Abstract
The cell surface receptor Notch is required during Drosophila embryogenesis for production of epidermal precursor cells. The secreted factor Wingless is required for specifying different types of cells during differentiation of tissues from these epidermal precursor cells. The results reported here show that the full-length Notch and a form of Notch truncated in the amino terminus associate with Wingless in S2 cells and in embryos. In S2 cells, Wingless and the two different forms of Notch regulate expression of Dfrizzled 2, a receptor of Wg; hairy, a negative regulator of achaete expression; shaggy, a negative regulator of engrailed expression; and patched, a negative regulator of wingless expression. Analyses of expression of the same genes in mutant N embryos indicate that the pattern of gene regulations observed in vitro reflects regulations in vivo. These results suggest that the strong genetic interactions observed between Notch and wingless genes during development of Drosophila is at least partly due to regulation of expression of cuticle patterning genes by Wingless and the two forms of Notch.
The transmembrane protein Notch (N) regulates cell fates in Drosophila melanogaster during the development of tissues from all three germ layers (6, 11, 14, 24, 42, 83, 88). For example, embryos without zygotically contributed N produce excess neuroblasts at the expense of epidermoblasts (11, 66, 90). In this instance, N appears to function by suppressing a default fate in some members of a population of physically interacting cells. Delta (Dl), also a transmembrane protein, has been identified as the ligand for this function of N, known as lateral inhibition (1, 14, 24, 32, 46, 50, 86).
The extracellular domain of N, where extracellular ligands or factors regulating intracellular N activities are expected to bind, is made up of 36 epidermal growth factor-like (EGF-like) repeats (42, 88). In vitro analyses of deletions affecting different segments of the extracellular domain of N have shown that Dl binds N in the region of EGF-like repeats 11 and 12 (68). Serrate (Ser), the only other identified ligand of N but with functions similar to that of Dl, also binds the same two EGF-like repeats (25, 29, 68). A single-amino-acid substitution in this region can produce an embryonic lethal phenotype (18). However, these two repeats are not sufficient for wild-type N function: loss of the remaining extracellular sequence blocks formation of the embryonic cuticle (52), and single-amino-acid substitutions affecting the 2nd (nd3), 14th (spl), 24th (Ax9, Ax59b, Ax59d), 25th (Ax1), 27th (Ax71d), 29th (Ax16, AxE2), or 32nd (Nts1) EGF-like repeats or the lin12/Notch repeats [l(1)NB] produce lethality or aberrant Notch function (41, 54, 91). Since most of these mutations alter the structure of N EGF-like repeats, it was likely that these extracellular regions mediate interactions with alternative ligands. These alternative ligands could be associated with other N functions, such as induction of cell fates observed in the development of the compound eye (2, 13, 28) or differentiation of the epidermis (15, 36). Therefore, I explored the functional significance of EGF-like repeats of N other than those involved in binding of Dl or Ser.
Interspecific sequence comparisons identified two possible ligand binding sites in the region containing EGF-like repeats 19 to 36. A cell surface screen of embryonic cDNA-derived peptides identified Wingless (Wg) as a possible ligand of N. In vitro analysis showed that Wg associates with N in this region. In vitro and in vivo gene expression analyses showed that Wg is associated with regulation of expression of the Dfrizzled2, patched, shaggy, and hairy genes through two distinct forms of N: the full-length form and a form of N lacking 18 or more of the amino-terminal EGF-like repeats (thereby also the Dl binding repeats). These two forms of N produce different ligand-independent and ligand-dependent gene activities in cells expressing them.
MATERIALS AND METHODS
Sequence analysis.
Extracellular N sequences of D. virilis and of D. pseudoobscura were obtained by reverse transcription-PCR with Taq polymerase and degenerate primers. Plots were generated by using the PILEUP and PLOTSIMILARITY programs in the Genetics Computer Group sequence analysis program (27). There are only 10 single-residue, 4 two-residue, and 1 eight-residue changes in the EGF-like repeats region between N and human Notch1.
Biopanning.
A 6- to 12-h Drosophila embryonic cDNA library was constructed in the Surfzap vector (Stratagene). Biopanning was performed as recommended by the manufacturer. Approximately 5 × 108 lambda phage (∼130 times the number of primary plaques) were used for mass excision of phagemids. About 6 × 108 excised phagemids were used for filamentous phage preparation (in the presence of 1% glucose). Phage precipitate was resuspended in balanced salt solution (BSS) (89). A total of 4 × 106 to 6 × 106 heat shock-induced S2-N cells were washed twice with BSS and blocked first for 30 min at 4°C with 1 ml of BSS–5% protease-free bovine serum albumin (BSA) and then for 30 min at 4°C with 1 ml of BSS–5% BSA–100 μl of ∼1014 M13mp8 phage per ml in BSS solution. Approximately 1013 to 1014 biopan-ready filamentous phage, mixed with 60 μl of M13mp8 phage solution, were added, and the tubes were shaken for 25 min at 4°C. The cells were washed twice with 10 ml of cold BSS–5% BSA followed by four times with 10 ml of cold BSS. The bound phages were eluted in 200 μl of 0.1 M triethylamine with protease inhibitors (1 μl each of benzamidine [0.1 mg/ml], trypsin inhibitor [10 mg/ml], pepstatin [10 mg/ml], leupeptin [10 mg/ml], and aprotinin [2.2 mg/ml] and 5 μl of phenylmethylsulfonyl fluoride [10 mg/ml]), the solution was neutralized with 200 μl of 1 M Tris-HCl (pH 7.5), and the phagemids were amplified in Escherichia coli. A total of ∼1012, ∼1010, and ∼108 amplified phage were used in the second, third, and fourth rounds of biopanning, respectively. In the fifth and sixth rounds, ∼107 and ∼106 phagemids, respectively, were biopanned four times for 30 min consecutively on ∼107 heat shock-induced S2 cells (each time), and the supernatant was subsequently biopanned once on heat shock-induced S2-N cells. Then 1,000 to 2,000 eluted, biopanned phagemids were screened with probes of various genes by standard procedures (73). Phages from purified wg-carrying phagemids were prepared as specified by the manufacturer (Stratagene).
Immunocytochemistry on cultured cells.
S2, S2-N, S2-NΔI, S2-NΔEGF19–36, and S2-NΔEGF1–18 cells were heat shock induced before use. S2-Dfz2 cells (8) were grown in the presence of copper for 12 to 14 h before use. The cells were washed twice with cold Shields and Sangs M3 medium (M3 medium; Sigma), resuspended with 400 μl of cold medium conditioned with growth of S2-Wg or S2 cells (see below), and incubated for 15 min at 4°C. The cells were washed twice with cold M3 medium, fixed in 4% paraformaldehyde–1× phosphate-buffered saline, and processed for immunofluorescence as described previously (24, 51). pv9 1.1 S2-Wg cells were used to make Wg conditioned M3 media as described previously (69). Unconcentrated medium was used for all experiments.
Immunoprecipitations. (i) From cultured cells.
N and Dl proteins were induced by heat shock. Dfz2 was induced by growing S2-Dfz2 cells for 12 to 14 h in the presence of copper. For N and Dl experiments, 107 cells each of S2-N and S2-Dl cells, respectively, were incubated in M3 medium for 15 min for formation of aggregates (51) and used for each immunoprecipitation (i.e., each lane). The number of S2-N or S2-Dl cells was approximately the same wherever a mixture of cell lines was used, with the remainder being made up by the other cell line or S2 cells. For N and Wg experiments, 3 × 107 S2 or S2-N cells were used per immunoprecipitation. For experiments with NΔEGF1–18, NΔEGF19–36, and S2-Dfz2 cells, 5 × 106 cells were used per immunoprecipitation. The cells were washed twice in cold serum-free M3 medium and resuspended in 250 μl of cold M3 medium conditioned with growth of S2 cells (S2 media) or pv9 1.1 S2-Wg cells (S2-Wg media) plus protease inhibitors (20 ng each of leupeptin, pepstatin, trypsin inhibitor, and E-64 per ml, 5 ng of aprotinin per ml, and 2 mM phenylmethylsulfonyl fluoride). Where required, EGTA was added to a final concentration of 15 mM. Then 10 μl of ∼1.25 mM bis(sulfosuccinimidyl suberate) (BS3) cross-linker resuspended in 1 ml of cold phosphate-buffered BSS (pbBSS: 55 mM NaCl, 40 mM KCl, 15 mM Mg2SO4, 10 mM CaCl2, 20 mM glucose, 50 mM sucrose, 0.74 mM KH2PO4, 0.35 mM Na2HPO4) was added to appropriate samples, and the cells were incubated for 30 min at 4°C. The cells were pelleted, resuspended in 1/10 pbBSS–10 mM Tris-HCl (pH 7.5) (to quench cross-linking)–protease inhibitors, and incubated on ice for 10 min. The membranes were pelleted, resuspended in 400 μl of cold pbBSS–60 mM Tris (pH 7.5)–0.8% Triton X-100–protease inhibitors, and incubated for 20 min on ice. A 20-μl volume of 10% deoxycholate was added, and incubation was continued for 90 min to 2 h. The extract was precleared with GammaBind Plus beads (Pharmacia) for ∼2 h at 4°C and incubated overnight at 4°C with the immunoprecipitation antibody where appropriate [100 μl of the monoclonal anti-Dl antibody, 1 μl of the polyclonal anti-Wg (rb) antibody, 1 μl of the polyclonal anti-Hedgehog antibody, 4 μl of the anti-Patched antibody, or 20 μl of the anti-Dfz2 monoclonal antibody]. Immunocomplexes were captured with GammaBind Plus beads, and the beads were rinsed four times with 1 ml of cold pbBSS–protease inhibitors–10 mM Tris (pH 7.5)–0.1% Triton X-100. Bound complexes were eluted with 40 μl of 1× Laemmli buffer–protease inhibitors, boiled for 6 min, and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 4% polyacrylamide gels (for anti-Dl or anti-Wg immunoprecipitations) or in 6% polyacrylamide gels (for anti-Dfz2 immunoprecipitations). Western blot analyses were performed by to standard procedures (30, 73), and signals were detected with the ECL kit (Amersham).
(ii) From embryos.
Approximately 800 μl of dechorionated embryos of appropriate ages and strains, laid by circadian cycle-entrained flies (to minimize age variance in embryos), was partially crushed, with a loose-fitting pestle in a 1-ml Wheaton Dounce grinder, in the presence of 400 μl of ice-cold pbBSS–protease inhibitors, with or without ∼2 mM BS3. After 45 min of incubation on ice, 12 μl of cold 2 M Tris-HCl (pH 7.5) was added to quench the cross-linking reaction. Membrane proteins were extracted in 0.75% Triton X-100–0.5% deoxycholate. The rest of the procedure was identical to that described for immunoprecipitation from cultured cells. Anti-Dl, anti-Wg, and anti-Ser immunoprecipitates (see Fig. 4A and B) were separated by SDS-PAGE in 4% polyacrylamide gels; anti-Wg, anti-Hh, and anti-Ptc immunoprecipitates (see Fig. 4C) were separated by SDS-PAGE in 6% polyacrylamide gels.
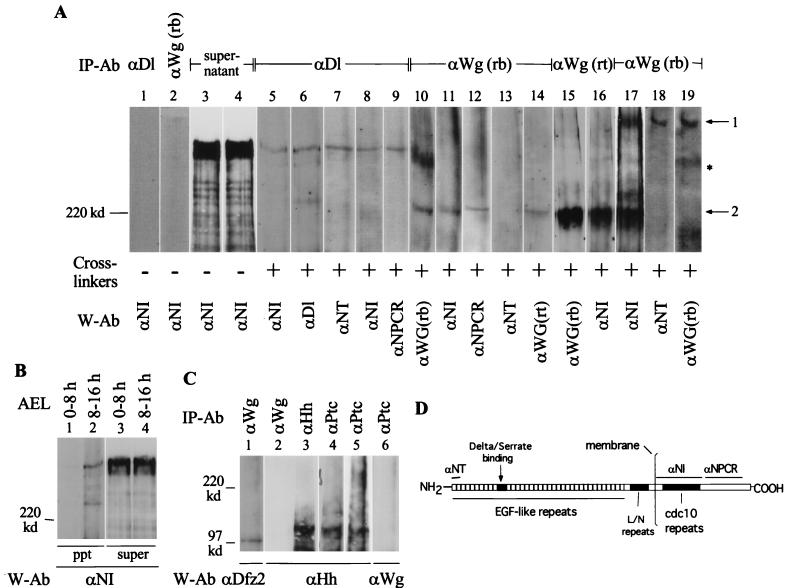
FIG. 4.
