Abstract
Background:
Residual rotational instability after isolated anterior cruciate ligament reconstruction (ACLR) has been a challenge for many years. Anterolateral extra-articular procedures (AEAPs), including anterolateral ligament reconstruction (ALLR) or lateral extra-articular tenodesis (LET), are performed as a surgical option for additional rotational stability, but clear evidence for their usefulness is lacking.
Purpose:
To conduct a systematic review and meta-analysis of the literature regarding the efficacy of AEAP in primary ACLR.
Study Design:
Systematic review; Level of evidence, 3.
Methods:
A literature search, data extraction, and quality assessment were conducted by 2 independent reviewers. MEDLINE, EMBASE, and the Cochrane Library were searched in April 2020, following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. A total of 3444 studies were screened, and 20 studies (11 randomized controlled trials and 9 nonrandomized studies) were evaluated. Functional outcomes, stability, and complications were compared between patients who underwent primary ACLR with AEAP and those who underwent isolated primary ACLR. For subgroup analysis, outcomes were compared according to AEAP technique (ALLR vs LET) and time from injury to surgery (≤12 vs >12 months). The methodological quality of the included studies was assessed using the Cochrane risk-of-bias tool, Jadad scale, and Newcastle-Ottawa Scale.
Results:
Compared with isolated ACLR, combined ACLR with AEAP led to improved pivot-shift grades and graft failure rates, regardless of the AEAP technique or of time from injury to surgery. A limited, marginal improvement in subjective function score was observed in patients who underwent AEAP combined with ACLR. In contrast to ALLR, patients who underwent LET combined with ACLR had an increased risk of knee stiffness and adverse events.
Conclusion:
Our review suggests that when there is a need to improve rotational stability and subjective function, AEAP combined with primary ACLR can be considered regardless of time from injury. ALLR appeared to be a better option for improving rotational stability compared with LET.
Keywords: primary anterior cruciate ligament reconstruction, lateral extra-articular tenodesis, anterolateral ligament reconstruction, pivot shift, graft failure
Anterior cruciate ligament (ACL) rupture is one of the most common sports injuries of the knee joint, affecting 68.6 per 100,000 individuals annually in the United States.54 Since the 1980s, arthroscopic ACL reconstruction (ACLR) techniques have continuously improved with the introduction of new surgical techniques, equipment, and materials.11 Although the long-term outcomes of ACLR have become satisfactory and reliable over time, the normal rotational stability of the knee is not fully restored.21,59,61 Furthermore, the rate of graft failure is high although it varies (17.1%-18%),34,46,65 the rate of return to preinjury sporting activity is low (44%-72%),7,43 and postoperative residual rotational instability is persistent in up to 25% to 30% of patients.8 Rotational instability increases the risk of meniscal and cartilaginous lesions and early secondary arthritic changes.33 To resolve these problems and improve knee stability, several strategies have been proposed, including more lateral positioning of the femoral tunnel,47,67 double-bundle reconstructions with an additional posterolateral bundle to control the rotation,2,32 and different types of autografts used.5,23 However, although there have been several randomized controlled trials (RCTs) and meta-analyses analyzing different strategies for treating rotational instability, no obvious differences in outcomes have been noted.20,42,52
Currently, since the “rediscovery” of the anterolateral ligament (ALL) of the knee in 2013, there is great interest in the role of the anterolateral structures of the knee in controlling rotational instability.10,53,56 Historically, several anterolateral extra-articular procedures (AEAPs) have been developed to reduce anterolateral rotational instability, including lateral extra-articular tenodesis (LET).38,39 However, according to previous studies, there are concerns over LET being nonanatomic and potentially overconstraining the joint because of altered biomechanics.17 In addition, overconstraint can potentially lead to graft overtensioning and elongation and, ultimately, to increased degenerative changes in the lateral tibiofemoral compartment.18,44 The recent renewed interest in the anterolateral structures of the knee has led to significant progress in understanding anatomy and biomechanics, which in turn has enabled the development of a relatively new procedure, ALL reconstruction (ALLR).55
The aims of this literature review were as follows: (1) to demonstrate the clinical efficacy of primary ACLR combined with LET or ALLR (ACLR+AEAP) compared with isolated ACLR, according to functional outcomes, stability, and complications; and (2) to compare ACLR+AEAP with isolated ACLR according to AEAP type (LET vs ALLR) and time from injury to surgery (≤12 vs >12 months).
Methods
This study was performed following the guidelines of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement.45
Search Strategy
In April 2020, two independent reviewers (B.-R.N. and J.-K.S.) searched the following databases: PubMed (MEDLINE), EMBASE, and the Cochrane Library. No restrictions were placed on language or year of publication. The databases were searched using the following keywords: (extra-articular or extra-articular or anterolateral or antero-lateral or ALL or anterior oblique band or iliotibial or IT band or IT tract or segond) and (tenodesis or plasty or augmentation or procedure or reconstruction or reconstructive or surgical or surgery or technique) and (ACL or anterior cruciate ligament). To supplement the electronic database search, the reference list of relevant articles was cross-checked to identify any additional references of interest. After removing the duplicates and excluding the articles by title, the full texts of the remaining articles were assessed.
Selection Criteria
The following inclusion criteria were applied to the selected studies:
Published peer-reviewed study: RCTs, nonrandomized comparative studies, and prospective or retrospective cohort studies
Outcome data of primary ACLR+AEAP
Skeletally mature patients of both sexes with ACL rupture who had undergone primary ACLR regardless of the graft type or reconstruction technique
Exclusion criteria were as follows:
Reports on guidelines, technical notes, reviews, and systematic reviews
Any biomechanical or radiological studies, ex vivo analysis (cadaveric, histological, or anatomical), case reports, and noncomparative studies
Patients with >2 surgically treated knee ligaments (posterior cruciate ligament, medial or lateral ligament, or posterolateral ligament surgery)
Extra-articular procedures performed in isolation
Patients who had undergone revision surgery after primary ACLR
Initially, we reviewed the title and abstract of each article when applying the selection criteria. On reviewing the title and abstract, if it was unclear whether the article was appropriate for inclusion, the full text of the article was assessed. Two reviewers (B.-R.N. and J.-K.S.) applied the selection criteria independently. Any differences of opinion between them on the importance and relevance of any identified article were resolved through discussion until consensus was reached. A third reviewer (H.-Y.S.) resolved any residual differences of opinion. For multiple reports with the same patient cohort and increasing duration of follow-up, only the latest publication (ie, the article with the longest follow-up) was included. There were 2 separate groups of authors with more than 1 study (Helito group27,28 and Zaffagnini group68,69), but they were included because there was no overlap of patients in the data analysis.
Data Extraction
Two reviewers (B.-R.N. and J.-K.S.) independently extracted data and entered them into a specifically designed spreadsheet containing headings of the selected outcomes. The following demographic data were extracted from the articles: study type, number of patients, sex, age, time from injury to surgery, type of extra-articular procedure (LET or ALLR), and follow-up time. Functional outcomes and stability were assessed using the mean values of subjective clinical scores (Lysholm, Tegner activity scale, and subjective International Knee Documentation Committee [IKDC]) and objective clinical examinations (Lachman test, pivot-shift test, and objective IKDC). Complications were assessed according to graft failure rate, adverse events (recurrent meniscal injury, infection, cyclops syndrome, screw loosening, and harvest-site pain), and knee stiffness (loss of full extension or flexion).
Quality Appraisal and Methodological Assessment
The methodological quality of the RCTs was assessed using the Cochrane risk-of-bias tool and the Jadad scale, which have been found to be reliable for quality assessment of RCTs.29,31 The nonrandomized studies were assessed using the Newcastle-Ottawa Scale,12,66 which is reliable for quality assessment of nonrandomized cohort studies and case-control studies. The methodological quality of each article was stratified. Any disagreements in the initial ratings of methodological quality between the 2 reviewers (B.-R.N. and J.-K.S.) were resolved through discussion until consensus was reached.
