Abstract
Objective
This retrospective multicentre observational study was performed to assess the predictors of acute kidney injury (AKI) in patients with acute decompensated heart failure (ADHF) in emergency departments in China.
Methods
In total, 1743 consecutive patients with ADHF were recruited from August 2017 to January 2018. Clinical characteristics and outcomes were compared between patients with and without AKI. Predictors of AKI occurrence and underdiagnosis were assessed in multivariate regression analyses.
Results
Of the 1743 patients, 593 (34.0%) had AKI. AKI was partly associated with short-term all-cause mortality and cost. Cardiovascular comorbidities such as coronary heart disease, diabetes mellitus, and hypertension remained significant predictors of AKI in the univariate analysis. AKI was significantly more likely to occur in patients with a lower arterial pH, lower albumin concentration, higher creatinine concentration, and higher N-terminal pro-brain natriuretic peptide (NT-proBNP) concentration. Patients treated with inotropic agents were significantly more likely to develop AKI during their hospital stay.
Conclusion
This study suggests that cardiovascular comorbidities, arterial pH, the albumin concentration, the creatinine concentration, the NT-proBNP concentration, and use of inotropic agents are predictors of AKI in patients with ADHF.
Keywords: Predictors, diagnosis, outcomes, acute kidney injury, acute decompensated heart failure, emergency department
Introduction
Acute decompensated heart failure (ADHF) is a growing global health problem affecting more than 26 million individuals worldwide.1 Twenty to forty percent of patients with ADHF develop acute kidney injury (AKI), known as acute cardiorenal syndrome, which is associated with significant mortality.2,3 In China, most patients with acute cardiorenal syndrome are treated in the emergency department (ED). However, the published data on acute cardiorenal syndrome are limited to small case series and single-centre studies,4,5 and no data is available from EDs in China. We conducted a 28-centre retrospective cohort study to investigate the predictors, diagnosis, and outcomes of AKI in patients with ADHF in EDs in China. To the best of our knowledge, this multicentre study of EDs is the largest-sample study of this kind in this geographical region to date. The findings provide important insight into the present situation of patients with ADHF who develop AKI in the emergency setting in China.
Methods
Study participants
We conducted a retrospective multicentre observational study of 28 tertiary hospitals throughout 11 provinces and municipalities in mainland China. Each ED had an average of 120,000 to 300,000 visits annually. The study complied with relevant EQUATOR Network guidelines (The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies34) and was approved by the institutional review board of Peking University Third Hospital and all participating hospitals (M2017166, Peking University Third Hospital Medical Science Research Ethics Committee, Beijing, 19 July 2017). All participants provided written informed consent for the study.
Adult patients (>18 years of age) with ADHF were consecutively enrolled from August 2017 to January 2018. All clinical data were collected from the electronic medical records. We de-identified the source data of all patients to ensure confidentiality. Data were manually entered via EpiData version 3.1 by dedicated research technicians. The patients’ records were de-identified and independently reviewed by two senior ED physicians with >5 years of clinical experience (HX.G. and Y.L.) on a case-by-case basis to confirm the diagnosis of AKI according to the Kidney Disease: Improving Global Outcomes (KDIGO) definition7 prior to analysis. Discrepancies between the reviewers were resolved through discussion. A third reviewer (QB.M.) was introduced if agreement was not reached after discussion. According to previous reports in the literature, the incidence of AKI in patients with ADHF is 29%.5 The sample size was estimated using the formula for calculation of the sample size of cross-sectional studies; the allowable error was ±2%, and α = 0.05 bilateral. The estimated sample size was 1978.
