify patients for clinical trials and escalation of supportive measures. In addition to patient characteristics such as age and severity of illness, various physiologic parameters associated with ARDS have been identified to stratify disease severity and outcomes. These physiologic parameters include driving pressure [3], PaO2:FiO2 ratio, and physiologic dead space [4]. In a prior study by Nuckton et al. [5], the dead-space fraction (Vd/Vt) was measured early in patients with ARDS and was independently correlated with survival. These investigators noted that mean dead-space fraction was markedly elevated (0.58 ± 0.09) early in the course of ARDS and was higher among patients who died than those who survived (0.63 ± 0.10 vs 0.54 ± 0.09; P < 0.001).
Unfortunately, measuring lung dead-space at the bedside is not easy. It requires collection of expired gas into a reservoir bag for several minutes and is not routinely employed outside research study circumstances. In contrast, arterial CO2 and end-tidal CO2 are both measured frequently in critically ill, mechanically ventilated patients. Continuous end-tidal CO2 monitoring provides insight into three main functions: metabolism, circulation, and ventilation [6, 7]. The pulmonary pathophysiology of ARDS includes increased dead space fraction in addition to shunt [8, 9]. There are several mechanisms by which increased dead space may occur in ARDS. These include alveolar capillary injury, in situ microvascular thrombosis, and small airways and/or alveolar epithelial injury with V/Q mismatch. Additionally, reduction in right ventricular function may lead to higher dead space by an increase in West zone I and II perfusion [10].
The arterial to end-tidal CO2 difference should follow the directional change of the dead space fraction. Accordingly, we hypothesized that the arterial-ET difference would predict mortality in mechanically ventilated patients with ARDS and sought to test this hypothesis with an exploratory analysis in a cohort from the ARDS Network public database (https://biolincc.nhlbi.nih.gov). Following the exploratory analysis, we sought to validate our hypothesis in a cohort of medical ICU patients at the University of Chicago.
Methods
Patient cohort
We used the ARDS Network database (https://biolincc.nhlbi.nih.gov) as our derivation cohort. In a review of the eight available ARDS Network databases, three contained both end-tidal CO2 and arterial CO2 measurements. These three databases (OMEGA [11], ALTA [12], and EDEN [13]) were included in the exploratory analysis.
For our validation cohort, we performed a retrospective analysis of patients with ARDS [4] at the University of Chicago from January 2010 through October 2019. With the assistance of the University of Chicago Center for Research Informatics, we queried our local Epic electronic medical record (Verona, WI) data warehouse using the following criteria: age > 18 years, inpatient or emergency encounters, index encounters, and ICD9/10 codes for ARDS, Acute Hypoxemic Respiratory Failure, Respiratory Failure, Ventilator Support, Hypoxia, and Hypoxemia. The study was approved by the University of Chicago Institutional Review Board (IRB) with a waiver of consent for this de-identified retrospective analysis. We used the STROBE cohort checklist when writing our report [14].
Exclusion criteria included: age < 18, pregnancy, extracorporeal membrane oxygenation (ECMO) during the first 24 h, and those with > 50 pack-year smoking history (to avoid patients with COPD and the inherent pre-existing impact on dead space fraction). We also excluded patients with COPD on pulmonary function tests and/or emphysema on chest high-resolution CT scans based upon a blinded review by a senior pulmonologist (JPK). Lastly, patients without reported end-tidal CO2 values within the first 24 h after ARDS diagnosis were excluded.
Measurements of the arterial-ET difference
Because both derivation and validation cohorts were retrospective analyses, simultaneous arterial and end-tidal CO2 measurements could not be collected by protocol. We recorded arterial blood gases measured during the first 24 h after the diagnosis of ARDS was established. In order to be included in the analyses, end-tidal CO2 must have been recorded within 1 h of the arterial blood gas with no ventilator changes between arterial and end tidal CO2 measurements. For all analyses, we used the arterial CO2 and end-tidal CO2 measured on day one of ARDS diagnosis only. The physiologic dead space was estimated using two methods. First by calculating the difference between arterial and end-title CO2 (PaCO2–PETCO2). Second, we substituted end-title CO2 for mean exhaled CO2 in the Enghoff modification of the Bohr equation. We will refer to this as the simplified Enghoff modification ((PaCO2–PETCO2)/PaCO2).
