Abstract
Background
It is unclear how preoperative neurodegeneration and postoperative changes in EEG delta power relate to postoperative delirium severity. We sought to understand the relative relationships between neurodegeneration and delta power as predictors of delirium severity.
Methods
We undertook a prospective cohort study of high-risk surgical patients (>65 yr old) to identify predictors of peak delirium severity (Delirium Rating Scale-98) with twice-daily delirium assessments (NCT 03124303). Participants (n=86) underwent preoperative MRI; 54 had both an MRI and a postoperative EEG. Cortical thickness was calculated from the MRI and delta power from the EEG.
Results
In a linear regression model, the interaction between delirium status and preoperative mean cortical thickness (suggesting neurodegeneration) across the entire cortex was a significant predictor of delirium severity (P<0.001) when adjusting for age, sex, and performance on preoperative Trail Making Test B. Next, we included postoperative delta power and repeated the analysis (n=54). Again, the interaction between mean cortical thickness and delirium was associated with delirium severity (P=0.028), as was postoperative delta power (P<0.001). When analysed across the Desikan–Killiany–Tourville atlas, thickness in multiple individual cortical regions was also associated with delirium severity.
Conclusions
Preoperative cortical thickness and postoperative EEG delta power are both associated with postoperative delirium severity. These findings might reflect different underlying processes or mechanisms.
Clinical trial registration
Keywords: cortical slowing, cortical thickness, delirium, neurodegeneration, surgery
Editor's key points.
-
•
Delirium is not a single entity, but is rather a constellation of features occurring with many different underlying pathological processes.
-
•
This study found that postoperative delirium severity was associated both with decreased cortical thickness and increased postoperative EEG delta power.
-
•
Interestingly, decreased cortical thickness is associated with decreased delta power; the associations of these phenomena with delirium severity are independent of each other.
-
•
This study generates interesting hypotheses regarding possible causal pathways towards delirium severity, but the relationships might be epiphenomenal and causality should not be inferred from these observational findings.
Delirium is associated with increased morbidity, mortality, loss of independence, and subsequent cognitive decline and dementia.1, 2, 3, 4 Unfortunately, there are limited therapies for delirium, largely because of the inadequately understood pathogenesis of the condition. Understanding the neuronal mechanisms through which risk factors may predispose to delirium, such as dementia or pre-existing neurodegeneration,1 is one approach to this issue.
One factor to consider is structural: synaptic loss correlates closely with dementia symptoms,5 and atrophy on MRI represents the cumulative loss and shrinkage of neuropil, which can be identified using cortical thickness.6, 7, 8 Prior data have shown that cortical atrophy in temporal regions is associated with delirium after surgery.9 Another study showed that reduced preoperative cortical thickness in Alzheimer's disease-associated regions was associated with increased delirium severity in patients who developed delirium (PD), but not in those who did not (PND).10
Another factor to consider is electrophysiological: it has been known for decades that the EEG slows and increases in amplitude in delirium,11 and we recently demonstrated that increased delta and theta power in the EEG correlates with increased delirium severity after surgery.12 Changes in these cortical dynamics were also associated with impaired connectivity and varied with plasma cytokines,12 providing a link to inflammatory hypotheses for delirium.13,14
Curiously, when considering these two factors together, the prominence of delta power in the EEG appears at odds with the evidence that neurodegeneration predisposes to delirium. The EEG reflects the summation of cortical postsynaptic potentials. Consequently, delta power in sleep15, 16, 17 and under anaesthesia18 varies proportionately to the thickness of the underlying grey matter. However, our recent data show that, in preclinical dementia and in older subjects, EEG power negatively correlates with structural, plasma, and CSF markers of neurodegeneration.19 Given that delta power correlates with delirium severity,12 extrapolation would suggest that increased thickness would be associated with increased severity. This logic is clearly inconsistent with the evidence regarding neurodegeneration predisposing to increased delirium severity.10
To disambiguate the relationships between neurodegeneration (mean cortical thickness) and cortical activity (EEG delta power) in relation to postoperative delirium, we investigated their relative associations with delirium severity. We hypothesised that there would be an orthogonal relationship between neurodegeneration (as measured by mean cortical thickness across all cortex) and EEG delta power. Based on prior work,10 we hypothesised that decreased cortical thickness would correlate with delirium severity. We also hypothesised that postoperative delta power would correlate with delirium severity, based on our recent work.12 Differentiating these alternate possible processes or mechanisms would provide insights into potential pathophysiology of delirium.