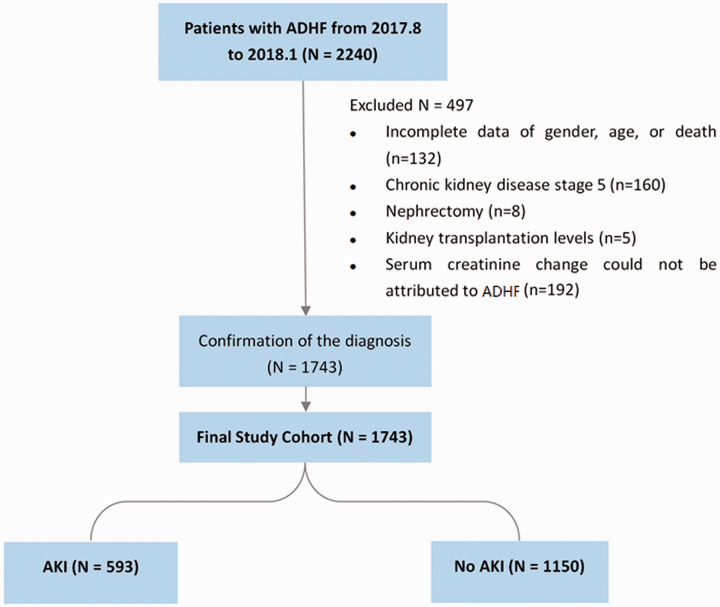
In total, 2240 patients with ADHF were initially enrolled in this study. Patients with incomplete data regarding sex, age, or clinical outcome (n = 132) were excluded. Patients with a history of stage 5 chronic kidney disease (CKD) (n = 160), nephrectomy (n = 8), and kidney transplantation (n = 5) were also excluded. Furthermore, because the aim of the study was to explore the interaction of ADHF and AKI, we excluded patients with AKI that was assumed to have been caused by drug toxicity, contrast medium, and confirmed severe sepsis; these patients were excluded because their serum creatinine change could not be definitively attributed to ADHF (n = 192). Finally, the remaining 1743 participants were entered the final analysis (Figure 1).
Figure 1.
From August 2017 to January 2018, 2240 patients with ADHF were enrolled. We excluded 497 patients because of incomplete data regarding sex, age, or death (n = 132); a history of stage 5 chronic kidney disease (n = 160), nephrectomy (n = 8), or kidney transplantation (n = 5); and a serum creatinine change that could not be attributed to ADHF (n = 192). The final study population comprised 1743 patients with ADHF (593 with AKI and 1150 without AKI)
ADHF, acute decompensated heart failure; AKI, acute kidney injury.
Definitions
ADHF was defined according to the definitions established in the 2013 American College of Cardiology Foundation/American Heart Association Guideline for the Management of Heart Failure.6 AKI in patients with ADHF was diagnosed according to the 2012 KDIGO clinical practice guideline for AKI.7 We used both creatinine and urine criteria if the source data were available in the electronic medical record. For patients whose urine data were missing, we used only creatinine criteria. According to the 2012 KDIGO clinical practice guidelines for AKI, patients were assumed to have a normal baseline creatinine concentration (estimated glomerular filtration rate (eGFR) of 75 mL/min/1.73 m2) when they had no history of CKD and unknown baseline kidney function. For patients with CKD and an unknown previous creatinine concentration, the diagnosis of AKI was mainly based on changes in the in-hospital serum creatinine concentration. The diagnosis of AKI was confirmed by the physicians’ clinical judgements during the chart review. However, we also performed a software analysis through Python based on the same criteria. Non-recognition of AKI was defined as satisfaction of the diagnostic criteria for AKI that failed to be recognised by clinical doctors. Based on the related guidelines, CKD was defined in this study as an eGFR of <60 mL/min/1.73 m2 for a 3-month duration. Hypertension was diagnosed by an increase in blood pressure exceeding 130/80 mmHg. Patients with hypertension were defined as those who had been diagnosed and required antihypertensive drugs. Coronary heart disease referred to coronary atherosclerotic heart disease. Patients with diabetes in this study were defined as those with a clear history of diabetes and long-term use of hypoglycaemic drugs. Considering the differences in blood lipid concentrations between Chinese and non-Chinese patients and based on the 2016 Chinese guidelines, hyperlipidaemia was diagnosed in patients with a total cholesterol concentration of >6.2 mmol/L, triglyceride concentration of >2.3 mmol/L, and low-density lipoprotein concentration of >4.1 mmol/L.
Study endpoints
The primary endpoints for the short-term prognosis were 7-day all-cause mortality and 30-day all-cause mortality. In-hospital all-cause mortality was also analysed and was calculated from arrival at the ED to hospital discharge. The secondary endpoints were the length of stay and the 30-day readmission rate. Emergency and hospitalisation cost data were collected from the financial department of each hospital and compared between patients with and without AKI. We also investigated predictors of the occurrence and underdiagnosis of AKI among patients with ADHF in the ED. The admission route for all patients was emergency admission followed by admission to the inpatient department for continuation of treatment. The data collected in this study began at the patient’s emergency visit and extended throughout the hospitalisation period.
Statistical analysis
Quantitative variables were expressed as mean ± standard deviation or median (25th–75th percentile) according to their distribution. The independent-samples Student’s t test or the Mann–Whitney U test was used for continuous variables. Categorical variables were expressed as count and frequency. Comparisons between groups were performed using Pearson’s chi-square test or Fisher’s exact test. Variables were identified in the univariate analyses as those with a p-value of <0.05 or those that were considered of clinical significance, including but not limited to age, sex, comorbidities, vital signs on arrival, arterial blood gas analysis, myocardial injury biomarkers, and use of inotropic agents. Collinearity was checked through Spearman’s correlation analysis. Multivariable analyses were then performed using binary logistic regression. A multivariable formula was generated through backward stepwise selection by maximum likelihood estimation. All analyses were two-sided with a significance level of 0.05 and were performed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA).