Statistical Analysis
All statistical analyses were performed using Review Manager (RevMan 5.3; The Nordic Cochrane Centre). Treatment effects were expressed as odds ratios (ORs) for binary outcomes and mean differences for continuous outcomes, with 95% CIs. A fixed-effects or random-effects model was used to combine the data according to the Mantel-Haenszel method.40 Both models provide similar results when interstudy heterogeneity is absent, but when the heterogeneity is high, the random-effects model is more appropriate. The heterogeneity of treatment effects was appraised visually by observing the overlapping confidence intervals on forest plots. In addition, I 2 statistics were calculated for objective assessment of heterogeneity. High heterogeneity was indicated by the absence of overlapping confidence intervals on forest plots and I 2 > 50%, and the reasons for heterogeneity were assessed. Subgroup analysis was performed when feasible. Publication bias was assessed by visual inspection of funnel plots of the primary outcomes.
Results
Search Results
The literature search across all databases yielded a total of 3441 articles, and an additional 3 articles were found on reviewing the reference lists. After the removal of duplicates, 2690 articles remained. This was reduced to 64 articles after screening titles and abstracts. After full-text review, 20 articles (2376 patients) met the eligibility criteria; 11 were RCTs (1294 patients) and 9 were nonrandomized studies (1082 patients). The literature search process is summarized in Figure 1.
Figure 1.
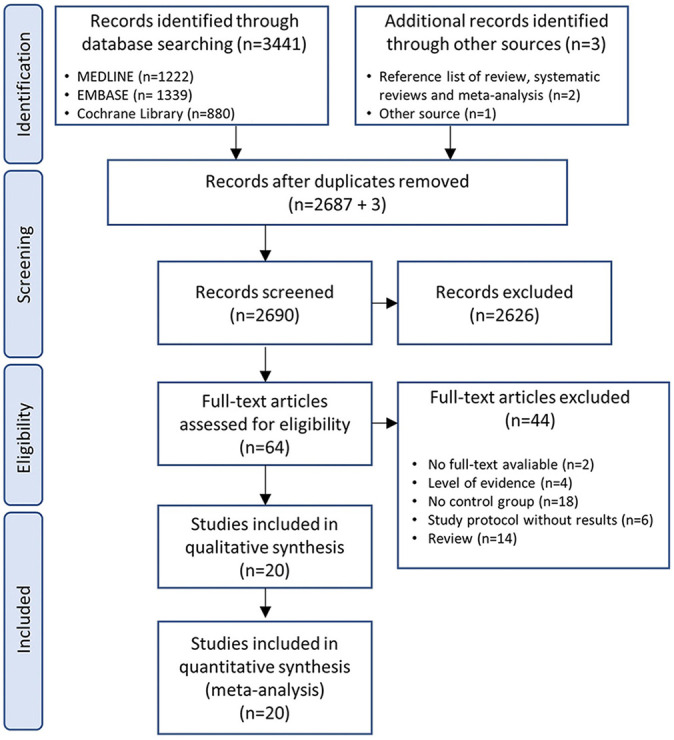
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram of the identification and selection of the studies included in the meta-analysis.
Demographic and Methodological Results
Of the 2376 patients, there were 1135 who had undergone ACLR+AEAP and 1241 patients who had undergone isolated ACLR (Appendix Table A1). The male-to-female ratio was 1.5:1 (1026:676); however, 8 articles did not provide patient sex. The mean age of patients at surgery was 25.1 years (range, 14-57 years), the mean time from injury to surgery was 13.5 months (range, 2 weeks–16 years), and the mean follow-up period was 42.0 months (range, 6 months–19.8 years).
The methodological quality of all the included studies was assessed. Most of the RCTs had an unclear to high risk of bias according to the Cochrane risk-of-bias tool (Figure 2), and all RCTs were of good quality (score range, 3-5 points) according to the Jadad scale (Table 1). All nonrandomized studies were of good quality according to the Newcastle-Ottawa Scale (score range, 7-9 stars) (Table 2).
Figure 2.
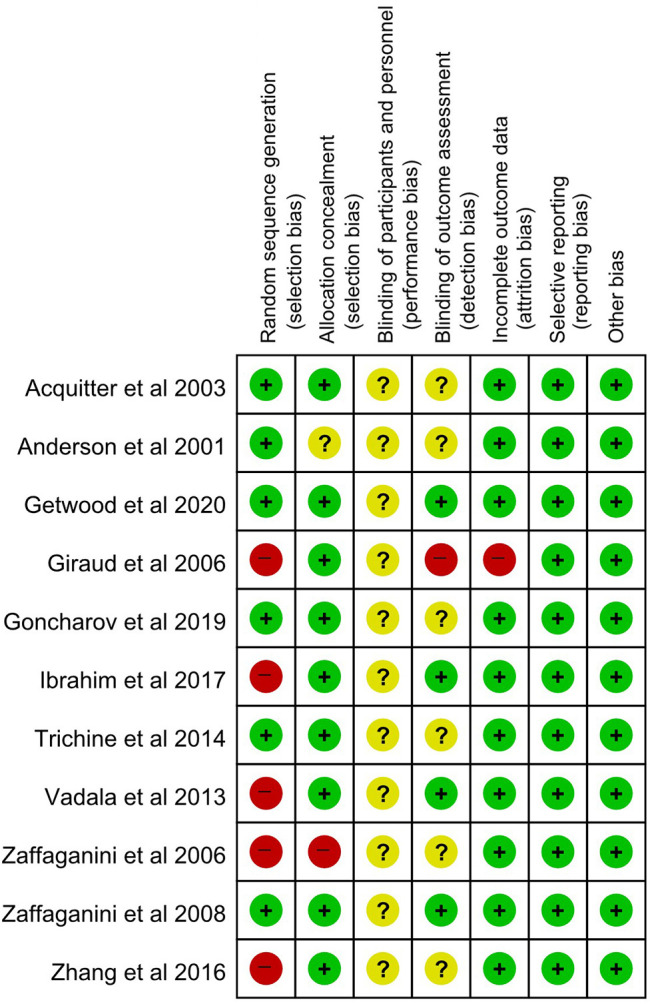
Risk-of-bias assessment of the included randomized controlled trials (Cochrane risk-of-bias tool). +, low risk of bias; −, high risk of bias; ?, unclear risk of bias.
TABLE 1.
Risk-of-Bias Assessment of Included Randomized Controlled Trials (Jadad Scale)
| Lead Author (Year) | Randomization | Double Blind | Withdrawals | Total (of 5)a |
|---|---|---|---|---|
| Acquitter (2003)1 | 2 | 0 | 1 | 3 |
| Anderson (2001)3 | 2 | 1 | 1 | 4 |
| Getgood (2020)22 | 2 | 1 | 1 | 4 |
| Giraud (2006)24 | 1 | 1 | 1 | 3 |
| Goncharov (2019)26 | 1 | 1 | 1 | 3 |
| Ibrahim (2017)30 | 2 | 1 | 1 | 4 |
| Trichine (2014)63 | 2 | 1 | 1 | 4 |
| Vadalà (2013)64 | 1 | 1 | 1 | 3 |
| Zaffagnini (2006)69 | 2 | 2 | 1 | 5 |
| Zaffagnini (2008)68 | 2 | 2 | 1 | 5 |
| Zhang (2016)70 | 1 | 1 | 1 | 3 |
aA score of ≤2 indicates a low-quality report and a score of ≥3 indicates a high-quality report.
TABLE 2.
