Abstract
Off-task thought has been found to occur at high rates and is related to impairment in ADHD. However, off-task thought is heterogenous and it remains unclear which specific dimensions of off-task thought are more prevalent in this disorder. It is therefore important to dissociate different aspects of off-task thought in order to better understand the mechanisms underlying impairment. The current study focused on the dimension of constrained (focused) to freely moving off-task thought. Self-report and neurophysiological measures during a computerized attention tasks provided convergent evidence that individuals with ADHD not only have more off-task thought than those without, but also engaged in a greater proportion of freely moving off-task thought than non-ADHD controls. Overall, this work demonstrated differences in both the quantity and type of off-task thought in adults with ADHD. It provides novel insight into both the phenomenology of off-task thought, as well as potential mechanisms underlying impairment in ADHD.
Keywords: ADHD, off-task thought, mind wandering, EEG
1. Introduction
Thought unrelated to the task at hand, or off-task thought, is a ubiquitous mental phenomenon, yet has only recently become subject to intensive empirical study. In many cases, off-task thought is beneficial for things like planning and creativity (Baird et al., 2012; Mooneyham & Schooler, 2013). Yet in other instances, it can be quite maladaptive (e.g., when the frequency is too great or the timing is inappropriate) (McVay & Kane, 2012; Mrazek et al., 2013). Further, higher levels of off-task thought have been tied to an array of psychiatric disorders (Franklin et al., 2017; Hoffmann et al., 2016; Mowlem et al., 2016; Seli et al., 2015; Smallwood, 2013), most notably ADHD (Biederman et al., 2017; Bozhilova et al., 2018; Franklin et al., 2017; Mowlem et al., 2016; Seli et al., 2015; Shaw & Giambra, 1993).
ADHD is a developmental disorder characterized by symptoms of inattention and hyperactivity/impulsivity (American Psychiatric Association, 2013). This disorder emerges in childhood, but impairment persists into adulthood in 40–60% of cases, even when full ADHD diagnostic criteria are not met (Fayyad et al., 2017; Rasmussen & Gillberg, 2000; van Lieshout et al., 2016). Accumulated impairments are substantial, including lower occupational attainment, as well as increased marital conflict, traffic accidents, drug and alcohol use, and comorbid mood and anxiety disorders (Adamou et al., 2013; J. Biederman et al., 1993; Joseph Biederman et al., 2006; Bioulac et al., 2016; R. C. Kessler et al., 2006).
1.1. ADHD and Dimensions of Off-Task Thought
Off-task thought is often referred to as “mind-wandering” in the broad literature. However, due to current definitional debates and a general disagreement about what the term “mind-wandering” refers to (see Christoff et al., 2016, 2018; Seli, Kane, Metzinger, et al., 2018; Seli, Kane, Smallwood, et al., 2018 for definitional debates), we use the more specific term “off-task thought” to encompass thought that is unrelated to the task at hand. Regardless of the term used, a majority of work investigating off-task thought commonly measures the construct as a homogenous experience and focus on quantifying the frequency of off-task thought (e.g., how often does your attention shift away from a task). However, we know that not all thought is alike and this approach misses qualitative differences (e.g., qualities or dimensions of thought such as content or movement) that can help clarify how off-task thought relates to impairment in psychopathologies (Christoff et al., 2016; Kam et al., 2021; Mills et al., 2018, 2021; Smith et al., 2021).
In the context of ADHD, off-task thought is a paramount feature that influences impairment in work/school, life skills, and interpersonal relationships (Biederman et al., 2019; Bozhilova et al., 2018, 2020; Franklin et al., 2017; Mowlem et al., 2016; Seli et al., 2015; Shaw & Giambra, 1993). Although it is clear that those with ADHD engage in more off-task thought that those without (Biederman et al., 2017, 2019; Bozhilova et al., 2021; Franklin et al., 2017; Mowlem et al., 2016; Seli et al., 2015; Shaw & Giambra, 1993) the nature of off-task within this population and whether it differs only quantitatively (i.e., increased frequency) or also qualitatively (i.e., is driven by distinct aspects of off-task thought) from typically developing populations is under studied. The few studies that have investigated qualities of off-task thought in ADHD have focused on the dimension of deliberate vs. automatic thought (Arabacı & Parris, 2018; Franklin et al., 2017; Seli et al., 2015). This work has found that those with ADHD engage in more automatic off-task thought than those without ADHD (Arabacı & Parris, 2018; Seli et al., 2015) and the degree to which they do this is related to functional impairment (Franklin et al., 2017). This work, although limited in scope, starts to suggest that off-task thought in ADHD may have distinct qualitative features that merit further investigation.