Results
Baseline patient characteristics
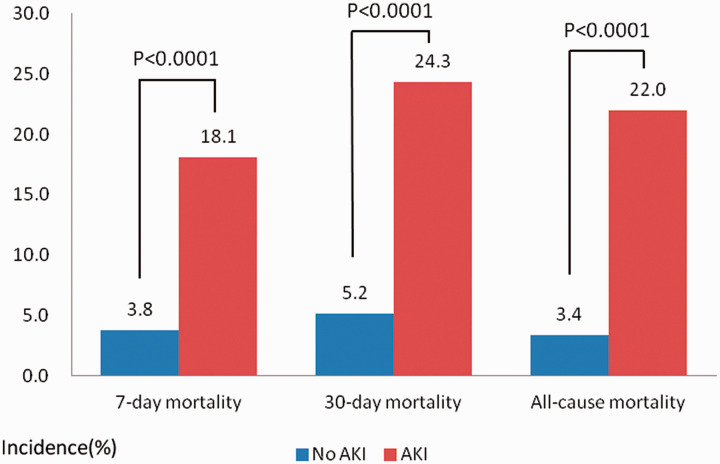
The study population comprised 1743 patients with ADHF, 593 (34.0%) of whom had AKI. The patients’ demographic and clinical characteristics are listed in Table 1. There were no significant differences in the age or sex composition between patients with and without AKI. Patients who developed AKI had a significantly higher rate of comorbidities, including hypertension (p = 0.001), coronary artery disease (p = 0.001), diabetes mellitus (p = 0.001), hyperlipidaemia (p = 0.011), and CKD (p = 0.001), than patients without AKI. The heart rate was significantly higher in patients with than without AKI (98 vs. 91 bpm, respectively; p = 0.003). There were no significant differences in the systolic or mean blood pressure between patients with and without AKI. The arterial blood gas analysis showed that patients with AKI presented with a significantly lower arterial pH (p < 0.001), base excess (p < 0.001), and ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2 ratio) (i.e., oxygenation index) (p = 0.001) and a higher lactate concentration (p = 0.007) than patients without AKI. In the AKI group, the serum creatinine concentration, blood urea nitrogen (BUN) concentration, and BUN/creatinine ratio were higher in accordance with a lower eGFR (p < 0.05). There was no significant difference in vasodilator use during treatment between the two groups. Invasive ventilator therapy was administered to a higher proportion of patients with than without AKI (22.3% vs. 5.1%, respectively; p = 0.001). The 7-day all-cause mortality, 30-day all-cause mortality, and in-hospital all-cause mortality rates were significantly higher in patients with than without AKI (p < 0.0001 for all) (Figure 2).
Table 1.
Demographic and clinical characteristics of the study population
| Total (n = 1743) | Non-AKI (n = 1150) | AKI (n = 593) | p | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 74 (63–82) | 74 (63–82) | 75 (63–84) | 0.069 |
| Male | 687 (51.8) | 496 (50.4) | 191 (55.7) | 0.103 |
| Comorbidities | ||||
| Hypertension | 853 (76.2) | 611 (73.3) | 242 (84.6) | 0.001 |
| Coronary heart disease | 706 (68.5) | 506 (65.1) | 200 (78.7) | 0.001 |
| Diabetes mellitus | 463 (48.7) | 326 (45.5) | 137 (58.5) | 0.001 |
| Hyperlipidaemia | 157 (19.7) | 108 (17.7) | 49 (26.5) | 0.011 |
| COPD | 164 (17.1) | 108 (16.4) | 56 (18.8) | 0.530 |
| CKD | 74 (10.0) | 39 (6.8) | 35 (20.7) | 0.001 |
| Vital signs | ||||
| Heart rate, bpm | 92 (78–112) | 91 (78–110) | 98 (80–117) | 0.003 |
| Systolic blood pressure, mmHg | 138 (120–161) | 138 (120–160) | 140 (120–164) | 0.250 |
| Mean blood pressure, mmHg | 99 (86–114) | 99 (88–114) | 98 (86–116) | 0.677 |
| Laboratory values | ||||
| pH | 7.41 (7.34–7.45) | 7.41 (7.36–7.45) | 7.38 (7.30–7.44) | <0.001 |
| pCO2, mmHg | 36 (30–42) | 36 (31–42) | 34 (28–41) | <0.001 |
| pO2, mmHg | 80 (61–100) | 80 (62–100) | 78 (60–100) | 0.440 |
| Lactate, mmol/L | 1.7 (1.1–2.7) | 1.7 (1.1–2.5) | 1.8 (1.2–3.2) | 0.007 |
| Base excess, mmol/L | −1.8 (−5.7 to 1.7) | −0.9 (−4.0 to 2.5) | −3.9 (−8.2 to −0.4) | <0.001 |
| PaO2/FiO2 ratio, mmHg | 245.8 (186.1–334.8) | 257 (196.2–343.8) | 228.3 (164.2–307.7) | 0.001 |
| Sodium, mmol/L | 138 (135–141) | 138 (135–141) | 137 (134–141) | 0.012 |
