Abstract
Systemic sclerosis, also known as scleroderma, is a rare multisystem autoimmune disease characterized by vascular lesions caused by collagen deposition in the skin and viscera and damage to the endothelium. Endothelial injury and microvascular occlusion result in Raynaud’s phenomenon, finger ischemia, pulmonary hypertension, and scleroderma renal crisis. Scleroderma itself is a rare disease with an incidence ranging from 0.1 to 14 per 100,000 people in the general population. Cerebral involvement is not considered a common manifestation of systemic sclerosis, although studies have shown that the brain can be involved. Therefore, to deepen the understanding of this disease, we herein report a case of cerebral infarction associated with systemic sclerosis.
Keywords: Cerebral infarction, systemic sclerosis, Raynaud’s phenomenon, computed tomography, magnetic resonance imaging, anti-nuclear antibody, case report
Introduction
The etiology of systemic sclerosis (SSC), also known as scleroderma, is unknown, but some scholars believe that it is influenced by genetic and environmental factors.1 The pathogenesis of SSC is complex. Research has indicated that the main pathogenesis of SSC is endothelial cell injury, followed by abnormal vascular and immune responses and then excessive deposition and accumulation of extracellular matrix. These changes eventually lead to progressive tissue remodeling, tissue structure destruction, vascular occlusion, and organ function loss.2 SSC is rare in the clinical setting; its reported prevalence rates range from 7.2 to 33.9 per 100,000 individuals in Europe and from 13.5 to 44.3 per 100,000 individuals in North America. In addition, the 10-year survival rate of patients with SSC reportedly ranges from 65% to 73% in Europe and from 54% to 82% in North America.3 Systemic lupus erythematosus and thyroid disease are autoimmune diseases that have been widely reported to increase the risk of cerebral infarction; however, ischemic cerebrovascular disease has rarely been reported in patients with scleroderma. We herein report a case of cerebral infarction associated with SSC.
Case report
A 46-year-old woman was admitted to our department of neurology because of a 2-day history of paroxysmal slurred speech with numbness of the right upper limb. Two days previously, she had developed tongue root stiffness and slurred speech without obvious inducement after meals and simultaneous numbness at the distal end of the right upper limb. These symptoms were relieved for approximately half an hour, and she experienced no dizziness, headache, nausea, vomiting, blurriness or rotation of objects, difficulty swallowing, choking or coughing while drinking water, or limb weakness. In the emergency department of our hospital, brain computed tomography (CT) showed no obvious hemorrhage. Cerebral infarction was considered, and the patient was admitted to our department. She had no history of hypertension, diabetes, or other diseases. She had no history of smoking, drinking, or drug allergies and had no relevant family history. The patient’s mental condition, sleep habits, diet, urine, and feces were normal. She had been found to have nephritis, thrombocytopenia, and elevated rheumatoid factor during physical examination at a local hospital 1 month previously but was not treated. Because of diagnostic limitations at the local hospital, the patient visited our hospital to obtain a clear diagnosis and begin appropriate treatment.
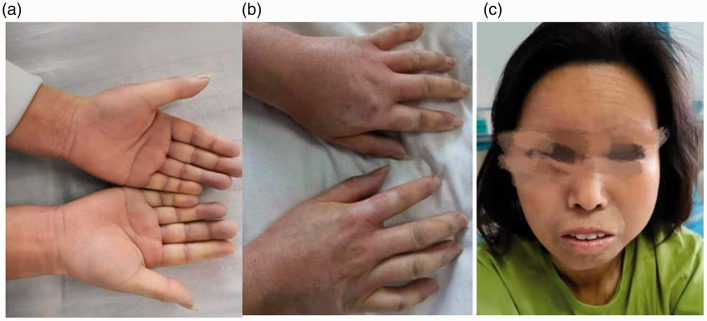
Physical examination after admission revealed a body temperature of 36.6°C, pulse rate of 90 beats/minute, respiratory rate of 19 breaths/minute, and blood pressure of 85/60 mmHg. A neurological examination revealed slurred speech. Her pupils were equal in size, and her pupillary reflexes were intact. She exhibited no diplopia or nystagmus, and her eye movements were normal. Her nasolabial groove was symmetrical, and the oral angle of her teeth was not skewed. Meningeal stimulation was negative. The muscle strength in her limbs was grade 5. Distal shallow hypoesthesia of the right upper limb was present. She had a stable finger-to-nose test, stable heel-knee-tibia test, positive extremity tendon reflexes (++), and negative bilateral Babinski sign. No obvious abnormalities were observed in the patient’s abdomen. The skin of her hands and lips was swollen, tight, and hard (Figure 1(a)–(c)).