Because investigation of specific dimensions of off-task thought has rarely been applied to empirical studies of disorders like ADHD, a new opportunity exists to clarify cognitive and neurophysiological mechanisms in this population. Doing so has the translational potential to both (a) increase understanding of the basic cognitive mechanisms of off-task thought in general, and (b) improve understanding of ADHD, possibly contributing to identification of new targets for intervention for this disorder. The current study contributed to these goals by using a neuroscientifically-informed model proposed by Christoff et al. (2016) that captures the potentially multifaceted nature of off-task thought. This model defines thought as varying on two dimensions: (a) the extent to which thought is deliberately constrained (goal-directed) and (b) the extent to which thoughts are automatically constrained (from sources like affect, habits, or sensory salience). When both types of constraints are low, the framework predicts that thoughts should be more unguided and freely moving (e.g., switching from topic to topic) and less constrained (e.g., focused on a single train of thought). Freely moving thought ebbs, flows, and switches topics (e.g., thinking about the uncomfortable chair you’re sitting in, wondering what you’ll eat later, remembering a funny thing someone said earlier). In contrast, constrained thoughts are relatively stable and fixated on a specific topic (e.g., going over what you’ll say in an upcoming meeting). Although previous work has focused on how ADHD relates to the off-task dimension, Christoff et al. (2016) would predict that it may not be off-task alone, but rather that the off-task thought has a specific “freely-moving” quality to it.
This freely moving to constrained dimension is of interest because ADHD is marked by enhanced attentional variability (i.e., a lack of attentional focus) during externally-focused attention (e.g., during cognitive tasks) (Huang-Pollock et al., 2012; Kuntsi & Klein, 2012). Hypotheses suggest individuals with ADHD may have difficulty stabilizing attention (i.e., maintaining attentional focus) during off-task thought as well (Christoff et al., 2016; Smallwood, 2013), but no work has investigated attentional variability/freedom of thought movement during internal cognition. Understanding the fluctuation (or lack thereof) of internalized attention in ADHD is important not only for gaining a greater understanding of attentional impairment in this disorder, but also for providing a framework for designing and implementing interventions that target the mechanisms driving aspects of attention that may be problematic for the individual.
1.2. Measuring Freely Moving Thoughts
Most literature on off-task thought has relied on self-report online experience sampling (Smallwood & Schooler, 2015). However, online experience sampling has rarely been used to distinguish between freely moving versus constrained thought. Recent work suggests such a distinction is possible using carefully framed questions, such as “how freely were your thoughts moving?” (Christoff et al., 2016; Mills et al., 2018). Mills et al. (2018) demonstrated that off-task thought in typically-developing adults is constrained only 40% of the time and freely moving the other 60% of the time, highlighting that off-task thought is not inherently freely moving and that this dimension may be an important source of individual differences.
While self-report measures are valuable and reliable measures for capturing nuances of off-task thought (Schubert et al., 2020; Varao-Sousa & Kingstone, 2019), they do come with limitations such as the impact of probe framing (Weinstein et al., 2018) and probe presentation rate (Seli et al., 2013). Combining subjective self-report measures and objective physiological measurements (e.g., neurophysiology) can aid in providing convergent evidence across measurement types for findings as well as elucidating underlying mechanisms. In the current study, EEG complexity was used to provide additional insight into the nature of off-task thought (Bob et al., 2011; Ibáñez-Molina & Iglesias-Parro, 2014, 2016; Liu et al., 2013; Mölle et al., 1996, 1999). As the name suggests, EEG complexity reflects the complexity, or chaos, of the underlying dynamic neural system (Stam, 2005). While the underlying processes leading to EEG signal complexity are widely debated (e.g., see Tononi et al., 1998), many measures of EEG complexity may reflect the number of cell assemblies (e.g., functional networks of neurons) contributing to the EEG signal, with more assemblies reflecting higher complexity (Elbert et al., 1994; Lutzenberger et al., 1995; Stam, 2005).
