The 2018 AHA/ACC Cholesterol Management Guideline identifies two risk groups for patients with atherosclerotic cardiovascular disease (ASCVD), each with a distinct set of lipid-lowering recommendations.1 The “very high-risk” ASCVD group has a class I recommendation for high-intensity statin therapy independent of age, a class IIa recommendation for ezetimibe, and a class IIa recommendation for a PCSK9 inhibitor (PCSK9i). Conversely, the “not very high-risk” ASCVD group has weaker recommendations for adults >75 years of age, a class IIb recommendation for ezetimibe, and no recommendation for PCSK9i use.1
We previously established that a strategy of adding high sensitivity troponin (hsTn) to the guideline’s ASCVD risk algorithm reclassifies a substantial portion of not very high-risk patients into a group whose risk profile is similar to the very high-risk group.2 In this analysis, we now assess the impact of more aggressive lipid-lowering in this reclassified cohort, hypothesizing that, despite not carrying a guideline recommendation, they would derive clinical benefit from a PCSK9i to a similar magnitude as that seen in the very high-risk group.
We tested this hypothesis with a nested prospective analysis within a randomized controlled trial of evolocumab (FOURIER3). The analysis includes 22,224 patients with stable ASCVD who consented for the biomarker substudy and had hsTnI results available. Individual informed consent was obtained and the trial protocol was approved by each site’s ethics committee. Data will not be publicly available; however, interested parties can contact the corresponding authors.
Based on guideline-derived ASCVD risk categories, each patient was assigned to either very high-risk or not very high-risk. Baseline hsTnI was measured using a high-sensitivity assay (Abbott ARCHITECT) at the TIMI Clinical Trials Laboratory (Boston, Massachusetts). A major vascular event (MVE) was defined as the composite of cardiovascular death, MI, stroke, unstable angina, or coronary revascularization. Median follow-up was 2.2 years. Absolute and relative risk reductions (ARR, RRR) with evolocumab were calculated in ASCVD and hsTn subgroups (< vs. ≥6 ng/L).
In this biomarker cohort, 19,866 had very-high risk ASCVD and 2,358 had not very high risk ASCVD. The median hsTnI level was 4 ng/L (25th-75th percentiles, 2-8 ng/L), with 7,398 (33%) having levels ≥6 ng/L. Higher levels of hsTnI were associated with older age and comorbidities including prior MI, kidney disease, and heart failure.
Plasma hsTnI levels predicted increased cardiovascular risk across both very high-risk and not very high-risk ASCVD categories (Figure, Panel-A). Not very high-risk ASCVD patients with hsTnI ≥6 ng/L had risk of MVE on par with very high-risk ASCVD (dashed lines). Among patients with very high-risk ASCVD and hsTn ≥6 ng/L, annualized MVE rate was >5%/year, a level of risk not achieved in the not very high-risk ASCVD group until hsTnI approached 26 ng/L, the 99% upper reference limit.