Figure 1.
The skin on the patient’s (a, b) hands and (c) face was swollen and hard.
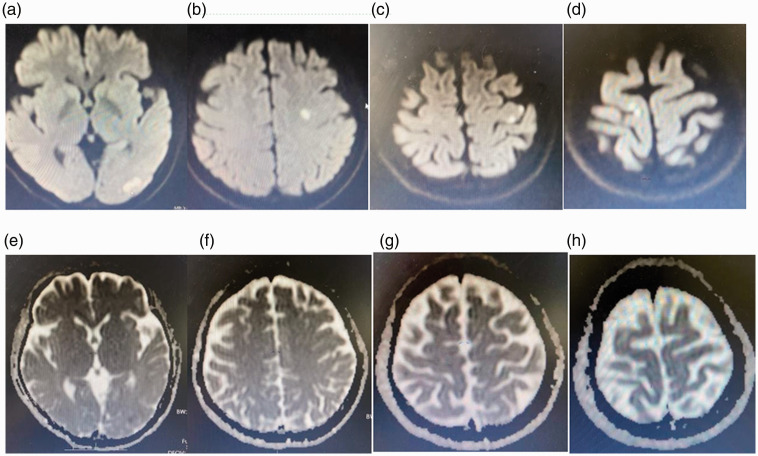
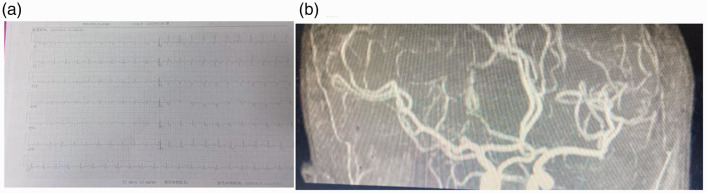
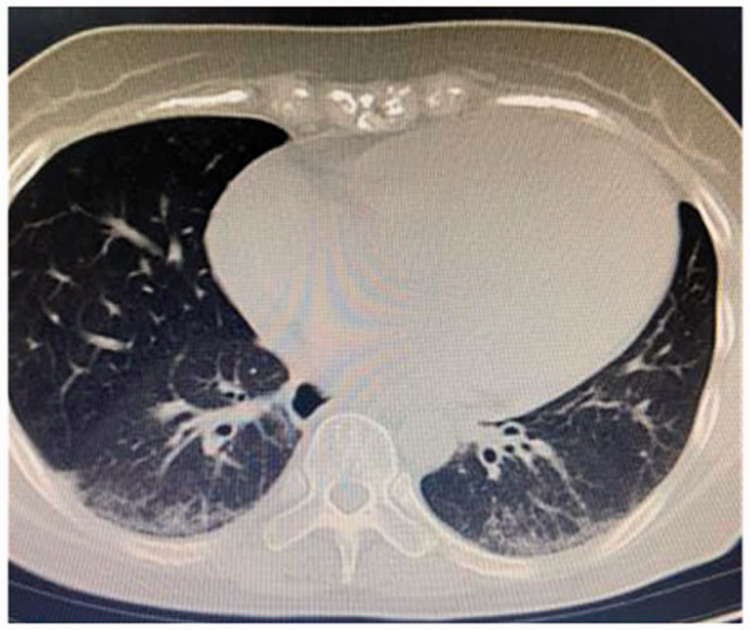
Laboratory and auxiliary examinations were then performed. Brain CT showed no obvious abnormalities. Brain magnetic resonance imaging showed an infarction in the left occipital lobe, left centrum semiovale, and bilateral cerebral parietal lobes (Figure 2). An electrocardiogram demonstrated sinus tachycardia, incomplete right bundle branch block, abnormal Q waves in V1 to V4, and right axis deviation (Figure 3(a)). No obvious abnormalities were observed on magnetic resonance angiography (Figure 3(b)). Thoracic CT showed bilateral interstitial pneumonia (Figure 4). The erythrocyte sedimentation rate was 2800 mm/hour, and routine blood test results were as follows: hemoglobin, 108 g/L; total protein, 60.3 g/L; albumin, 34.48 g/L; glucose, 3.88 mmol/L; and rheumatoid factor, 156.7 IU/mL. A full set of antinuclear antibodies (qualitative) was evaluated, revealing the following: anti-Sjogren’s syndrome A, strongly positive (+++); antiscleroderma 70, strongly positive (+++); antinuclear antibody, positive (+) at 1:320; anti-RO-52, strongly positive (+++); and anticardiolipin antibody quantification, 53.71 RU/mL. Normal results were obtained for an antineutrophil cytoplasmic antibody series (qualitative); immunoglobulins G, A, and M; and anticyclic citrullinate peptide antibody (quantitative). Normal results were also obtained for HbA1c, routine urinalysis, novel coronavirus nucleic acid (qualitative) (negative), five coagulation items, blood lipids, and eight immunity items.