Methods
These data were collected through the prospective study entitled, Interventions for Postoperative Delirium: Biomarker-3, registered with ClinicalTrials.gov (NCT 03124303) and approved by the University of Wisconsin–Madison Institutional Review Board (2015–0374). In this study, 162 adult patients, who were 65 yr of age or older and scheduled to undergo an elective major surgery with an estimated hospital length of stay of 2 days or greater, were recruited. Of these 162 subjects, 86 had an MRI scan and 54 had both an MRI scan and an EEG. The discordance in numbers of subjects with imaging, or imaging and EEG, is attributable to EEG being added to the protocol (15/2/2017) after imaging (14/7/15). Surgery types included were cardiovascular, spinal, general, and urological. Individuals unable to communicate with the research staff because of language or sensory barriers, had a documented history of dementia, or resided at a skilled nursing facility were excluded. All participants completed full written informed consent before study participation.
Data collection
The participants completed a baseline preoperative visit within 2 weeks of their scheduled surgery. This visit included data collection on patient characteristics (age, sex, race, and education) and past medical history characteristics, cognitive testing, a blood draw, EEG recording, and MRI imaging. Preoperative cognitive tests administered included the Trail Making Test B (TMTB). The TMTB measures executive function and cognitive flexibility, and predicts delirium severity.20 It is a timed assessment score, and this score indicates the time needed to complete the assessment correctly. Higher scores mean longer completion time, which suggests declining executive function. Clinical data collection included variables listed in Supplementary Table 1.
Primary outcome
The outcome variable of delirium symptom severity (which we refer to as delirium severity henceforth) was assessed twice daily on postoperative day (POD) 1–4 using the Delirium Rating Scale-98 (DRS).21 This scale provides an index of the severity of symptoms of delirium even in PND. In addition, we assessed for diagnoses of delirium status (yes/no) using the Confusion Assessment Method (CAM was replaced by 3D-CAM when it became available),22 or the CAM-ICU and Richmond Agitation Sedation Scale if the patient was intubated.23
Imaging
MRI scans were completed on a 3.0 T whole-body scanner (General Electric Medical Systems) with an eight-channel head coil. T1-weighted magnetization-prepared rapid gradient-echo pulse sequence parameters included the following: repetition time (TR)=2530 ms, echo time (TE)=3.09 ms, flip angle=10°, 256×256 matrix, 208 coronal slices, 1 mm isotropic resolution, and total acquisition time=4:07 min. High-resolution 3D fast-spin-echo sagittal T2-weighted Cube fluid-attenuated inversion recovery (FLAIR) image sequence parameters included the following: TR=6000 ms, TE=132 ms, inversion time=1709 ms, field of view 25×25×(118×0.16) cm providing a slice thickness of 0.16 cm, matrix size 256×256×118, excitation flip angle α=90°, and total acquisition time=5:33 min, using parallel imaging (autocalibrating reconstruction for Cartesian imaging). Cortical thickness was analysed using surface-based cortical thickness measures from the software FreeSurfer version 6.0. Based on our recent testing of different FreeSurfer parcellation protocols showing enhanced detection of age-related cortical thickness changes,24 we used the T1+T2-FLAIR multimodal recon-all processing stream. This includes motion correction, skull stripping, registration, segmentation, smoothing, and parcellation mapping. After the scans were processed, coronal slices were manually inspected and quality checked for any errors. Next, the cortical parcellation statistics were extracted using the Desikan–Killiany–Tourville (DKT) atlas,25 which contains 68 regions, 34 per hemisphere. Each of these regions was averaged with left and right hemispheres to give the overall average cortical thickness for all 34 regions of interest (ROI).
Note that this approach differs from a prior study of preoperative scanning and postoperative delirium,10 as (i) we used a T1+T2-FLAIR multimodal processing approach; (ii) we concentrated on global atrophy using mean cortical thickness as our primary measure of neurodegeneration, rather than averaging across a restricted subset of regions; (iii) we next looked across all cortical brain regions rather than restricting ourselves to regions that may be associated with degeneration in Alzheimer's disease10; and (iv) we included greater adjustment for confounding, including preoperative cognition.