Complexity measures have been a valuable tool for evaluating brain dynamics during various attentional states (Bob et al., 2011; Ibáñez-Molina & Iglesias-Parro, 2014, 2016; Iglesias-Parro et al., 2020; Liu et al., 2013; Lutzenberger et al., 1992; Mölle et al., 1996, 1999). Studies have demonstrated greater complexity during rest (where attention is free to wander) (Bob et al., 2011; Liu et al., 2013) and off-task thought (Ibáñez-Molina & Iglesias-Parro, 2014, 2016; Iglesias-Parro et al., 2020) than in focused attention tasks. Constrained thinking tasks (e.g., doing mental arithmetic) are associated with less complexity across all EEG sites as compared to less-constrained thinking tasks (e.g., coming up with as many unusual uses for a credit card as you can) (Mölle et al., 1996, 1999). Further, simulation studies have shown that EEG complexity increases with increased default mode network activity (Ibáñez-Molina & Iglesias-Parro, 2016), a pattern of activation often associated with off-task or more freely moving thought (Christoff et al., 2016; Kucyi, 2018; Mittner et al., 2014). EEG complexity is a non-linear measure of the underlying EEG signal. While other non-linear EEG metrics have been used to investigate aspects of thought/attention (e.g., detrended fluctuation analysis (Irrmischer et al., 2018), EEG complexity is used here as one of the few metrics that has demonstrated the ability to distinguish between constrained and freely moving thought (Mölle et al., 1996, 1999).
1.3. The Current Study
The current study tested whether individuals with ADHD show differences in amount and/or type of off-task thought compared to those without ADHD using self-report, cognitive performance, and EEG measures. Hypotheses were that those with ADHD would 1) self-report more off-task thought overall and a greater proportion of freely moving off-task thought, specifically, 2) show more variable reaction times and more errors on the attention task overall as well as a greater increase in reaction time variability and errors during off vs on-task trials than controls, 3) have a larger increase in EEG-measured complexity during off-task vs. on-task thought as compared to controls, consistent with more freely moving off-task thought. Although the primary goal of this study was to examine group-based differences in freely moving off-task thought, exploratory analyses were also run to investigate whether freely moving thought in ADHD predicted ADHD-related impairment. If so, this would suggest that this aspect of thought could become a potential target for intervention.
2. Methods
2.1. Participant Recruitment
Adults (ages 18–40) were recruited via public advertisements as part of an ongoing study of adults with ADHD. Participants were told they were partaking in a study to better understand attention in those with and without ADHD and were not explicitly told it was a study on off-task thought. Participants provided written informed consent and all procedures were approved by the Institutional Review Board at Oregon Health & Science University.
After an initial screening phone call, enrolled adults reported on their ADHD symptoms. Self-report of current and childhood ADHD symptoms was assessed with a semi-structured interview with a masters-degree level clinician (DSM-5 Adult ADHD Clinical Diagnostic Scale [ACDS] (Adler & Spencer, 2004)) as well as standardized questionnaires (Barkley Adult ADHD Rating Scale (BAARS) and Connors’ Adult ADHD Rating Scale (CAARS)) (Barkley, 2011). Informant measures of clinical symptoms were also obtained for diagnostic assignment (Kooij et al., 2008; Zucker, Morris, Ingram, Morris, & Bakeman, 2002). To further validate past childhood symptoms of ADHD, a parent or former guardian of the participant was asked to complete the BAARS (Barkley, 2011) rating symptoms of the participant during childhood. An additional informant (e.g., spouse or close friend) was also asked to complete the BAARS (Barkley, 2011) rating current ADHD symptoms. This method conforms to best practices for determining validity of ADHD in adults (Sibley et al., 2012).
The Mini International Neuropsychiatric Interview (MINI (Sheehan et al., 1998)), a structured clinical interview, was also administered by a masters-degree-level clinician to assess other psychiatric disorder symptoms.
2.1.1. Exclusion criteria.
Participants were excluded from the current study if they had a self-reported history of neurological impairment such as seizures or head injury with loss of consciousness; a prior diagnosis of intellectual disability, autism spectrum disorder, fetal alcohol syndrome, Tourette’s disorder, PTSD, or psychosis; left-handedness; and any non-stimulant psychotropic medications (with the exception of SSRIs and SNRIs for those with an ADHD assignment). Additional exclusionary criteria determined by a clinical interview and neuropsychological testing included currently experiencing a major depressive episode, meeting criteria for substance use disorder, or having an estimated IQ<80..
2.2. Determining Diagnostic Grouping
In line with DSM-5 criteria, in order to be assigned to the ADHD group, participants needed to have ≥ 5 current inattentive or hyperactivity symptoms, at least mild impairment resulting from these symptoms, as well as evidence that the onset of symptoms occurred prior to the age of 12 (American Psychiatric Association, 2013). Symptom counts were determined using the following rules: (a) at least one reporter (informant or self) must have endorsed ≥ 5 current symptoms on either the BAARS or ACDS; (b) and at least one reporter must endorse ≥ 3 childhood inattentive or hyperactive symptoms also on either the BAARS or ACDS; (c) the participant must report at least mild impairment on the ACDS; (d) for both the child and adult symptom domains, both reporters must report a minimum of 2 symptoms within the inattentive or hyperactive domain on either the BAARS or ACDS; and (e) participants must have a T-score > 65 on at least one ADHD related scale on the CAARS.