Figure 2.
Diffusion-weighted magnetic resonance imaging of the brain showed a high signal in the (a) left occipital lobe, (b) left centrum semiovale, and (c, d) bilateral parietal lobes. Brain magnetic resonance imaging showed low or equal signals (apparent diffusion coefficients) in the (e) left occipital lobe, (f) left centrum semiovale, and (g, h) bilateral parietal lobes.
Figure 3.
(a) An electrocardiogram showed sinus tachycardia, incomplete right bundle branch block, abnormal Q waves in V1 to V4, and right axis deviation. (b) Magnetic resonance angiography showed no obvious abnormalities.
Figure 4.
Chest computed tomography showed bilateral interstitial pneumonia.
The patient was treated with enteric-coated aspirin tablets (0.1 g once a day), clopidogrel hydrogen sulfate (75 mg once a day), atorvastatin calcium (20 mg once per night), and betastastine and triclutin to improve her microcirculation. She was also prescribed a prednisone tablet (50 mg once a day), cyclophosphamide tablets (0.5 g once a week), and nifedipine (30 mg once a day).
Discussion
The imaging examination of this patient after admission revealed acute cerebral infarction in the left occipital lobe, left centrum semiovale, and bilateral parietal lobes; the diagnosis of cerebral infarction was clear. Laboratory examination showed that anti-Sjogren’s syndrome A, antiscleroderma 70, and antinuclear antibody were all positive. Her erythrocyte sedimentation rate and rheumatoid factor were increased. Other abnormalities included bilateral interstitial pneumonia, arrhythmia, nephritis, thrombocytopenia, and increased rheumatoid factor. Physical examination of such patients can reveal distal limb involvement, stiff facial skin, swelling, and Raynaud’s phenomenon combined with pulmonary interstitial lesions and sinus tachycardia; these findings may indicate the merging of immune abnormalities but also conform to the 1980 American Rheumatism Association criteria for SSC. The patient had no history of thrombosis, fetal loss, or pregnancy complications. When she visited the outpatient department of our hospital for re-examination 12 weeks after discharge, antibody quantification showed a result of 9.81 RU/mL. Because the patient’s anticardiolipin antibody was normal after re-examination, we excluded antiphospholipid syndrome. Ultimately, we diagnosed cerebral infarction and SSC.