Statistical analysis
The primary outcome variable for both cohorts was in-hospital mortality. Logistic-regression analysis was used to examine multiple variables in order to determine independent association(s) with mortality. Independent variables were chosen on the basis of prior ARDS studies as well as biological plausibility. All variables associated with mortality by univariable analysis (P < 0.10) were included in the multivariable models. We began with our derivation analysis using the 124 patient cohort gathered from the ARDS network database. Demographics, comorbidities, physiological characteristics, ventilator parameters, and outcomes were compared using Chi-square test for categorical variables and Mann–Whitney U for continuous variables.
The following independent variables were considered: age, gender, APACHE score, tidal volume per ideal body weight, arterial-ET difference, positive end expiratory pressure (PEEP), and ECMO use during hospitalization. A second model was created where the simplified Enghoff equation ((PaCO2–PETCO2)/PaCO2) was substituted for the arterial to end-tidal difference. P values less than 0.05 were considered statistically significant for all comparisons. All analyses were performed using Stata 16.1 (College Station, TX) and R [15].
Results
The ARDS Network database yielded a total of 124 patients to serve as the derivation cohort. Our original query from the University of Chicago medical records found 499 patients for our validation cohort. After review of each record, we identified 302 patients who met our a priori inclusion criteria. The most common reasons that patients were excluded were: COPD/emphysema (N = 52), the absence of PaCO2 and ETCO2 data within an hour of each other (N = 112), and other (N = 33).
Patient characteristics are summarized in Table 1. When comparing clinical characteristics, the University of Chicago cohort was significantly older, had more severe ARDS, and a higher mortality.
Table 1.
Clinical characteristics of ARDS patients
| Total | ARDSnet | University of Chicago | P value comparing ARDSnet and U of Chicago | |
|---|---|---|---|---|
| N = 426 | N = 124 | N = 302 | ||
| Age | 55.5 (42.1–66.6) | 51.0 (40.5–63.0) | 57.7 (42.4–67.7) | 0.007 |
| Gender | ||||
| Male | 227 (53.3%) | 68 (54.8%) | 159 (52.6%) | 0.68 |
| Female | 199 (46.7%) | 56 (45.2%) | 143 (47.4%) | |
| Race | < 0.001 | |||
| White | 188 (44.1%) | 88 (71.0%) | 100 (33.1%) | |
| Hispanic | 21 ( 4.9%) | 11 ( 8.9%) | 10 ( 3.3%) | |
| African American | 196 (46.0%) | 25 (20.2%) | 171 (56.6%) | |
| Asian | 9 (2.1%) | 0 (0.0%) | 9 (3.0%) | |
| Other | 12 (2.8%) | 0 (0.0%) | 12 (4.0%) | |
| SOFA | 9.0 (7.0–12.0) | 10.0 (7.5–13.0) | 9.0 (7.0–12.0) | 0.085 |
| APACHE II | NA | NA | 29 (23–36) | |
| APACHE III | NA | 90 (72–113)* | NA | |
| PaO2:FiO2 | 136.0 (92.8–197.5) | 176.6 (134.0–235.8) | 120.0 (81.0–178.0) | < 0.001 |
| Tidal volume | 420.0 (362.0–460.0) | 427.5 (360.0–480.0) | 420.0 (370.0–450.0) | 0.71 |
| TV ml/kg IBW | 6.4 (5.8–7.2) | 6.5 (6.0–7.4) | 6.3 (5.7–7.1) | 0.002 |
| Hospital mortality | 199 (46.7%) | 26 (21.0%) | 173 (57.3%) | < 0.001 |
Data are presented as median and interquartile range (IQR) for continuous measures and n (%) for categorical measures
PaO2:FiO2 Ratio of arterial partial pressure of oxygen to fractional inspired oxygen, SOFA sequential organ failure assessment, APACHE acute physiology and chronic health evaluation, IBW ideal body weight
Gender, tidal volume per ideal body weight, PEEP, and ECMO use during hospitalization were not significant in univariable analyses for either derivation or validation cohorts. Accordingly, these independent variables were not introduced into the multiple logistic-regression models for either analysis.
In the ARDS Network cohort, four variables were associated with mortality by univariable analysis: arterial-ET difference, age, vasopressor requirement, and APACHE III. By multivariable analysis, only the arterial-ET difference remained significant (Table 2). A model substituting the simplified Enghoff equation in place of the arterial-ET difference also demonstrated the estimation of dead space to be the only significant variable (Table 3).