Figure:
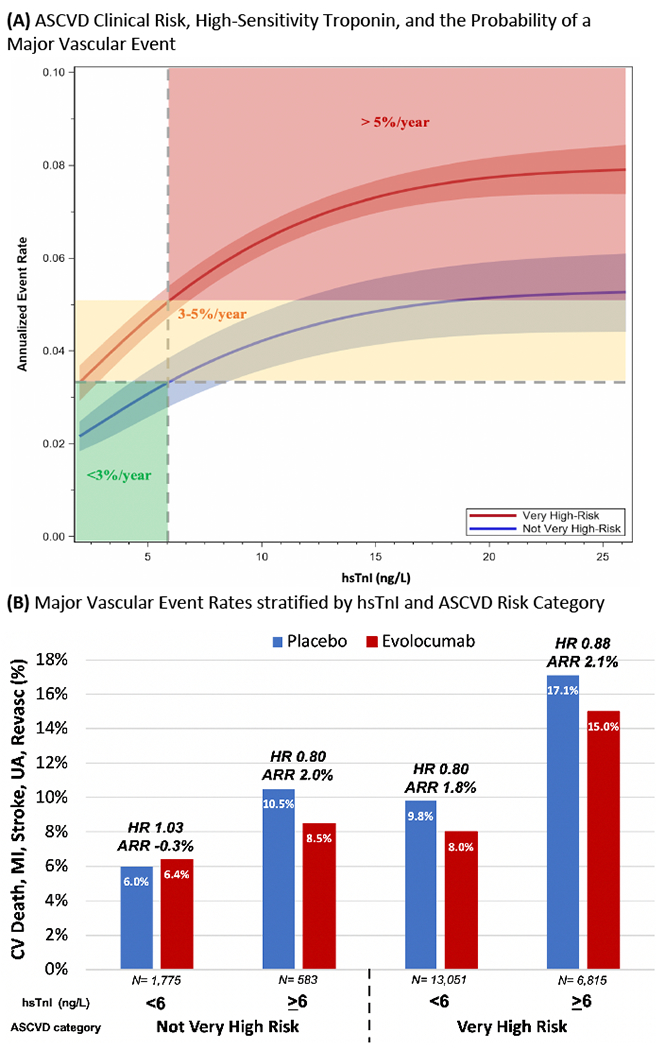
(A) ASCVD Clinical Risk, High-Sensitivity Troponin, and the Probability of a Major Vascular Event. A spline was created by using the Cox model with natural cubic spline effect and hsTnI data truncated outside 1st percentile and 99th percentile before modeling. Assay’s Limit of Detection = 2 ng/L and 99th percentile/MI Threshold = 26 ng/L. (B) Major Vascular Event Rates stratified by hsTnI and ASCVD Risk Category. Median follow up of 2.2 years. Hazard ratios with 95% confidence intervals from left to right are 1.03 (0.71-1.50), 0.80 (0.47-1.36), 0.80 (0.71-0.90), 0.88 (0.78-0.99).
Very high-risk patients benefited from evolocumab, with an ARR of 1.9% (95% CI 1.0-2.7) and RRR of 16% (HR 0.84, 95% CI 0.77-0.91) for MVE. In not very high-risk ASCVD, the ARR appeared to vary by hsTnI level. Specifically, there was no apparent benefit in those with hsTnI <6 ng/L, whereas in the 25% with hsTnI ≥6 ng/L the ARR (2.0%) and RRR (HR 0.80, 95% CI 0.47-1.36) were similar to those in the very high-risk ASCVD group (p-interaction=0.94) (Figure, Panel-B).
An association between hsTn and cardiovascular risk in stable ASCVD is well established;4, 5 however, hsTn has not yet been integrated into clinical practice because of unclear treatment implications. In this analysis of >22,000 ASCVD patients, we demonstrate that hsTn can identify patients who benefit from PCSK9i, specifically those in an ostensibly lower clinical risk category who would not have otherwise been offered this therapy.
The 2018 AHA/ACC Cholesterol Management Guideline only recommends PCSK9i for patients with very high-risk ASCVD using a defined set of characteristics. Our prior work demonstrated that approximately 25% of patients characterized as having not very high-risk ASCVD, actually have a similar event rate to the very high-risk group when selected using hsTnI. Our present analysis goes one step further, to show that these patients reclassified by hsTnI ≥6 ng/L do appear to derive benefit from evolocumab similar to the very high-risk group with a RRR of 20% and an ARR of 2.0%.
These findings have potential clinical implications. While FOURIER was enriched for very high-risk ASCVD, not very high-risk ASCVD is more common in the general population. Therefore, this biomarker-driven strategy for more risk-appropriate selection for treatment with PCSK9i could ultimately reduce the cardiovascular disease burden in a substantial proportion of the ASCVD population and help physicians personalize therapy for ASCVD.
Conclusions
Among patients with ASCVD, either hsTn or ASCVD clinical criteria can identify patients who benefit from evolocumab. Specifically, hsTnI identifies a significant cohort of “not very high-risk” ASCVD patients who are at greater risk than otherwise appreciated and derive risk reductions with evolocumab on par with clinically very high-risk ASCVD patients.