Risk-of-Bias Assessment of Included Nonrandomized Trials (Newcastle-Ottawa Scale for Cohort Studies)a
| Lead Author (Year) | Selection | Comparability | Outcome | Total (of 9)b | |||||
|---|---|---|---|---|---|---|---|---|---|
| Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | Item 6 | Item 7 | Item 8 | ||
| Branch (2015)6 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Dejour (2013)13 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Ferretti (2016)19 | ★ | ★ | ★ | ★ | ★ | — | ★ | ★ | 7 |
| Goertzen (1993)25 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Helito (2018)27 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Helito (2019)28 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Noyes (1991)49 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Sonnery-Cottet (2017)57 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Strum (1989)60 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
aA dash denotes ineligibility. Items are defined as follows: 1, Representativeness of the exposed cohort; 2, Selection of the nonexposed cohort; 3, Ascertainment of exposure; 4, Demonstration that outcome of interest was not present at start of study; 5, Comparability of cohorts on the basis of the design or analysis controlled for confounders; 6, Assessment of outcome; 7, Was follow-up long enough for outcomes to occur; 8, Adequacy of follow-up of cohorts.
bA study can be awarded a maximum of 1 star for each numbered item within the Selection and Outcome categories. A maximum of 2 stars can be given for the Comparability category.
Functional Outcomes and Stability
Subjective Clinical Scores (Lysholm Score, Tegner Activity Scale, and Subjective IKDC)
Of the 20 included studies, 9 reported the Lysholm score (1018 patients), 5 reported the Tegner score (645 patients), and 11 reported the subjective IKDC score (1644 patients). All 3 scores were higher in the ACLR+AEAP group than in the isolated ACLR group (mean difference: Lysholm score, 3.02 [95% CI, 1.31-4.74]; P = .0006; I 2 = 88%; Tegner score, 0.80 [95% CI, 0.08-1.52]; P = .03; I 2 = 94%; and subjective IKDC, 2.65 [95% CI, 0.91-4.49]; P = 0.005; I 2 = 96%).
Objective Clinical Examination (Lachman Test, Pivot-Shift Test, and Objective IKDC)
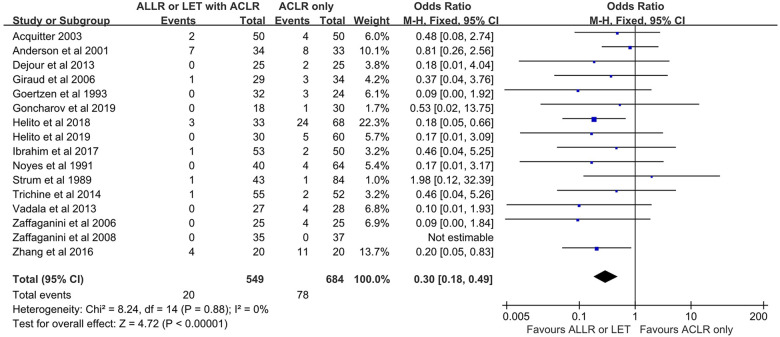
Of the 20 included studies, 7 reported the Lachman test (479 patients). A Lachman test grade of 2 or 3 indicates poor anterior knee stability. The proportion of patients with Lachman grade 2 or 3 was lower in the ACLR+AEAP group than in the isolated ACLR group (OR, 0.42 [95% CI, 0.20-0.89]; P = .02; I 2 = 0%). Of the 20 included studies, 16 reported the pivot-shift test (1233 patients). A grade 2 or 3 on the pivot-shift test indicates poor knee rotational stability. The proportion of patients with grade 2 or 3 pivot shift was lower in the ACLR+AEAP group than in the isolated ACLR group (OR, 0.30 [95% CI, 0.18-0.49]; P < .00001; I 2 = 0%). Of the 19 included studies, 10 reported the objective IKDC results (798 patients), in which scores are reported as A (normal), B (nearly normal), C (abnormal), or D (severely abnormal). The proportion of patients with an IKDC score of C or D did not differ between the 2 groups (OR, 0.76 [95% CI, 0.50-1.16]; P = .21; I 2 = 7%) (Figure 3).
Figure 3.
Comparison of pivot-shift test results. The forest plot shows that a significantly lower proportion of patients who underwent an additional extra-articular procedure combined with ACLR had grade 2 or 3 pivot shift compared with those who underwent isolated ACLR. ACLR, anterior cruciate ligament reconstruction; ALLR, anterolateral ligament reconstruction; LET, lateral extra-articular tenodesis; M-H, Mantel-Haenszel.
Complications (Graft Failure, Adverse Events, Knee Stiffness)
Of the 20 included studies, 14 reported graft failure (2099 patients). The rate of graft failure was lower in the ACLR+AEAP group than in the isolated ACLR group (OR, 0.31 [95% CI, 0.20-0.48]; P < .00001; I 2 = 0%). Of the 20 included studies, 14 reported adverse events (1972 patients). The rates of overall adverse events did not differ between the 2 groups (OR, 1.20 [95% CI, 0.68-2.09]; P = .53; I 2 = 53%). Of the 20 included studies, 10 reported knee stiffness (1284 patients), indicated as loss of full extension or flexion of >5°. The rates of knee stiffness did not differ between the 2 groups (OR, 1.65 [95% CI, 0.52-5.23]; P = .39; I 2 = 71%) (Figure 4).
Figure 4.
Comparison of graft failure rates. The forest plot shows a significantly lower rate of graft failure in patients who underwent an additional extra-articular procedure combined with ACLR than in those who underwent isolated ACLR. ACLR, anterior cruciate ligament reconstruction; ALLR, anterolateral ligament reconstruction; LET, lateral extra-articular tenodesis; M-H, Mantel-Haenszel.
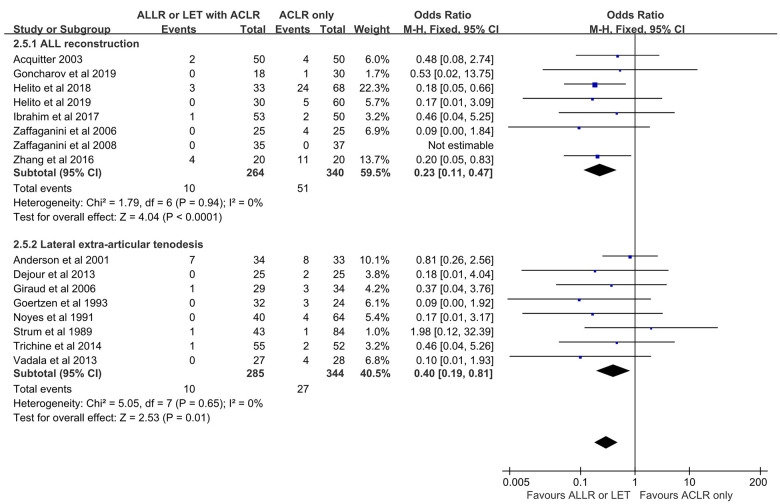
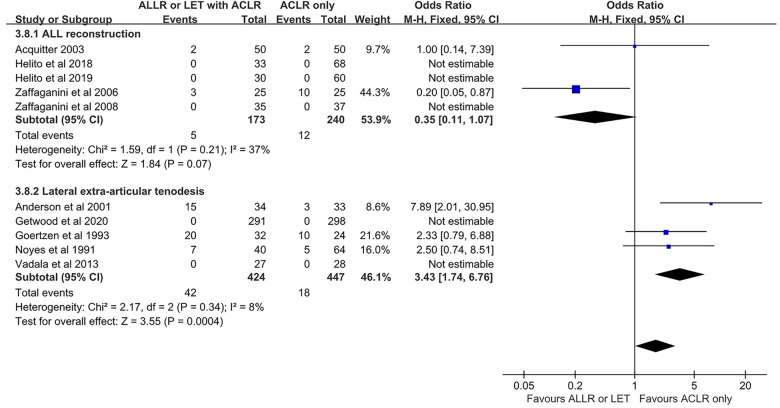
Subgroup Analysis: AEAP Technique (ALLR vs LET)
Analyses were performed to determine whether outcomes after ACLR varied according to the AEAP technique. Use of ALLR combined with ACLR (ALLR group) was reported in 10 of 20 included studies. The Lysholm and subjective IKDC scores were higher in the ALLR group than in the isolated ACLR group; however, the Tegner and objective IKDC scores, Lachman test grade, and the rate of knee stiffness were not different between the 2 groups. The proportion of patients with grade 2 or 3 pivot shift and the rates of graft failure and adverse events were lower in the ALLR group than in the isolated ACLR group.