Wg and N form complexes during embryogenesis. (A) Two Wg- and N-containing cross-linked complexes, similar to those recovered from cultured cells, are immunoprecipitated from Canton S embryonic extracts. Anti-Dl, is monoclonal antibody MAb 202; anti-NT is described in reference 43; anti-NPCR is described in reference 52; and anti-Wg(rt) was kindly provided by A. Martinez-Arias (See panel D for epitope regions for N antibodies). I used 0- to 3-h embryos for lanes 1 to 16, 6- to 12-h embryos for lane 17, and 10- to 16-h embryos for lanes 18 and 19. Arrow 1 shows Wg complexed with full-length N (lanes 17, 18, and 19); arrow 2 shows Wg complexed with a truncated N (lanes 10 to 17). The asterisk marks the Wg complex not containing N (lanes 10 and 19). A single blot was probed sequentially with the indicated antibodies to form lanes 10 and 11; 12, 13, and 14; 15 and 16; and 19 and 18 (numbers also indicate the sequence of probing). The same embryonic extract was used for lanes 1 to 4 and 7 to 14; lanes 5 to 6, 15, 17, and 18 are derived from different embryonic extracts. (B) Ser-N cross-linked complexes are also recovered from cross-linked embryonic extracts. Complexes were immunoprecipitated with anti-Ser antibody (kindly provided by Elizabeth Knust). Complexes migrating faster than a ∼120-kDa marker protein were not analyzed in panels A and B. (C) The procedure recovering Wg-N, Dl-N, and Ser-N complexes also recovers Wg-Dfz2 (lane 1) and Hh-Ptc complexes from cross-linked embryonic extracts (lanes 3 to 6). For lanes 5 and 6, equal volumes of anti-ptc immunoprecipitate was separated in two different lanes and probed with the indicated antibodies. IP-Ab, immunoprecipitation antibody; cross-linker, BS3; W-Ab, Western blotting antibody; AEL, after egg laying. For panels A and B, 4% polyacrylamide gels were used; for panel C, 6% polyacrylamide gels were used. The tops of all the blots shown in panels A, B, and C coincide with the top of the resolving gel of the discontinuous SDS-PAGE gels. (D) Diagram showing the N epitopes used to produce the N antibodies used in the study.
Northern analyses.
Total RNAs were extracted from cultured cells or embryos by using RNAzol B (Tel-test, Inc.). I used 0- to 20-h dechorionated embryos, collected at the indicated temperatures (with appropriate corrections for developmental times). A total of 2 × 107 cells of different cell lines (grown to confluence) were heat shocked and incubated for 2 h at room temperature before use. S2-Dfz2 cells were grown in either the presence or absence of copper for 12 to 14 h before use. The cells were washed twice with serum-free M3 medium with antibiotics, and equal volumes were aliquoted to two 1.5-ml Eppendorf tubes, pelleted, and resuspended in 600 μl of S2 medium or S2-Wg medium. After 2 h of gentle shaking at room temperature, RNAs were extracted. Then 40 μg of total RNA was loaded in each lane. Standard Northern blot procedures were used (73). For generation of NΔEGF1–18/Ax59d molecules, the fragment including NheI (nucleotide 4324) (42) and BglII (nucleotide 5160) was PCR amplified (with Pfu enzyme) from Ax59d/FM7 flies, cut with NheI and BglII, and used to replace the same fragment in NΔEGF1–18. Samples were sequenced fully in the replaced region, and clones carrying the Ax59d mutation was transfected into S2 cells. Expression of protein was confirmed by Western blotting and immunocytochemistry.
Western blot analyses.
For assessment of Armadillo protein in the cytoplasm, about 3 × 106 heat shock- or metal-induced cells were processed as described by Bhanot et al. (8). The same blot was stripped and stained for ∼16 h with India ink. For analyses of proteins from embryos, proteins were extracted with 0.75% Triton X-100–0.5% deoxycholate or with SDS lysis buffer (43). The amounts of proteins in different embryonic extracts were standardized by using absorbance values at 280 nm and the Bio-Rad DC protein assay kit. The proteins were separated by SDS-PAGE in 4% polyacrylamide gels. For analysis of S2-N, S2-NΔEGF1–18, S2-NΔEGF19–36 and LN rpts, and S2-NΔEGF1–36 proteins, heat shock-induced cells were dissolved in 1× Laemmli buffer and separated by SDS-PAGE in 4% polyacrylamide gels. Western blotting in all cases was performed by standard procedures (30, 73) and signals were detected with the ECL kit.
In situ RNA hybridization.
Batches of wild-type and mutant embryos were grown under identical conditions at 25 or 18°C and processed simultaneously under the same conditions at all steps of the procedure. For each comparative experiment, the batches of wild-type and mutant embryos were divided just before addition of probe, and different probes were added to the divided batches of embryos. A double RNA-protein hybridization procedure, described by Corbin et al. (14), was used. An anti-β-galactosidase antibody made in mouse was used to sort out FM7 or TM6 chromosome carrying embryos laid by N264-47/FM7, spl Ax59d/FM7, Ax9B/FM7, Ax59d/FM7, or DlX/TM6 flies.
RESULTS
EGF-like repeats 23 to 27 and 31 to 34 are potential ligand binding sites.
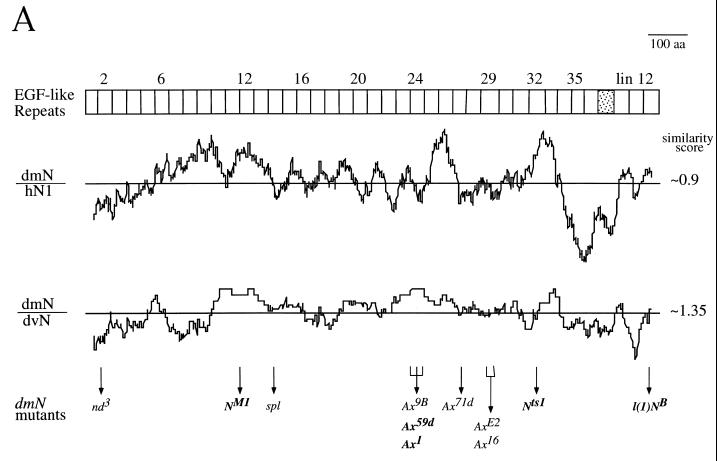
A chimeric Drosophila N protein containing EGF-like repeats 10 to 13 from Xenopus physically associates with the Drosophila Dl protein (68). Therefore, the Dl binding region in N is expected to be conserved between homologous sequences. If there are additional ligands associated with different functions of Notch and if they interact with regions other than the Dl binding region, these regions are expected to be conserved between homologous sequences also. To determine whether such conserved regions exist in the extracellular domain of N, the DNA sequences coding for the extracellular portions of N proteins in D. virilis and D. pseudoobscura were compared by plotting the running averages of conservation between sequences of these invertebrate homologs and between sequences of D. melanogaster N and the human homolog, hNotch 1 (Fig. 1A). Figure 1 shows that, as expected, a peak with high conservation is centered on EGF-like repeats 11, 12, and 13, which include the Dl binding region. Besides that region, there are two additional regions that are as conserved as EFG-like repeats 11 to 13 in both comparisons. In the D. melanogaster-D. virilis comparison, the comparison relevant to N function in Drosophila, one region extends from EGF-like repeats 23 to 27 and encompasses most of the domain affected by the Ax mutations of N (17, 41). A second conserved region extends from EGF-like repeats 31 to 34. The functional importance of all these conserved regions is underscored by inclusion of at least one lethal mutation in each (boldface type in Fig. 1A). As EGF-like repeats 11 and 12 bind Dl (68) and are evolutionarily conserved (Fig. 1A), the regions containing EGF-like repeats 23 to 27 and 31 to 34 were hypothesized to be conserved because they are additional ligand binding regions in the extracellular domain of N.
FIG. 1.
Search for new N ligands. (A) Interspecific sequence comparisons reveal possible ligand binding sites in the extracellular domain of N. Each plot represents a running average of sequence conservation between two homologs, over sliding blocks of 40 amino acids (aa). At the top, the corresponding EGF-like repeats of N are graphically represented. lin12 = lin12/N repeats (42, 88). The line in the middle of each plot represents average similarity between the two sequences compared (the maximum value is 1.5). This average would include sequence conservation due to sequence elements common to all EGF-like repeats. The plot of D. melanogaster N and its D. pseudoobscura homolog is similar to that of N and the D. virilis homolog (differing only in the level of overall conservation). The between-lineage comparison identifies evolutionarily stable conserved regions. dmN, D. melanogaster N; hN1, human homolog of N (23); dvN, D. virilis N. Sites of mutations in nd3 and l(1)NB are from reference 54, NM1 are from reference 18, spl and the Ax alleles are from reference 41, and Nts1 are from 91. Lethal alleles are in bold letters. (B) Schematic representation of the biopanning screen used for identification of potential N ligands (see Materials and Methods).
Cell surface biopanning screen suggests a physical affinity between N and Wg.
The two identified ligands, Dl and Ser, bind N only at the region including EGF-like repeats 11 and 12 (68). This specificity suggested that if the regions including EGF-like repeats 23 to 27 and 31 to 34 bound ligands, these are likely to be novel ligands. To identify such novel N ligands, if any, phagemid biopanning (55, 74) was performed on the surfaces of live S2 cells expressing full-length N proteins (S2-N). The procedure used is schematically shown in Fig. 1B. Phagemids encoding the known N ligands, Dl and Ser, were specifically enriched by this biopanning. Enrichment was also observed for Wg, N, Big Brain, Pecanex, and Fringe phagemids but not for Scabrous and Star phagemids (Table 1). Genes encoding these proteins are known to genetically interact with N (5, 15, 22, 31, 40, 47, 56, 64, 67). Enrichments were not detected for hedgehog (hh), patched (ptc), and slit genes, whose products function on cell surfaces (35, 45, 49, 57, 70, 81), or for several genes whose products are cytoplasmic proteins (Table 1 footnote). The high enrichment of Pecanex phagemids (from 0.3/105 before biopanning to 25,600/105 after biopanning), enrichment of phagemids of known ligands of N (Dl and Ser), enrichment of phagemids representing only a subset of genes showing interaction with the N gene, and lack of enrichment of EGF-like repeat sequence containing Slit phagemids indicated specificity in the enrichment process (the after-biopanning phagemid population was estimated to be composed of phagemids representing only about 15 genes).
TABLE 1.
Change in frequency of test phagemids following biopanning of Drosophila embryonic cDNA carrying filamentous phages on the surfaces of S2-N cellsa
| Gene | Phagemid frequency (10−5) in:
|
|
|---|---|---|
| Biopan 0 | Biopan 6 | |
| Delta | 5 | 2,000 |
| wingless | 1 | 1,500 |
| Serrate | 5 | 1,300 |
| Notch | 0.6 | 4,700 |
| big brain | 6 | 6,200 |
| pecanex | 0.3 | 25,600 |
| fringe | 0.6 | 4,500 |
| scabrous | 0.9 | <0.1 |
| Star | 4.8 | <0.1 |
| hedgehog | 2 | <0.1 |
| patched | 0.5 | <0.1 |
| slit | 0.6 | <0.1 |
Phage enrichment was initially determined by restriction enzyme analysis of a random sample of phagemids and colony hybridization with some of the fragments. All clones in a random sample of 46 phagemids from the biopan 0 population contained inserts of different sizes, indicating a library complexity of ∼1. A sample of 43 phagemid clones from the biopan 6 population was found to be composed of five sequences, indicating a maximal complexity of ∼0.25. Enrichment levels of Dl, wg, sca phages were confirmed in several one-round biopanning experiments. Biopan 0 is the frequency before biopanning and is based on a sample of 105 to 106 phagemids; biopan 6 is the frequency after completion of the full procedure of biopanning and is based on a sample of 1,000 to 2,000 phagemids. The same membranes were also probed with gene sequences of cytoplasmic proteins cactus, alcohol dehydrogenase, and glucose 6 phosphate dehydrogenase. Each gene was present in biopan 0 but failed to show enrichment after biopanning.
Among the genes showing enrichment, wg was of particular interest. wg and Ax alleles of N genetically interact, and both produce similar “antineurogenic” phenotypes; N− alleles, or the NM1 allele carrying a mutation in the Dl binding region, produce the other phenotypes (10, 15, 17, 18). Thus, it seemed possible that Wg physically interacts with the conserved Ax domain of N including EGF-like repeats 23 to 27 (Fig. 1). Therefore, further studies were focused on wg. Six of the Wg phagemids selected by biopanning were further analyzed by sequencing and Western blotting. The inserts in all six were of wg coding DNA, and all six phages produced Wg protein as part of their cpIII coat protein (data not shown), suggesting that the enrichment of Wg phagemids was due to physical interactions between Wg and N.