| NT-proBNP, pg/mL | 4386 (1048–11635) | 3435 (938–9000) | 7690 (1299–21235) | <0.001 |
| Troponin I, ng/mL | 0.08 (0.03–0.50) | 0.06 (0.03–0.46) | 0.13 (004–0.63) | <0.001 |
| Haemoglobin, g/L | 120 (100–137) | 123 (105–139) | 112 (90–133) | <0.001 |
| ALB, g/L | 36.8 (33.0–40.0) | 37.0 (33.1–40.0) | 36.0 (32.0–40.0) | 0.003 |
| BUN, mg/dL | 8.7 (6.05–14.6) | 7.4 (5.6–10.7) | 12.7 (8.1–21.2) | <0.001 |
| BUN/Cr ratio | 20 (15–26) | 21 (16–27) | 18 (13–24) | <0.001 |
| Cr, mmol/L | 99 (74–165) | 85 (67–115) | 164 (104–292) | <0.001 |
| eGFR, mL/min/1.73 m2 | 47 (26–71) | 57 (39–79) | 27 (16–45) | <0.001 |
| Treatment | ||||
| Invasive MV | 72 (9.3) | 30 (5.1) | 42 (22.3) | 0.001 |
| Inotropic agent | 145 (8.8) | 62 (5.7) | 83 (14.8) | <0.001 |
| Vasodilators* | 593 (79.9) | 422 (71.0) | 129 (70.5) | 0.926 |
| Outcome | ||||
| All-cause 7-day mortality | 98 (7.4) | 37 (3.8) | 61 (18.1) | 0.001 |
| All-cause 30-day mortality | 132 (10.1) | 51 (5.2) | 81 (24.3) | 0.001 |
| In-hospital all-cause mortality | 100 (8.1) | 31 (3.4) | 69 (22.0) | 0.001 |
| Length of hospital stay, days | 12.7 ± 32.2 | 11.64 ± 25.5 | 15.62 ± 46.4 | 0.606 |
| 30-day readmission | 138 (10.8) | 96 (10.1) | 42 (12.7) | 0.217 |
| Cost*, ¥ | 14322 ± 19947 | 12092 ± 15767 | 18510 ± 25622 | 0.030 |
Data are presented as median (25th–75th percentiles), n (%), or mean ± standard deviation.
*Number of patients with data was limited; for reference only.
AKI, acute kidney injury; ALB, serum albumin; BUN, blood urea nitrogen; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; Cr, creatinine; eGFR, estimated glomerular filtration rate; MV, mechanical ventilation; NT-proBNP, N-terminal pro-brain natriuretic peptide; PaO2/FiO2 ratio, ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (oxygenation index); pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen.
Figure 2.
Incidence of ADHF with and without AKI in terms of 7-day mortality, 30-day mortality, and all-cause mortality
ADHF, acute decompensated heart failure; AKI, acute kidney injury.
Multivariate predictors of AKI in patients with ADHF
Given the significant differences in baseline characteristics between patients with and without AKI, a logistic regression model was used to adjust for potential confounders. Age, sex, cardiovascular comorbidities, arterial blood gas analysis, haemoglobin concentration, albumin concentration, creatinine concentration, BUN concentration, BUN/creatinine ratio, eGFR, troponin concentration, N-terminal pro-brain natriuretic peptide (NT-proBNP) concentration, and use of inotropic agents on arrival at the ED were recognised as significant predictive factors for AKI during the hospital stay (p < 0.05). Furthermore, hypertension and coronary heart disease remained significant independent predictors of AKI, with adjusted odds ratios (ORs) of 1.771 and 1.543 (p = 0.008 and 0.018), respectively). AKI was more likely to occur and develop in patients with a lower arterial pH, lower albumin concentration, and higher NT-proBNP concentration with ORs of 0.058, 0.966, and 1.202 (p = 0.002, 0.021, and 0.009), respectively. Patients treated with inotropic agents were more likely to develop AKI during their hospital stay (OR, 2.879; p < 0.001) (Table 2).