LET combined with ACLR (LET group) was reported in 10 of the 20 included studies. The Lysholm, Tegner, and subjective IKDC scores were higher in the LET group than in the isolated ACLR group; however, the Lachman test grade and objective IKDC score were not different between the 2 groups. The proportion of patients with grade 2 or 3 pivot shift and graft failure were lower in the LET group than in the isolated ACLR group, however the rates of adverse events and knee stiffness were higher in the LET group.
The Tegner scores and the rates of adverse events and knee stiffness were statistically different between the ALLR and LET groups (Figures 5 –7). The rates of adverse events were lower in the ALLR group, and higher in the LET group, compared with the isolated ACLR group. The rate of knee stiffness was higher in the LET group compared with the isolated ACLR group, and there was no significant difference between the ALLR group and the isolated ACLR group..
Figure 5.
Comparison of pivot-shift results after ACLR according to additional procedure. The forest plot shows that compared with isolated ACLR, a significantly lower proportion of ACLR+ALLR and ACLR+LET patients had grade 2 or 3 pivot shift. ACLR, anterior cruciate ligament reconstruction; ALLR, anterolateral ligament reconstruction; LET, lateral extra-articular tenodesis; M-H, Mantel-Haenszel.
Figure 6.
Comparison of adverse event rates after ACLR according to additional procedure. The forest plot shows that compared with isolated ACLR, the ACLR+ALLR group had a significantly lower rate of adverse events and the ACLR+LET group had a significantly higher rate of adverse events. ACLR, anterior cruciate ligament reconstruction; ALLR, anterolateral ligament reconstruction; LET, lateral extra-articular tenodesis; M-H, Mantel-Haenszel.
Figure 7.
Comparison of knee stiffness rates after ACLR according to additional procedure. The forest plot shows a significantly higher rate of knee stiffness in the ACLR+LET group than in the isolated ACLR group. In contrast, the rate of knee stiffness was not significantly different between the ACLR+ALLR and isolated ACLR groups. ACLR, anterior cruciate ligament reconstruction; ALLR, anterolateral ligament reconstruction; LET, lateral extra-articular tenodesis; M-H, Mantel-Haenszel.
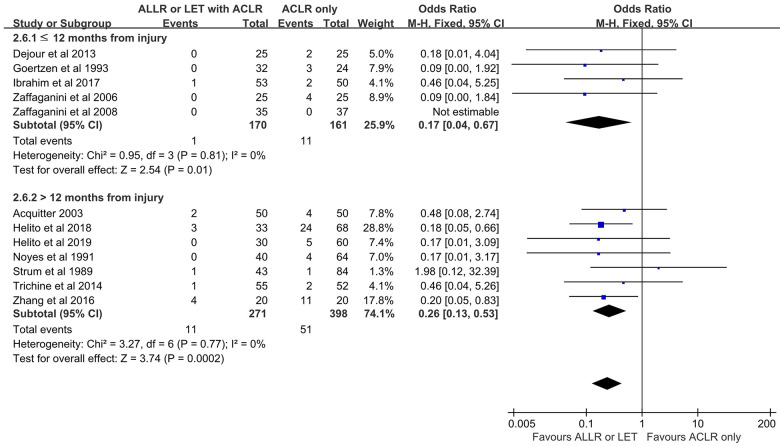
Subgroup Analysis: Time From Injury to Surgery (≤12 vs >12 Months)
Analyses were performed to determine whether outcomes after ACLR varied according to time from injury to surgery. In 8 of 20 included studies, ACLR was performed within 12 months of the index ACL injury. The Lysholm score was higher in the ACLR+AEAP group than in the isolated ACLR group. There were no differences in Tegner, subjective IKDC, and objective IKDC scores; Lachman test grade; or adverse event and knee stiffness rates between the 2 treatment groups. The proportion of patients with grade 2 or 3 pivot shift and the rate of graft failure were lower in the ACLR+AEAP group compared with isolated ACLR.
In 7 of the 20 included studies, ACLR was performed >12 months after the index ACL injury. The Lysholm, Tegner, and subjective IKDC scores were higher in the ACLR+AEAP group versus the isolated ACLR group. The Lachman test grades, objective IKDC, and knee stiffness rates were not different between the 2 treatment groups. The proportion of patients with grade 2 or 3 pivot shift and the rate of graft failure were lower in the ACLR+AEAP group versus the isolated ACLR group; this was true regardless of early or delayed reconstruction (Figures 8 and 9). However, the rate of adverse events was higher in the ACLR+AEAP group.
Figure 8.
Comparison of pivot shift after ACLR according to time from injury to surgery. The forest plot shows that regardless of whether the procedure was early (≤12 months) or delayed (>12 months), a significantly lower proportion of patients with grade 2 or 3 pivot shift was seen after additional extra-articular procedure combined with ACLR versus isolated ACLR. ACLR, anterior cruciate ligament reconstruction; ALLR, anterolateral ligament reconstruction; LET, lateral extra-articular tenodesis; M-H, Mantel-Haenszel.
Figure 9.
Comparison of graft failure rate after ACLR according to time from injury to surgery. The forest plot shows that regardless of whether the procedure was early (≤12 months) or delayed (>12 months), a significantly lower graft failure rate was seen in patients after additional extra-articular procedure combined with ACLR versus isolated ACLR. ACLR, anterior cruciate ligament reconstruction; ALLR, anterolateral ligament reconstruction; LET, lateral extra-articular tenodesis; M-H, Mantel-Haenszel.
Discussion
This systematic literature review indicated that performing ALLR or LET with ACLR led to a statistically significant reduction in the pivot-shift grade and graft failure rate compared with ACLR alone. No difference was observed in objective IKDC, adverse event rate, or knee stiffness rate between isolated ACLR and ACLR+AEAP. In contrast to ALLR, the LET procedure was associated with increased knee stiffness and adverse events. Subjective clinical scores and Lachman test grade were only marginally superior in the ACLR+AEAP group. Some advantages in knee stability were observed with AEAP; however, it remains unclear whether these advantages are justified at the cost of an additional procedure.
Subjective clinical scores (Lysholm, Tegner, and subjective IKDC) were only marginally improved in the ACLR+AEAP group, indicating that the added stability obtained with the extra-articular procedure did not greatly influence the final subjective clinical results. Previous studies have demonstrated an association between objective clinical examinations of ligament stability and subjective functional outcomes.35 Studies have also shown that statistically significant differences may not be clinically significant. Therefore, performing or not performing AEAP is not a major concern in all ACLR surgery and is only an additional procedure to be considered for improving rotational instability. The primary aspects of ACLR surgery, such as fixation method, correct tunnel placement, and graft type, possibly have a greater influence on the final clinical outcome and should be addressed before considering an additional procedure.