EEG analyses
EEG data were collected on POD 1 using a 256-channel high-density EEG with a sampling frequency of 250 Hz. EEG data included 15 min of eyes-closed resting state. Data collection and analysis methods were the same as in our recent report.12 Data from 39 of 54 participants were reported previously; data from 15 participants were added to complete the data set. Preprocessing included finite impulse response filtering from 0.1 to 50 Hz using a Hamming window. Artifacts and bad channels were identified visually and removed. Independent component analysis (EEGLAB) was used to remove artifact of eye movement and muscle movement. Before analysis, any removed channels were interpolated, and then average referenced. For our regression models, we concentrated on electrode Oz that we previously showed was sensitive to the EEG slowing in delirium and correlated with delirium severity.12 Delta power was calculated as the power spectral density in the 0.5–4 Hz range using the MATLAB function pwelch.
Statistical analysis
We hypothesised that a biologically important relationship should be strong (Cohen's effect size: 0.59). Assuming our model would include seven predictors with an alpha of 0.05 and power of 0.8, we required 49 subjects to show what we consider a biologically relevant effect. We hypothesised that there would be a strong relationship of delta power and delirium severity,12 and the interaction of mean cortical thickness with delirium status on the outcome of delirium severity, whilst additionally adjusting for age, sex, and cognition. Linear regressions and Pearson correlations were conducted in RStudio. The ROI analysis involved false discovery rate (FDR) correction over all regions (P<0.05).
Results
Patient characteristics
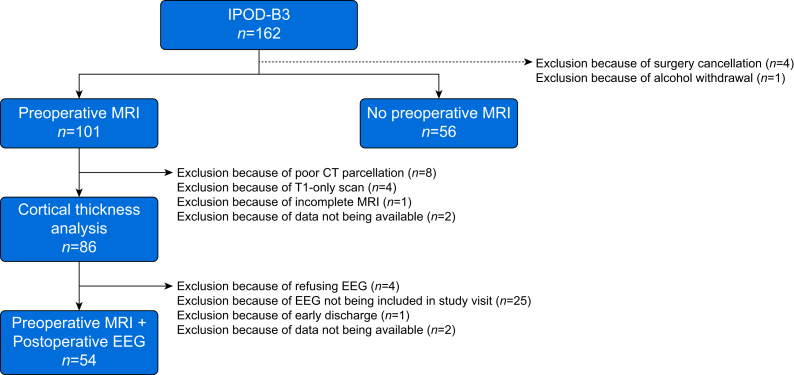
Of the 162 participants in the study, one patient was excluded, as the patient had alcohol withdrawal, and thus may have a different mechanism for delirium symptoms (Fig. 1). One hundred and one participants had a preoperative MRI. Fifteen scans were excluded because of poor cortical thickness parcellation, incomplete scan, or T2 scan not collected. Therefore, 86 MRI scans were analysed in total. Fifty-four participants had both a preoperative MRI and a postoperative EEG. This discordance is largely attributable to EEG being added after preoperative MRI scanning to the cohort protocol and the need for subjects to have both EEG and MRI for inclusion in this subgroup.
Fig 1.
Strengthening the Reporting of Observational studies in Epidemiology diagram for the Interventions for Postoperative Delirium: Biomarker-3 (IPOD-B3), registered with ClinicalTrials.gov (NCT 03124303). The diagram shows exclusions based on surgery cancellations, outlier analysis, poor cortical thickness parcellation, T1-only scans, incomplete scans or unavailable scans, refusal of EEG, EEG not included in the study at the time of visit, early discharge, or unavailable data.
The cohort characteristics are reported in Supplementary Table 1. There was a 26% delirium incidence rate in the MRI cohort (n=22/86) and a 24% delirium incidence rate in the MRI+EEG cohort (n=13/54). The PD were more likely to have a longer operative time (Wilcoxon rank-sum test; P=0.002) and a higher National Surgical Quality Improvement Program risk of death score (Wilcoxon rank-sum test; P=0.030) than the PND.