To be in the control group, (a) both reporters (participant and informant) must agree that the participant has ≤ 3 total current inattentive and hyperactive symptoms on the ACDS and BAARS (b) and < 3 total childhood inattentive and hyperactive symptoms on the ACDS or BAARS; (c) the participant must report no impairment on the ACDS; and (d) the participant must have T-scores < 60 on all ADHD related scales on the CAARs.
Only participants who fell into ADHD or Control groups were eligible for the study.
2.3. Experimental Procedure
Participants with ADHD taking stimulant medications (51%) were included in the study but were required to be off medication for 24 hours prior to testing. All participants were assessed for state sleepiness (Stanford Sleepiness Scale) (Hoddes et al., 1972) and if they indicated they were considerably tired (denoted as a 5 or higher on the 8 point scale), their visit was rescheduled. Participants were also asked to refrain from recreational drug use and discontinue stimulant medication use for at least 24 hours prior to their visit. Abstinence was confirmed through oral report as well as through a urine screen (iCUP). If the urine screen came back positive, the visit was rescheduled. An exception was made for THC if the participant reported abstinence for the last 24 hours.
2.3.1. Sustained attention to response task (SART).
The SART (Robertson et al., 1997) is a well-established attentional task (Smallwood et al., 2008; Smilek et al., 2010). The task was programmed using Python 2.7. Participants responded with a button press to a pseudorandom series of digits 1–9 and withheld that response to the digit, “3”, appearing 11% of the time. Figure 1 depicts an example of an experimental run. Each digit appeared one at a time for 250 ms followed by a centrally located fixation cross presented for 900–1100 ms. 500 Hz tones (distractors) were presented 400–650ms after the offset of each visual stimuli for 75% of the trials. Participants were told to ignore these tones. The auditory distractors were included to address questions about the stimulus-dependence of off-task thought and are not included in analyses in the current study. A total of 45 blocks were presented, each varying in length from 30–90 seconds. The task was ~60 minutes in duration including short voluntary breaks.
Figure 1.
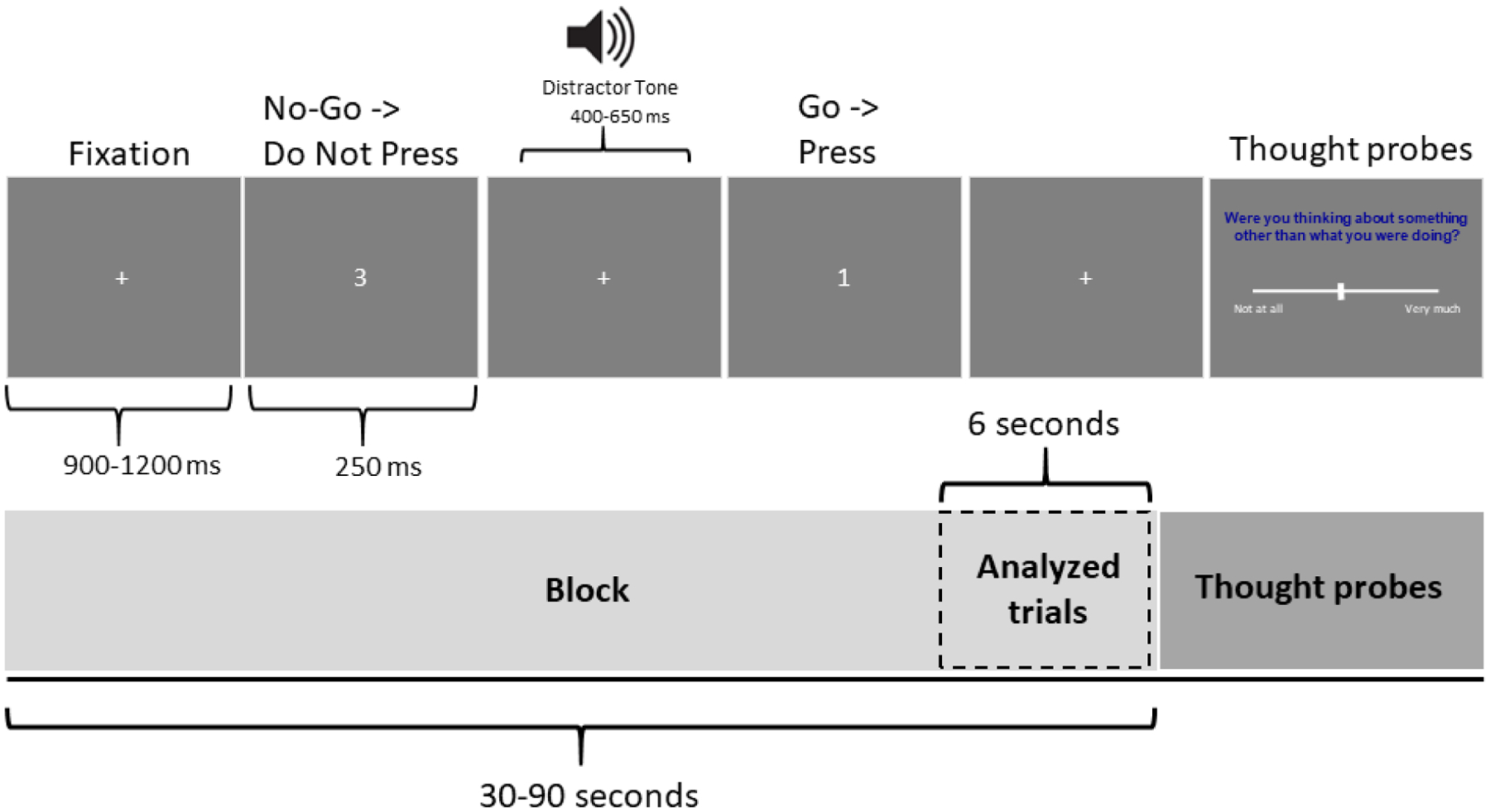
Depiction of a SART experimental run
Performance measures on the SART demonstrated high internal consistency using split-half correlation methods on odd versus even trials for RT, SDRT, and errors of commission. Spearman-Brown Coefficients were 0.99, 0.99, 0.94 respectively. Coefficients remained high when assessing internal consistency within each group as well (all coefficients > 0.92).
2.3.1.1. Thought probes.