Objective clinical examinations (Lachman test, pivot-shift test, and objective IKDC) generally demonstrated improved outcomes. The proportion of patients with grade 2 or 3 pivot shift was significantly reduced in the ACLR+AEAP group, suggesting that the extra-articular procedure played a role of secondary restraint to rotational stability. Some ACL injuries damage only the central structure, but sometimes in pivot-shift injuries a combination of central and peripheral structures is damaged. Previous studies have demonstrated that AEAP can reduce rotational instability.16,41 Therefore, it is thought that AEAP should be considered during ACLR in patients diagnosed with rotational instability on preoperative pivot-shift test, as well as in patients with damage to the peripheral structures on magnetic resonance imaging.37,51 The Lachman test grade was improved in the ACLR+AEAP group; however, this appeared to be a secondary effect, and the interpretation requires attention. AEAP does not directly improve anterior stability after ACLR; however, it reduces the deformation of the graft by dissipating forces during ligamentization. The protective effect during ligamentization may lead to a reduction in graft elongation and eventually to a reduction in graft failure.9,50 The results of the current study confirm these findings, as the rate of graft failure decreased in the ACLR+AEAP group.
The finding of reduced graft failure rates in the ACLR+AEAP group held despite different surgical techniques being performed across the included studies. Because we found similar results across the various series of studies, it can be concluded that the general control of rotation and the protective effect of the graft is more important than the specific AEAP technique itself.
The results of subgroup analyses according to ALLR versus LET techniques showed increased knee stiffness and adverse events in the LET group. One possible explanation for this finding could be that more nonanatomic reconstruction was performed in LET than in ALLR. These nonanatomic procedures were almost completely discontinued in the United States after 1989, and additional ALLR procedures were attempted to compensate for the concerns of nonanatomic graft placement. A review of recent biomechanical studies revealed inconsistencies depending on the anatomical attachment site of ALLR,14 but several studies indicated that there was no concern for overconstraint in ALLR.58,62 These concepts are consistent with the results of our meta-analysis—no increase in knee stiffness and adverse events were observed with ALLR. Although the protective effect of the graft is more important than the specific technique, ALLR appears to be a better option for rotational stability.
The results of the subgroup analyses according to time from injury to surgery showed that ACLR+AEAP led to improved pivot-shift grade and graft failure compared with isolated ACLR, regardless of the timing of reconstruction. Similar findings were reported in a recent systematic review on this topic.15 Devitt et al15 concluded that although LET did not provide additional benefit in early primary ACLR, it was effective in delayed ACLR. The findings on delayed reconstruction are consistent with those of our meta-analysis; however, the findings on early reconstruction are different. This difference may be partially explained as differently defined pivot-shift results. Devitt et al defined a positive pivot shift as a grade of 1, 2, or 3 and a negative pivot-shift as a grade of 0. However, we defined a high-grade pivot shift as a grade of 2 or 3 and a low-grade pivot shift as a grade 0 or 1. Our interpretation was based on several studies that focused on the association between the amount of lateral compartment translation and the clinical grade of the pivot shift.4,36 In a cadaveric study, isolated ACL transection rarely produced a pivot shift of grade 2 or 3 in a laboratory setting.4 Mostly, when an ACL deficiency combined with damage to the anterolateral structures is present, it produces a pivot shift of grade 2 or 3.
Limitations
This review has some limitations. First, a limited number of studies with different study designs were evaluated. This could raise serious concerns on the quality of data and possible overlap of patients. The heterogeneity of studies, including ACLR graft selection, ALLR graft selection, attachment points, and surgical techniques, limit direct comparisons when evaluating clinical outcomes. Second, an uncertainty on the degree of preoperative rotational instability and a potential for injury to other structures that may affect rotational instability existed. Third, although the pivot-shift test is considered to be one of the most common and sensitive methods for evaluating rotational instability, it is subjective, and interexaminer variability has been observed previously.48 However, although many attempts have been made to objectify the pivot-shift test with mechanical and optical tracking devices, it is still the most widely used method to assess rotational stability. Therefore, despite the amount of variability present, we had to compare these indicators in our meta-analysis. Despite these limitations, this review highlights the lack of literature on the use of the additional procedures in primary ACLR and provides encouraging results for future studies.
Conclusion
Patients who underwent AEAP combined with ACLR had improved pivot-shift grades and graft failure rates compared with isolated ACLR, regardless of early or delayed reconstruction. ALLR combined with ACLR appeared to be a better option for treating rotational instability, as LET combined with ACLR led to an increased risk of knee stiffness and adverse events.