MRI-only cohort: regression of mean cortical thickness with delirium severity
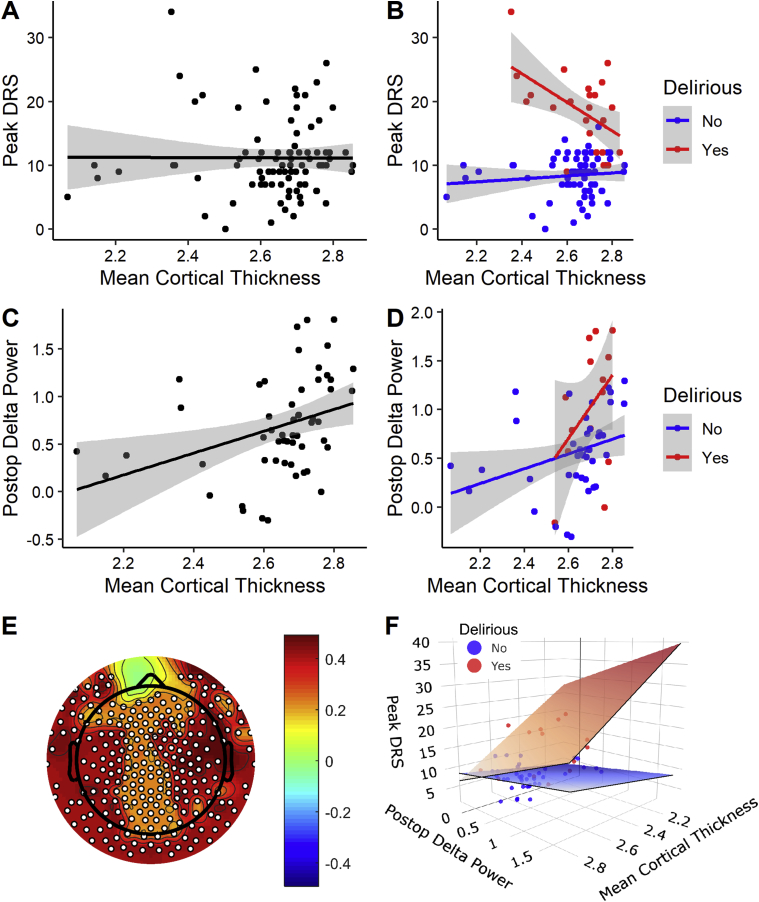
First, we investigated whether the interaction of mean cortical thickness with delirium status was negatively associated with delirium severity (Supplementary Table 2). Our data show a significant negative estimate for the interaction of mean preoperative cortical thickness with delirium status on the outcome of delirium severity (P<0.001). In this model, delirium status (yes/no) (P<0.001) and preoperative TMTB (P=0.001) also predicted delirium severity. Age (P=0.340), sex (P=0.509), and mean cortical thickness (P=0.069) did not. To graphically display these results, we plotted correlations of preoperative mean cortical thickness with delirium severity, finding no significant relationship (r=–0.00458; P=0.967; Fig. 2a), similar to prior work.10 However, when divided by delirium status, mean cortical thickness was correlated with delirium severity in the PD (r=–0.525; P=0.010) but not in the PND (r=0.114; P=0.377; Fig. 2b). Amongst those who developed delirium, thinner preoperative mean cortical thickness was associated with greater delirium severity.
Fig 2.
Descriptive univariate correlations between imaging and EEG variables and delirium severity. (a) Across the whole cohort, there is no correlation between delirium severity (peak DRS) and mean cortical thickness (n=86). (b) When divided by delirium status, a significant negative correlation is noted between delirium severity (peak DRS) and mean cortical thickness (n=86). (c) Postoperative delta power correlates with mean cortical thickness in the MRI+EEG cohort (n=54) with (d) similar relationships evident in PD and PND (n=54). (e) Topographic plot showing the scalp-based correlations of postoperative delta power and mean cortical thickness. Across the MRI+EEG cohort, postoperative delta power correlates with mean cortical thickness across the entire scalp using threshold-free cluster enhancement for multiple comparison across electrodes. White dot signifies a significant electrode. (f) Data from panels (b) and (d) were plotted in three dimensions to give an index of the influence of the different parameters. To orient the reader to the 3D space, the edges of the regression planes in panel (f) nearest the reader have been outlined in black, whereas the edges receding into the page have been left plain. DRS, Delirium Severity Rating Scale-98; PD, patients who developed delirium; PND, patients who did not develop delirium.