Table 2.
Multivariable analysis of derivation cohort using arterial-ET difference: ARDS net database
| Mortality | Odds ratio | P value | [95% conf. interval] | |
|---|---|---|---|---|
| Arterial-ET difference | 1.10 | 0.047 | 1.00 | 1.21 |
| Age | 1.01 | 0.423 | 0.98 | 1.04 |
| APACHE III | 1.02 | 0.093 | 1.00 | 1.04 |
| Vasopressor requirement | 1.30 | 0.623 | 0.45 | 3.84 |
Arterial-ET Difference arterial end-tidal CO2 difference, APACHE acute physiology and chronic health evaluation
Table 3.
Multivariable analysis of derivation cohort using simplified Enghoff equation: ARDS net database
| Mortality | Odds ratio | P value | [95% conf. interval] | |
|---|---|---|---|---|
| Simplified Enghoff equation | 1.05 | 0.024 | 1.00 | 1.10 |
| Age | 1.01 | 0.462 | 0.98 | 1.04 |
| APACHE III | 1.02 | 0.136 | 1.00 | 1.03 |
| Vasopressor requirement | 1.21 | 0.716 | 0.41 | 3.64 |
APACHE acute physiology and chronic health evaluation
In the University of Chicago validation cohort, five variables were associated with mortality by univariable analysis: arterial-ET difference, ventilator mode, vasopressor requirement, age, and APACHE II. By multivariable analysis, age, APACHE, vasopressor requirement, and the arterial-ET difference remained significant (Table 4). Similarly, replacing the arterial-ET difference with the simplified Enghoff equation, these variables, including estimation of deadspace, continued to be significant variables in the model (Table 5).
Table 4.
Multivariable analysis of validation cohort: University of Chicago
| Mortality | Odds ratio | P value | [95% conf. interval] | |
|---|---|---|---|---|
| Arterial-ET difference | 1.03 | 0.031 | 1.01 | 1.06 |
| Age | 1.02 | 0.012 | 1.01 | 1.04 |
| APACHE II | 1.07 | < 0.001 | 1.04 | 1.10 |
| Vasopressor requirement | 0.029 | |||
| Ventilator mode* | ||||
| Assist control | – | – | – | |
| APRV | 897,712.43 | 0.992 | 8.55 × 10–102–NA | |
| Pressure control | 8,997,732.21 | 0.984 | 6.72 × 10–49–NA | |
| Pressure support | 8,997,732.21 | 0.992 | 2.08 × 10−02–NA | |
| SIMV | 38.97 | 0.410 | 0.0193–2.75 | |
Arterial-ET Difference arterial end-tidal CO2 difference, APACHE acute physiology and chronic health evaluation
Table 5.
Multivariable analysis of derivation cohort using simplified Enghoff equation: University of Chicago
| Mortality | Odds ratio | P value | [95% conf. interval] | |
|---|---|---|---|---|
| Simplified Enghoff equation | 1.02 | 0.003 | 1.01 | 1.09 |
| Age | 1.02 | 0.017 | 1.00 | 1.04 |
| APACHE III | 1.07 | < 0.001 | 1.04 | 1.10 |
| Vasopressor Requirement | 1.81 | 0.044 | 1.02 | 3.22 |
| Ventilator mode* | ||||
| Assist control | – | – | – | |
| APRV | 9,517,519.45 | 0.992 | 4.8 × 10–104–NA | |
| Pressure control | 6,336,025.64 | 0.988 | 7.89 × 10–50–NA | |
| Pressure support | 1,046,002.43 | 0.992 | 6.3 × 10 −106–NA | |
| SIMV | 0.047 | 0.51 | 1.01–1.04 | |
APACHE acute physiology and chronic health evaluation
In the ARDS net cohort, the arterial-ET difference was significantly higher in non-survivors (median 10 mmHg, IQR 7–12) compared to those who survived (median 5 mmHg, IQR 2–11) by univariable analysis (P < 0.005). The same was seen in the University of Chicago cohort (median 15 mmHg, IQR 10–23 vs median 12 mmHg, IQR 8–17, P < 0.001) (Fig. 1). Importantly, the arterial-ET difference was independently associated with an increased risk of death in the multiple regression analysis in both cohorts using either arterial-ET difference or simplified Enghoff estimation of dead space. For every increase of 1 mmHg in the arterial-end tidal CO2 difference, the odds of death were 1.10 and 1.03 times higher in the ARDS Network and the University of Chicago cohorts, respectively (Tables 2, 4).
