Abstract
Background:
With the knowledge of oligometastases, primary surgery plays an increasingly vital role in metastatic non-small cell lung cancer. We aimed to evaluate the survival benefit of primary surgery based on metastatic patterns.
Materials and Methods:
The selected patients with stage IV extrathoracic metastatic (m1b) non-small cell lung cancer between 2010 and 2015 were included in a retrospective cohort study from the Surveillance, Epidemiology, and End Results (SEER) database. Multiple imputation was used for the missing data. Patients were divided into 2 groups depending on whether surgery was performed. After covariate balancing propensity score (CBPS) weighting, multivariate Cox regression models and Kaplan-Meier survival curve were built to identify the survival benefit of different metastatic patterns.
Results:
Surgery can potentially increase the overall survival (OS) (adjusted HR: 0.68, P < 0.001) of non-small cell lung cancer. The weighted 3-year OS in the surgical group was 16.9%, compared with 7.8% in the nonsurgical group. For single organ metastasis, surgery could improve the survival of metastatic non-small cell lung cancer. Meanwhile, no significant survival improvements in surgical group were observed in patients with multiple organ metastases.
Conclusion:
The surgical survival benefits for extrathoracic metastatic non-small cell lung cancer could be divided by metastatic pattern.
Keywords: non-small cell lung cancer, extrathoracic metastases, primary surgery, metastatic patterns, survival benefit
Introduction
Based on the Surveillance, Epidemiology, and End Results (SEER) database, 57% patients were diagnosed with metastatic disease.1,2 According to traditional therapeutic notions, surgery is not considered to be suitable for metastatic non-small cell lung cancer (NSCLC) over chemoradiotherapy. With the knowledge of the tumor environment and biology, targeted therapies and immunotherapy have made a breakthrough.3 In the latest National Comprehensive Cancer Network (NCCN) guidelines, the systemic treatment of metastatic NSCLC are prescribed according to mutational and biological characteristics.2 However, targeted therapies and immunotherapy may not be suitable for everyone due to the low mutation positive rate of targeted gene. Moreover, some people don’t benefit from the targeted therapy and drug resistance is also the nonnegligible weakness of targeted therapy and immunotherapy. And, local consolidative therapy prolonged the OS of patients with metastatic NSCLC who did not benefit from front-line systemic therapy.4
Recently, oligometastatic NSCLC has gradually entered the field of vision of many clinicians and has been enlarged to a maximum of 5 metastases and 3 organs. Moreover, a radical form of treatment may reduce the tumor burden and improve the disease course.5,6 Therefore, primary surgery, as a main method of local treatment, plays an increasingly vital role in oligometastatic NSCLC. In addition, the tumor resection of primary sites may benefit patients with metastatic NSCLC, especially single organ metastasis in some retrospective cohort studies.7-11 However, inconsistent results emerged in controversial studies due to the small number of people included in the metastatic NSCLC study.7,12 The age, regional lymph node status, tumor size, and histology type of patients are some important indicators; however, metastatic sites are often overlooked in surgical decision-making. While some studies have suggested that the location of metastases can predict different outcomes.13-15 Although Sun et al confirmed that local surgery benefited patients with metastatic NSCLC, regardless of whether there were single or multiple metastases, distant lymph nodes were ignored and an excessive number of cases were excluded. In addition, multiple metastatic patterns are difficult to analyze.7 Therefore, we aimed to evaluate the survival benefit of primary surgery using metastatic patterns in this real-world observational study.
Materials and Methods
Patient Population
We selected potential patients who might be included in a retrospective cohort study from an incidence-SEER 18 population-based registries comprising approximately 28% of the US population using the National Cancer Institute SEER*Stat software version 8.3.6 (seer.cancer.gov/seerstat). A total of 82,506 patients who were first primarily diagnosed with malignant tumors of the lung and bronchus with M1b stage according to the third edition of the International Classification of Diseases for Oncology (ICD-O-3) and the seventh edition of TNM staging of NSCLC between 2010 and 2015 were screened. We excluded patients who were aged younger than 18 years or only diagnosed by autopsy or death certification. In addition, patients who received surgery for metastatic or unknown sites, as well as patients with unknown metastatic sites were excluded. Patients with unknown treatment, including primary surgery and radiation, were necessarily excluded. Moreover, cases with T0, TX, NX, or 0 survival months were excluded. Based on the ICD-O-3 histology codes, the histological subtype was classified as adenocarcinoma (8140, 8144, 8230, 8250 to 8255, 8260, 8310, 8323, 8480, 8481, 8490, and 8550), squamous cell carcinoma (8052, 8070 to 8075, 8083, and 8084), larger cell carcinoma (8012 to 8014, 8082, and 8123), and adenosquamous carcinoma (8560). Finally, a total of 33,612 patients were included in the cohort study, and 1,074 patients underwent primary surgery (code 12-90). Because the data was derived from the SEER database, it was unnecessary to obtain patient consent.
Patient identification, age of diagnosis, race/ethnicity, marital status, primary site, distant metastatic site, T stage, N stage, histology type, nuclear grade, surgery, chemotherapy, radiation therapy, cause-specific death classification, vital status, and survival month were collected from the SEER database. Metastatic patterns were divided into single organ and multiple metastasis. Single organ metastasis was further classified into distant lymph node-only, bone-only, liver-only, brain-only, and other-only (excepting bone, brain, liver, and distant lymph node) metastasis. Multiple metastases were further divided according to the analysis results. The overall survival (OS) and lung cancer specific survival (LCSS) were considered to be the main endpoints of this study.
Statistical Analysis
A Pearson’s chi-square and t-test were respectively used to compare the baseline data and clinical characteristics of patients between the surgery and non-surgery groups according to the data types and structures. The R package “effectsize” was used to calculate the effect size.16 There were some unknown data in Race, Marital, Grade variables (Table S2). Multiple imputation was conducted to deal with the missing data by building a polytomous regression model based on patient age (continuous), race (white, black, or other), sex, marital status (single, married, and other), year of diagnose, nuclear grade (I, II, III, and IV), histology type, and primary site using “mice” R package.17 In addition, the procedure was repeated for 10 cycles to produce a final data set (Table S3).18 For further analysis, patient age was classified in 5-year age ranges; however, everything else remained the same.