MRI-only cohort: regression of cortical regions with delirium severity
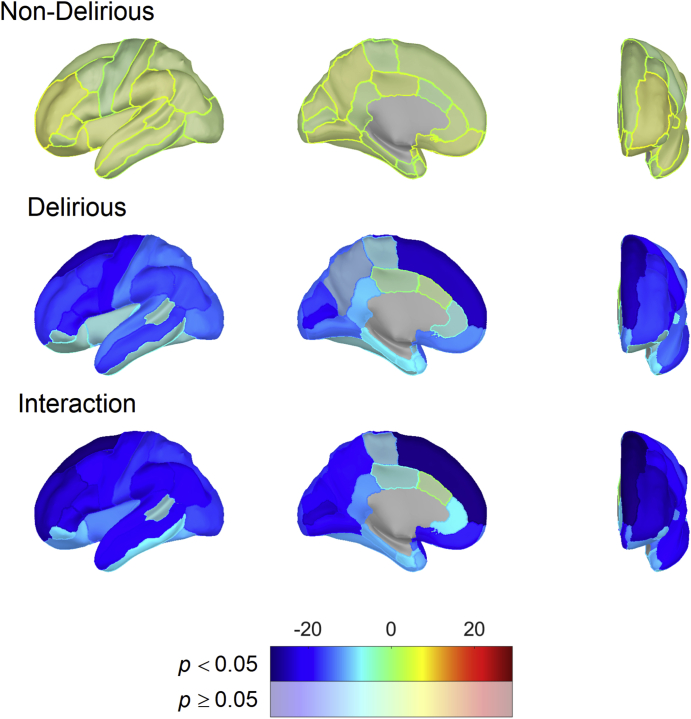
Next, we conducted an unbiased ROI analysis across all cortical regions, with FDR correction across all regions. We re-ran the regression model, including an interaction between the ROI and delirium status, and found that 30 of 34 regions showed the same effect as mean cortical thickness (Fig. 3; Supplementary Table 3).
Fig 3.
Hemispheric plots showing regional associations between cortical thickness and delirium severity based on linear model results in the whole cohort (n=86). Colours indicate model effect-size estimates (beta values) and are masked by statistical significance with significant results (FDR corrected for multiple comparisons) being opaque. Significant results for PD and PND groups indicate an association between DRS and each ROI that is different from zero. Significant interaction results indicate non-zero differences in effect size between PD and PND. DRS, Delirium Severity Rating Scale-98; FDR, false discovery rate; PD, patients who developed delirium; PND, patients who did not develop delirium; ROI, region of interest.
Correlation of preoperative mean cortical thickness with postoperative Oz delta power
Next, we investigated the relationships of preoperative mean cortical thickness and postoperative Oz delta power. Similar to prior data from sleep15, 16, 17 and anaesthesia,18 and consistent with our recent work on structural and CSF markers of neurodegeneration,19 postoperative delta power positively correlated with preoperative mean cortical thickness in the whole cohort (r=0.359; P=0.008; Fig. 2c). Similar correlation strengths were also evident when we divided the groups by delirium status in both PD (r=0.410; P=0.135) and PND (r=0.327; P=0.0394) (Fig. 2d).
We also correlated preoperative mean cortical thickness with postoperative delta power across the scalp of electrodes using threshold-free cluster enhancement for multiple comparison correction26 across all electrodes (P<0.05). A scalp-wide effect of positive correlation of mean preoperative cortical thickness with postoperative delta power was evident (Fig. 2e).
To understand the importance of neurodegeneration as a predictor of postoperative delta power, we next identified predictors of postoperative delta power using multivariable linear regression. In this model, increased preoperative mean cortical thickness and undergoing cardiac/vascular surgery predicted greater postoperative delta power (Supplementary Table 4).
Correlation of preoperative ROI cortical thickness with postoperative Oz delta power
Next, we correlated ROI with postoperative Oz delta power, identifying multiple positive correlations that survived FDR multiple comparison correction (Supplementary Table 5). Unsurprisingly, many of these regions are in posterior cortex (consistent with our approach of correlations with an occipital electrode) or have a putative role in the generation of slow waves in sleep (e.g. insula).
Primary outcome: linear regression for the associations of preoperative mean cortical thickness and delta power with delirium severity in the MRI+EEG cohort
Overall, our data suggest a series of interesting potential contradictory observations that (i) decreased preoperative cortical thickness was associated with increased delirium severity in PD, (ii) increased postoperative delta power correlated with increased delirium severity, and (iii) increased preoperative cortical thickness correlated with increased postoperative delta power. To resolve whether these processes might be statistically and biologically independent, we ran a linear regression for predictors of delirium severity using both EEG and imaging metrics. This model included preoperative mean cortical thickness, delirium status, interaction of preoperative mean thickness with delirium status, postoperative delta power, age, sex, and preoperative TMTB. As hypothesised, the interaction between preoperative mean thickness with delirium was associated with delirium severity (P=0.028), whilst postoperative delta power was also associated with delirium severity (P<0.001; Table 1). Statistical independence combined with opposing directionality of the effects argues for biologically independent processes.