Fig. 1.
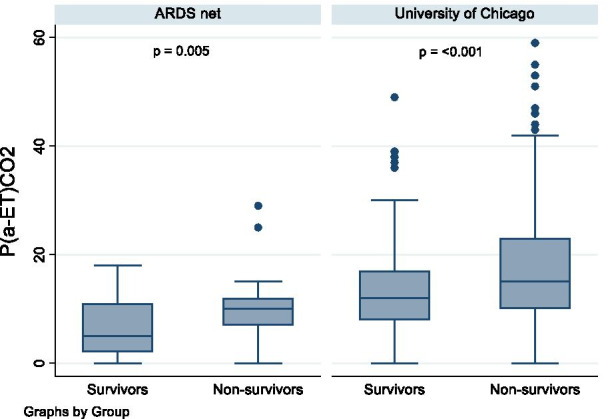
Box plot of arterial end-tidal CO2 difference stratified by mortality in the ARDS Net and University of Chicago cohorts
Discussion
ARDS is a common reason for critical illness and respiratory failure with high mortality [16], making routinely collected measurements that accurately predict outcomes of utmost importance. They may assist in clinical management and prognostication as well as stratification of patients enrolled in clinical trials. Several physiologic parameters available at the bedside have been described to serve these purposes including P/F ratio [4], oxygenation index [17], driving pressure [3], and dead space [5]. Dead space can be measured by the Bohr method [18], but this requires collection of expired gas and measurement of expired PCO2 and arterial PCO2. Here, we present that the estimation of dead space by either the arterial-ET difference or the simplified Enghoff modification equation is independently predictive of mortality in critically ill patients with ARDS.
A potential alternative to the direct measurement of dead space is the use of the arterial to end-tidal PCO2 difference, which theoretically should be a reliable estimate of dead space. To date, there are limited data supporting this inference. Very early studies by Severinghaus, et al. [19] and others [20, 21] showed that this difference tracked with changes in dead space and the degree of ventilation/perfusion (V/Q) mismatch in healthy animal models. Nunn et al. [22] obtained arterial-ET differences in 12 healthy anesthetized patients and proposed this measurement as the simplest method of demonstrating the existence of V/Q mismatch. Shetty et al. evaluated 215 patients presenting to the emergency department (ED) and the arterial-ET difference modestly predicted adverse outcomes in patients presenting with suspected sepsis due to non-respiratory causes. Those with normal arterial-ET differences were noted to have much lower risk for hospital mortality and prolonged ICU length of stay [23]. Yamanaka et al. [24] studied 17 patients requiring endotracheal intubation and mechanical ventilation using an average of exhaled PCO2 at the end of several breaths over a duration of 30 s and found that the difference between arterial and exhaled CO2 correlated closely with physiological dead space (r = 0.80, P < 0.05). Similarly, a prospective study in 106 trauma patients requiring emergency surgery noted that the arterial-ET difference was lower during all phases of surgery in survivors (5.8 ± 4.5 vs. 16.5 ± 14.7 mm Hg) (P < 0.001) [25]. In another study that included 412 patients presenting to the ED with shortness of breath, ETCO2 was measured with a sampling cannula. A difference > 10 mm Hg was strongly predictive of the need for positive pressure ventilation via face mask or endotracheal tube (AUC 0.91 [95% CI 0.87–0.94]) [7]. In our current study, high estimations of deadspace measured within the first 24 h of ARDS onset were significantly associated with in-hospital mortality. These statistically significant associations persisted with multiple cohorts and when using either the artieral-ET difference or simplified Enghoff equation. Accordingly, we believe that the arterial-ET difference and the simplified Enghoff equation can be useful for early prognostication in ARDS patients in a highly generalizable manner.