Soluble Wg proteins form two molecular complexes with N proteins.
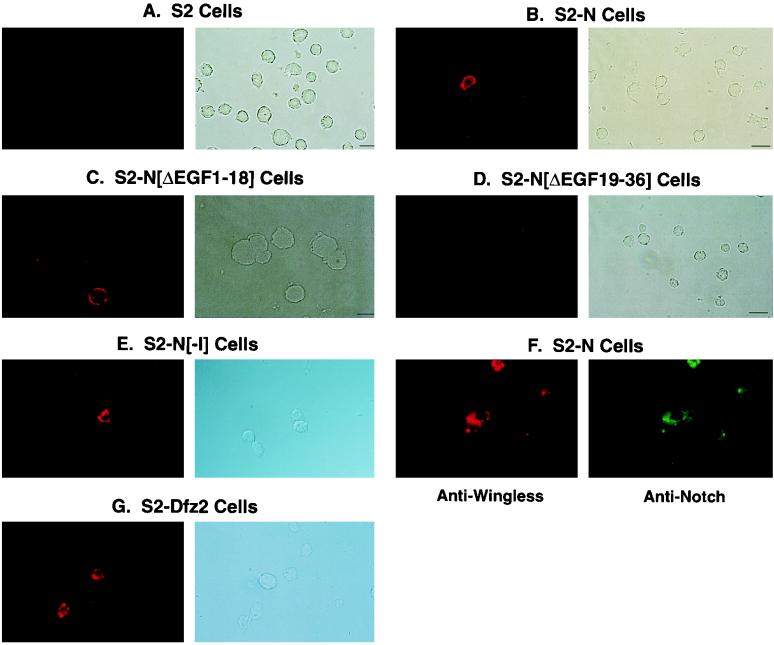
To confirm that enrichment of wg phagemids on S2-N cells was due to binding of Wg protein (expressed on the surfaces of phagemids) to the surfaces of S2-N cells, immunocytochemical analyses was performed with soluble Wg and S2 cells expressing the following N molecules (see reference 52 for a complete description of these molecules): full-length N (S2-N), N lacking EGF-like repeats 19 to 36 (S2-NΔEGF19–36), N lacking EGF-like repeats 1 to 18 (S2-NΔEGF1–18), and N lacking the intracellular domain (S2-NΔI). Wg was detected on the surfaces of S2-N cells (Fig. 2B), S2-NΔEGF1–18 cells (Fig. 2C), and S2-NΔI cells (Fig. 2E) but not on surfaces of S2 cells (Fig. 2A) and S2-NΔEGF19–36 cells (Fig. 2D). More than 2 × 106 cells were processed on each slide, and several such slides were examined for each cell type. Immunofluorescence signals were not detected on S2 and S2-NΔEGF19–36 cell slides. Double staining with antibodies against N and Wg showed that only N-expressing cells bound Wg (Fig. 2F). Comparable frequencies of Wg-positive cells were obtained with S2-Dfz2 cells (8) treated in the same way as N-expressing cells (Fig. 2G). Dfz2 is known to bind Wg (8).
FIG. 2.
Soluble Wg binds N in the region containing EGF-like repeats 19 to 36. Wg binds surfaces of S2-N (B), S2-NΔEGF1–18 (C), and S2-NΔI cells (E) but not surfaces of S2 (A) and S2-NΔEGF19–36 cells (D). Only N-expressing cells bind Wg (F), and the frequency of N-expressing cells binding Wg is comparable to the frequency of S2-Dfz2 cells binding Wg (G). (A to E and G) The photograph on the left shows anti-Wg immunofluorescence in a microscopic field of cells, and the photograph on the right shows Nomarski illumination of the same microscopic field of cells. (F) The photograph on the left shows immunofluorescence generated by the anti-Wg antibody, and the photograph on the right shows immunofluorescence generated in the same microscopic field of cells by an anti-N antibody. Cells were treated with unconcentrated culture medium conditioned by growth of S2-Wg cells. Wg on cell surfaces was detected immunocytochemically with anti-Wg (rb), an antibody made in rabbit (69), and a rhodamine-conjugated secondary antibody. N on cell surfaces was detected with αNI (52) and a fluorescein-conjugated secondary antibody. None of the cells incubated with culture medium conditioned by growth of S2 cells showed any detectable signals. Only cells expressing high levels of N are apparent in the photographs. An actual count of all immunofluorescent cells indicates a Wg-positive frequency of ∼40% (N is expressed by only 50% of the cells stably cotransfected with the hygromycin gene). A short binding period was used because N was found to be lost from the cell surfaces within minutes of treatment with Wg.
To determine whether Wg bound N or NΔEGF1–18 on S2 cells expressing these proteins, immunoprecipitations were performed. In experiments with S2-N/S2-Dl cell aggregates, the anti-Dl antibody used failed to immunoprecipitate either the full-length or the extracellular domain of N. This may have been due to the particular antibody used or the disruption of N-Dl complexes during lysis and washes. Fehon et al. (24) considered disruption of the N-Dl complexes, as a consequence of the disruption of the physiological conformation of the extremely cysteine-rich extracellular domain of N, the reason for their low recovery of full-length N in Dl coimmunoprecipitations. To overcome the disruption of interaction between N and its ligands when cells are lysed for immunoprecipitation, a membrane insoluble cross-linker, BS3, was used to covalently link proteins interacting at the cell surfaces. The activity of cross-linkers was quenched prior to lysis of cells so that cross-linking was limited to proteins interacting on the cell surfaces. BS3 and related cross-linkers have been used successfully in studies of several cell surface protein interactions (80, 81, 87).
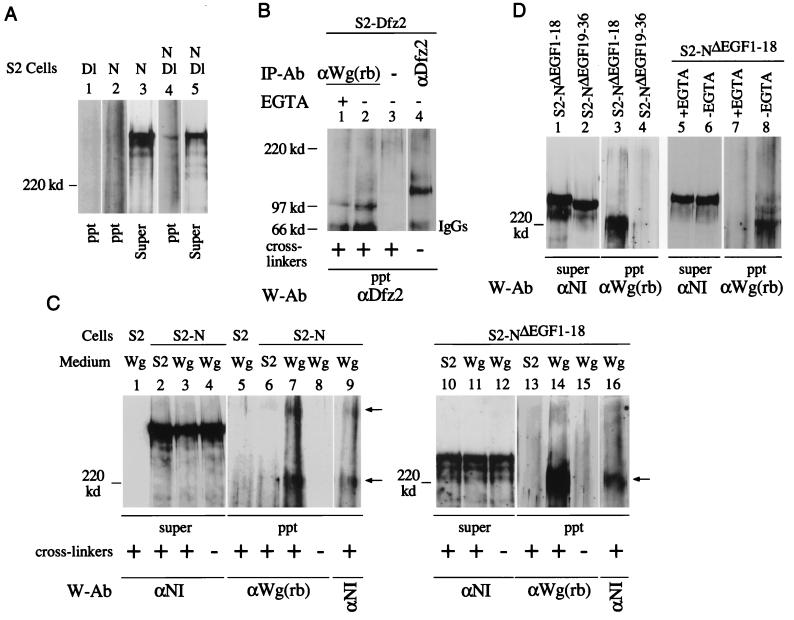
Immunoprecipitation of Dl from cross-linked protein extracts of S2-N/S2-Dl cell aggregates recovered a complex containing N and Dl (Fig. 3A, lane 4). The Dl-N complex was not recovered from S2-N cells in the absence of S2-Dl cells (lane 2) or from S2-Dl cells alone (lane 1). The levels of N in the supernatants of immunoprecipitates loaded in lanes 2 and 4 were comparable (lanes 3 and 5). Anti-Dl antibody failed to recover N when purified membranes of S2-N/S2-Dl cell aggregates or lysates of S2-N/S2-Dl cell aggregates were used, suggesting that the integrity of cells is indeed important for recovery of the Dl-N complex.
FIG. 3.
Two different Wg- and N-containing complexes are recovered from N-expressing S2 cell surfaces. (A) N- and Dl-containing complexes are recovered from S2-N and S2-Dl cell aggregates in the presence of cross-linkers. Dl-containing cross-linked complexes were immunoprecipitated by the monoclonal anti-Dl antibody, MAb 202 (24) and analyzed by Western blotting with anti-NI antibody. (B) Wg- and Dfz2-containing cross-linked complexes are recovered from S2-Dfz2 cells treated with Wg medium containing cross-linkers, in the presence or absence of EGTA (lanes 1 and 2). A mouse monoclonal anti-Dfz2 antibody (kindly provided by R. Nusse) was used for immunoprecipitation (lane 4) and for detection of Wg-Dfz2 complexes by Western blotting. Wg-Dfz2 complexes were not recovered from S2-Dfz2 cells treated with medium conditioned by growth of S2 cells (not shown). (C) Two Wg- and N-containing cross-linked complexes (arrows) are immunoprecipitated from S2-N cells (lanes 7 and 9), and only one is immunoprecipitated from S2-NΔEGF1–18 cells (lanes 14 and 16), treated with Wg-containing medium. N- and Wg-containing complexes were immunoprecipitated with anti-Wg(rb) and detected by Western blotting with the indicated antibodies (W-Ab). Lanes 7 and 9 and lanes 14 and 16 show reaction of the same blots with anti-Wg(rb) and anti-NI antibodies. (D) Wg and N containing cross-linked complexes are recovered from S2-NΔEGF1–18 cells (lane 3) in the absence of EGTA (lane 8) but not from S2-NΔEGF19–36 cells (lane 4). S2-NΔEGF1–18 or S2-NΔEGF19–36 cells were treated with Wg medium containing cross-linkers, immunoprecipitation was performed with anti-Wg(rb) antibody, and the Western blots were probed with the indicated antibodies. ppt, immunoprecipitated complexes eluted from GammaBind beads; Super, an aliquot of the protein extract after the last pelleting of the GammaBind beads (see Materials and Methods); IP-Ab, immunoprecipitation antibody; W-Ab, Western blotting antibody; cross-linker, BS3. Wg, medium conditioned by growth of S2-Wg cells; S2, medium conditioned by growth of S2 cells. For panels A, C, and D, 4% polyacrylamide gels were used; for panel B, 6% polyacrylamide gels were used. Only proteins or protein complexes migrating slower than a 120-kDa marker protein are resolved in panels A, C, and D. The tops of all the blots shown coincide with the top of the resolving gel of the discontinuous SDS-PAGE gels.
The capability of the cross-linking and immunoprecipitation procedure to recover proteins interacting at the cell surface was further tested with S2-Dfz2 cells treated with Wg. A Wg-Dfz2 complex, migrating at the rate of a ∼100-kDa protein, was recovered (Fig. 3B, lane 2). Unlinked Dfz2 migrated at ∼130 kDa (lane 4). The Wg-Dfz2 complex was not recovered in the absence of the Wg antibody (lane 3). The same Wg-Dfz2 complex was obtained when EGTA was used to chelate calcium in the medium (lane 1), indicating that calcium is not required for Wg and Dfz2 interaction.
The cross-linking and immunoprecipitation procedure that recovered the Dl-N and Wg-Dfz2 complexes was applied to S2-N and S2-NΔEGF1–18 cells treated with Wg. Two N- and Wg-containing complexes, with different electrophoretic mobilities, were recovered from S2-N cells (Fig. 3C, lane 7). Only one was recovered from S2-NΔEGF1–18 cells (lane 14). The same complexes were recognized by both anti-Wg and anti-N antibodies (lanes 7 and 9 and lanes 14 and 16), confirming that they contain both N and Wg. Wg- and N-containing complexes were not recovered from immunoprecipitations from S2-N or S2-NΔEGF1–18 cells treated with medium that does not contain Wg (lanes 6 and 13), from S2 cells treated with Wg medium (lane 5), or from S2-N or S2-NΔEGF1–18 cells treated with Wg medium without cross-linkers (lanes 8 and 15). The levels of N were similar in the protein extracts used for all these immunoprecipitations (lanes 2 to 4 and lanes 10 to 12). Note also that S2 cells do not produce N (lane 1). Since proteins or protein complexes analyzed in 4% gels migrate slower than a ∼120-kDa protein, neither the ∼45-kDa Wg monomers nor the Wg-Dfz2 complex migrating at ∼100 kDa are expected to be retained in the gel. Wg- and N-containing complexes were not recovered from either S2-NΔEGF19–36 cells treated with Wg medium (Fig. 3D, lanes 1 to 4) or S2-NΔEGF1–18 cells treated with Wg medium containing EGTA (lanes 5 to 8), indicating that Wg associates with N in the region containing EGF-like repeats 19 to 36 and requires calcium for this association.