To balance the clinicopathological characteristics between 2 groups, the covariate balancing propensity score (CBPS) weighting was performed using the “CBPS” R package.19 We calculated the propensity scores using covariate-balancing propensity scores including the following variables: patient age, race, sex, marital status, year of diagnose, primary site, nuclear grade, histology type, T stage, N stage, radiation therapy, chemotherapy, and metastatic pattern. After CBPS, Log-rank test and Cox proportional hazards models were performed to evaluate the hazard ratios (HRs) with 95% confidence interval (CI) of OS (LCSS) between the surgery and non-surgery groups. Multivariate Cox regression models were fit adjusting for patient age, race, sex, marital status, year of diagnose, primary site, nuclear grade, histology type, T stage, N stage, radiation therapy, and chemotherapy and was weighted according to CBPS. After making interaction tests, similar methods were conducted to further analyze the survival benefit of different subgroups. Moreover, we used random survival forest methodology instead of multiple imputation to access the consistency of the results. All statistical analyses were performed using R software (version 3.6.3) with 2-sided testing and P < 0.05 was considered significant.
Results
Patients Characteristics
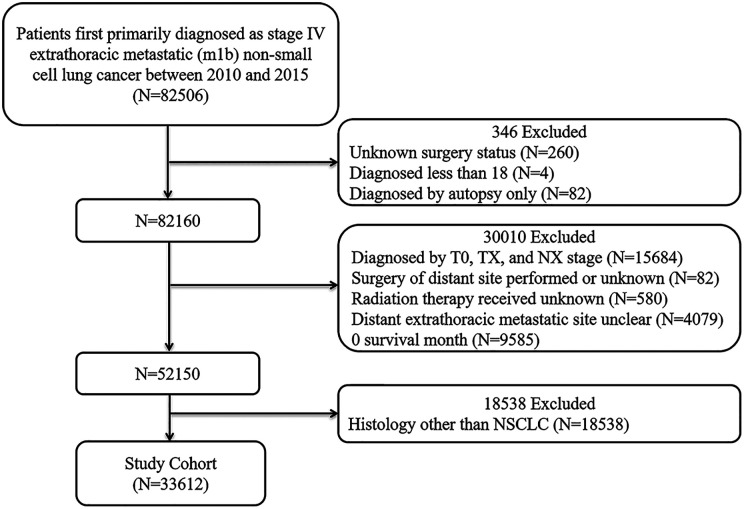
A total of 33,612 patients with stage IV extrathoracic metastases were included in our study based on exclusion and inclusion criteria (Figure 1). Table 1 shows the comparison of the clinicopathological characteristics of 1,074 patients who underwent surgery and 32,538 patients who did not undergo surgery. As shown in Table 2, the patient clinicopathological characteristics were adequately balanced after multiple imputation and propensity score adjustments were performed to estimate the average treatment effect. The standard mean difference (SMD) was all less than 0.1, which indicated that the baseline data of the 2 groups were adequately balanced. As shown in Table 1, all of clinicopathological characteristics were statistically associated with surgical selection, except for sex. Compared with the non-surgery group, more individuals who underwent surgery were white, had an earlier diagnosis, were younger, or married. In addition, patients with upper lobe lesions, patients with a higher nuclear grade, patients with an earlier T stage, patients with an earlier N stage, and patients with large cell carcinoma or adenosquamous carcinoma had access to receiving surgery. In which, patients with higher nuclear grade and earlier N stage were more likely to undergo surgery.
Figure 1.
Flow diagram of patient population.
Table 1.
Clinicopathologic Characteristics of Patients by Receipt of Primary Surgery.
| Characteristic | No. of patients (%) | P-value | Effect size | |
|---|---|---|---|---|
| Non-surgery group (N = 32538) | Surgery group (N = 1074) | |||
| Year of diagnosis | <0.001 | 0.03 | ||
| 2010 | 4892 (15.0) | 199 (18.5) | ||
| 2011 | 4753 (14.6) | 190 (17.7) | ||
| 2012 | 5336 (16.4) | 175 (16.3) | ||
| 2013 | 5710 (17.5) | 163 (15.2) | ||
| 2014 | 5862 (18.0) | 177 (16.5) | ||
| 2015 | 5985 (18.4) | 170 (15.8) | ||
| Sex | 0.577 | - | ||
| Male | 17958 (55.2) | 583 (54.3) | ||
| Female | 14580 (44.8) | 491 (45.7) | ||
| Age, years | <0.001 | 0.06 | ||
| 18-49 | 2115 (6.5) | 113 (10.5) | ||
| 50-54 | 2940 (9.0) | 109 (10.1) | ||
| 55-59 | 4390 (13.5) | 191 (17.8) | ||
| 60-64 | 5200 (16.0) | 187 (17.4) | ||
| 65-69 | 5666 (17.4) | 197 (18.3) | ||
| 70-74 | 4848 (14.9) | 130 (12.1) | ||
| 75-79 | 3754 (11.5) | 107 (10.0) | ||
| 80+ | 3625 (11.1) | 40 (3.7) | ||
| Race | 0.017 | 0.02 | ||
| White | 24975 (76.8) | 864 (80.4) | ||
| Black | 4332 (13.3) | 125 (11.6) | ||
| Othera | 3183 (9.8) | 82 (7.6) | ||
| Unknown | 48 (0.1) | 3 (0.3) | ||
| Marital | <0.001 | 0.02 | ||
| Single | 5217 (16.0) | 172 (16.0) | ||
| Married | 17088 (52.5) | 629 (58.6) | ||
| Otherb | 8935 (27.5) | 231 (21.5) | ||
| Unknown | 1298 (4.0) | 42 (3.9) | ||
| Site | 0.01 | 0.02 | ||
| Lower lobe, lung | 8551 (26.3) | 286 (26.6) | ||
| Lung, NOS | 3140 (9.7) | 71 (6.6) | ||
| Main bronchus | 1531 (4.7) | 50 (4.7) | ||
| Middle lobe, lung | 1352 (4.2) | 49 (4.6) | ||
| Overlapping lesion of lung | 314 (1.0) | 17 (1.6) | ||
| Upper lobe, lung | 17650 (54.2) | 601 (56.0) | ||
| Grade | <0.001 | 0.14 | ||
| Ⅰ | 644 (2.0) | 48 (4.5) | ||
| Ⅱ | 3919 (12.0) | 312 (29.1) | ||