Our study has strengths and limitations. Our validation cohort was derived from a single-center analysis. Mortality in the University of Chicago cohort group was significantly higher than the ARDS Network group. This difference is likely explained by the University of Chicago cohort being significantly older and having dramatically worse gas exchange by P/F ratio. Both derivation and validation cohorts found the arterial-ET difference or simplified Enghoff equation to be an independent predictor of mortality, which speaks to the generalizability of our findings. This is a retrospective study with no precise timing between difference measurements. In order to minimize changes in the difference between arterial and end-tidal CO2, we required that the two measurements occurred no more than 1 h apart and with no modifications of ventilator settings. Given that arterial blood-gas and ETCO2 measurements are largely dependent on temporal hemodynamic and respiratory factors, our study is limited by potential disparities due to the rapid, time-dependent fluctuations of the measured variables. The use of volumetric capnometry may be a more accurate measurement than ETCO2, given that it allows the separation of physiologic dead space from apparent changes in dead space due to shunt and thus can give a more precise indication of physiological mechanism; however, it is often not practical in the ICU setting. Optimization of future investigation of the association between dead space fraction and mortality could include measuring the arterial blood gas and ETCO2 at the same time to reduce the risk of hemodynamic changes potentially skewing the data.
Conclusions
In summary, we identified the arterial-ET difference or the simplified Enghoff equation as independently associated with ARDS mortality. Bedside estimation of dead space early after the diagnosis of ARDS may provide useful prognostic information for ICU care providers.
Acknowledgements
Not applicable.
Authors' contributions
PL contributed to the conception, design of the work; the acquisition, analysis, interpretation of data; drafted the work, approved the submitted version and agreed to be personally accountable for the author’s own contributions. SP contributed to the analysis, interpretation of data; drafted the work, approved the submitted version and agreed to be personally accountable for the author's own contributions. CP contributed to the analysis, interpretation of data; drafted the work, approved the submitted version and agreed to be personally accountable for the author’s own contributions. MS contributed to the interpretation of data; drafted the work, approved the submitted version and agreed to be personally accountable for the author's own contributions. BP contributed to the acquisition, interpretation of data; drafted the work, approved the submitted version and agreed to be personally accountable for the author's own contributions. KW contributed to the analysis of data, drafted the work, approved the submitted version and agreed to be personally accountable for the author's own contributions. AP contributed to the acquisition of data, drafted the work, approved the submitted version and agreed to be personally accountable for the author's own contributions. JL contributed to the acquisition, analysis, interpretation of data; drafted the work, approved the submitted version and agreed to be personally accountable for the author’s own contributions. JH contributed to the acquisition, analysis, interpretation of data; drafted the work, approved the submitted version and agreed to be personally accountable for the author’s own contributions. YH contributed to the acquisition, analysis, interpretation of data; approved the submitted version and agreed to be personally accountable for the author’s own contributions. PB contributed to the analysis of data, drafted the work, approved the submitted version and agreed to be personally accountable for the author’s own contributions. SD contributed to the analysis, interpretation of data; drafted the work, approved the submitted version and agreed to be personally accountable for the author’s own contributions. JK contributed to the conception, design of the work; the acquisition, analysis, interpretation of data; drafted the work, approved the submitted version and agreed to be personally accountable for the author’s own contributions. All authors read and approved the final manuscript.
Funding
Research supported by NIH 5T-32 HL 007605-36 and NIH/NHLBI K23 HL14 8387.
Availability of data and materials
The datasets generated and analyzed for the derivation cohort during the current study are available in the ARDS Network Public Database repository, [https://biolincc.nhlbi.nih.gov]. The datasets used and analyzed during the current study for the validation cohort are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the University of Chicago Institutional Review Board (IRB) with waiver of consent for this de-identified retrospective analysis.