SSC is a rare chronic autoimmune connective tissue disease with unique pathological features, a complex pathogenesis, and multiple organ involvement. It is characterized by microvascular lesions, immune system activation, and tissue fibrosis4 that can involve multiple systems of the body, such as the heart, lungs, kidneys, and digestive tract; nervous system involvement is comparatively rare. SSC was previously believed to be a microvascular disease. However, recent studies have shown that SSC also causes damage to large vessels. It can also further increase the occurrence of ischemic vascular disease and atherosclerosis, which accounts for 20% to 30% of the causes of death among patients with SSC.5,6 In addition, meta-analyses and large national cohort studies have shown that SSC is independently associated with a higher risk of cerebral infarction,7,8 although the specific mechanism is unclear. SSC is reportedly associated with blood hypercoagulability, which may also be one of the mechanisms underlying the development of cerebral infarction in patients with SSC.9 Nervous system involvement in SSC has long been reported10–12: central nervous system damage occurs in approximately 26% of patients, and peripheral nervous system involvement occurs in 39.1% of patients. Our patient had numbness in her right upper extremity and no history of hyperthyroidism or diabetes. Therefore, we considered that SSC might have involved her peripheral nerves. In addition, SSC-related interstitial lung disease is a common complication of SSC; it occurs in the early stage of the disease and is a main cause of death in patients with SSC.13 The chest CT examination of our patient indicated interstitial pneumonia, which was also consistent with the evolution of SSC disease.
Among patients with cerebral infarction, some have risk factors for cerebral infarction while others do not. Patients who do not have risk factors for cerebral infarction often have other systemic diseases, such as autoimmune diseases. Among the various autoimmune diseases, systemic lupus erythematosus and thyroid disease have been more frequently reported to be associated with cerebral infarction, but scleroderma with cerebral infarction has been less frequently reported. After considering the patient’s history, related auxiliary examinations, and TOAST classification, we ultimately determined that the patient’s cerebral infarction had been caused by autoimmune vascular disease, which is consistent with a report by Lucivero et al.14 The mechanisms by which SSC can cause cerebral infarction may include vasculitis, vasospasm, vascular fibrosis, microvascular injury, hypertension, thrombosis, and hemodynamic changes.15,16 However, the pathogenesis of cerebral infarction caused by SSC remains unclear.
There are no clear guidelines for the treatment of SSC with cerebral infarction. The literature indicates that anti-inflammatory and immunoregulatory therapy, such as glucocorticoids, cyclophosphamide, cyclosporine A, azathioprine, and methotrexate, are mainly effective for this condition. For SSC-related Raynaud’s phenomenon, the patient should quit smoking and keep his or her hands and feet warm to avoid cold. Nifedipine is often used as a first-line treatment for SSC-associated Raynaud’s phenomenon. Studies have shown that methotrexate can improve skin sclerosis in patients with early diffuse SSC.17 Treatment of patients with cerebral infarction mainly involves secondary prevention of cerebrovascular disease. Some studies have indicated that patients with SSC who develop cerebral infarction do not have a poor prognosis.18
The clinical spectrum of SSC is wide and diverse, and the common features include Raynaud’s phenomenon, skin sclerosis, joint contracture deformity, pulmonary fibrosis, renal crisis, and nervous system effects. Nervous system abnormalities in these patients can lead to serious dysfunction and failure of almost all internal organs, resulting in great harm and seriously affecting patients’ quality of life and safety. Nervous system involvement is not common; once it occurs, however, the consequences are serious. The mechanism of its occurrence remains unclear. Further clinical studies are needed to explore the nervous system manifestations related to SSC and to understand the clinical specificity of this condition.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605211044045 for Cerebral infarction caused by systemic sclerosis: a case report by Qingqing Wang, Mengen Zhang, Mingfeng Zhai and Zongyou Li in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605211044045 for Cerebral infarction caused by systemic sclerosis: a case report by Qingqing Wang, Mengen Zhang, Mingfeng Zhai and Zongyou Li in Journal of International Medical Research
Footnotes
Ethics statement and informed consent: The requirement for ethics approval to publish this case report was waived by the Institutional Review Board of The Affiliated Fuyang Hospital of Bengbu Medical College, Fuyang City, Anhui Province. The patient provided written informed consent for treatment and publication of this case, including all images. The reporting of this study conforms to the CARE guidelines, and we have completed the CARE checklist.