Two Wg- and N-containing complexes were recovered from Wg-treated S2-N cells (Fig. 3C, lane 7). S2-N cells are designed to produce full-length N, and most N produced by this cell line is of the size expected from full-length N (∼350 kDa [lanes 2 to 4]). The Wg-N complex migrating near a ∼220-kDa marker protein probably included a truncated N because S2-NΔEGF1–18 cells also produce it (lane 14). NΔEGF1–18 encodes a protein that lacks about half of the N extracellular domain EGF-like repeats (52). If N in the faster-migrating Wg-N complex is a truncated form, it was preferentially enriched or generated by Wg since a truncated form of N was not detectable in S2-N cell protein extracts (Fig. 3C, lanes 2 and 3; see also Fig. 6E, lane 4). A faster-migrating Dl-N complex was not recovered in Dl immunoprecipitations (Fig. 3A).
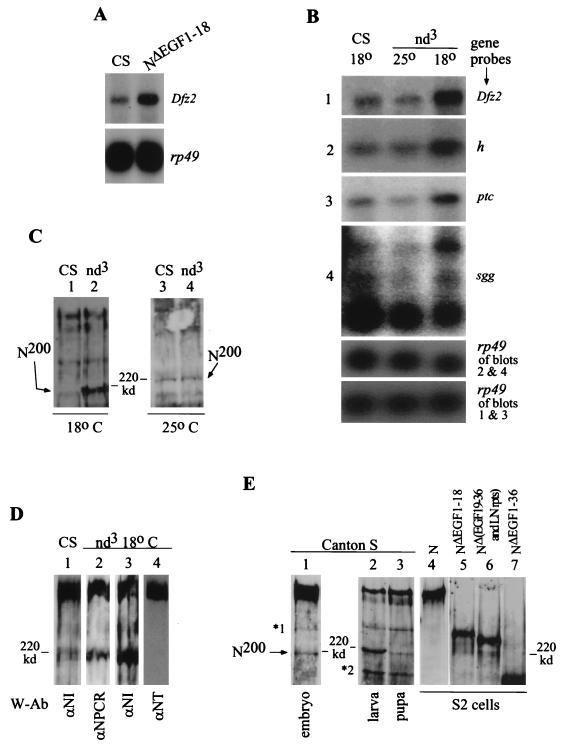
FIG. 6.
NΔEGF1–18 and nd3 embryos show increased expression of genes regulated by NΔEGF1–18 in vitro, and an endogenous form of N resembling NΔEGF1–18 is overproduced in nd3 embryos. (A) Embryos carrying the heat shock-inducible NΔEGF1–18 transgene also overexpress Dfz2. We heat shocked 0- to 20-h Canton S (CS) (yw strain) and NΔEGF1–18 (in Canton S, yw strain) embryos for 30 min, allowed them to recover at room temperature for 45 min, and extracted total RNAs for Northern blot analysis. rp49 indicates relative levels of RNA in different lanes. (B) Dfz2, h, ptc, and sgg are overexpressed in nd3 embryos at 18°C, the temperature at which the overt mutant phenotype is observed. At 25°C, expression of these genes in nd3 does not differ from that in Canton S. Levels of expression in Canton S at 25 and 18°C are indistinguishable (expression at 18°C is shown). Total RNAs were extracted from 0- to 20-h Canton S and nd3 embryos, reared at 25 or 18°C (with appropriate correction for developmental times), and analyzed by Northern blotting. Gene sequences used as probes are shown at the right. Exposure times: rp49 < Dfz2 < ptc < sgg < h. (C) nd3 embryos at 18°C overproduce a ∼200-kDa form of N, N200. Because signals from high-molecular-weight forms of N interfere with assessment of levels of the less abundant N200, total embryonic proteins extracted from 0- to 12-h Canton S and nd3 embryos (at 18 or 25°C) were incubated with anti-NT and cleared prior to SDS-PAGE. Anti-NT does not react with N200 (see below). Extracts containing equivalent levels of ∼350-kDa N were used for lanes 1 and 2. N is detected with anti-NI. (D) N200 is truncated in the amino terminus. N was immunoprecipitated from 0- to 12-h Canton S or nd3 embryos (reared at 18°C) by using anti-NI and separated by SDS-PAGE (4% polyacrylamide), and the Western blots were probed with the indicated antibodies (W-Ab). See Fig. 4D for epitopes for N antibodies. nd3 embryos were used to determine missing epitopes because they produce higher levels of N200 than do Canton S embryos (compare lanes 1 and 3). (E) Different developmental stages of Canton S flies express N200, and N200 lacks more than 18 of the amino-terminal EGF-like repeats. Aliquots of total proteins extracted from Canton S embryos (0 to 3 h), one Canton S larva, one Canton S pupa, S2-N cells, S2-NΔEGF1–18 cells, S2-NΔEGF19–36 and LN rpts cells, and S2-NΔEGF1–36 cells were separated by SDS-PAGE (4% polyacrylamide) and analyzed by Western blotting with anti-NI. ∗1 is recognized by all of the N antibodies studied and is therefore considered to be the partially denatured form of N (43); ∗2 is not recognized by anti-NT and anti-NPCR (not shown) but is recognized by anti-NI.
Wg-N complexes similar to those recovered from cultured cells are recovered from Canton S embryonic extracts.
To determine whether the two Wg-N complexes produced in cultured cells treated with Wg are also produced in vivo, immunoprecipitations were performed with proteins extracted from Canton S embryos. The most frequent and predominant Wg-N complex recovered from young embryos (0 to 3 h or 0 to 6 h) was Wg complexed with a truncated N (N lacking the anti-NT epitope) (Fig. 4A, lanes 10 to 13, see Fig. 4D for epitopes of N antibodies). The electrophoretic mobility of this complex was similar to that of the faster-migrating Wg-N complex (i.e., near the ∼220-kDa marker protein) recovered from cultured cells (Fig. 3C). From aliquots of the same embryonic extracts, Dl was found complexed with N recognized by all three N antibodies (Fig. 4A, lanes 7 to 9). This N in the Dl-N complex is apparently the full-length N.
Wg complexed with the truncated N was also recognized by an independently generated anti-Wg antibody (Fig. 4A, lane 14). This independent antibody (made in the rat) also immunoprecipitated the same Wg-N complex (lane 15). Probing of the blots with anti-N and anti-Wg antibodies showed that this Wg-N complex contains both Wg and N (lanes 10 and 11 and lanes 15 and 16). Similar probings showed that the Dl-N complex actually contains both N and Dl (lanes 5 and 6). Anti-N antibodies do not recognize unlinked Wg, Dl, or Ser; anti-Wg antibodies do not recognize unlinked N, Dl, or Ser; anti-Dl antibodies do not recognize unlinked N, Wg, or Ser; and anti-Ser antibodies do not recognize unlinked N, Dl, or Wg (data not shown). Therefore, recognition of the same complex by two different antibodies indicates the presence of both of the proteins in the complex. Similar mobilities of the N-Dl complexes or N-Ser complexes (see below) and unlinked full-length N might be due to the resolution limitations of SDS–4% polyacrylamide gels or to anomalous mobilities of cross-linked complexes (Dfz2-Wg complexes migrate faster than unlinked Dfz2 [Fig. 3B]).
As with cultured cells, Dl was not recovered with truncated N (Fig. 4A, lanes 7 to 9) suggesting that truncated N specifically associated with Wg. The slower-migrating Wg-N complex was generally recovered at low levels in extracts prepared from 0- to 6-h embryos. Higher levels of the slower-migrating Wg-N complex were recovered from extracts prepared from 6- to 12-h embryos (lane 17), and this was the only Wg-N complex recovered from extracts prepared from 10- to 16-h embryos (lanes 18 and 19). Unlike the N-Wg complex migrating near the ∼220-kDa marker protein, this slower-migrating complex reacted with all anti-N antibodies, anti-NI (lane 17), anti-NT (lane 18), and anti-NPCR (data not shown), indicating that it contains the full-length N. Neither Dl nor Wg recovered any N molecules in the absence of cross-linkers (lanes 1 to 4). All immunoprecipitations were repeatedly confirmed. A Wg-containing complex migrating at the rate of a 250- to 300-kDa protein was recovered at all times during embryogenesis (lanes 10 and 19). This complex appeared not to contain N, since none of the anti-N antibodies recognized it: lanes 10 and 11 and lanes 18 and 19 are the same blots probed with an anti-Wg antibody and two different anti-N antibodies (anti-NPCR also does not recognize it [lane 12]).
The immunoprecipitation procedure used to recover Wg-N and Dl-N complexes also recovered the expected Ser-N and Patched (Ptc)-Hedgehog (Hh) complexes from Drosophila embryonic extracts (Fig. 4B and C, lanes 3 and 4). Note that anti-Wg immunoprecipitates fail to recover Hh or Ptc (Fig. 4C, lanes 2, 5, and 6). Anti-Wg immunoprecipitates separated by SDS-PAGE in 6% polyacrylamide gels showed the Wg-Dfz2 complex migrating at ∼100 kDa, similar to the complex recovered from S2-Dfz2 cells treated with Wg (Fig. 4C, lane 1). This complex will not be retained in 4% polyacrylamide gels and therefore is not seen in Fig. 4A. These results confirm that the cross-linking and immunoprecipitation procedure used to recover Wg-N complexes also recovers other complexes expected to be formed during embryogenesis. All the immunoprecipitation experiments together argue strongly in favor of the simplest proposal that Wg binds N directly.
The above-described experiments showed that (i) two forms of N associate with Wg under in vitro and in vivo conditions, (ii) one form of N lacks a portion of the amino-terminal EGF-like repeats, and (iii) the association of Wg with N is dependent on the region of N containing EGF-like repeats 19 to 36.
Wg regulates expression of Dfrizzled2, hairy, patched, and shaggy genes in S2 cells expressing N and NΔEGF1–18.
The cell surface binding and immunoprecipitation experiments described above showed that N and Wg form physical complexes in vitro and in vivo. But does Wg alter the physiological state of cells through N? This question is not easily answered for the in vivo case because N is required for production of epidermal precursor cells through lateral inhibition functions associated with Dl (11, 66, 79, 90) and is also subsequently required for production of epidermis from these epidermal precursor cells (15, 36). Of these two successive developmental events, Wg is required only for production of epidermis from the epidermal precursor cells (4, 7). Any perturbation of the lateral inhibition functions of N (associated with Dl) is expected to mask epidermal functions of N associated with Wg. Therefore, I explored the response of N-expressing S2 cells to Wg in the medium and tested wild-type and mutant N embryos for comparable responses. Since regulation of endogenous gene activities in response to exogenous factors in the medium is a good indicator of the signaling effects of cell surface ligand-receptor interactions, the expression of a selected sample of genes that are linked to the N and Wg signaling pathways was assessed in N-expressing S2 cells treated with Wg.