Consent for publications
The study was approved by the University of Chicago Institutional Review Board (IRB) with a waiver of consent for this de-identified retrospective analysis.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rezoagli E, Fumagalli R, Bellani G. Definition and epidemiology of acute respiratory distress syndrome. Ann Transl Med. 2017;5(14):282. doi: 10.21037/atm.2017.06.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Network ARDS, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 3.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372(8):747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 4.Force ADT, Ranieri V, Rubenfeld G, Thompson B, Ferguson N, Caldwell E. Acute respiratory distress syndrome. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 5.Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet J-F, Eisner MD, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346(17):1281–1286. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 6.Siobal MS. Monitoring exhaled carbon dioxide. Respir Care. 2016;61(10):1397–1416. doi: 10.4187/respcare.04919. [DOI] [PubMed] [Google Scholar]
- 7.Shetty AL, Lai KH, Byth K. The CO2 GAP Project–CO2 GAP as a prognostic tool in emergency departments. Emerg Med Australas. 2010;22(6):524–531. doi: 10.1111/j.1742-6723.2010.01349.x. [DOI] [PubMed] [Google Scholar]
- 8.Radermacher P, Maggiore SM, Mercat A. Fifty years of research in ARDS. Gas exchange in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;196(8):964–984. doi: 10.1164/rccm.201610-2156SO. [DOI] [PubMed] [Google Scholar]
- 9.Ferluga M, Lucangelo U, Blanch L. Dead space in acute respiratory distress syndrome. Ann Transl Med. 2018;6(19):388. doi: 10.21037/atm.2018.09.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jardin F, Vieillard-Baron A. Right ventricular function and positive pressure ventilation in clinical practice: from hemodynamic subsets to respirator settings. Intensive Care Med 2003;29(9):1426–34. [DOI] [PubMed]
- 11.Rice TW, Wheeler AP, Thompson BT, DeBoisblanc BP, Steingrub J, Rock P. Enteral omega-3 fatty acid, γ-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306(14):1574–1581. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Heart L, Network BIARDSCT. Randomized, placebo-controlled clinical trial of an aerosolized β2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184(5):561–568. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Heart L, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N. Rice TW, Wheeler AP, Thompson BT, Steingrub J, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307(8):795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poorolajal J, Cheraghi Z, Irani AD, Rezaeian S. Quality of cohort studies reporting post the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. Epidemiol Health. 2011;33:e2011005. doi: 10.4178/epih/e2011005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X. Applied ordinal logistic regression using Stata: from single-level to multilevel modeling. Thousand Oaks: Sage Publications; 2015. [Google Scholar]
- 16.Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5(1):1–22. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seeley E, McAuley DF, Eisner M, Miletin M, Matthay MA, Kallet RH. Predictors of mortality in acute lung injury during the era of lung protective ventilation. Thorax. 2008;63(11):994–998. doi: 10.1136/thx.2007.093658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bohr C. Ueber die lungenathmung. Skand Arch Physiol. 1891;2(236):68. [Google Scholar]
- 19.Severinghaus J, Stupfel M. Alveolar dead space as an index of distribution of blood flow in pulmonary capillaries. J Appl Physiol. 1957;10(3):335–348. doi: 10.1152/jappl.1957.10.3.335. [DOI] [PubMed] [Google Scholar]
- 20.Mosing M, Böhm SH, Rasis A, Hoosgood G, Auer U, Tusman G, et al. Physiologic factors influencing the arterial-to-end-tidal CO2 difference and the alveolar dead space fraction in spontaneously breathing anesthetised horses. Front Vet Sci. 2018;5:58. doi: 10.3389/fvets.2018.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer RE, Short CE. Arterial to end-tidal CO2 tension and alveolar dead space in halothane-or isoflurane-anesthetized ponies. Am J Vet Res. 1985;46(3):597–599. [PubMed] [Google Scholar]
- 22.Nunn J, Hill D. Respiratory dead space and arterial to end-tidal CO2 tension difference in anesthetized man. J Appl Physiol. 1960;15(3):383–389. doi: 10.1152/jappl.1960.15.3.383. [DOI] [PubMed] [Google Scholar]
- 23.Shetty A, Sparenberg S, Adams K, Selvedran S, Tang B, Hanna K, et al. Arterial to end-tidal carbon dioxide tension difference (CO2 gap) as a prognostic marker for adverse outcomes in emergency department patients presenting with suspected sepsis. Emerg Med Australas. 2018;30(6):794–801. doi: 10.1111/1742-6723.13095. [DOI] [PubMed] [Google Scholar]
- 24.Yamanaka MK, Sue DY. Comparison of arterial-end-tidal PCO2 difference and dead space/tidal volume ratio in respiratory failure. Chest. 1987;92(5):832–835. doi: 10.1378/chest.92.5.832. [DOI] [PubMed] [Google Scholar]
- 25.Tyburski JG, Collinge JD, Wilson RF, Carlin AM, Albaran RG, Steffes CP. End-tidal CO2-derived values during emergency trauma surgery correlated with outcome: a prospective study. J Trauma Acute Care Surg. 2002;53(4):738–743. doi: 10.1097/00005373-200210000-00020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed for the derivation cohort during the current study are available in the ARDS Network Public Database repository, [https://biolincc.nhlbi.nih.gov]. The datasets used and analyzed during the current study for the validation cohort are available from the corresponding author on reasonable request.