Table 2.
Independent predictors of acute kidney injury by multivariable logistic regression models.
| Variable | Univariate |
Multivariate |
||||||
|---|---|---|---|---|---|---|---|---|
| p-value | OR | 95% CI | p-value | OR | 95% CI | |||
| Age (years) | 0.184 | 1.148 | 0.936 | 1.408 | 0.226 | 1.009 | 0.994 | 1.024 |
| Male sex | 0.275 | 1.004 | 0.997 | 1.011 | 0.065 | 1.493 | 0.976 | 2.284 |
| Hypertension | <0.001 | 2.026 | 1.514 | 2.711 | 0.008 | 1.771 | 1.162 | 2.699 |
| Diabetes mellitus | <0.001 | 1.740 | 1.369 | 2.213 | 0.223 | 1.260 | 0.868 | 1.829 |
| Coronary heart disease | 0.001 | 1.526 | 1.184 | 1.968 | 0.018 | 1.543 | 1.078 | 2.209 |
| Heart rate (bpm) | 0.025 | 1.005 | 1.001 | 1.008 | 0.197 | 1.005 | 0.998 | 1.012 |
| pH | <0.001 | 0.066 | 0.023 | 0.192 | 0.002 | 0.058 | 0.010 | 0.348 |
| Haemoglobin (g/L) | <0.001 | 0.989 | 0.985 | 0.992 | 0.875 | 0.999 | 0.993 | 1.006 |
| ALB (g/L) | <0.001 | 0.965 | 0.949 | 0.983 | 0.021 | 0.966 | 0.938 | 0.995 |
| Cr (µmol/L) | <0.001 | 1.003 | 1.003 | 1.004 | 0.004 | 1.001 | 1.000 | 1.002 |
| NT-proBNP/10,000 (pg/mL) | <0.001 | 1.373 | 1.244 | 1.515 | 0.009 | 1.202 | 1.047 | 1.380 |
| Inotropic agent | <0.001 | 2.879 | 2.036 | 4.072 | <0.001 | 0.233 | 0.127 | 0.428 |
ALB, serum albumin; CI, confidence interval; Cr, creatinine; NT-proBNP, N-terminal pro-brain natriuretic peptide; OR, odds ratio.
Risk factors for underdiagnosis of AKI in patients with ADHF
We recorded a very high rate of non-recognition of AKI by the physicians in the ED; the AKI in 318 (53.7%) of 593 patients with identifiable AKI remained unrecognised by the physicians in charge in the ED. At the hospital level, 22 (78.5%) hospitals did not give a correct diagnosis of AKI. Twelve clinical characteristics were entered into multivariate logistic regression models to assess their possible predictive value for underdiagnosis of AKI. Among these variables, the PaO2/FiO2 ratio (OR, 2.007; 95% confidence interval, 1.020–3.018; p = 0.001) was the strongest predictor of missed diagnosis of AKI. A history of hypertension or diabetes mellitus markedly differed between the two groups (p = 0.007), but neither was an independent risk factor for missed diagnosis of AKI (Tables 3 and 4).
Table 3.
Recognition of acute kidney injury by physicians in charge in emergency departments.
| Unrecognition of AKI | Recognition of AKI | p-value | |
|---|---|---|---|
| Total (N = 593) | 318 (53.7) | 275 (46.3) | |
| Hospital level | 22 (78.5) | 6 (21.5) | 0.001 |
| PaO2/FiO2 ratio, mmHg | 271.1 ± 113.4 | 239.3 ± 110.6 | 0.004 |
| Disease factors | 0.007 | ||
| Hypertension (n = 330) | 169 (51.2) | 161 (48.8) | |
| No hypertension (n = 105) | 70 (66.7) | 35 (33.3) | |
| Diabetes mellitus (n = 186) | 95 (51.1) | 91 (48.9) | |
| No diabetes mellitus (n = 187) | 115 (61.5) | 72 (38.5) |
Data are presented as n (%) or mean ± standard deviation.
AKI, acute kidney injury; PaO2/FiO2 ratio, ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (oxygenation index).
Table 4.