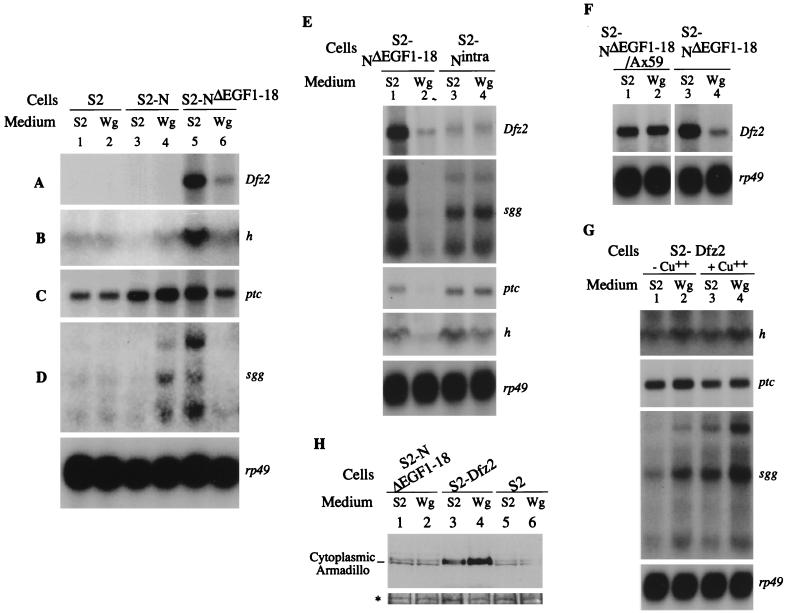
The expression patterns of m5 and m8 genes of the Enhancer of split Complex [E(spl)C; genes associated with Dl-mediated functions of N], wg, ac, en, hh, h, ptc, Dfz2, sgg, and several housekeeping genes were tested in S2-N and S2-NΔEGF1–18 cells in the presence and absence of Wg. Only Dfz2, ptc, sgg, and h expression was affected in these experiments. Interestingly, the full-length N and N truncated in the amino terminus (NΔEGF1–18), the two types of N molecules found associated with Wg in cultured cells and embryos, affected the expression of these genes differently. Expression of full-length N in S2 cells did not affect expression of Dfz2, ptc, sgg, and h (Fig. 5A to D, lanes 3). However, expression of NΔEGF1–18 in S2 cells strongly induced expression of these four genes, independent of any ligands (lanes 5). This difference indicated that the expression of N with a truncated extracellular domain results in ligand-independent induction of expression of Dfz2, ptc, h, and sgg. Treatment of S2-N cells with Wg induced or increased the expression of ptc and sgg (Fig. 5C and D, lanes 4). Treatment of S2-NΔEGF1–18 cells, on the other hand, suppressed the expression of genes that were induced independently of ligands (Fig. 5A to D, lanes 6). Among the four genes, expression of Dfz2 and h were affected solely by NΔEGF1–18 (Fig. 5A and B). The level of sgg and ptc expression observed in S2-N cells treated with Wg is likely to be the net expression level due to an increase in expression promoted by the full-length N- and Wg-containing complex and to a decrease in expression promoted by the truncated N- and Wg-containing complex (also formed on S2-NΔEGF1–18 cells). However, it is also possible that there is no contribution from the truncated N formed in S2-N cells, because Wg is always associated with it under the experimental conditions and prevented ligand-independent induction of gene expression.
FIG. 5.
N and NΔEGF1–18 have different ligand-independent activities and respond differently to Wg. (A to D) Expression of Dfz2, h, ptc, and sgg in S2-N and S2-NΔEGF1–18 cells are regulated by Wg. en, wg, ac, hh, and m5 and m8 of E(spl)C were not detected in any of the experiments. The sizes of transcripts of all genes were similar to published reports. Dfz2 (8), ptc (35), h (37), rp49 (61), and sgg (77). The largest sgg RNA corresponds in size to the embryonic transcript, while the smallest sgg RNA has a size expected for the ovarian transcript (77). sgg, wg, ac, and m5 and m8 of E(spl)C are known to genetically interact with N (15, 33, 71, 72, 85); Dfz2, ptc, wg, and sgg, are involved in epidermal patterning (4, 7, 8, 20, 35, 57, 65); h is a negative regulator of ac (38, 79, 84). (E) The extracellular domain of NΔEGF1–18 is required for regulation of Dfz2, sgg, ptc, and, to a lesser extent, h expression. (F) Ax59d mutation in NΔEGF1–18 abolishes Wg-mediated down regulation of Dfz2 expression in S2-NΔEGF1–18 cells. The two autoradiographs were derived from the same blot with different exposure times. (G) S2-Dfz2 cells (S2-pMK 33 cells [8]) do not down regulate expressions of ptc, sgg, and h in response to Wg, with or without copper induction. (A to G) Total RNAs were extracted from the indicated cells treated with M3 medium conditioned by growth of S2 cells (S2 medium) or S2-Wg cells (Wg medium) and analyzed by Northern blotting. The same batch of S2 or Wg medium was used for all of the studies. Gene sequences used as probes are indicated on the right of each panel. rp49 was used to indicate the relative levels of RNA in different lanes. The individual blots are exposed to film for different periods. Exposure times: rp49 < Dfz2 < ptc < sgg < h. (H) S2-NΔEGF1–18 cells do not accumulate Arm in the cytoplasm in response to Wg. The bottom panel (∗) shows a India ink-stained protein band visible in all lanes of the blot to indicate the amount of samples loaded. An anti-Arm antibody made in rabbits (kindly provided by Laurent Ruel) was used to detect Arm.
S2-N cells do not express any other gene(s) known to bind Wg. However, S2-NΔEGF1–18 cells express Dfz2, whose product is known to bind Wg (8). To determine whether the downregulation of genes in S2-NΔEGF1–18 cells is due to NΔEGF1–18 or Dfz2, Dfz2, sgg, ptc, and h expression were assessed in S2-Nintra cells in the presence and absence of Wg. S2-Nintra cells produce N without the extracellular domain (52). If association of Wg with NΔEGF1–18 is required for suppression of the activities of these genes, the level of Dfz2, sgg, ptc, and h expression in S2-Nintra cells should not be altered by the presence of Wg in the medium. S2-Nintra cells were found to express these genes at lower levels than were S2-NΔEGF1–18 cells. However, the levels of Dfz2, sgg, and ptc expression in these cells were not affected by Wg in the medium, and the level of h expression was only slightly reduced (Fig. 5E), indicating that the down regulation of gene expression in Fig. 5A to D, lanes 6, was due to association of Wg with NΔEGF1–18. Nintra behaves as an activated N receptor with respect to Dl signaling (52, 82). Since N and NΔEGF1–18 respond oppositely to Wg, and since NΔEGF1–18 has a strong ligand-independent activity, Nintra activity shown in Fig. 5E is possibly a combination of N and NΔEGF1–18 activities, with and without Wg.
The requirement of the extracellular domain of NΔEGF1–18 for suppression of gene activities by Wg was also tested in a stable cell line transfected with NΔEGF1–18 carrying the Ax59d mutation. Ax59d is a lethal allele of N because of a mutation in EGF-like repeat 24 (41). This allele manifests antineurogenic phenotypes and shows strong genetic interaction with wg (10, 15, 17, 18). Figure 4F shows that unlike S2-NΔEGF1–18 cells, S2-NΔEGF1–18/Ax59d cells treated with Wg did not suppress expression of Dfz2 (Fig. 5F). Thus, the region containing EGF-like repeat 24 appears to be important for Wg-mediated suppression of gene activities in S2-NΔEGF1–18 cells. This region is as conserved as the Dl binding region of N (Fig. 1A).
To further clarify the roles of NΔEGF1–18 and Dfz2 in suppression of gene expression in S2-NΔEGF1–18 cells, the gene expression pattern was determined in S2-Dfz2 cells (pMK 33 S2-Dfz2 cells [8]). Expression of endogenous Dfz2 cannot be assessed in this cell line because of induction of the transfected Dfz2 gene through an ectopic promoter. Instead, the expression levels of genes that were coregulated with Dfz2, namely, ptc, sgg, and h, were determined with and without metal induction of Dfz2 expression. S2-Dfz2 cells are responsive to Wg both with and without metal induction (8). The expression of ptc, sgg, and h in response to Wg was not reduced in S2-Dfz2 cells (Fig. 5G). The expression of these genes seemed to be slightly but consistently increased in response to Wg. Since Wg promotes the accumulation of Armadillo (Arm) in the cytoplasm of S2-Dfz2 cells (8), the cytoplasmic level of Arm in S2-NΔEGF1–18 cells was also assessed to determine the level of Dfz2 activity in S2-NΔEGF1–18 cells. The level of Arm in the cytoplasm of S2-NΔEGF1–18 cells did not change in response to Wg (Fig. 5H, lanes 1 to 2). In several repetitions of the experiment, the response of S2-NΔEGF1–18 cells was no different from that of S2 cells (lanes 5 and 6). On the other hand, S2-Dfz2 cells accumulated Arm in the cytoplasm (lanes 3 and 4).
The experiments with S2-Nintra, S2-NΔEGF1–18/Ax59d, and S2-Dfz2 cells indicated that the suppression of Dfz2, ptc, sgg, and h expression by S2-NΔEGF1–18 cells in response to Wg was mediated by NΔEGF1–18 rather than Dfz2. It is also likely that the increase in ptc and sgg expression in S2-N cells treated with Wg is due to N and not any other (unknown) receptor induced by N.
N lacking amino-terminal EGF-like repeats is associated with Dfz2 expression in vivo.
Full-length N and a truncated N were associated with Wg in S2 cells and embryos (Fig. 3 and 4). In S2 cells, the full-length N and the truncated NΔEGF1–18 were active and responsive to Wg in different ways: N did not induce gene expression in the absence of ligands, whereas NΔEGF1–18 did; N up regulated the expression of genes in response to Wg, whereas NΔEGF1–18 down regulated the expression of genes in response to Wg (Fig. 5A to D). Thus, the two N-Wg complexes formed in embryos (Fig. 4) appear to have the potential to trigger different intracellular activities in vivo. To determine whether NΔEGF1–18 shows the same activity in vivo that it showed in vitro, expression of Dfz2 was assessed in embryos expressing the transgenic NΔEGF1–18. Dfz2 expression was assessed because in S2 cells it was regulated only by NΔEGF1–18; S2-N cells did not induce or regulate Dfz2 expression (Fig. 5A). Figure 6A shows that NΔEGF1–18 embryos overexpress Dfz2, indicating that NΔEGF1–18 behaves similarly in vitro and in vivo.
To find out whether endogenous N affects Dfz2 expression in embryos, Dfz2 RNA levels were compared between N mutant and Canton S embryos. Embryos of nd3, a homozygous viable temperature-sensitive allele of N (75), were found to overexpress RNA of Dfz2 and other genes coregulated with Dfz2 in vitro, i.e., ptc, sgg, and h. These RNAs were overexpressed at 18°C (the restrictive temperature) but not at 25°C (Fig. 6B). The overexpression was specific, since the levels of RNAs of wg, ac, and the E(spl)C genes m5 and m8 were not increased in nd3 embryos at 18°C (data not shown; these genes were also not regulated by N or NΔEGF1–18 in vitro). That a temperature-sensitive N allele accumulates Dfz2 in a temperature-sensitive manner indicates that endogenous N also affects Dfz2 expression in embryos.
Since (i) expression of Dfz2 was associated only with NΔEGF1–18 in S2 cell experiments, (ii) expression of NΔEGF1–18 in embryos resulted in overexpression of Dfz2, and (iii) the mutation in nd3 is not in the region mediating suppression of Dfz2 expression (Fig. 1A and 5), it was likely that nd3 embryos overproduced the endogenous form of N affecting Dfz2 expression in vivo. Western blotting analyses revealed that nd3 embryos indeed produced higher than Canton S levels of a ∼200-kDa form of N. Just like expression of the Dfz2, ptc, sgg, and h genes, this ∼200-kDa form, designated N200, was overexpressed in nd3 embryos at 18 but not 25°C (Fig. 6C). spl, N264-47, spl Ax59d, Ax59d, Ax9B, and other N alleles did not show increased level of N200 (data not shown). N200 lacks the amino-terminal EGF-like repeats, since it is not recognized by anti-NT (Fig. 6D). It is produced at low levels in embryos (relative to the full-length ∼350-kDa form of N) but is expressed at much higher levels in larvae and pupae (Fig. 6E, lanes 1 to 3). N200 migrates between NΔEGF1–18 and NΔEGF1–36 (lanes 4 to 7), indicating that it is lacking more than 18 of the amino-terminal EGF-like repeats but includes a significant fraction of the carboxy-terminal half of the EGF-like repeats. The association of overexpression of Dfz2 with overexpression of N200 in nd3 embryos suggests that N200 is the form of N that affects Dfz2 expression in vivo. A fine-scale developmental analysis indicated that the full-length N was also overexpressed in nd3 embryos at certain periods of embryogenesis (data not shown). Therefore, overexpression of Dfz2 in nd3 embryos, and possibly that of h as well, is most probably due to overproduction of N200 (since full-length N is not associated with Dfz2 expression in vitro) and overexpression of ptc and sgg is most probably due to overproduction of both N and N200 (since both N and NΔEGF1–18 regulate ptc and sgg in vitro [Fig. 5A to D]). nd3 embryos show the same level and size of N RNA as Canton S embryos do (data not shown). Thus, it appears that EGF-like repeat 2 (the site of mutation in the nd3 allele [Fig. 1A]) is important for posttranslational production of N200 from the full-length N or for regulation of the levels of full-length N during development. However, the actual mechanism of generation of the truncated N200 is unknown.