| Ⅲ | 8432 (25.9) | 474 (44.1) | ||
| Ⅳ | 306 (0.9) | 21 (2.0) | ||
| Unknown | 19237 (59.1) | 219 (20.4) | ||
| Histology | <0.001 | 0.03 | ||
| Adenocarcinoma | 23935 (73.6) | 776 (72.3) | ||
| Squamous cell carcinoma | 7112 (21.9) | 212 (19.7) | ||
| Larger cell carcinoma | 907 (2.8) | 48 (4.5) | ||
| Adenosquamous carcinoma | 584 (1.8) | 38 (3.5) | ||
| T stage | <0.001 | 0.04 | ||
| T1 | 4014 (12.3) | 189 (17.6) | ||
| T2 | 9196 (28.3) | 363 (33.8) | ||
| T3 | 8567 (26.3) | 270 (25.1) | ||
| T4 | 10761 (33.1) | 252 (23.5) | ||
| N stage | <0.001 | 0.11 | ||
| N0 | 6776 (20.8) | 467 (43.5) | ||
| N1 | 2615 (8.0) | 146 (13.6) | ||
| N2 | 15250 (46.9) | 349 (32.5) | ||
| N3 | 7897 (24.3) | 112 (10.4) | ||
| Radiation therapy | 0.867 | - | ||
| No | 13595 (41.8) | 452 (42.1) | ||
| Yes | 18943 (58.2) | 622 (57.9) | ||
| Chemotherapy | 0.191 | - | ||
| No | 11738 (36.1) | 366 (34.1) | ||
| Yes | 20800 (63.9) | 708 (65.9) | ||
| Distant metastatic site | ||||
| Distant lymph nodes | <0.001 | 0.03 | ||
| No | 26602 (81.8) | 956 (89.0) | ||
| Yes | 5936 (18.2) | 118 (11.0) | ||
| Bone | <0.001 | 0.08 | ||
| No | 15344 (47.2) | 762 (70.9) | ||
| Yes | 17194 (52.8) | 312 (29.1) | ||
| Brain | <0.001 | 0.03 | ||
| No | 20721 (63.7) | 590 (54.9) | ||
| Yes | 11817 (36.3) | 484 (45.1) | ||
| Liver | <0.001 | 0.05 | ||
| No | 25444 (78.2) | 962 (89.6) | ||
| Yes | 7094 (21.8) | 112 (10.4) | ||
a American Indian/AK Native, Asian/Pacific Islander.
b Widowed/Divorced/Separated/Unmarried or Domestic Partner.
Table 2.
Clinicopathologic Characteristics of Patients After the Covariate Balancing Propensity Score Weighting.
| Characteristic | No. of patients (%) | SMD | |
|---|---|---|---|
| Non-surgery group (N = 32538) | Surgery group (N = 1074) | ||
| Year of diagnosis | 0.067 | ||
| 2010 | 4925 (15.1) | 166 (15.5) | |
| 2011 | 4785 (14.7) | 163 (15.1) | |
| 2012 | 5331 (16.4) | 159 (14.8) | |
| 2013 | 5688 (17.5) | 184 (17.2) | |
| 2014 | 5839 (17.9) | 183 (17) | |
| 2015 | 5971 (18.3) | 219 (20.4) | |
| Sex | 0.046 | ||
| Male | 17946 (55.2) | 568 (52.9) | |
| Female | 14592 (44.8) | 506 (47.1) | |
| Age, years | 0.068 | ||
| 18-49 | 2156 (6.6) | 75 (7.0) | |
| 50-54 | 2948 (9.1) | 87 (8.1) | |
| 55-59 | 4435 (13.6) | 151 (14.1) | |
| 60-64 | 5209 (16.0) | 161 (15.0) | |
| 65-69 | 5676 (17.4) | 189 (17.6) | |
| 70-74 | 4826 (14.8) | 179 (16.7) | |
| 75-79 | 3738 (11.5) | 119 (11.1) | |
| 80+ | 3550 (10.9) | 112 (10.4) | |
| Race | 0.044 | ||
| White | 25039 (77.0) | 808 (75.2) | |
| Black | 4331 (13.3) | 158 (14.7) | |
| Othera | 3168 (9.7) | 108 (10.1) | |
| Marital | 0.021 | ||
| Single | 5462 (16.8) | 172 (16) | |
| Married | 17837 (54.8) | 596 (55.5) | |
| Otherb | 9239 (28.4) | 306 (28.5) | |
| Site | 0.064 | ||
| Lower lobe, lung | 8558 (26.3) | 273 (25.4) | |
| Lung, NOS | 3115 (9.6) | 107 (10.0) | |
| Main bronchus | 1538 (4.7) | 65 (6.1) | |
| Middle lobe, lung | 1357 (4.2) | 45 (4.2) | |
| Overlapping lesion of lung | 319 (1.0) | 10 (1.0) | |
| Upper lobe, lung | 17651 (54.2) | 573 (53.3) | |
| Grade | 0.047 | ||
| Ⅰ | 1650 (5.1) | 59 (5.5) | |
| Ⅱ | 9690 (29.8) | 300 (27.9) | |
| Ⅲ | 20448 (62.8) | 693 (64.5) | |
| Ⅳ | 750 (2.3) | 23 (2.1) | |
| Histology | 0.045 | ||
| Adenocarcinoma | 23916 (73.5) | 773 (71.9) | |
| Squamous cell carcinoma | 7101 (21.8) | 253 (23.6) | |
| Larger cell carcinoma | 923 (2.8) | 31 (2.9) | |
| Adenosquamous carcinoma | 598 (1.8) | 17 (1.6) | |
| T stage | 0.042 | ||
| T1 | 4062 (12.5) | 126 (11.7) | |
| T2 | 9245 (28.4) | 291 (27.1) | |
| T3 | 8555 (26.3) | 289 (27) | |
| T4 | 10676 (32.8) | 367 (34.2) | |
| N stage | 0.059 | ||
| N0 | 6992 (21.5) | 209 (19.4) | |
| N1 | 2670 (8.2) | 89 (8.3) | |
| N2 | 15111 (46.4) | 500 (46.6) | |
| N3 | 7765 (23.9) | 277 (25.8) | |
| Radiation therapy | 0.023 | ||
| No | 13596 (41.8) | 461 (42.9) | |
| Yes | 18942 (58.2) | 613 (57.1) | |
| Chemotherapy | 0.012 | ||
| No | 11708 (36.0) | 375 (35.4) | |
| Yes | 20830 (64.0) | 694 (64.6) | |
| Distant metastatic site | |||
| Distant lymph nodes | 0.005 | ||
| No | 26672 (82.0) | 878 (81.8) | |
| Yes | 5866 (18.0) | 196 (18.2) | |
| Bone | 0.023 | ||
| No | 15573 (47.9) | 502 (46.7) | |
| Yes | 16965 (52.1) | 572 (53.3) | |
| Brain | 0.043 | ||
| No | 20639 (63.4) | 703 (65.5) | |
| Yes | 11899 (36.6) | 371 (34.5) | |
| Liver | 0.011 | ||
| No | 25552 (78.5) | 848 (79.0) | |
| Yes | 6986 (21.5) | 226 (21.0) | |
Abbreviation: SMD, standard mean difference.