Multivariable logistic regression analysis of failure to diagnose acute kidney injury
| Variable | Failure to diagnose acute kidney
injury |
||||
|---|---|---|---|---|---|
| B | p-value | OR | 95% CI | ||
| PaO2/FiO2 ratio (mmHg) | 0.002 | 0.001 | 2.007 | 1.020 | 3.018 |
| Hypertension | −0.685 | 0.085 | 0.504 | 0.264 | 0.963 |
| Diabetes mellitus | −0.179 | 0.141 | 0.836 | 0.427 | 1.637 |
CI, confidence interval; OR, odds ratio; PaO2/FiO2 ratio, ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (oxygenation index).
Discussion
Main findings
The two major findings of the current investigation of EDs in China were that (1) hypertension, coronary artery disease, arterial pH, the albumin concentration, the creatinine concentration, the NT-proBNP concentration, and use of inotropic agents were likely to be the strongest predictors of AKI in patients with ADHF during their hospital stay and (2) the PaO2/FiO2 ratio might be a predictor of failure to diagnose AKI in patients with ADHF.
ADHF with AKI and predictors
The development of AKI in patients with ADHF has been an area of significant interest. Nonetheless, relevant information from EDs of developing countries is scarce. Our study showed that among EDs in China, about 34% of patients with ADHF had AKI, which was higher than the proportion of such patients in developed countries (29%).5 Therefore, AKI in patients with ADHF is a burden for emergency physicians in China. In the present study, there was no significant difference in the age or sex composition between patients with and without AKI. The median age of the whole patient cohort was 74 years, which is similar to that in most studies of AKI in patients with heart failure.33 As shown in previous studies, the patients in the current study presented with cardiovascular comorbidities such as hypertension, diabetes, hyperlipidaemia, and coronary heart disease, and patients with CKD were more likely to develop AKI.12–14,31,32 A low arterial pH and base excess and a high lactate concentration indicate the presence of acidosis, which is closely associated with renal injury.28 Moreover, invasive mechanical ventilation therapy and use of inotropic agents were more frequently observed in patients with ADHF who developed AKI, and both were significant indicators of critical illness as well as higher in-hospital mortality.29 These findings are consistent with prior reports of hospitalised patients with ADHF.4,8–10 Unfortunately, the complex pathophysiology of acute cardiorenal syndrome is still poorly understood and likely involves interrelated haemodynamic and neurohormonal mechanisms.9,11 These findings emphasise the need to identify modifiable predictors of AKI in patients with ADHF. In our study, hypertension, coronary artery disease, arterial pH, albumin, creatinine, NT-proBNP, and use of inotropic agents were likely to be the strongest predictors of AKI in patients with ADHF during their hospital stay. CKD was not included in the logistic regression for independent predictors in our study because of potential collinearity. However, several large-cohort studies have demonstrated that patients with AKI are also likely to have risk factors for CKD12–14 and that patients with known background CKD are likely to develop AKI.15–17 The reason for their mutual influence might be the close interactions among the underlying pathophysiology, pathology, definitions, and risk factors of the two conditions.18 The economic impact of AKI in patients with ADHF was also noteworthy, although it was not the main purpose of this study. Our analysis demonstrated that AKI conferred a ≥50% increase in the costs of emergency care and hospitalisation.30
Underdiagnosis of AKI and independent risk factors
The present study revealed an extremely high rate of AKI underdiagnosis among Chinese hospitals (78.5%). The underdiagnosis of AKI is the main contributor to the high mortality of patients with ADHF. In the ED, the condition of patients with ADHF is often critical and complicated by their older age and numerous underlying diseases. Prompt diagnosis and appropriate treatment for all diseases are needed to improve patients’ outcomes.22 ED physicians tend to pay more attention to the care of critically ill patients in urgent situations when enhanced computed tomography or medications that induce renal toxicity are inevitable for further diagnosis or treatment of these patients’ primary diseases, either ignoring the possibility of AKI or instituting such measures before acknowledging the occurrence of AKI. Thus, the diagnosis of AKI can be very challenging for ED physicians. In our study, the rate of missed diagnosis of AKI in patients with ADHF was as high as 53.7%, and the diagnosis tended to be missed in patients with a higher PaO2/FiO2 ratio. Heart failure itself can cause increased pulmonary oedema, which decreases the PaO2/FiO2 ratio and might partly represent the severity of heart failure. Both human and preclinical experimental studies have demonstrated a kidney–lung interaction in which AKI leads to lung injury and inflammation while acute lung injury with its attendant hypoxaemia may also worsen renal function.23–25 Thus, ED physicians should be alert to the development of AKI in patients with ADHF, even if the PaO2/FiO2 ratio is only slightly reduced. Moreover, patients without hypertension and diabetes mellitus are prone to be underdiagnosed. Awareness of these characteristics may help to recognise AKI. Further studies are needed to thoroughly assess the preventable causes and underdiagnosis of AKI in patients with ADHF.