Declaration of competing interests: The authors declare that there are no conflicts of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Qingqing Wang https://orcid.org/0000-0001-7298-1811
References
- 1.Hoffmann-Vold AM, Midtvedt Ø, Molberg Ø, et al. Prevalence of systemic sclerosis in south-east Norway. Rheumatology (Oxford) 2012; 9: 1600–1605. [DOI] [PubMed] [Google Scholar]
- 2.Ho YY, Lagares D, Tager AM, et al. Fibrosis-a lethal component of systemic sclerosis. Nat Rev Rheumatol 2014; 10: 390–402. [DOI] [PubMed] [Google Scholar]
- 3.Bergamasco A, Hartmann N, Wallace L, et al. Epidemiology of systemic sclerosis and systemic sclerosis-associated interstitial lung disease. Clin Epidemiol 2019; 11: 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orlandi M, Barsotti S, Lepri G, et al. One year in review 2018: systemic sclerosis. Clin Exp Rheumatol 2018; 36: 3–23. [PubMed] [Google Scholar]
- 5.Nordin A, Jensen-Urstad K, Björnådal L, et al. Ischemic arterial events and atherosclerosis in patients with systemic sclerosis: a population-based case-control study. Arthritis Res Ther 2013; 15: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belch JJF, Mcswiggan S, Lau C.Macrovascular disease in systemic sclerosis: the tip of an iceberg? Rheumatology (Oxford) 2008; 47: 16–17. [DOI] [PubMed] [Google Scholar]
- 7.Chiang CH, Liu CJ, Huang CC, et al. Systemic sclerosis and risk of ischaemic stroke: a nationwide cohort study. Rheumatology (Oxford) 2013; 52: 161–165. [DOI] [PubMed] [Google Scholar]
- 8.Patompong U, Anawin S, Sikarin U.Risk of ischemic stroke in patients with systemic sclerosis: a systematic review and meta-analysis. Mod Rheumatol 2016; 26: 128–131. [DOI] [PubMed] [Google Scholar]
- 9.Renard D, Heroum C.Carotid thrombus formation and extension during anticoagulation: a case report of large vessel disease and hypercoagulable state in systemic sclerosis. Acta Neurol Belg 2007; 107: 55–57. [PubMed] [Google Scholar]
- 10.Averbuch-Heller L, Steiner I, Abramsky O.Neurologic manifestations of progressive systemic sclerosis. Arch Neurol 1992; 49: 1292–1295. [DOI] [PubMed] [Google Scholar]
- 11.Morer IC, Marco JV, Aznar JLH, et al. [Neurological involvement in systemic sclerosis]. Rev Clin Esp 2003; 203: 373–377. [DOI] [PubMed] [Google Scholar]
- 12.Clark P.Trigeminal neuropathy in progressive systemic sclerosis. Am J Med 1982; 73: 57–62. [DOI] [PubMed] [Google Scholar]
- 13.Elhai M, Meune C, Boubaya M, et al. Mapping and predicting mortality from systemic sclerosis. Ann Rheum Dis 2017; 76: 1897–1905. [DOI] [PubMed] [Google Scholar]
- 14.Lucivero V, Mezzapesa DM, Petruzzellis M, et al. Ischaemic stroke in progressive systemic sclerosis. Neurol Sci 2004; 25: 230–233. [DOI] [PubMed] [Google Scholar]
- 15.Chizzolini C, Boin F.The role of the acquired immune response in systemic sclerosis. Semin Immunopathol 2015; 37: 519–528. [DOI] [PubMed] [Google Scholar]
- 16.Zulian F, Vallongo C, Woo P, et al. Localized scleroderma in childhood is not just a skin disease. Arthritis Rheum 2014; 52: 2873–2881. [DOI] [PubMed] [Google Scholar]
- 17.Asano Y, Jinnin M, Kawaguchi Y, et al. Diagnostic criteria, severity classification and guidelines of systemic sclerosis. J Dermatol 2018; 45: 633–691. [DOI] [PubMed] [Google Scholar]
- 18.Edigin E, Eseaton P, Kaul S, et al. Systemic sclerosis is not associated with worse outcomes of patients admitted for ischemic stroke: analysis of the National Inpatient Sample. Cureus 2020; 12: e9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605211044045 for Cerebral infarction caused by systemic sclerosis: a case report by Qingqing Wang, Mengen Zhang, Mingfeng Zhai and Zongyou Li in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605211044045 for Cerebral infarction caused by systemic sclerosis: a case report by Qingqing Wang, Mengen Zhang, Mingfeng Zhai and Zongyou Li in Journal of International Medical Research