APPENDIX
TABLE A1.
Demographic Data of Included Studiesa
| Lead Author (Year) | Study Type | (1) Study Group (2) Control Group |
N (Sex, M: F) | Age,b y | Time From Injury,b mo | Follow-up,b mo | Anterolateral Procedure Technique (Graft) | ACLR Technique (Graft) |
|---|---|---|---|---|---|---|---|---|
| Anterolateral Ligament Reconstruction | ||||||||
| Acquitter (2003)1 | RCT | (1) BPTB+ALLR | 50 | 27.2 (16-59) | 23 ± 29 (3-156) | 58 | SB (ST+gracilis) | BPTB (patellar) |
| (2) BPTB | 50 | 27 (16-59) | 35 ± 43 (3-156) | 58 | — | BPTB (patellar) | ||
| Branch (2015)6 | Prosp | (1) BPTB+ALLR | 6 | 40 (26-48) | NR | 108 (99-237) | SB (gracilis) | BPTB (patellar) |
| (2) BPTB | 12 | 40 (26-48) | NR | 108 (99-237) | — | BPTB (patellar) | ||
| Goncharov (2019)26 | RCT | (1) BPTB+ALLR | 18 | NR (16-40) | NR | 24 | SB (ST+gracilis) | BPTB (patellar) |
| (2) BPTB | 30 | NR (16-40) | NR | 24 | — | BPTB (patellar) | ||
| Helito (2018)27 | Retrosp | (1) SB+ALLR | 33 (30:3) | 33.1 ± 8.8 | 15 (13-18) | 25 (24-28) | SB (ST+gracilis) | SB (ST+gracilis) |
| (2) SB | 68 (59:9) | 33.9 ± 6.1 | 14 (12-30.5) | 26 (24-29) | — | SB (ST+gracilis) | ||
| Helito (2019)28 | Retrosp | (1) SB+ALLR | 30 (13:17) | 27.0 ± 9.1 | 13.1 ± 12.8 | 28.1 ± 4.2 | SB (ST+gracilis) | SB (ST+gracilis) |
| (2) SB | 60 (28:32) | 29.9 ± 8.1 | 12.4 ± 14.2 | 29.6 ± 6.2 | — | SB (ST+gracilis) | ||
| Ibrahim (2017)30 | RCT | (1) SB+ALLR | 53 | 26 ± 2.5 (20-30) | 3 (2.0-4.6) | 27 (25-30) | SB (gracilis) | SB (ST+gracilis) |
| (2) SB | 50 | 26 (21-32) | 3 (2.0-4.6) | 27 (25-30) | — | SB (ST+gracilis) | ||
| Sonnery-Cottet (2017)57 | Prosp | (1) SB+ALLR | 221 (152:59) | 21.8 ± 4.0 (16-30) | 5.3 ± 9.0 | 35.4 ± 8.4 | SB (gracilis) | SB (ST) |
| (2) SB | 176 (116:60) | 23.5 ± 4.0 (16-30) | 4.5 ± 6.2 | 41.6 ± 7.0 | — | SB (ST) | ||
| Zaffagnini (2006)69 | RCT | (1) SB+ALLR | 25 (18:7) | 26.7 ± 7.25 (15-49) | 11 (1-14) | 60 | SB (ST+gracilis) | SB (ST+gracilis) |
| (2) SB | 25 (15:10) | 31.3 (26-49) | 9 (2-12) | 60 | — | SB (ST+gracilis) | ||
| Zaffagnini (2008)68 | RCT | (1) SB+ALLR | 35 (20:15) | 26 ± 10.2 (19-45) | 8.2 ± 10.4 (1-48) | 46.8 (36-60) | SB (ST+gracilis) | SB (ST+gracilis) |
| (2) DB | 37 (20:17) | 27 ± 9.0 (21-46) | 6.9 ± 10.4 (1-48) | 46.8 (36-60) | — | DB (ST+gracilis) | ||
| Zhang (2016)70 | RCT | (1) SB+ALLR | 20 (12:8) | 26.3 ± 6.8 | 16.3 ± 3.6 | 12 | SB (ST+gracilis) | SB (ST+gracilis) |
| (2) SB | 20 (13:7) | 22.3 ± 5.3 | 12.3 ± 4.3 | 12 | — | SB (ST+gracilis) | ||
| Lateral Extra-articular Tenodesis | ||||||||
| Anderson (2001)3 | RCT | (1) SB+Losee | 34 | 22 (14-40) | · <2 wk (n = 13) · 2-12 wk (n = 10) · >12 wk (n = 12) |
35.7 ± 12.1 | Losee (ITB) | SB (ST+gracilis) |
| (2) SB | 33 | 20.1 (14-38) | · <2 wk (n = 9) · 2-12 wk (n = 18) · >12 wk (n = 8) |
35.9 ± 11.7 | — | SB (ST+gracilis) | ||
| Dejour (2013)13 | Prosp | (1) BPTB+Lemaire | 25 (20:5) | 21.4 ± 5 (14-34) | 10.78 (3-30) | 25.6 (14-33) | Modified Lemaire (gracilis) | BPTB (patellar) |
| (2) BPTB | 25 (17:8) | 27.5 (14-42) | 12.96 (1-84) | 25.4 (18-30) | — | BPTB (patellar) | ||
| Ferretti (2016)19 | Retrosp | (1) SB+MacIntosh | 68 (56:12) | 25.7 ± 7 (18-46) | 3.4 | 126 ± 2.4 (122-130) | MacIntosh (ITB) | SB (ST+gracilis) |
| (2) SB | 71 (51:20) | 27.3 (18-50) | 4.4 | 125 (121-128) | — | SB (ST+gracilis) | ||
| Getgood (2020)22 | RCT | (1) SB + Lemaire | 291 (151:140) | 18.8 ± 3.2 | 8.1 ± 18.9 | 24 | Modified Lemaire (ITB) | SB (ST+gracilis) |
| (2) SB | 298 (151:147) | 19.1 ± 3.3 | 9.3 ± 16.7 | 24 | — | SB (ST+gracilis) | ||
| Giraud (2006)24 | RCT | (1) BPTB+Mac-In Jones | 29 (22:7) | 28.8 ± 12.4 | NR | 93 | Mac-In Jones (ITB) | BPTB (patellar) |
| (2) BPTB | 34 (25:9) | 26.4 ± 7.3 | NR | 102 | — | BPTB (patellar) | ||
| Goertzen (1993)25 | Retrosp | (1) SB+Jäger-Wirth | 32 (21:11) | 28.4 (17-46) | 9.6 (3-25) | 14.2 | Jäger-Wirth (ITB) | SB (ST+gracilis) |
| (2) SB | 24 (16:8) | 26.7 (19-43) | 9.6 (3-25) | 14.2 | — | SB (ST+gracilis) | ||
| Noyes (1991)49 | Retrosp | (1) BPTB+Losee | 40 | 23 (14-40) | 41 (4-223) | 36 (23-54) | Losee (ITB) | BPTB (allograft) |
| (2) BPTB | 64 | 24 (14-40) | 54 (3-282) | 34 (23-53) | — | BPTB (allograft) | ||
| Strum (1989)60 | Retrosp | (1) Various+tenodesis | 43 | 27.8 (17-57) | 31.5 (2.0-122.0) | 53.3 (28-90) | 25 Ellison or modified Ellison; 11 MacIntosh; 7 Lemaire; 15 pes anserinus transplant (various) | 9 BPTB; 3 Marshall (patellar); 31 meniscal (patellar or meniscal) |
| (2) Various | 84 | 25.2 (16-42) | 25.3 (0.5-192.0) | 41.3 (24-85) | — | 7 BPTB; 2 Marshall (patellar); 75 meniscal (patellar or meniscal) | ||
| Trichine (2014)63 | RCT | (1) Kenneth Jones+ITB tenodesis | 55 | 28.6 ± 4.69 | 35.4 | 23.4 (6-45) | ITB tenodesis (ITB) | BPTB - Kenneth Jones (patellar) |
| (2) Kenneth Jones | 52 | 27.7 ± 4.75 | 37.8 | 24.5 (6-63) | — | Kenneth Jones (patellar) | ||
| Vadalà (2013)64 | RCT | (1) SB+MacIntosh | 27 (0:27) | 26 (15-40) | 2-mo minimum | 45.2 (38-50) | MacIntosh, modified Cocker-Arnold (ITB) | SB (ST+gracilis) |
| (2) SB | 28 (0:28) | 28 (15-40) | 2-mo minimum | 43.1 (36-50) | — | SB (ST+gracilis) | ||
aDashes indicate not applicable. ACLR, anterior cruciate ligament reconstruction; ALLR, anterolateral ligament reconstruction; BPTB, bone–patellar tendon–bone; DB, double bundle; F, female; ITB, iliotibial band; M, male; NR, not reported; Prosp, prospective study; RCT, randomized controlled trial; Retrosp, retrospective study; SB, single bundle; ST, semitendinosus tendon.
bData are reported as mean (range).
Footnotes
Final revision submitted January 26, 2021; accepted February 24, 2021.