Study limitations
This study had several limitations. First, a considerable number of patients presented without data regarding their baseline creatinine concentration and urine volume. Moreover, some patients (although a minority) underwent repeated measurements of their serum creatinine concentration (more than twice). Therefore, the diagnosis of AKI might have been underestimated. However, we included all available information to reduce bias originating from establishment of the diagnosis, which was the basis of this study. Second, because this was a retrospective study, the data for several key indicators were missing; this limited the sample size in the final logistic regression. Finally, the study was performed mainly in EDs of tertiary hospitals, which might have impacted the generalisability of the results to patients in community hospitals or other study settings. Despite these limitations, however, this study provides insight into the predictors and under-recognition of AKI in patients with ADHF in Chinese EDs.
Conclusions
AKI might be partly associated with short-term mortality and cost in patients with ADHF in Chinese EDs. Cardiovascular comorbidities, arterial pH, albumin, creatinine, NT-proBNP, and use of inotropic agents are probably the strongest independent predictors of AKI in patients with ADHF. The PaO2/FiO2 ratio is also a potential predictor of underdiagnosis of AKI. Further studies are needed to identify preventive and management strategies to reduce the burden of AKI in the ED.
Perspectives
Competency in Medical Knowledge: Patients with concurrent ADHF and AKI might have higher mortality and increased medical costs. Cardiovascular comorbidities, arterial pH, albumin, creatinine, NT-proBNP, and use of inotropic agents could be significant risk factors for AKI in patients with ADHF. The rate of missed diagnosis of AKI is high among patients with ADHF. The PaO2/FiO2 ratio may be a risk factor for underdiagnosis of AKI.
Competency in Patient Care: Patients with ADHF admitted to EDs should be monitored in terms of their urine output and serum creatinine concentration, and ED physicians should be alert to the occurrence of AKI.
Translational Outlook 1: This was a relatively short-term study (median of 3 months); therefore, longer-term follow-up of patients will lead to better identification of outcomes and underdiagnosis of AKI combined with ADHF.
Translational Outlook 2: Despite the quite sufficient sample size of this study and the difficulty in collecting emergency data, a larger sample size and more complete data from multiple hospitals in China might be helpful in detecting the risk factors for AKI and the underdiagnosis of AKI in patients with ADHF across China.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a grant from the Discovery Cardiovascular Research Fund, Chinese Medical Doctor Association, P.R. China.
ORCID iDs: Yang Liang https://orcid.org/0000-0002-4666-9653
Guoqiang Zhang https://orcid.org/0000-0003-1728-3847
References
- 1.Ponikowski P, Anker SD, AlHabib KF, et al. Heart failure: preventing disease and death worldwide. ESC Heart Failure 2014; 1: 4–25. [DOI] [PubMed] [Google Scholar]
- 2.Murray PT, Wettersten N, van Veldhuisen DJ, et al. Utility of urine neutrophil gelatinase-associated lipocalin for worsening renal function during hospitalization for acute heart failure: primary findings for urine N-gal Acute Kidney Injury N-gal Evaluation of Symptomatic heart faIlure Study (AKINESIS). J Card Fail 2019; 25: 654–665. [DOI] [PubMed] [Google Scholar]
- 3.Ronco C, Haapio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol 2008; 52: 1527–1539. [DOI] [PubMed] [Google Scholar]
- 4.Zhou LZ, Yang XB, Guan Y, et al. Development and validation of a risk score for prediction of acute kidney injury in patients with acute decompensated heart failure: a prospective cohort study in China. J Am Heart Assoc 2016; 5: e004035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breidthardt T, Socrates T, Drexler B, et al. Plasma neutrophil gelatinase-associated lipocalin for the prediction of acute kidney injury in acute heart failure. Crit Care 2012; 16: R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147–239. [DOI] [PubMed] [Google Scholar]
- 7.James M, Bouchard J, Ho J, et al. Canadian Society of Nephrology commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis 2013; 61: 673–685. [DOI] [PubMed] [Google Scholar]
- 8.Metra M, Nodari S, Parrinello G, et al. Worsening renal function in patients hospitalised for acute heart failure: clinical implications and prognostic significance. Eur J Heart Fail 2008; 10: 188–195. [DOI] [PubMed] [Google Scholar]
- 9.Liang KV, Williams AW, Greene EL, et al. Acute decompensated heart failure and the cardiorenal syndrome. Crit Care Med 2008; 36(1 Suppl): S75–S88. [DOI] [PubMed] [Google Scholar]