ACKNOWLEDGMENTS
DISCLOSURES
NAM reports grant support from the National Institutes of Health and involvement in clinical trials with Amgen, Pfizer, Novartis, and AstraZeneca without personal fees, payments, or increase in salary. KO reports grant support from JSPS Overseas Research Fellowships. PJ reports research support from Abbott Laboratories, Amgen, Inc., AstraZeneca, LP, Daiichi-Sankyo, Inc., Eisai, Inc., GlaxoSmithKline, Merck & Co., Inc., Regeneron Pharmaceuticals, Inc., Roche Diagnostics Corporation, Siemens Healthineers, Takeda Global Research and Development Center, and Waters Technologies Corporation, and consulting fees from Roche Diagnostics Corporation. MT reports no disclosures. PS reports research grants and honoraria for speakers bureau- Amgen and Pfizer. ACK reports grants and personal fees from Abbott, personal fees from Amgen, personal fees from AstraZeneca, grants and personal fees from Mylan, personal fees from Pfizer, grants from Sanofi, grants from Novartis, personal fees from Bayer, outside the submitted work. ALP was previously employed by Amgen, now employed by Arrowhead Pharmaceutical. HW is employed at Amgen. RPG reports grants from Amgen and Daiichi Sankyo, during the conduct of the study; personal fees from Akcea, grants and personal fees from Amarin, personal fees from American College of Cardiology, grants and personal fees from Amgen, personal fees personal fees from Bristol Myers Squibb, personal fees from CVS Caremark, grants and personal fees from Daiichi Sankyo, personal fees from GlaxoSmithKline, personal fees from Janssen, personal fees from Lexicon, grants and personal fees from Merck, personal fees from Pfizer, personal fees from Servier, outside the submitted work; and Institutional research grant to the TIMI Study Group at Brigham and Women’s Hospital for research he is not directly involved in from Abbott, Amgen, Aralez, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., BRAHMS, Daiichi Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen, MedImmune, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Roche, Takeda, The Medicines Company, Zora Biosciences. MSS reports research grant support through Brigham and Women’s Hospital from Amgen; AstraZeneca; Bayer; Daiichi-Sankyo; Eisai; GlaxoSmithKline; Intarcia; Janssen Research and Development; Medicines Company; MedImmune; Merck; Novartis; Pfizer; Poxel; Quark Pharmaceuticals; Takeda (All >$10,000 per year); Consulting for Amgen; Anthos Therapeutics; AstraZeneca; Bristol-Myers Squibb; CVS Caremark; DalCor; Dyrnamix; Esperion; IFM Therapeutics; Intarcia; Ionis; Janssen Research and Development; Medicines Company; MedImmune; Merck; Novartis (all ≤$10,000 per year except Amgen, Esperion & Ionis); Dr. Sabatine is a member of the TIMI Study Group, which has also received institutional research grant support through Brigham and Women’s Hospital from: Abbott, Aralez, Roche, and Zora Biosciences. DAM reports grants to Brigham and Women’s Hospital from Abbott Laboratories, AstraZeneca, BRAHMS, Daiichi-Sankyo, Eisai, GlaxoSmithKline, Medicines Company, Merck, Novartis, Pfizer, Quark Pharmaceuticals, Roche Diagnostics, Takeda, Zora Biosciences. He has received consulting fees from Aralez, AstraZeneca, Bayer Pharma, InCarda, Novartis, and Roche Diagnostics.
FUNDING
The FOURIER trial was sponsored by Amgen. Reagent support was provided by Abbott Laboratories.
References
- 1.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marston NA, Bonaca MP, Jarolim P, Goodrich EL, Bhatt DL, Steg PG, Cohen M, Storey RF, Johanson P, Wiviott SD, et al. Clinical Application of High-Sensitivity Troponin Testing in the Atherosclerotic Cardiovascular Disease Framework of the Current Cholesterol Guidelines. JAMA Cardiol. 2020;5:1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 4.Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski MJ, Gersh BJ, Rouleau JL, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361:2538–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavender MA, White WB, Jarolim P, Bakris GL, Cushman WC, Kupfer S, Gao Q, Mehta CR, Zannad F, Cannon CP, et al. Serial Measurement of High-Sensitivity Troponin I and Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus in the EXAMINE Trial (Examination of Cardiovascular Outcomes With Alogliptin Versus Standard of Care). Circulation. 2017;135:1911–1921. [DOI] [PubMed] [Google Scholar]