Table 1.
Linear regression model for the MRI+EEG cohort (n=54). DRS, Delirium Severity Rating Scale-98; TMTB, Trail Making Test B.
| Regression variable | Mean thickness effect on peak DRS |
||
|---|---|---|---|
| Estimate | t-Value | P-value | |
| Mean thickness | 5.268 | 1.590 | 0.119 |
| Delirium status | 78.493 | 2.485 | 0.017∗ |
| Postoperative delta power | 3.784 | 3.803 | <0.001∗ |
| Age | 0.094 | 0.996 | 0.324 |
| Sex | –1.008 | –1.085 | 0.283 |
| TMTB | 0.019 | 1.724 | 0.091 |
| Mean thickness:delirium status | –26.782 | –2.276 | 0.028∗ |
∗Denotes statistical significance at p<0.05.
Linear regression for the associations of regional cortical thickness and delta power with delirium severity in the MRI+EEG cohort
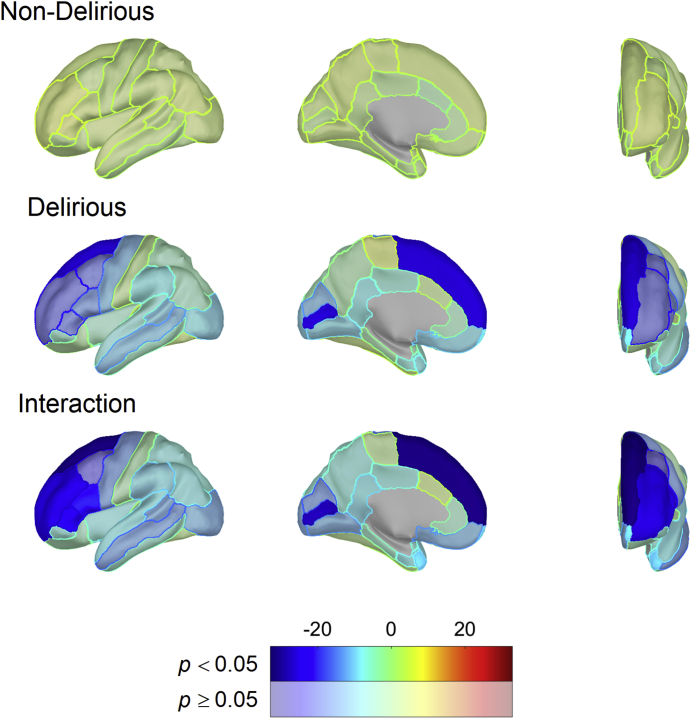
We then ran this same model of a linear regression across all ROI (replacing mean cortical thickness), looking at the interaction between the ROI and delirium status, with delirium severity. In Fig. 4 and Supplementary Table 6, we show the ROI and delirium status interactions that are significantly associated with delirium severity after adjusting for delta power, delirium status, age, sex, and TMTB in the linear regression model. Notably, the interaction of preoperative ROI cortical thickness and delirium status was significantly associated with delirium severity in several (largely frontal) regions (Fig. 4; Supplementary Table 6).
Fig 4.
Hemispheric plots showing regional associations between cortical thickness and delirium severity based on linear model results in the MRI+EEG cohort (n=54). Colours indicate model effect-size estimates (beta values) and are masked by statistical significance with significant results (FDR corrected for multiple comparisons) being opaque. Significant results for PD and PND groups indicate an association between DRS and each ROI that is different from zero. Significant interaction results indicate non-zero differences in effect size between PD and PND. DRS, Delirium Severity Rating Scale-98; FDR, false discovery rate; PD, patients who developed delirium; PND, patients who did not develop delirium; ROI, region of interest.
Discussion
These data dissociate two associations with delirium severity: cortical neurodegeneration (decreased cortical thickness) and EEG slowing. They show a linear relationship between EEG delta power and delirium severity, and an independent, non-linear relationship between neurodegeneration and delirium severity. It is intriguing that, independent of EEG slowing, pre-existing neurodegeneration is associated with delirium severity.
Overall, these data support the concept that EEG slowing might be a modifiable risk factor for postoperative delirium, as it is independent of neurodegeneration and preoperative cognition (which are not likely to be modifiable in the short term, preoperatively). We propose that modifying inflammation-related changes in EEG slowing may be one approach to reducing postoperative delirium severity. Alternative investigations will be required to identify therapeutic approaches to mitigate the risk from neurodegeneration.