The authors declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1.Acquitter Y, Hulet C, Locker B, et al. [Patellar tendon-bone autograft reconstruction of the anterior cruciate ligament for advanced-stage chronic anterior laxity: is an extra-articular plasty necessary? A prospective randomized study of 100 patients with five year follow-up]. Rev Chir Orthop Reparatrice Appar Mot. 2003;89(5):413–422. [PubMed] [Google Scholar]
- 2.Aglietti P, Giron F, Losco M, et al. Comparison between single-and double-bundle anterior cruciate ligament reconstruction: a prospective, randomized, single-blinded clinical trial. Am J Sports Med. 2010;38(1):25–34. [DOI] [PubMed] [Google Scholar]
- 3.Anderson AF, Snyder RB, Lipscomb AB, Jr. Anterior cruciate ligament reconstruction: a prospective randomized study of three surgical methods. Am J Sports Med. 2001;29(3):272–279. [DOI] [PubMed] [Google Scholar]
- 4.Bedi A, Musahl V, Lane C, et al. Lateral compartment translation predicts the grade of pivot shift: a cadaveric and clinical analysis. Knee Surg Sports Traumatol Arthrosc. 2010;18(9):1269–1276. [DOI] [PubMed] [Google Scholar]
- 5.Bourke HE, Salmon LJ, Waller A, Patterson V, Pinczewski LA. Survival of the anterior cruciate ligament graft and the contralateral ACL at a minimum of 15 years. Am J Sports Med. 2012;40(9):1985–1992. [DOI] [PubMed] [Google Scholar]
- 6.Branch T, Lavoie F, Guier C, et al. Single-bundle ACL reconstruction with and without extra-articular reconstruction: evaluation with robotic lower leg rotation testing and patient satisfaction scores. Knee Surg Sports Traumatol Arthrosc. 2015;23(10):2882–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40(11):2517–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambat P, Guier C, Sonnery-Cottet B, Fayard JM, Thaunat M. The evolution of ACL reconstruction over the last fifty years. Int Orthop. 2013;37(2):181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claes S, Verdonk P, Forsyth R, Bellemans J. The “ligamentization” process in anterior cruciate ligament reconstruction: what happens to the human graft? A systematic review of the literature. Am J Sports Med. 2011;39(11):2476–2483. [DOI] [PubMed] [Google Scholar]
- 10.Claes S, Vereecke E, Maes M, et al. Anatomy of the anterolateral ligament of the knee. J Anat. 2013;223(4):321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davarinos N, O’Neill B, Curtin W. A brief history of anterior cruciate ligament reconstruction. Adv Orthop Surg. 2014;2014:706042. [Google Scholar]
- 12.Deeks JJ, Dinnes J, D’Amico R, et al. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7(27):iii-x, 1–173. doi:10.3310/hta7270 [DOI] [PubMed] [Google Scholar]
- 13.Dejour D, Vanconcelos W, Bonin N, Saggin PR. Comparative study between mono-bundle bone-patellar tendon-bone, double-bundle hamstring and mono-bundle bone-patellar tendon-bone combined with a modified Lemaire extra-articular procedure in anterior cruciate ligament reconstruction. Int Orthop. 2013;37(2):193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DePhillipo NN, Cinque ME, Chahla J, Geeslin AG, LaPrade RF. Anterolateral ligament reconstruction techniques, biomechanics, and clinical outcomes: a systematic review. Arthroscopy. 2017;33(8):1575–1583. [DOI] [PubMed] [Google Scholar]
- 15.Devitt BM, Bell SW, Ardern CL, et al. The role of lateral extra-articular tenodesis in primary anterior cruciate ligament reconstruction: a systematic review with meta-analysis and best-evidence synthesis. Orthop J Sports Med. 2017;5(10):23259.67117731767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dodds AL, Gupte CM, Neyret P, Williams AM, Amis AA. Extra-articular techniques in anterior cruciate ligament reconstruction: a literature review. J Bone Joint Surg Br. 2011;93(11):1440–1448. [DOI] [PubMed] [Google Scholar]
- 17.Draganich LF, Reider B, Ling M, Samuelson M. An in vitro study of an intraarticular and extraarticular reconstruction in the anterior cruciate ligament deficient knee. Am J Sports Med. 1990;18(3):262–266. [DOI] [PubMed] [Google Scholar]
- 18.Engebretsen L, Lew WD, Lewis JL, Hunter RE, Benum P.Anterolateral rotatory instability of the knee. Cadaver study of extraarticular patellar-tendon transposition. Acta Orthop Scand. 1990;61(3):225–230. [DOI] [PubMed] [Google Scholar]
- 19.Ferretti A, Monaco E, Ponzo A, et al. Combined intra-articular and extra-articular reconstruction in anterior cruciate ligament-deficient knee: 25 years later. Arthroscopy. 2016;32(10):2039–2047. [DOI] [PubMed] [Google Scholar]
- 20.Forster MC, Forster IW. Patellar tendon or four-strand hamstring? A systematic review of autografts for anterior cruciate ligament reconstruction. Knee. 2005;12(3):225–230. [DOI] [PubMed] [Google Scholar]
- 21.Georgoulis AD, Ristanis S, Chouliaras V, Moraiti C, Stergiou N. Tibial rotation is not restored after ACL reconstruction with a hamstring graft. Clin Orthop Relat Res. 2007;454:89–94. [DOI] [PubMed] [Google Scholar]
- 22.Getgood AMJ, Bryant DM, Litchfield R, et al. Lateral extra-articular tenodesis reduces failure of hamstring tendon autograft anterior cruciate ligament reconstruction: 2-year outcomes from the STABILITY study randomized clinical trial. Am J Sports Med. 2020;48(2):285–297. [DOI] [PubMed] [Google Scholar]
- 23.Gifstad T, Sole A, Strand T, et al. Long-term follow-up of patellar tendon grafts or hamstring tendon grafts in endoscopic ACL reconstructions. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):576–583. [DOI] [PubMed] [Google Scholar]
- 24.Giraud B, Besse JL, Cladière F, et al. Influence d’une ligamentoplastie extra-articulaire latérale sur les résultats de la reconstruction du ligament croisé antérieur avec le ligament patellaire avec 7 ans de recul. Rev Chir Orthop Reparatrice Appar Mot. 2006;92(8):788–797. [DOI] [PubMed] [Google Scholar]
- 25.Goertzen M, Schulitz K. [Comparison of combined extra-and intra-articular stabilization versus isolated arthroscopic semitendinosus repair after rupture of the anterior cruciate ligament]. Sportverletz Sportschaden. 1993;7(1):7–12. [DOI] [PubMed] [Google Scholar]
- 26.Goncharov EN, Koval OA, Dubrov VE, et al. Clinical experience with combined reconstruction of the anterior cruciate and anterolateral ligaments of the knee in sportsmen. Int Orthop. 2019;43(12):2781–2788. [DOI] [PubMed] [Google Scholar]
- 27.Helito CP, Camargo DB, Sobrado MF, et al. Combined reconstruction of the anterolateral ligament in chronic ACL injuries leads to better clinical outcomes than isolated ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018;26(12):3652–3659. [DOI] [PubMed] [Google Scholar]
- 28.Helito CP, Sobrado MF, Giglio PN, et al. Combined reconstruction of the anterolateral ligament in patients with anterior cruciate ligament injury and ligamentous hyperlaxity leads to better clinical stability and a lower failure rate than isolated anterior cruciate ligament reconstruction. Arthroscopy. 2019;35(9):2648–2654. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:D5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibrahim SA, Shohdy EM, Marwan Y, et al. Anatomic reconstruction of the anterior cruciate ligament of the knee with or without reconstruction of the anterolateral ligament: a randomized clinical trial. Am J Sports Med. 2017;45(7):1558–1566. [DOI] [PubMed] [Google Scholar]
- 31.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. [DOI] [PubMed] [Google Scholar]
- 32.Järvelä T. Double-bundle versus single-bundle anterior cruciate ligament reconstruction: a prospective, randomize clinical study. Knee Surg Sports Traumatol Arthrosc. 2007;15(5):500–507. [DOI] [PubMed] [Google Scholar]
- 33.Jonsson H, Riklund-Ahlstrom K, Lind J. Positive pivot shift after ACL reconstruction predicts later osteoarthrosis: 63 patients followed 5-9 years after surgery. Acta Orthop Scand. 2004;75(5):594–599. [DOI] [PubMed] [Google Scholar]
- 34.Kamath GV, Murphy T, Creighton RA, et al. Anterior cruciate ligament injury, return to play, and reinjury in the elite collegiate athlete: analysis of an NCAA Division I cohort. Am J Sports Med. 2014;42(7):1638–1643. [DOI] [PubMed] [Google Scholar]
- 35.Kocher MS, Steadman JR, Briggs KK, Sterett WI, Hawkins RJ. Relationships between objective assessment of ligament stability and subjective assessment of symptoms and function after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(3):629–634. [DOI] [PubMed] [Google Scholar]