a American Indian/AK Native, Asian/Pacific Islander.
b Widowed/Divorced/Separated/Unmarried or Domestic Partner.
Distant Metastatic Site
As shown in Figure S1, bone metastasis was the most common metastatic site, followed by brain metastasis, liver metastasis, and distant lymph node metastasis. The metastatic patterns before and after CBPS between the non-surgery and surgery group is shown in Table 3. Patients with single organ metastasis had an inevitable selection bias for receiving surgery. There were 947 out of 23,479 patients who had undergone surgery with single metastasis. Brain only metastasis was more likely to receive the primary surgery. Simultaneous bone and brain metastases had access to undergo surgery in cases of multiple metastases.
Table 3.
Patterns of Distant Metastases of Patient Cohort Before and After the Covariate Balancing Propensity Score Weighting.
| Metastatic pattern | No. of patients before CBPS (%) | No. of patients after CBPS (%) | ||||
|---|---|---|---|---|---|---|
| Total | Non-surgery | Surgery | Total | Non-surgery | Surgery | |
| One site of distant metastasis | ||||||
| Liver | 2025 (6.0) | 1965 (6.0) | 60 (5.6) | 2033 (6.0) | 1963 (6.0) | 70 (6.5) |
| Distant lymph node | 2453 (7.3) | 2379 (7.3) | 74 (6.9) | 2459 (7.3) | 2376 (7.3) | 83 (7.8) |
| Othera | 3416 (10.2) | 3217 (9.9) | 199 (18.5) | 3400 (10.1) | 3298 (10.1) | 102 (9.5) |
| Bone | 9037 (26.9) | 8830 (27.1) | 207 (19.3) | 9065 (27.0) | 8755 (26.9) | 310 (28.9) |
| Brain | 6548 (19.5) | 6141 (18.9) | 407 (37.9) | 6520 (19.4) | 6324 (19.4) | 196 (18.2) |
| Two sites of distant metastasis | ||||||
| Liver + Distant lymph node | 338 (1.0) | 334 (1.0) | 4 (0.4) | 342 (1.0) | 328 (1.0) | 14 (1.3) |
| Brain + Liver | 580 (1.7) | 573 (1.8) | 7 (0.7) | 577 (1.7) | 562 (1.7) | 15 (1.4) |
| Brain + Distant lymph node | 650 (1.9) | 640 (2.0) | 10 (0.9) | 648 (1.9) | 629 (1.9) | 19 (1.8) |
| Bone + Distant lymph node | 1212 (3.6) | 1196 (3.7) | 16 (1.5) | 1213 (3.6) | 1174 (3.6) | 39 (3.7) |
| Bone + Liver | 2284 (6.8) | 2260 (6.9) | 24 (2.2) | 2283 (6.8) | 2214 (6.8) | 68 (6.3) |
| Bone + Brain | 2594 (7.7) | 2548 (7.8) | 46 (4.3) | 2598 (7.7) | 2514 (7.7) | 84 (7.8) |
| Three sites of distant metastases | ||||||
| Brain + Liver + Distant lymph node | 96 (0.3) | 95 (0.3) | 1 (0.1) | 96 (0.3) | 93 (0.3) | 3 (0.3) |
| Bone + Brain + Distant lymph node | 496 (1.5) | 493 (1.5) | 3 (0.3) | 495 (1.5) | 481 (1.5) | 14 (1.3) |
| Bone + Brain + Liver | 1074 (3.2) | 1068 (3.3) | 6 (0.5) | 1075 (3.2) | 1042 (3.2) | 33 (3.1) |
| Bone + Liver + Distant lymph node | 546 (1.6) | 540 (1.7) | 6 (0.5) | 545 (1.6) | 529 (1.6) | 16 (1.5) |
| Four sites of distant metastases | 263 (0.8) | 259 (0.8) | 4 (0.4) | 262 (0.8) | 255 (0.8) | 7 (0.7) |
Abbreviation: CBPS, covariate balancing propensity score weighting.
a Distant metastasis except for bone, brain, liver, and distant lymph node.