If only heterodimeric N receptors are present on cell surfaces of S2 cells and embryos (9, 53, 63; see also reference 43), the cross-linkers used in immunoprecipitations must have covalently linked the two fragments composing each of the three cell surface receptors, i.e., the full length N, NΔEGF1–18, and N200, and their ligands (Fig. 3 and 4). The reported ∼110-kDa intracellular product is not retained in the gels used in Fig. 6C to E, and the extracellular product would not be recognized by anti-NI or anti-NPCR (Fig. 4D).
N− and Ax mutant embryos show altered expression patterns of epidermal patterning genes that are consistent with expression patterns observed in vitro.
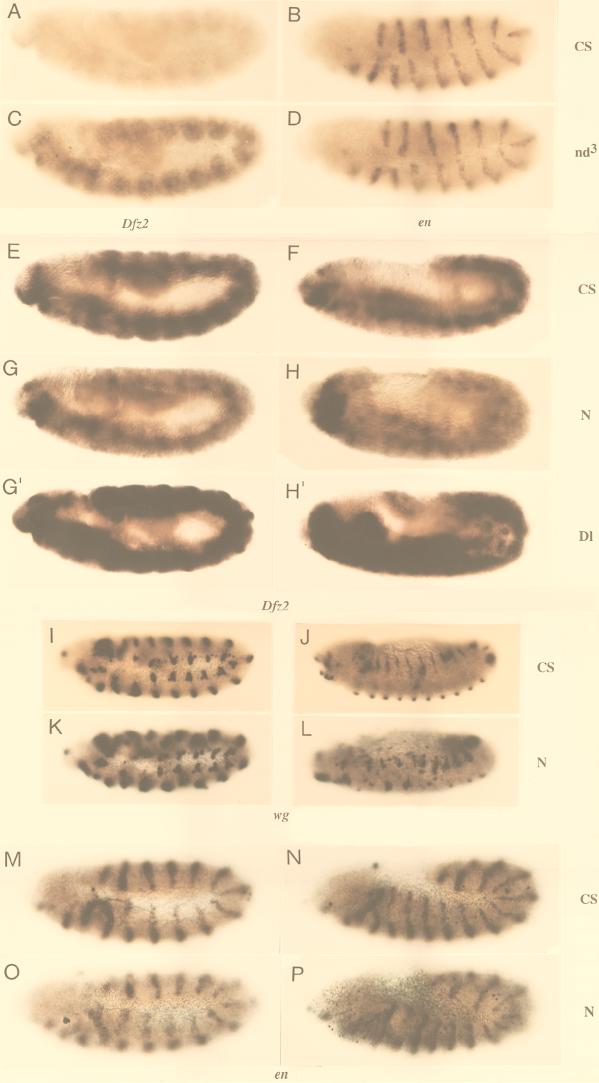
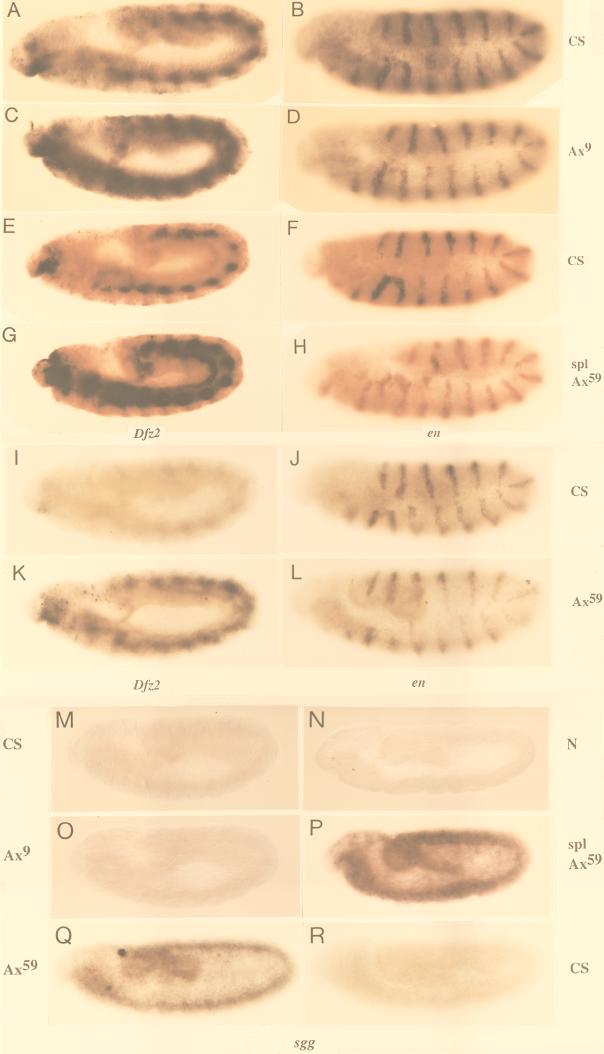
A further test of involvement of N in expression of cuticle-patterning genes in vivo would be that N− and Ax embryos (which do not overproduce N200) show low and high expression, respectively, of the same genes. Since N− and Ax alleles are homozygous lethal, in situ hybridization rather than Northern blotting was used. In situ hybridization of nd3 embryos with Dfz2 and en probes (the latter serving as a control for levels of RNA in embryo) show that the results are qualitatively comparable to results obtained by Northern blotting (Fig. 7A to D).
FIG. 7.
Expression of Dfz2 is reduced in zygotic N− embryos, while expression of en and wg are unaffected. (A to D) In situ hybridization of Canton S and nd3 embryos corroborates results from Northern blotting that Dfz2 is overexpressed in nd3 embryos but en is not (E to H, G′, H′) Dfz2 expression is lost in N264-47/Y embryos but not in comparable stages of Canton S or DlX/DlX embryos. (I to L) wg expression is not lost in N264-47/Y. (M to P) en expression is similar in Canton S and N264-47/Y embryos. CS, Canton S; nd3, nd3; N, N264-47/Y; Dl, DlX/DlX embryos; genes used as probes are indicated below the appropriate sets of embryos. Anterior is to the left of each embryo. N264-47/Y and DlX/DlX embryos were identified by lack of β-galactosidase staining associated with the FM7 or TM6 balancer chromosomes (see Materials and Methods). Embryos A to D were processed simultaneously, and so were embryos E to P.
Stage-specific comparisons of Canton S and zygotic N− or Ax embryos showed that levels of Dfz2 and sgg RNA are indeed as expected from in vitro results. N264-47/Y embryos expressed lower levels of Dfz2 RNA than did Canton S embryos and DlX embryos (Fig. 7E to H). The levels of wg and en RNA did not differ significantly between N264-47/Y and Canton S embryos (Fig. 7I to L, wg M-P, en), indicating that the loss of Dfz2 transcripts is not due to a general suppression of RNA accumulation in N264-47/Y embryos or loss of wg expression. Conversely, Ax59d, Ax9B (both carrying mutations in EGF-like repeat 24 [41]), and spl Ax59d embryos overproduced Dfz2 RNA but not en RNA (Fig. 8A to L; spl embryos do not overexpress Dfz2 [data not shown]). A similar pattern of expression was manifest with sgg as well (Fig. 8M to R).
FIG. 8.
Ax embryos overproduce Dfz2 and sgg RNA. (A to L) Ax embryos overproduce Dfz2 RNA (A, C, E, G, I, and K) but not en RNA (B, D, F, H, J, and L). (M to R) Ax embryos overproduce sgg RNA (O to Q) but not Canton S (M and R) and N264-47/Y embryos (N). Due to low-level of expression in a general pattern, reduced sgg expression (as in panel N) and weak sgg overexpression (as in panel O) are more obvious in pools of embryos than in individual embryos. CS, Canton S; Ax9, Ax9B/Y; spl Ax59, spl Ax59d/Y; Ax59, Ax59d/Y; N, N264-47/Y. Anterior is to the left of each embryo. Homozygous Ax or N embryos were identified by the lack of β-galactosidase staining associated with the FM7 balancer chromosomes (see Materials and Methods). Embryos A to H and M to P were processed simultaneously, and so were embryos I to L and Q to R.
DISCUSSION
N Regions within EGF-like repeats 19 to 36 mediate interactions with Wg.
Wg was identified as a putative ligand of N in a cell surface screen with phagemids carrying cDNA sequences from Drosophila embryos. Further analyses showed that Wg and N form molecular complexes, both in vitro and in vivo. EGF-like repeats 19 to 36 of N are required for the association of these two proteins (Fig. 2 to 4). These repeats of N include two strongly conserved regions, those containing EGF-like repeats 23 to 27 and EGF-like repeats 31 to 34 (Fig. 1A). Experiments with the Ax59d allele (Fig. 5F and 8) showed that EGF-like repeat 24 is important for Wg-mediated down regulation of gene expression through NΔEGF1–18. N200 is identified as the in vivo equivalent of NΔEGF1–18. Since the nd3 embryos (overproducing N200), Ax59d embryos, and S2-NΔEGF1–18/Ax59d cells overexpress the same genes down regulated by Wg and NΔEGF1–18 (Fig. 5F and 8), the region containing EGF-like repeats 23 to 27 might be involved in down regulation of gene expression by Wg and N molecules in vivo. The evolutionary conservation of this region in homologous N molecules might be due to the Wg-associated functions of N. Whether Wg associates with the same region in full-length N, for induction of sgg and ptc expression, is not known. Preliminary results suggest that Wg associates at a second site within the region containing EGF-like repeats 19 to 36 of N.
Two forms of Notch regulate expression of epidermal patterning genes.
Immunoprecipitations from cultured cells and embryos recovered Wg complexed with the full-length N and a form of N lacking EGF-like repeats in the amino terminus (Fig. 3 and 4). In vitro experiments showed that two forms of N regulate expression of cuticle-patterning genes, i.e., sgg and ptc by the full-length N in response to Wg, and Dfz2, sgg, h, and ptc by a form of N lacking 18 amino-terminal EGF-like repeats, NΔEGF1–18, both independent of ligands and in response to Wg. Thus, Wg behaves as a ligand for two different forms of N in vitro, eliciting different responses from cells expressing these two forms of N. The significant difference between N and NΔEGF1–18 is the lack of the Dl binding region in NΔEGF1–18. This appears to be true of N200 as well. N and N200 might therefore regulate genes in vivo in a manner comparable to gene regulations by N and NΔEGF1–18 in vitro. The in vivo relative levels of the full-length N (capable of associating with Dl and Wg) and N200 (capable of associating with Wg) may therefore represent a differential commitment of N to Dl or Wg signaling during embryogenesis and a differential commitment of N to different kinds of Wg signaling. Since N is required not only for different developmental functions but also for sequential developmental functions (13, 76), it is quite possible that N and N200 constitute important components of the mechanism of N function at successive steps of differentiation. There is some indication that the activity of NΔEGF1–18 requires the activity of N in a preceding step: expression of NΔEGF1–18 in N264-47/Y embryos prior to onset of the neurogenic phenotype results in overexpression of h and sgg (as expected, in the expected stage-specific pattern for h), but this does not occur in the neurogenic embryos (data not shown).
The intracellular pathways associated with transduction of signals by N and NΔEGF1–18 do not appear to involve the expression of the m5 and m8 genes of E(spl)C that are associated with activation of N by Dl (26, 33, 39, 48, 85) or Wg-mediated stabilization of Arm in the cytoplasm (Fig. 5H) observed with Wg and Dfz2 receptor (8, 34, 60, 65). Therefore, novel pathways appear to be transducing signals to the nucleus of S2-NΔEGF1–18 or S2-N cells, in both the presence and absence of Wg.
In conclusion, the strong genetic interaction between N and wg functions during Drosophila development (10, 15, 16, 19, 21, 44, 58, 59, 72) could be due to regulation of wg expression by N (19, 72), suppression of lateral inhibition signaling of N by Dsh (3), and physical associations of Wg with the two forms of N for the purpose of regulation of cuticle patterning genes. Regulation of negative regulators of en and ac expression by Wg, namely, sgg and h, respectively (38, 62, 65, 78, 79, 84), and the involvement of two different forms of N with different activities could explain previous contradictory results regarding the role of N in Wg signaling. NΔEGF1–18 induces the expression of sgg and h in S2 cells (Fig. 5B and D). Loss of N and therefore loss of N200 (the putative in vivo equivalent of NΔEGF1–18) would result in loss of sgg and h expression, leading to loss of inhibition of en and ac expression, consistent with the results of Rulifson and Blair (72) and Cadigan and Nusse (12). On the other hand, overexpression of sgg (Fig. 8M to R) and h (not shown) in Ax mutants could interfere with the stabilization of en and ac expression, consistent with the results of Couso and Martinez-Arias (15).