- 36.Lane CG, Warren RF, Stanford FC, Kendoff D, Pearle AD. In vivo analysis of the pivot shift phenomenon during computer navigated ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2008;16(5):487–492. [DOI] [PubMed] [Google Scholar]
- 37.Leblanc MC, Kowalczuk M, Andruszkiewicz N, et al. Diagnostic accuracy of physical examination for anterior knee instability: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2015;23(10):2805–2813. [DOI] [PubMed] [Google Scholar]
- 38.Lemaire M. Ruptures anciennes du ligament croise anterieur du genou. J Chir. 1967;93(3):311–320. [Google Scholar]
- 39.MacIntosh D.Lateral substitution reconstruction. In: Proceedings of the Canadian Orthopaedic Association. J Bone Joint Surg. 1976;58:142. [DOI] [PubMed] [Google Scholar]
- 40.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 41.Marcacci M, Zaffagnini S, Giordano G, Iacono F, Presti ML. Anterior cruciate ligament reconstruction associated with extra-articular tenodesis: a prospective clinical and radiographic evaluation with 10- to 13-year follow-up. Am J Sports Med. 2009;37(4):707–714. [DOI] [PubMed] [Google Scholar]
- 42.Mascarenhas R, Cvetanovich GL, Sayegh ET, et al. Does double-bundle anterior cruciate ligament reconstruction improve postoperative knee stability compared with single-bundle techniques? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(6):1185–1196. [DOI] [PubMed] [Google Scholar]
- 43.Mascarenhas R, Tranovich MJ, Kropf EJ, Fu FH, Harner CD. Bone-patellar tendon-bone autograft versus hamstring autograft anterior cruciate ligament reconstruction in the young athlete: a retrospective matched analysis with 2-10 year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1520–1527. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto H, Seedhom BB. Treatment of the pivot-shift intraarticular versus extraarticular or combined reconstruction procedures. A biomechanical study. Clin Orthop Relat Res. 1994;299:298–304. [PubMed] [Google Scholar]
- 45.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morgan MD, Salmon LJ, Waller A, Roe JP, Pinczewski LA. Fifteen-year survival of endoscopic anterior cruciate ligament reconstruction in patients aged 18 years and younger. Am J Sports Med. 2016;44(2):384–392. [DOI] [PubMed] [Google Scholar]
- 47.Musahl V, Plakseychuk A, VanScyoc A, et al. Varying femoral tunnels between the anatomical footprint and isometric positions: effect on kinematics of the anterior cruciate ligament-reconstructed knee. Am J Sports Med. 2005;33(5):712–718. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura K, Koga H, Sekiya I, et al. Evaluation of pivot shift phenomenon while awake and under anaesthesia by different manoeuvres using triaxial accelerometer. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2377–2383. [DOI] [PubMed] [Google Scholar]
- 49.Noyes FR, Barber SD. The effect of an extra-articular procedure on allograft reconstructions for chronic ruptures of the anterior cruciate ligament. J Bone Joint Surg Am. 1991;73(6):882–892. [PubMed] [Google Scholar]
- 50.Pauzenberger L, Syré S, Schurz M. “Ligamentization” in hamstring tendon grafts after anterior cruciate ligament reconstruction: a systematic review of the literature and a glimpse into the future. Arthroscopy. 2013;29(10):1712–1721. [DOI] [PubMed] [Google Scholar]
- 51.Puzzitiello RN, Agarwalla A, Zuke WA, Garcia GH, Forsythe B. Imaging diagnosis of injury to the anterolateral ligament in patients with anterior cruciate ligaments: association of anterolateral ligament injury with other types of knee pathology and grade of pivot-shift examination: a systematic review. Arthroscopy. 2018;34(9):2728–2738. [DOI] [PubMed] [Google Scholar]
- 52.Riboh JC, Hasselblad V, Godin JA, Mather RC, 3rd. Transtibial versus independent drilling techniques for anterior cruciate ligament reconstruction: a systematic review, meta-analysis, and meta-regression. Am J Sports Med. 2013;41(11):2693–2702. [DOI] [PubMed] [Google Scholar]
- 53.Roessler PP, Schuttler KF, Heyse TJ, Wirtz DC, Efe T. The anterolateral ligament (ALL) and its role in rotational extra-articular stability of the knee joint: a review of anatomy and surgical concepts. Arch Orthop Trauma Surg. 2016;136(3):305–313. [DOI] [PubMed] [Google Scholar]
- 54.Sanders TL, Maradit Kremers H, Bryan AJ, et al. Incidence of anterior cruciate ligament tears and reconstruction: a 21-year population-based study. Am J Sports Med. 2016;44(6):1502–1507. [DOI] [PubMed] [Google Scholar]
- 55.Sonnery-Cottet B, Daggett M, Helito CP, Fayard JM, Thaunat M. Combined anterior cruciate ligament and anterolateral ligament reconstruction. Arthrosc Tech. 2016;5(6):e1253–e1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sonnery-Cottet B, Lutz C, Daggett M, et al. The involvement of the anterolateral ligament in rotational control of the knee. Am J Sports Med. 2016;44(5):1209–1214. [DOI] [PubMed] [Google Scholar]
- 57.Sonnery-Cottet B, Saithna A, Cavalier M, et al. Anterolateral ligament reconstruction is associated with significantly reduced ACL graft rupture rates at a minimum follow-up of 2 years: a prospective comparative study of 502 patients from the SANTI Study Group. Am J Sports Med. 2017;45(7):1547–1557. [DOI] [PubMed] [Google Scholar]
- 58.Spencer L, Burkhart TA, Tran MN, et al. Biomechanical analysis of simulated clinical testing and reconstruction of the anterolateral ligament of the knee. Am J Sports Med. 2015;43(9):2189–2197. [DOI] [PubMed] [Google Scholar]
- 59.Stergiou N, Ristanis S, Moraiti C, Georgoulis AD. Tibial rotation in anterior cruciate ligament (ACL)-deficient and ACL-reconstructed knees: a theoretical proposition for the development of osteoarthritis. Sports Med. 2007;37(7):601–613. [DOI] [PubMed] [Google Scholar]
- 60.Strum GM, Fox JM, Ferkel RD, et al. Intraarticular versus intraarticular and extraarticular reconstruction for chronic anterior cruciate ligament instability. Clin Orthop Relat Res. 1989;245:188–198. [PubMed] [Google Scholar]
- 61.Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(4):975–983. [DOI] [PubMed] [Google Scholar]
- 62.Tavlo M, Eljaja S, Jensen JT, Siersma VD, Krogsgaard MR. The role of the anterolateral ligament in ACL insufficient and reconstructed knees on rotatory stability: a biomechanical study on human cadavers. Scand J Med Sci Sports. 2016;26(8):960–966. [DOI] [PubMed] [Google Scholar]
- 63.Trichine F, Alsaati M, Chouteau J, et al. Patellar tendon autograft reconstruction of the anterior cruciate ligament with and without lateral plasty in advanced-stage chronic laxity. A clinical, prospective, randomized, single-blind study using passive dynamic X-rays. Knee. 2014;21(1):58–65. [DOI] [PubMed] [Google Scholar]
- 64.Vadalà AP, Iorio R, De Carli A, et al. An extra-articular procedure improves the clinical outcome in anterior cruciate ligament reconstruction with hamstrings in female athletes. Int Orthop. 2013;37(2):187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Webster KE, Feller JA. Exploring the high reinjury rate in younger patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(11):2827–2832. [DOI] [PubMed] [Google Scholar]
- 66.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Accessed April 10, 2018. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 67.Yamamoto Y, Hsu WH, Woo SL, et al. Knee stability and graft function after anterior cruciate ligament reconstruction: a comparison of a lateral and an anatomical femoral tunnel placement. Am J Sports Med. 2004;32(8):1825–1832. [DOI] [PubMed] [Google Scholar]
- 68.Zaffagnini S, Bruni D, Russo A, et al. ST/G ACL reconstruction: double strand plus extra-articular sling vs double bundle, randomized study at 3-year follow-up. Scand J Med Sci Sports. 2008;18(5):573–581. [DOI] [PubMed] [Google Scholar]
- 69.Zaffagnini S, Marcacci M, Lo Presti M, et al. Prospective and randomized evaluation of ACL reconstruction with three techniques: a clinical and radiographic evaluation at 5 years follow-up. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1060–1069. [DOI] [PubMed] [Google Scholar]
- 70.Zhang H, Qiu M, Zhou A, Zhang J, Jiang D. Anatomic anterolateral ligament reconstruction improves postoperative clinical outcomes combined with anatomic anterior cruciate ligament reconstruction. J Sports Sci Med. 2016;15(4):688–696. [PMC free article] [PubMed] [Google Scholar]