Survival Benefit of Surgery Compared With the Non-Surgery Group
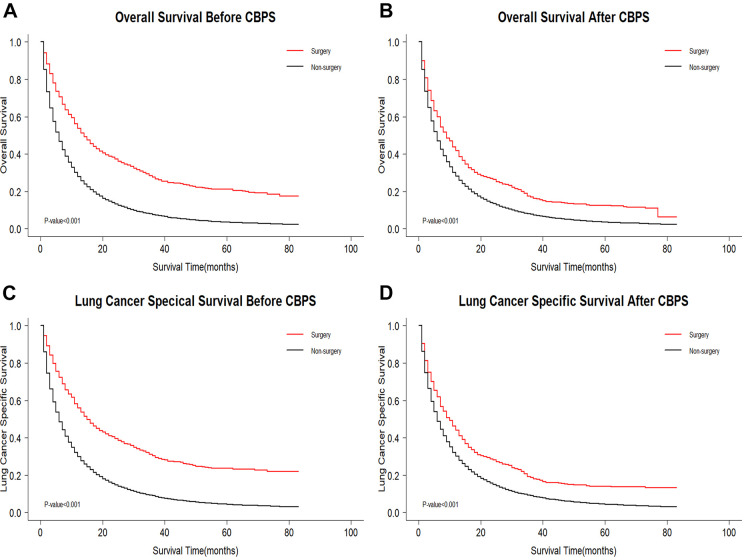
The median survival time of the surgery group was 14 months compared with the non-surgery group at 6 months (Log-rank test: P OS < 0.001 and P LCSS < 0.001). We found that there was obvious statistical significance of the OS (LCSS) between the surgery and the non-surgery group according to the Log-rank test (Figure 2) and the Cox regression hazard model adjusted by patient age, race, sex, marital status, year of diagnosis, primary site, nuclear grade, histology type, T stage, N stage, radiation therapy, chemotherapy, and metastatic pattern (OS: adjusted HR: 0.68; 95% CI, 0.67 to 0.70, P < 0.001; LCSS: adjusted HR 0.68; 95% CI, 0.67 to 0.70, P < 0.001). The weighted 3-year OS rate of the surgery and non-surgery group was 16.9% and 7.8%, respectively. Moreover, early mortality rate of the postoperative patients was significantly lower than that of non-operative patients (Table S1). Interaction testing was performed using a likelihood ratio test, which confirmed that the metastatic site discriminates the survival benefit of primary surgery (P < 0.001). We then further analyzed the survival benefit of primary surgery variation across the subgroups for single organ metastasis and multiple organ metastases.
Figure 2.
Kaplan-Meier survival curves comparing OS/LCSS between surgery group and non-surgery group. (A and C) KM curves for overall survival (OS) (P < 0.001) and lung cancer specific survival (LCSS) (P < 0.001) before CBPS; (B and D) KM curves for overall survival (OS) (P < 0.001) and lung cancer specific survival (LCSS) (P < 0.001) after CBPS. OS indicates overall survival; LCSS, lung cancer specific survival; CBPS, covariate balancing propensity score weighting.
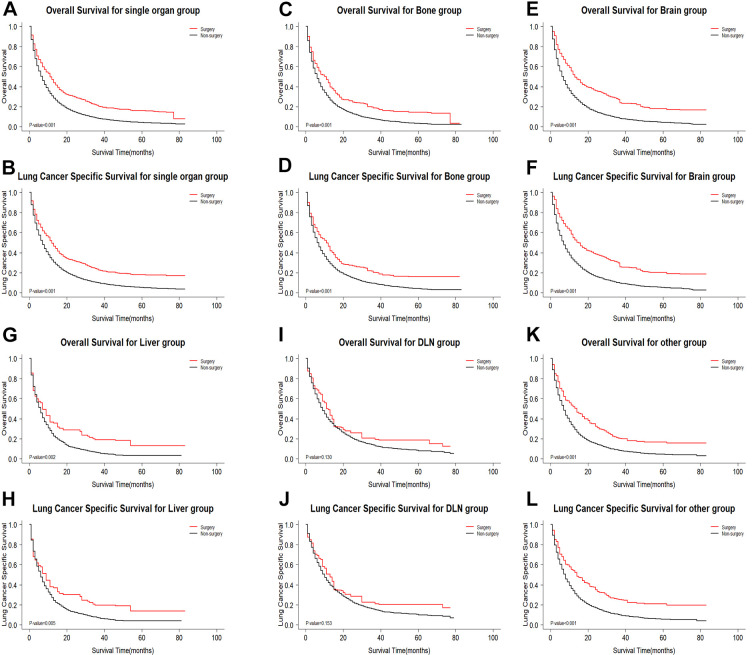
Single Organ Metastasis
There were 23,479 patients with single organ metastasis of NSCLC, for which there were 9,037 patients with only bone metastasis, 6,548 patients with only brain metastasis, 2,453 patients with distant lymph node metastasis, 2,025 patients with only liver metastasis, 3,416 patients with only other (except bone, brain, liver, and distant lymph node) metastases. First, primary surgery could benefit patients with single organ metastasis of NSCLC (OS: weighted 3-year rate difference: 12.6%, adjusted Log-rank test: P < 0.001; LCSS: weighted 3-year rate difference: 13.3%, adjusted Log-rank test: P < 0.001; Figure 3A and B). Then, the degree of survival benefit for receiving surgery was associated with the metastatic site (interaction testing: P OS < 0.001 and P LCSS < 0.001). We found that patients with brain, bone, liver, distant lymph node, and other metastases were demonstrated to have a survival benefit from primary surgery, among which brain metastasis benefit the most (OS: weighted 3-year rate difference: 18.5%, adjusted Log-rank test: P < 0.001; LCSS: weighted 3-year rate difference: 19.5%, adjusted Log-rank test: P < 0.001, Figure 3E and F), followed by other metastasis (OS: weighted 3-year rate difference: 11.9%, adjusted Log-rank test: P < 0.001; LCSS: weighted 3-year rate difference: 15.3%, adjusted Log-rank test: P < 0.001, Figure 3K and L), bone metastasis (OS: weighted 3-year rate difference: 10.8%, adjusted Log-rank test: P < 0.001; LCSS: weighted 3-year rate difference: 10.9%, adjusted Log-rank test: P < 0.001, Figure 3C and D), liver metastases (OS: weighted 3-year rate difference: 12.8%, adjusted Log-rank test: P = 0.002; LCSS: weighted 3-year rate difference: 12.4%, adjusted Log-rank test P = 0.005, Figure 3G and H), and distant lymph node metastasis have no a survival benefit from primary surgery (OS: weighted 3-year rate difference: 6.3%, adjusted Log-rank test: P = 0.130; LCSS: weighted 3-year rate difference: 6.1%, adjusted Log-rank test: P = 0.153, Figure 3I and J).
Figure 3.