These findings suggest revision of our working cognitive disintegration model27 for delirium pathogenesis to incorporate neurodegeneration more thoroughly. This model focuses on the delirium that results from a sudden loss of integration of information across cortical regions, driven by changes in EEG delta power. Previous results based on EEG12,28 and imaging connectivity12,29,30 have been consistent with this model. However, as described in the introduction, the findings of Racine and colleagues10 appeared prima facie contrary to the importance of EEG slowing. Herein, we have reconciled these views and found similar results to the prior study of cortical thickness (although we did not restrict ourselves to Alzheimer's disease-affected regions). We have also significantly extended these findings to show statistical independence from EEG slowing. However, although intuitive, the biological mechanism through which neurodegeneration would predispose to increases in delirium severity remains unclear. Understanding how neurodegeneration exacerbates delirium severity independent of measurable synaptic effects (on the EEG) offers a novel paradigm for understanding the biological basis of cognitive and brain reserve. Reduced cortical thickness may reflect loss of synapses, neurones, or glia (or all three). Loss of any of these could influence the integration of information. Of course, it is unclear how these factors may be modifiable, although it is conceivable that environmental enrichment through provision of glasses and hearing aids, and attention to circadian rhythm to improve sleep hygiene and appropriate synaptic downscaling31 could modulate changes in synaptic structure and function.
Limitations
Although we have identified statistically significant changes in this cohort, it will be important to validate these findings in larger cohorts and with different aetiologies for delirium. Our approach also used a rigorous correction for multiple comparisons using FDR across the DKT atlas brain regions and threshold-free cluster enhancement across electrodes. However, in larger cohorts, voxel-wise analysis may identify subregions or additional regions that may be associated with delirium severity. Future cohorts should also test the predictive value of preoperative measures of neurodegeneration and EEG more thoroughly. This should include analysis of subcortical regions. We did not further investigate subcortical regions, as EEG poorly samples neuronal activity from these deep structures.
Conclusion
This cohort study has identified that neurodegeneration (suggested by decreased cortical thickness) and EEG slowing are independently associated with delirium severity, with non-linear and linear relationships, respectively.
Authors' contributions
Research design: RDS, RL, RAP
Data collection: MFW, ST, CC, MP, AB, DK, RL, RDS
Cortical thickness analysis: HL, MFW, CC, VN, VP, RDS
EEG analyses: ST, CC, RAP, RDS
Data analysis: MFW, CC, RDS
Data interpretation: all authors
Writing of paper: all authors
Declarations of interest
The authors declare that they have no conflict of interest.
Funding
National Institute of Health, USAR01 AG063849-01.
Handling editor: Michael Avidan
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2021.02.028.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Inouye S.K., Westendorp R.G., Saczynski J.S. Delirium in elderly people. Lancet. 2014;383:911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inouye S.K., Marcantonio E.R., Kosar C.M. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 2016;12:766–775. doi: 10.1016/j.jalz.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Witlox J., Eurelings L.S., de Jonghe J.F., Kalisvaart K.J., Eikelenboom P., van Gool W.A. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304:443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 4.Sanders R.D., Pandharipande P.P., Davidson A.J., Ma D., Maze M. Anticipating and managing postoperative delirium and cognitive decline in adults. BMJ. 2011;343:d4331. doi: 10.1136/bmj.d4331. [DOI] [PubMed] [Google Scholar]
- 5.Jack C.R., Jr., Bennett D.A., Blennow K. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakkour A., Morris J.C., Dickerson B.C. The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology. 2009;72:1048–1055. doi: 10.1212/01.wnl.0000340981.97664.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickerson B.C., Stoub T.R., Shah R.C. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011;76:1395–1402. doi: 10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knopman D.S., Lundt E.S., Therneau T.M. Joint associations of beta-amyloidosis and cortical thickness with cognition. Neurobiol Aging. 2018;65:121–131. doi: 10.1016/j.neurobiolaging.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shioiri A., Kurumaji A., Takeuchi T., Nemoto K., Arai H., Nishikawa T. A decrease in the volume of gray matter as a risk factor for postoperative delirium revealed by an atlas-based method. Am J Geriatr Psychiatry. 2016;24:528–536. doi: 10.1016/j.jagp.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Racine A.M., Fong T.G., Travison T.G. Alzheimer’s-related cortical atrophy is associated with postoperative delirium severity in persons without dementia. Neurobiol Aging. 2017;59:55–63. doi: 10.1016/j.neurobiolaging.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engel G.L., Romano J. Delirium, a syndrome of cerebral insufficiency. J Chronic Dis. 1959;9:260–277. doi: 10.1016/0021-9681(59)90165-1. [DOI] [PubMed] [Google Scholar]