ACKNOWLEDGMENTS
I thank Michael Young for his support, advice, experimental suggestions, and review of the manuscript. I thank Lino Saez for research materials, technical advice, and critical assessment of data; Alfonso Martinez-Arias for research materials, advice, encouragement, and review of the manuscript; Toby Lieber for research materials and suggestions; Simon Kidd, Frieda Reichsman, Susan Cumberledge, Stephen Pronovost, Roel Nusse, Armen Manoukian, Vuk Stambolic, Laurent Ruel, Mark Muskavitch, Keiko Sawai, Claude Desplan, Anthony Brown, and Michael Caudy for research materials; and Amy Bejsovec for review of the manuscript.
This work was supported by NIH grant GM 25103 to Michael W. Young.
REFERENCES
- 1.Alton A K, Fechtel K, Kopczynski C C, Shepard S B, Kooh P J, Muskavitch M A T. Molecular genetics of Delta, a locus required for ectodermal differentiation in Drosophila. Dev Genet. 1989;10:261–272. doi: 10.1002/dvg.1020100315. [DOI] [PubMed] [Google Scholar]
- 2.Artavanis-Tsakonas S, Matsuno K, Fortini M E. Notch signaling. Science. 1995;268:225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- 3.Axelrod J D, Matsuno K, Artavanis-Tsakonas S, Perrimon N. Interaction between Wingless and Notch signaling pathways mediated by Dishevelled. Science. 1996;271:1826–1832. doi: 10.1126/science.271.5257.1826. [DOI] [PubMed] [Google Scholar]
- 4.Baker N E. Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: the spatial distribution of a transcript in embryos. EMBO J. 1987;6:1765–1773. doi: 10.1002/j.1460-2075.1987.tb02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker N E, Mlodzik M, Rubin G M. Spacing differentiation in the developing Drosophila eye: a fibrinogen-related lateral inhibitor encoded by scabrous. Science. 1990;250:1370–1377. doi: 10.1126/science.2175046. [DOI] [PubMed] [Google Scholar]
- 6.Bate M, Rushton E, Frash M. A dual requirement for neurogenic genes in Drosophila myogenesis. Dev Suppl. 1993;119:149–161. [PubMed] [Google Scholar]
- 7.Bejsovec A, Martinez-Arias A. Roles of wingless in patterning the larval epidermis of Drosophila. Development. 1991;113:471–485. doi: 10.1242/dev.113.2.471. [DOI] [PubMed] [Google Scholar]
- 8.Bhanot P, Brink M, Samos C H, Hsieh J-C, Wang Y, Macke J P, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- 9.Blaumueller C M, Qi H, Zagouras P, Artavanis-Tsakonas S. Intracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane. Cell. 1997;90:281–291. doi: 10.1016/s0092-8674(00)80336-0. [DOI] [PubMed] [Google Scholar]
- 10.Brennan K, Tateson R, Lewis K, Martinez-Arias A. A functional analysis of Notch mutations in Drosophila. Genetics. 1997;147:177–188. doi: 10.1093/genetics/147.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabrera C V. Lateral inhibition and cell fate during neurogenesis in Drosophila: the interactions between scute, Notch and Delta. Development. 1990;109:733–742. [PubMed] [Google Scholar]
- 12.Cadigan K M, Nusse R. wingless signaling in the Drosophila eye and embryonic epidermis. Development. 1996;122:2801–2812. doi: 10.1242/dev.122.9.2801. [DOI] [PubMed] [Google Scholar]
- 13.Cagan R L, Ready D F. Notch is required for successive cell decisions in the developing Drosophila retina. Genes Dev. 1989;3:1099–1112. doi: 10.1101/gad.3.8.1099. [DOI] [PubMed] [Google Scholar]
- 14.Corbin V, Michelson A M, Abmayr S M, Neel V, Alcamo E, Maniatis T, Young M W. A role for the Drosophila neurogenic genes in mesoderm differentiation. Cell. 1991;67:311–323. doi: 10.1016/0092-8674(91)90183-y. [DOI] [PubMed] [Google Scholar]
- 15.Couso J P, Martinez-Arias A. Notch is required for wingless signaling in the epidermis of Drosophila. Cell. 1994;79:259–272. doi: 10.1016/0092-8674(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 16.Couso J P, Knust E, Martinez-Arias A. Serrate and wingless cooperate to induce vestigial gene expression and wing formation in Drosophila. Curr Biol. 1995;5:1437–1448. doi: 10.1016/s0960-9822(95)00281-8. [DOI] [PubMed] [Google Scholar]
- 17.de Celis J F, Garcia-Bellido A. Modifications of the Notch function by Abruptex mutations in Drosophila melanogaster. Genetics. 1994;136:183–194. doi: 10.1093/genetics/136.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Celis J F, Barrio R, del Arco A, Garcia-Bellido A. Genetic and molecular characterization of a Notch mutation in its Delta- and Serrate-binding domain in Drosophila. Proc Natl Acad Sci USA. 1993;90:4037–4041. doi: 10.1073/pnas.90.9.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz-Benjumea F J, Cohen S M. Serrate signals through Notch to establish a wingless-dependent organizer at the dorsal/ventral compartment boundary of the Drosophila wing. Development. 1995;121:4215–4225. doi: 10.1242/dev.121.12.4215. [DOI] [PubMed] [Google Scholar]
- 20.DiNardo S, Sher E, Heemskerk-Jongens J, Kassis J A, O’Farrell P A. Two-tiered regulation of spatially patterned engrailed gene expression during drosophila embryogenesis. Nature. 1988;332:604–609. doi: 10.1038/332604a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doherty D, Feger G, Younger-Sheperd S, Jan L Y, Jan Y N. Delta is a ventral to dorsal signal complementary to Serrate, another Notch ligand, in Drosophila wing formation. Genes Dev. 1996;10:421–434. doi: 10.1101/gad.10.4.421. [DOI] [PubMed] [Google Scholar]
- 22.Doherty D, Jan L Y, Jan Y N. The Drosophila neurogenic gene big brain, which encodes a membrane-associated protein, acts cell autonomously and can act synergistically with Notch and Delta. Development. 1997;124:3881–3893. doi: 10.1242/dev.124.19.3881. [DOI] [PubMed] [Google Scholar]
- 23.Ellisen L W, Bird J, West D C, Soreng A L, Reynolds T C, Smith S D, Sklar J. Tan-1, the human homologue of the Drosophila Notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649–661. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- 24.Fehon R G, Kooh P J, Rebay I, Regan C L, Xu T, Muskavitch M, Artavanis-Tsakonas S. Molecular interaction between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell. 1990;61:523–534. doi: 10.1016/0092-8674(90)90534-l. [DOI] [PubMed] [Google Scholar]
- 25.Fleming R J, Scottgale T N, Diedrich R J, Artavanis-Tsakonas S. The gene Serrate encodes a putative EGF-like transmembrane protein essential for proper ectodermal development in Drosophila melanogaster. Genes Dev. 1990;4:2188–2201. doi: 10.1101/gad.4.12a.2188. [DOI] [PubMed] [Google Scholar]
- 26.Fortini M E, Artavanis-Tsakonas S. The suppressor of Hairless protein participates in Notch receptor signaling. Cell. 1994;79:273–282. doi: 10.1016/0092-8674(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 27.Genetics Computer Group. Manual for the Wisconsin package, version 8. Madison, Wis: Genetic Computer Group; 1993. [Google Scholar]
- 28.Greenwald I, Rubin G M. Making a difference: The role of cell-cell interactions in establishing separate identities for equivalent cells. Cell. 1992;68:271–281. doi: 10.1016/0092-8674(92)90470-w. [DOI] [PubMed] [Google Scholar]
- 29.Gu Y, Hukriede N A, Fleming R J. Serrate expression can functionally replace Delta activity during neuroblast segregation in the Drosophila embryo. Development. 1995;121:855–865. doi: 10.1242/dev.121.3.855. [DOI] [PubMed] [Google Scholar]
- 30.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 31.Heberlein U I, Hariharan K, Rubin G M. Star is required for neuronal differentiation in the Drosophila retina and displays dosage-sensitive interaction with Ras 1. Dev Biol. 1993;160:51–63. doi: 10.1006/dbio.1993.1285. [DOI] [PubMed] [Google Scholar]
- 32.Heitzler P, Simpson P. The choice of cell fate in the epidermis of Drosophila. Cell. 1991;64:1083–1092. doi: 10.1016/0092-8674(91)90263-x. [DOI] [PubMed] [Google Scholar]
- 33.Heitzler P, Bourouis M, Ruel L, Carteret C, Simpson P. Genes of the Enhancer of split and achaete-scute complexes are required for a regulatory loop between Notch and Delta during lateral signalling in Drosophila. Development. 1996;122:161–171. doi: 10.1242/dev.122.1.161. [DOI] [PubMed] [Google Scholar]
- 34.Heslip T R, Theisen H, Walker H, Marsh J L. SHAGGY and DISHEVELLED exert opposite effects on wingless and decapentaplegic expression and on positional identity in imaginal discs. Development. 1997;124:1069–1078. doi: 10.1242/dev.124.5.1069. [DOI] [PubMed] [Google Scholar]
- 35.Hooper J E, Scott M P. The Drosophila patched gene encodes a putative membrane protein required for segmental patterning. Cell. 1989;59:751–765. doi: 10.1016/0092-8674(89)90021-4. [DOI] [PubMed] [Google Scholar]
- 36.Hoppe P E, Greenspan R J. The Notch locus of Drosophila is required in epidermal cells for epidermal development. Development. 1990;109:875–885. doi: 10.1242/dev.109.4.875. [DOI] [PubMed] [Google Scholar]
- 37.Ish-Horowicz D, Howard K R, Pinchin S M, Ingham P W. Molecular and genetic analysis of the hairy locus in Drosophila. Cold Spring Harbor Symp Quant Biol. 1985;50:135–144. doi: 10.1101/sqb.1985.050.01.019. [DOI] [PubMed] [Google Scholar]
- 38.Ishibashi M, Ang S-L, Shiota K, Nakanishi S, Kageyama R, Guillemot F. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 1995;9:3136–3148. doi: 10.1101/gad.9.24.3136. [DOI] [PubMed] [Google Scholar]
- 39.Jennings B, de Celis J, Delidakis C, Preiss A, Bray S. Role of Notch and achaete-scute complex in the expression of Enhancer of split bHLH proteins. Development. 1995;121:3745–3752. [Google Scholar]
- 40.Johnston S H, Rauskolb C, Wilson R, Prabhakaran B, Irvine K D, Vogt T F. A family of mammalian Fringe genes implicated in boundary determination and the Notch pathway. Development. 1997;124:2245–2254. doi: 10.1242/dev.124.11.2245. [DOI] [PubMed] [Google Scholar]
- 41.Kelley M R, Kidd S, Deutsch W A, Young M W. Mutations altering the structure of epidermal growth factor-like coding sequences at the Drosophila Notch locus. Cell. 1987;51:539–548. doi: 10.1016/0092-8674(87)90123-1. [DOI] [PubMed] [Google Scholar]
- 42.Kidd S, Kelley M R, Young M W. Sequence of the Notch locus of Drosophila melanogaster: relationship of the encoded protein to mammalian clotting and growth factors. Mol Cell Biol. 1986;6:3094–3108. doi: 10.1128/mcb.6.9.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kidd S, Baylies M K, Gasic G P, Young M W. Structure and distribution of the Notch protein in developing Drosophila. Genes Dev. 1989;3:1113–1129. doi: 10.1101/gad.3.8.1113. [DOI] [PubMed] [Google Scholar]
- 44.Klein T, Martinez-Arias A. Different spatial and temporal interactions between Notch, wingless, and vestigial specify proximal and distal pattern elements of the wing in Drosophila. Dev Biol. 1998;194:196–212. doi: 10.1006/dbio.1997.8829. [DOI] [PubMed] [Google Scholar]
- 45.Knust E, Dietrich U, Tepass U, Bremer K A, Weigel D, Vassin H, Campos-Ortega J A. EGF homologous sequences encoded in the genome of Drosophila melanogaster and their relation to neurogenic genes. EMBO J. 1987;6:761–766. doi: 10.1002/j.1460-2075.1987.tb04818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kopczynski C C, Alton A K, Fechtel K, Kooh P J, Muskavitch M A T. Delta, a Drosophila neurogenic gene, is transcriptionally complex and encodes a protein related to blood coagulation factors and epidermal growth factor of vertebrates. Genes Dev. 1988;2:1723–1735. doi: 10.1101/gad.2.12b.1723. [DOI] [PubMed] [Google Scholar]