Kaplan-Meier survival curves comparing OS/LCSS between surgery group and non-surgery group in single organ metastasis subgroups after CBPS. (A and B) Single organ metastasis group; (C and D) bone metastasis group; (E and F) brain metastasis group; (G and H) liver metastasis group; (I and J) distant lymph node metastasis group; (K and L) other site metastasis group. OS indicates overall survival; LCSS, lung cancer specific survival; CBPS, covariate balancing propensity score weighting; DLN, distant lymph node.
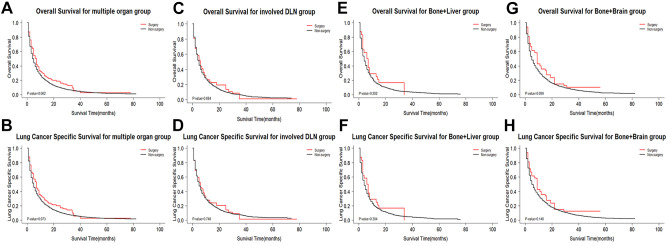
Multiple Organ Metastases
Patients with multiple organ metastases were demonstrated to not benefit from primary surgery (OS: weighted 3-year rate difference: −0.5%, adjusted Log-rank test: P = 0.062; LCSS: weighted 3 year rate difference: −0.8%, adjusted Log-rank test: P = 0.073; Figure 4A and B). Due to the negative weighted 3-year rate difference, it was necessary to further analyze the survival benefit from primary surgery in the different subgroups. In single organ metastasis, patients with distant lymph node metastasis did not benefit from surgery, thus multiple organ metastatic patients with involved distant lymph node metastases were divided into a group. According to the results shown in Table S4, we divided multiple organ metastases into 5 groups: distant lymph node ± liver ± bone ± brain, liver + bone + brain, liver + brain, liver + bone, and bone + brain. Since less patient cases in surgery group, we only further analyses 3 subgroups. As shown in Figure 4, the survival benefit from primary surgery was no significant for patients with multiple organ metastases. In the sensitivity analysis, random survival forest methodology instead of multiple imputation did not change our findings.
Figure 4.
Kaplan-Meier survival curves comparing OS/LCSS between surgery group and non-surgery group in multiple organ metastases subgroups after CBPS. (A and B) Multiple organ metastases group; (C and D) involved distant lymph node metastases group; (E and F) bone and liver metastases group; (G and H) bone and brain metastases group. OS indicates overall survival; LCSS, lung cancer specific survival; CBPS, covariate balancing propensity score weighting; DLN, distant lymph node.
Discussion
This is the first large population-based retrospective cohort study to distinguish the survival benefit of primary surgery based on metastatic patterns for patients with stage IV extrathoracic metastatic NSCLC. Moreover, our observation that local primary surgery could benefit patients with metastatic NSCLC is consistent with prior studies.7,20,21 Furthermore, patients with single organ metastasis (bone, brain, and liver) experienced a substantially improved survival benefit from primary surgery. In multiple organ metastasis, primary surgery increased the risk for patients with a high tumor burden. Our findings suggested that metastatic patterns distinguish the survival benefit of primary surgery for stage IV extrathoracic metastatic NSCLC.
Metastatic NSCLC has been widely studied and it has recently been confirmed that different metastatic sites can predict different clinical prognostic values under the background of big data.14,22 It has been well-established that the bone is the most common metastatic site of NSCLC, followed by the lung, brain, liver, and adrenal glands, with the best prognosis associated with brain-only metastasis.22 Surgical treatment initially focused on patients with synchronous solitary brain or adrenal metastasis and improved the clinical outcome.23 Recently, Takahashi and colleagues found that primary lung cancer resection improved the survival rates of patients with synchronous isolated bone metastasis.24 The above research findings are consistent with our findings; however, distant lymph node metastasis as a vital metastatic site has often been overlooked and it was discovered to be difficult to benefit from primary surgery in single and multiple organ metastases. Therefore, surgical management for patients with distant lymph node metastasis is required to consider other risk factors, including age, physical state, and lymph node status.
We found that patients with multiple organ metastases did not benefit from primary surgery. While previous studies have rarely focused on multiple organ metastases in metastatic NSCLC, some have demonstrated that primary surgery is beneficial for patients with multiple organ metastases.7,9,25 With an accumulation in research studies and the implementation of prospective clinical trials, additional subgroups that can be used for surgery will be identified.
An increasing number of studies have found that oligometastatic NSCLC is no longer single lesion or organ metastasis.23 The definition of oligometastatic NSCLC is heterogeneous in both retrospective and prospective studies, and the maximum number of metastases range from 1 to 8.26 Recently, the European Organization for the Research and Treatment of Cancer (EORTC) lung cancer group (LCG) considered the maximum number of metastases to be variable and 42% of responders identified 3 metastases to be the correct definition.27,28 The members of the consensus meeting enlarged this number to a maximum of 5 metastases and 3 organs, and considered it changes with local radical treatment (LRT) strategy.5 Thus, more patients with multiple organ metastases will be included in future studies or clinical trials.
At present, multimodal comprehensive therapy represents the optimal treatment management strategy for metastatic NSCLC based on the results discussed within a multidisciplinary tumor board. In the most recent European Society of Medical Oncology (EMSO) Clinical Practice Guidelines for metastatic NSCLC, the first line treatment remains the targeted therapy or immunotherapy based on genetic testing.29 In addition, surgical management is suitable for single brain metastasis according to the EMSO guidelines of metastatic NSCLC. In recent years, studies found that the local ablative treatment is an important method to prolong the survival of patients with NSCLC. Especially for patients with oligometastatic or oligoprogressive NSCLC, the local ablative treatment can reduce significantly the burden of tumor compared with radiochemotherapy. Moreover, the local ablative treatment is safer for patients with drug resistant clones than traditional surgery.30,31 Although some retrospective studies have demonstrated that primary surgery could improve survival time and be even better than radiochemotherapy for metastatic NSCLC, current evidence is limited according to prospective studies for surgery in metastatic NSCLC.7-11,32 Prospective series suggest that primary surgical resection as a part of local consolidative therapy is necessary to prolong survival time but can be affected by mediastinal nodal status.33-37 Similarly, distant lymph node metastasis for single and multiple organ metastases was also a risk factor in our study. We hope that more prospective studies will be performed to verify the clinical significance of primary surgical resection for metastatic NSCLC. The EORTC-LCG consensus meeting proposed the inclusion criteria for future prospective studies.5
There are some unavoidable limitations associated with our retrospective study. First, other selection bias (e.g., better performance status) may affect surgical management, which can influence the reliability of our findings. Second, mediastinal lymph node staging, surgery timing, and performance status may affect the surgical results. We cannot control these potential modifier effects due to the lack of these data in the SEER database. Third, the number of metastases from the same organ, a vital confounding factor, cannot be controlled. Finally, although some common metastatic sites, like adrenal metastasis, were not recorded in the SEER database, we were not able to group them in detail.