- 12.Tanabe S., Mohanty R., Lindroth H. Cohort study into the neural correlates of postoperative delirium: the role of connectivity and slow-wave activity. Br J Anaesth. 2020;125:55–66. doi: 10.1016/j.bja.2020.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerejeira J., Firmino H., Vaz-Serra A., Mukaetova-Ladinska E.B. The neuroinflammatory hypothesis of delirium. Acta Neuropathol. 2010;119:737–754. doi: 10.1007/s00401-010-0674-1. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham C., Maclullich A.M. At the extreme end of the psychoneuroimmunological spectrum: delirium as a maladaptive sickness behaviour response. Brain Behav Immun. 2013;28:1–13. doi: 10.1016/j.bbi.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Latreille V., Gaubert M., Dube J., Lina J.M., Gagnon J.F., Carrier J. Age-related cortical signatures of human sleep electroencephalography. Neurobiol Aging. 2019;76:106–114. doi: 10.1016/j.neurobiolaging.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Saletin J.M., van der Helm E., Walker M.P. Structural brain correlates of human sleep oscillations. Neuroimage. 2013;83:658–668. doi: 10.1016/j.neuroimage.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varga A.W., Ducca E.L., Kishi A. Effects of aging on slow-wave sleep dynamics and human spatial navigational memory consolidation. Neurobiol Aging. 2016;42:142–149. doi: 10.1016/j.neurobiolaging.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ni Mhuircheartaigh R., Warnaby C., Rogers R., Jbabdi S., Tracey I. Slow-wave activity saturation and thalamocortical isolation during propofol anesthesia in humans. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3006007. 208ra148. [DOI] [PubMed] [Google Scholar]
- 19.Tanabe S., Bo A., White M. Cohort study of electroencephalography markers of amyloid-tau-neurodegeneration pathology. Brain Commun. 2020;2 doi: 10.1093/braincomms/fcaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindroth H., Bratzke L., Twadell S. Predicting postoperative delirium severity in older adults: the role of surgical risk and executive function. Int J Geriatr Psychiatry. 2019;34:1018–1028. doi: 10.1002/gps.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trzepacz P.T., Mittal D., Torres R., Kanary K., Norton J., Jimerson N. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13:229–242. doi: 10.1176/jnp.13.2.229. [DOI] [PubMed] [Google Scholar]
- 22.Marcantonio E.R., Ngo L.H., O’Connor M. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med. 2014;161:554–561. doi: 10.7326/M14-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ely E.W., Inouye S.K., Bernard G.R. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 24.Lindroth H., Nair V.A., Stanfield C. Examining the identification of age-related atrophy between T1 and T1 + T2-FLAIR cortical thickness measurements. Sci Rep. 2019;9:11288. doi: 10.1038/s41598-019-47294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desikan R.S., Segonne F., Fischl B. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 27.Sanders R.D. Hypothesis for the pathophysiology of delirium: role of baseline brain network connectivity and changes in inhibitory tone. Med Hypotheses. 2011;77:140–143. doi: 10.1016/j.mehy.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 28.van Dellen E., van der Kooi A.W., Numan T. Decreased functional connectivity and disturbed directionality of information flow in the electroencephalography of intensive care unit patients with delirium after cardiac surgery. Anesthesiology. 2014;121:328–335. doi: 10.1097/ALN.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 29.Cavallari M., Dai W., Guttmann C.R. Neural substrates of vulnerability to postsurgical delirium as revealed by presurgical diffusion MRI. Brain. 2016;139:1282–1294. doi: 10.1093/brain/aww010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shioiri A., Kurumaji A., Takeuchi T., Matsuda H., Arai H., Nishikawa T. White matter abnormalities as a risk factor for postoperative delirium revealed by diffusion tensor imaging. Am J Geriatr Psychiatry. 2010;18:743–753. doi: 10.1097/JGP.0b013e3181d145c5. [DOI] [PubMed] [Google Scholar]
- 31.de Vivo L., Bellesi M., Marshall W. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science. 2017;355:507–510. doi: 10.1126/science.aah5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.