- 10.Alkandari O, Eddington KA, Hyder A, et al. Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Crit Care 2011; 15: R146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ronco C, Cicoira M, McCullough PA.Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol 2012; 60: 1031–1042. [DOI] [PubMed] [Google Scholar]
- 12.Ishani A, Xue JL, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 2009; 20: 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Triverio PA, Martin PY, Romand J, et al. Long-term prognosis after acute kidney injury requiring renal replacement therapy. Nephrol Dial Transplant 2009; 24: 2186–2189. [DOI] [PubMed] [Google Scholar]
- 14.Mehta RL, Pascual MT, Soroko S, et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int 2004; 66: 1613–1621. [DOI] [PubMed] [Google Scholar]
- 15.Stevens PE, Levin A.Evaluation and management of chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158: 825–830. [DOI] [PubMed] [Google Scholar]
- 16.Lafrance JP, Djurdjev O, Levin A.Incidence and outcomes of acute kidney injury in a referred chronic kidney disease cohort. Nephrol Dial Transplant 2010; 25: 2203–2209. [DOI] [PubMed] [Google Scholar]
- 17.James MT, Hemmelgarn BR, Wiebe N, et al. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet 2010; 376: 2096–2103. [DOI] [PubMed] [Google Scholar]
- 18.Bedford M, Farmer C, Levin A, et al. Acute kidney injury and CKD: chicken or egg. Am J Kidney Dis 2012; 59: 485–491. [DOI] [PubMed] [Google Scholar]
- 19.Iglesias JI, DePalma JA, Levine JS.Risk factors for acute kidney injury following orthotopic liver transplantation: the impact of changes in renal function while patients await transplantation. BMC Nephrol 2010; 11: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medve L, Antek C, Paloczi B, et al. Epidemiology of acute kidney injury in Hungarian intensive care units: a multicenter, prospective, observational study. BMC Nephrol 2011; 12: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Akker JP, Egal M, Groeneveld AB.Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: a systematic review and meta-analysis. Crit Care 2013; 17: R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di SS, Gori CS, Salvatori E.How to manage cardiorenal syndromes in the emergency room. Contrib Nephrol 2010; 165: 93–100. [DOI] [PubMed] [Google Scholar]
- 23.Kuiper JW, Groeneveld AB, Slutsky AS, et al. Mechanical ventilation and acute renal failure. Crit Care Med 2005; 33: 1408–1415. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Hassoun HT, Santora R, et al. Organ crosstalk: the role of the kidney. Curr Opin Crit Care 2009; 15: 481–487. [DOI] [PubMed] [Google Scholar]
- 25.Husain-Syed F, McCullough PA, Birk HW, et al. Cardio-pulmonary-renal interactions: a multidisciplinary approach. J Am Coll Cardiol 2015; 65: 2433–2448. [DOI] [PubMed] [Google Scholar]
- 26.Charat T, Wisit C, Mao M A, et al. U-shape association of serum albumin level and acute kidney injury risk in hospitalized patients. Plos One 2018; 13: e0199153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiedermann C J, Wiedermann W, Joannidis M.Hypoalbuminemia and acute kidney injury: a meta-analysis of observational clinical studies. Intensive Care Med 2010; 36: 1657–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaber S, Paugam C, Futier E, et al. Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the intensive care unit (BICAR-ICU): a multicentre, open-label, randomised controlled, phase 3 trial. Lancet 2018; 392: 31–40. [DOI] [PubMed] [Google Scholar]
- 29.Liao X, Cheng Z, Wang L, et al. Analysis of the risk factors of acute kidney injury in patients receiving extracorporeal membrane oxygenation. Clin Nephrol 2018; 90: 270–275. [DOI] [PubMed] [Google Scholar]
- 30.Ostermann M, Cerdá J.The burden of acute kidney injury and related financial issues. Contrib Nephrol 2018; 193: 100–112. [DOI] [PubMed] [Google Scholar]
- 31.Saja MF, Cook HT, Ruseva MM, et al. A triglyceride-rich lipoprotein environment exacerbates renal injury in the accelerated nephrotoxic nephritis model. Clin Exp Immunol 2018; 192: 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Go AS, Hsu C-Y, Yang J, et al. Acute kidney injury and risk of heart failure and atherosclerotic events. Clin J Am Soc Nephrol 2018; 13: 833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kevin D, Valente MAE, Voors AA, et al. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J 2014; 35: 455–469. [DOI] [PubMed] [Google Scholar]
- 34.Elm EV, Altmann DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting of observational studies. Internist (Berl) 2008; 23: 688–693. https://www.equator-network.org/reporting-guidelines/strobe/. [DOI] [PubMed] [Google Scholar]