Conclusion
In conclusion, the metastatic pattern distinguished the survival benefit of primary surgery for stage IV extrathoracic metastatic non-small cell lung cancer. For single organ metastasis, primary surgery benefited patients with metastatic NSCLC compared to the non-surgery group. The surgical management for patients with distant lymph node metastasis is required to seriously consider. Surgical management of metastatic NSCLC should be adjusted according to metastatic patterns.
Supplemental Material
Supplemental Material, sj-pdf-1-tct-10.1177_15330338211033064 for Surgical Survival Benefits With Different Metastatic Patterns for Stage IV Extrathoracic Metastatic Non-Small Cell Lung Cancer: A SEER-Based Study by Ce Chao, Yongxiang Qian, Xihao Li, Chen Sang, Bin Wang and Xiao-ying Zhang in Technology in Cancer Research & Treatment
Supplemental Material, sj-tif-1-tct-10.1177_15330338211033064 for Surgical Survival Benefits With Different Metastatic Patterns for Stage IV Extrathoracic Metastatic Non-Small Cell Lung Cancer: A SEER-Based Study by Ce Chao, Yongxiang Qian, Xihao Li, Chen Sang, Bin Wang and Xiao-ying Zhang in Technology in Cancer Research & Treatment
Abbreviations
- NSCLC
non-small cell lung cancer
- SEER
the Surveillance, Epidemiology, and End Results
- CBPS
covariate balancing propensity score
- OS
overall survival
- LCSS
lung cancer specific survival
- NCCN
National Comprehensive Cancer Network
- ICD-O-3
the 3rd edition of the International Classification of Diseases for Oncology
- SMD
standard mean difference
- HRs
hazard ratios
- CI
confidence interval
- EORTC
the European Organization for the Research and Treatment of Cancer
- LCG
lung cancer group
- LRT
local radical treatment
- EMSO
European Society of Medical Oncology
Footnotes
Authors’ Note: Ce Chao and Yongxiang Qian contributed equally to this work. Conception and design: Ce Chao, Bin Wang, and Xiao-ying Zhang; administrative support: Bin Wang and Xiao-ying Zhang; provision of study materials or patients: Ce Chao, Yongxiang Qian, and Chen Sang; collection and assembly of data: Ce Chao, Xihao Li, and Yongxiang Qian; data analysis and interpretation: Ce Chao, Yongxiang Qian, Xihao Li, and Chen Sang; manuscript writing: all authors; final approval of manuscript: all authors. Because the data was derived from the SEER database, it was unnecessary to obtain patient consent.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding from Young Talent Development Plan of Changzhou Health Commission (CZQM2020004), Basic Research Project of Changzhou Science and Technology Bureau (CJ20200104), and Social Development Projects of Changzhou Science and Technology Bureau (CE20205039).
ORCID iD: Ce Chao  https://orcid.org/0000-0003-2396-1536
https://orcid.org/0000-0003-2396-1536
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. [DOI] [PubMed] [Google Scholar]
- 2.Ettinger DS, Wood DE, Aggarwal C, et al. NCCN guidelines insights: non-small cell lung cancer, Version 1.2020. J Natl Compr Canc Netw. 2019;17(12):1464-1472. [DOI] [PubMed] [Google Scholar]
- 3.Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446-454. [DOI] [PubMed] [Google Scholar]
- 4.Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37(18):1558-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dingemans AC, Hendriks LEL, Berghmans T. Definition of synchronous oligometastatic non-small cell lung cancer—a consensus report. J Thorac Oncol. 2019;14(12):2109-2119. [DOI] [PubMed] [Google Scholar]
- 6.Giaj-Levra N, Giaj-Levra M, Durieux V, et al. ; European Organization for Research and Treatment of Cancer-Lung Cancer Group. Defining synchronous oligometastatic non-small cell lung cancer: a systematic review. J Thorac Oncol. 2019;14(12):2053-2061. [DOI] [PubMed] [Google Scholar]
- 7.Sun Z, Sui X, Yang F, Wang J. Effects of primary tumor resection on the survival of patients with stage IV extrathoracic metastatic non-small cell lung cancer: a population-based study. Lung Cancer. 2019;129:98-106. [DOI] [PubMed] [Google Scholar]
- 8.Casiraghi M, Bertolaccini L, Sedda G, et al. Lung cancer surgery in oligometastatic patients: outcome and survival. Eur J Cardiothorac Surg. 2020;57(6):1173-1180. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi M, Edagawa M, Suzuki Y, et al. Pulmonary resection for synchronous M1b-cStage IV non-small cell lung cancer patients. Ann Thorac Surg. 2017;103(5):1594-1599. [DOI] [PubMed] [Google Scholar]
- 10.Congedo MT, Cesario A, Lococo F, et al. Surgery for oligometastatic non-small cell lung cancer: long-term results from a single center experience. J Thorac Cardiovasc Surg. 2012;144(2):444-452. [DOI] [PubMed] [Google Scholar]
- 11.Hanagiri T, Takenaka M, Oka S, et al. Results of a surgical resection for patients with stage IV non-small-cell lung cancer. Clin Lung Cancer. 2012;13(3):220-224. [DOI] [PubMed] [Google Scholar]
- 12.Opitz I, Patella M, Payrard L, et al. Prognostic factors of oligometastatic non-small-cell lung cancer following radical therapy: a multicentre analysis. Eur J Cardiothorac Surg. 2020;57(6):1166-1172. [DOI] [PubMed] [Google Scholar]
- 13.Gibson AJW, Li H, D’Silva A, et al. Impact of number versus location of metastases on survival in stage IV M1b non-small cell lung cancer. Med Oncol. 2018;35(9):117. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Zhu H, Sun L, Xu W, Wang X. Prognostic value of site-specific metastases in lung cancer: a population based study. J Cancer. 2019;10(14):3079-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu Y, Zhang J, Zhou Z, et al. Metastasis patterns and prognosis of octogenarians with NSCLC: a population-based study. Aging Dis. 2020;11(1):82-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben-Shachar MS, Makowski D, Lüdecke D. Effectsize: estimation of effect size indices and standardized parameters. J Open Source Softw. 2020;5(56):2815. [Google Scholar]