- 47.LaBonne S G, Sunitha I, Mahowald A P. Molecular genetics of pecanex, a maternal-effect neurogenic locus of Drosophila melanogaster that potentially encodes a large transmembrane protein. Dev Biol. 1989;136:1–16. doi: 10.1016/0012-1606(89)90127-9. [DOI] [PubMed] [Google Scholar]
- 48.Lecourtois M, Schweisguth F. The neurogenic Suppressor of Hairless DNA-binding protein mediates the transcriptional activation of the Enhancer of split Complex genes triggered by Notch signaling. Genes Dev. 1995;9:2598–2608. doi: 10.1101/gad.9.21.2598. [DOI] [PubMed] [Google Scholar]
- 49.Lee J J, von Kessler D P, Parks S, Beachy P A. Secretion and localized transcription suggest a role in positional signaling for products of the segmentation gene hedgehog. Cell. 1992;71:33–50. doi: 10.1016/0092-8674(92)90264-d. [DOI] [PubMed] [Google Scholar]
- 50.Lehmann R, Jimenez F, Dietrich U, Campos-Ortega J A. On the phenotype and development of mutants of early neurogenesis in Drosophila melanogaster. Roux’s Arch Dev Biol. 1983;192:62–74. doi: 10.1007/BF00848482. [DOI] [PubMed] [Google Scholar]
- 51.Lieber T, Wesley C S, Alcamo E, Hassel B, Krane J F, Campos-Ortega J A, Young M W. Single amino acid substitutions in EGF-like elements of Notch and Delta modify Drosophila development and affect cell adhesion in vitro. Neuron. 1992;9:847–859. doi: 10.1016/0896-6273(92)90238-9. [DOI] [PubMed] [Google Scholar]
- 52.Lieber T, Kidd S, Alcamo E, Corbin V, Young M W. Antineurogenic phenotypes induced by truncated Notch proteins indicate a role in signal transduction and may point to a novel function for Notch in nuclei. Genes Dev. 1993;7:1949–1965. doi: 10.1101/gad.7.10.1949. [DOI] [PubMed] [Google Scholar]
- 53.Logeat F, Bessia C, Brou C, LeBail O, Jarriault S, Seidah N G, Israel A. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc Natl Acad Sci USA. 1998;95:8108–8112. doi: 10.1073/pnas.95.14.8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lyman D, Young M W. Further evidence for function of the Drosophila Notch protein as a transmembrane receptor. Proc Natl Acad Sci USA. 1993;90:10395–10399. doi: 10.1073/pnas.90.21.10395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marks J D, Ouwehand W H, Bye J M, Finnern R, Gorick B D, Voak D, Thorpe S J, Hughes-Jones N C, Winter G. Human antibody fragments specific for human blood group antigens from a phage display library. Bio/Technology. 1993;11:1145–1149. doi: 10.1038/nbt1093-1145. [DOI] [PubMed] [Google Scholar]
- 56.Mlodzik M, Baker N E, Rubin G M. Isolation and expression of scabrous, a gene regulating neurogenesis in Drosophila. Genes Dev. 1990;4:1848–1861. doi: 10.1101/gad.4.11.1848. [DOI] [PubMed] [Google Scholar]
- 57.Nakano Y, Guerrero I, Hidalgo A, Taylor A, Whittle J R S, Ingham P W. A protein with several possible membrane-spanning domains encoded by the Drosophila segment polarity gene patched. Nature. 1989;341:508–513. doi: 10.1038/341508a0. [DOI] [PubMed] [Google Scholar]
- 58.Neumann C J, Cohen S M. A hierarchy of cross-regulation involving Notch, wingless, vestigial, and cut organizes the dorsal/ventral axis of the Drosophila wing. Development. 1996;122:3477–3485. doi: 10.1242/dev.122.11.3477. [DOI] [PubMed] [Google Scholar]
- 59.Ng M, Diaz-Benjumea F J, Vincent J-P, Wu J, Cohen S M. Specification of the wing by localized expression of wingless protein. Nature. 1996;381:316–318. doi: 10.1038/381316a0. [DOI] [PubMed] [Google Scholar]
- 60.Noordermeer J, Kligensmith J, Perrimon N, Nusse R. dishevelled and armadillo act in the wingless signaling pathway in Drosophila. Nature. 1994;367:80–83. doi: 10.1038/367080a0. [DOI] [PubMed] [Google Scholar]
- 61.O’Connell P, Rosbash M. Sequence, structure, and codon preference of the Drosophila ribosomal protein 49 gene. Nucleic Acids Res. 1984;12:5495–5513. doi: 10.1093/nar/12.13.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohsako S, Hyer J, Panganiban G, Oliver I, Caudy M. hairy function as a DNA-binding helix-loop-helix repressor of Drosophila sensory organ formation. Genes Dev. 1994;8:2743–2755. doi: 10.1101/gad.8.22.2743. [DOI] [PubMed] [Google Scholar]
- 63.Pan D, Rubin G. Kuzbanian controls proteolytic processing of Notch and mediates lateral inhibition during Drosophila and vertebrate neurogenesis. Cell. 1997;90:271–280. doi: 10.1016/s0092-8674(00)80335-9. [DOI] [PubMed] [Google Scholar]
- 64.Panin V M, Papayannopoulos V, Wilson R, Irvine K D. Fringe modulates Notch-ligand interactions. Nature. 1997;387:908–912. doi: 10.1038/43191. [DOI] [PubMed] [Google Scholar]
- 65.Peifer M, Sweeton D, Casey M, Wieschaus E. wingless and Zeste-white 3 kinase trigger opposing changes in the intracellular distribution of Armadillo. Development. 1994;120:369–380. doi: 10.1242/dev.120.2.369. [DOI] [PubMed] [Google Scholar]
- 66.Poulson D. Chromosomal deficiencies and embryonic development of Drosophila melanogaster. Proc Natl Acad Sci USA. 1937;23:133–137. doi: 10.1073/pnas.23.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rao Yi, Jan L Y, Jan Y N. Similarity of the product of the Drosophila neurogenic gene big brain to transmembrane channel proteins. Nature. 1990;345:163–167. doi: 10.1038/345163a0. [DOI] [PubMed] [Google Scholar]
- 68.Rebay I, Fleming R J, Fehon R G, Cherbas L, Cherbas P, Artavanis-Tsakonas S. Specific repeats of Notch mediate interactions with Delta and Serrate: implications for Notch as a multifunctional receptor. Cell. 1991;67:687–699. doi: 10.1016/0092-8674(91)90064-6. [DOI] [PubMed] [Google Scholar]
- 69.Reichsman F, Smith L, Cumberledge S. Glycosaminoglycans can modulate extracellular localization of the wingless protein and promote signal transduction. J Cell Biol. 1996;135:819–827. doi: 10.1083/jcb.135.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rothberg J M, Hartley D A, Walther Z, Artavanis-Tsakonas S. slit: an EGF-homologous locus of Drosophila melanogaster involved in the development of the embryonic central nervous system. Cell. 1988;55:1047–1059. doi: 10.1016/0092-8674(88)90249-8. [DOI] [PubMed] [Google Scholar]
- 71.Ruel L, Bourouis M, Heitzler P, Pantesco V, Simpson P. Drosophila shaggy kinase and rat glycogen synthase kinase-3 have conserved activities and act downstream of Notch. Nature. 1993;362:557–560. doi: 10.1038/362557a0. [DOI] [PubMed] [Google Scholar]
- 72.Rulifson E J, Blair S S. Notch regulates wingless expression and is not required for reception of the paracrine wingless signal during wing margin neurogenesis in Drosophila. Development. 1995;121:2813–2824. doi: 10.1242/dev.121.9.2813. [DOI] [PubMed] [Google Scholar]
- 73.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 74.Scott J K, Smith G P. Searching for peptide ligands with an epitope library. Science. 1990;249:386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- 75.Shellenbarger D L, Mohler J D. Temperature sensitive mutations of the Notch locus in Drosophila melanogaster. Genetics. 1975;81:143–162. doi: 10.1093/genetics/81.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shellenbarger D L, Mohler J D. Temperature-sensitive periods and autonomy of pleiotropic effects of l(1)Nts1, a conditional Notch lethal in Drosophila. Dev Biol. 1978;62:432–446. doi: 10.1016/0012-1606(78)90226-9. [DOI] [PubMed] [Google Scholar]
- 77.Siegfried E, Perkins L A, Capaci T M, Perrimon N. Putative protein kinase product of the Drosophila segment polarity gene zeste-white3. Nature. 1990;345:825–829. doi: 10.1038/345825a0. [DOI] [PubMed] [Google Scholar]
- 78.Siegfried E, Chou T-B, Perrimon N. wingless signaling acts through zeste-white 3, the Drosophila homolog of glycogen synthase kinase-3, to regulate engrailed and establish cell fate. Cell. 1992;71:1167–1179. doi: 10.1016/s0092-8674(05)80065-0. [DOI] [PubMed] [Google Scholar]
- 79.Skeath J B, Carroll S B. Regulation of achaete-scute gene expression and sensory organ pattern formation in the Drosophila wing. Genes Dev. 1991;5:984–995. doi: 10.1101/gad.5.6.984. [DOI] [PubMed] [Google Scholar]
- 80.Staros J V, Kakkad B P. Crosslinking and chymotropic digestion of the extracytoplasmic domain of the anion exchange channel in intact human erythrocytes. J Membr Biol. 1983;74:247–254. doi: 10.1007/BF02332127. [DOI] [PubMed] [Google Scholar]
- 81.Stone D M, Hynes M, Armanini M, Swanson T A, Gu Q, Johnson R L, Scott M P, Pennica D, Goddard A, Phillips H, Noll M, Hooper J E, de Sauvage F, Rosenthal A. The tumor-suppressor gene patched encodes a candidate receptor for sonic hedgehog. Nature. 1996;384:129–134. doi: 10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- 82.Struhl G, Fitzgerald K, Greenwald I. Intrinsic activity of the Lin-12 and Notch intracellular domains in vivo. Cell. 1993;74:331–345. doi: 10.1016/0092-8674(93)90424-o. [DOI] [PubMed] [Google Scholar]
- 83.Tepass U, Hartenstein V. Neurogenic and proneural genes control cell fate specification in the Drosophila endoderm. Development. 1995;121:393–405. doi: 10.1242/dev.121.2.393. [DOI] [PubMed] [Google Scholar]
- 84.Van Doren M, Bailey A M, Esnayra J, Ede K, Posakony J W. Negative regulation of proneural gene activity: hairy is a direct transcriptional repressor of achaete. Genes Dev. 1994;8:2729–2742. doi: 10.1101/gad.8.22.2729. [DOI] [PubMed] [Google Scholar]
- 85.Vassin H, Vielmetter J, Campos-Ortega J A. Genetic interactions in early neurogenesis of Drosophila melanogaster. J Neurogenet. 1985;2:291–308. doi: 10.3109/01677068509102325. [DOI] [PubMed] [Google Scholar]
- 86.Vassin H, Bremer K A, Knust E, Campos-Ortega J A. The neurogenic gene Delta of Drosophila melanogaster is expressed in neurogenic territories and encodes a putative transmembrane protein with EGF-like repeats. EMBO J. 1987;6:3431–3440. doi: 10.1002/j.1460-2075.1987.tb02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Waugh S M, DiBella E E, Pilch P F. Isolation of a proteolytically derived domain of the insulin receptor containing the major site of crosslinking/binding. Biochemistry. 1989;28:3448–3455. doi: 10.1021/bi00434a045. [DOI] [PubMed] [Google Scholar]
- 88.Wharton K A, Johansen K, Xu T, Artavanis-Tsakonas S. Nucleotide sequence from the neurogenic locus Notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell. 1985;43:567–581. doi: 10.1016/0092-8674(85)90229-6. [DOI] [PubMed] [Google Scholar]
- 89.Wilcox M. Cell surface antigens. In: Roberts D B, editor. Drosophila, a practical approach. Oxford, United Kingdom: IRL Press; 1986. pp. 243–276. [Google Scholar]
- 90.Wright T R F. The genetics of embryogenesis in Drosophila. Adv Genet. 1970;15:261–395. doi: 10.1016/s0065-2660(08)60075-9. [DOI] [PubMed] [Google Scholar]
- 91.Xu T, Caron L A, Fehon R G, Artavanis-Tsakonas S. The involvement of the Notch locus in Drosophila oogenesis. Development. 1992;115:913–922. doi: 10.1242/dev.115.4.913. [DOI] [PubMed] [Google Scholar]