- 17.Van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Software. 2011;45(1):1-67. [Google Scholar]
- 18.Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyss R, Ellis AR, Brookhart MA, et al. The role of prediction modeling in propensity score estimation: an evaluation of logistic regression, bCART, and the covariate-balancing propensity score. Am J Epidemiol. 2014;180(6):645-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao Y, Fan X, Wang X. Effects of different metastasis patterns, surgery and other factors on the prognosis of patients with stage IV non-small cell lung cancer: a surveillance, epidemiology, and end results (SEER) linked database analysis. Oncol Lett. 2019;18(1):581-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chikaishi Y, Shinohara S, Kuwata T, et al. Complete resection of the primary lesion improves survival of certain patients with stage IV non-small cell lung cancer. J Thorac Dis. 2017;9(12):5278-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Z, Yang Q, Chen X, et al. Clinical associations and prognostic value of site-specific metastases in non-small cell lung cancer: a population-based study. Oncol Lett. 2019;17(6):5590-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schanne DH, Heitmann J, Guckenberger M, Andratschke NHJ. Evolution of treatment strategies for oligometastatic NSCLC patients—a systematic review of the literature. Cancer Treat Rev. 2019;80:101892. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi Y, Adachi H, Mizukami Y, Yokouchi H, Oizumi S, Watanabe A. Patient outcomes post-pulmonary resection for synchronous bone-metastatic non-small cell lung cancer. J Thorac Dis. 2019;11(9):3836-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, Zhang Y, Sun X, et al. The prognostic value of multiorgan metastases in patients with non-small cell lung cancer and its variants: a SEER-based study. J Cancer Res Clin Oncol. 2018;144(9):1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giaj-Levra N, Giaj Levra M, Berghmans T, et al. Oligometastatic non-small cell lung cancer (NSCLC): does number of metastasis matter? Lung Cancer. 2020;139:216-218. [DOI] [PubMed] [Google Scholar]
- 27.Levy A, Hendriks LEL, Berghmans T, et al. ; EORTC Lung Cancer Group. EORTC Lung Cancer Group survey on the definition of NSCLC synchronous oligometastatic disease. Eur J Cancer. 2019;122:109-114. [DOI] [PubMed] [Google Scholar]
- 28.Hendriks LEL, Dooms C, Berghmans T, et al. ; European Organisation for Research and Treatment of Cancer (EORTC) Lung Cancer Group. Defining oligometastatic non-small cell lung cancer: a simulated multidisciplinary expert opinion. Eur J Cancer. 2019;123:28-35. [DOI] [PubMed] [Google Scholar]
- 29.Planchard D, Popat S, Kerr K, et al. ; ESMO Guidelines Committee. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(suppl 4):iv192-iv237. [DOI] [PubMed] [Google Scholar]
- 30.Jairam V, Park HS, Decker RH. Local ablative therapies for oligometastatic and oligoprogressive non-small cell lung cancer. Cancer J. 2020;26(2):129-136. [DOI] [PubMed] [Google Scholar]
- 31.Kim C, Hoang CD, Kesarwala AH, Schrump DS, Guha U, Rajan A. Role of local ablative therapy in patients with oligometastatic and oligoprogressive non-small cell lung cancer. J Thorac Oncol. 2017;12(2):179-193. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Gao SG, Xue Q, et al. Surgery of primary non-small cell lung cancer with oligometastasis: analysis of 172 cases. J Thorac Dis. 2018;10(12):6540-6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Ruysscher D, Wanders R, van Baardwijk A, et al. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: long-term results of a prospective phase II trial (Nct01282450). J Thorac Oncol. 2012;7(10):1547-1555. [DOI] [PubMed] [Google Scholar]
- 34.Gomez DR, Blumenschein GR, Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17(2):1672-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Downey RJ, Ng KK, Kris MG, et al. A phase II trial of chemotherapy and surgery for non-small cell lung cancer patients with a synchronous solitary metastasis. Lung Cancer. 2002;38(2):193-197. [DOI] [PubMed] [Google Scholar]
- 36.De Ruysscher D, Wanders R, Hendriks LE, et al. Progression-free survival and overall survival beyond 5 years of NSCLC patients with synchronous oligometastases treated in a prospective phase II trial (NCT 01282450). J Thorac Oncol. 2018;13(12):1958-1961. [DOI] [PubMed] [Google Scholar]
- 37.David EA, Clark JM, Cooke DT, Melnikow J, Kelly K, Canter RJ. The role of thoracic surgery in the therapeutic management of metastatic non-small cell lung cancer. J Thorac Oncol. 2017;12(11):1636-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-tct-10.1177_15330338211033064 for Surgical Survival Benefits With Different Metastatic Patterns for Stage IV Extrathoracic Metastatic Non-Small Cell Lung Cancer: A SEER-Based Study by Ce Chao, Yongxiang Qian, Xihao Li, Chen Sang, Bin Wang and Xiao-ying Zhang in Technology in Cancer Research & Treatment
Supplemental Material, sj-tif-1-tct-10.1177_15330338211033064 for Surgical Survival Benefits With Different Metastatic Patterns for Stage IV Extrathoracic Metastatic Non-Small Cell Lung Cancer: A SEER-Based Study by Ce Chao, Yongxiang Qian, Xihao Li, Chen Sang, Bin Wang and Xiao-ying Zhang in Technology in Cancer Research & Treatment