At the end of each block, participants answered questions assessing their attentional state (Mills et al., 2018). The following questions were analyzed in the current study:
Were you thinking about something other than what you were doing?
Were your thoughts moving about freely?
Participants responded using a slider bar on a continuous scale from (1) not at all to (7) very much for each question. Prior to the task, participants were provided with an explanation of each of the thought probes with examples. See Supplementary Material for the full script used. After completing the thought probes1, the task continued to the next block. Participants were told they could take short breaks to drink water or stretch after responding to the thought probes and before continuing on to the next block.
No-go trials (i.e. trials with a “3” presented) did not occur within 5 trials (6 seconds) preceding a thought probe. This was in order to be able to analyze only go trials preceding thought probes (more details provided in the analysis section).
2.3.2. Thought probe confidence.
After completing the SART, participants completed a single thought probe confidence rating scale which asked about their confidence in responding to the thought probes. Participants answered each question on a 4-point likert scale with (1) not confident (2) somewhat confident (3) confident and (4) very confident. All participants were included in all primary analyses; however, sensitivity analyses were conducted only including those who rated their confidence as a 2, 3 or 4.2
2.3.1. Functional Impairment.
Functional impairment was assessed using the Weiss functional impairment rating scale self-report (WFIRS-S). This 70-item questionnaire assesses seven domains of functioning (family, work, school, life skills, self-concept, social, and risk). For each of the items, participants indicated how much difficulty they have had in that area using a 4-point scale with responses ranging from never to very often. Global impairment was used in the current study which is an average of scores across the seven domains. All scales have been previously shown to have adequate internal consistency (Cronbach’s alpha = 0.84–0.93) as did the global impairment score (average of all scales) (Cronbach’s alpha = 0.96). Construct, predictive, and discriminant validity have also all been previously documented (Canu et al., 2016; Weiss et al., 2018).
2.4. EEG Recording and Preprocessing
EEG was recorded during the SART from each adult at a sampling rate of 500 Hz with 96 Ag-AgCl active electrodes using the open source software PyCorder v1.0.9. The electrode array is based on the international 10–20 system centered at Cz. EEG signals were amplified by a BrainVision actiCHamp2 amplifier.
EEG data was analyzed using ERPLAB (Lopez-Calderon & Luck, 2014) and EEGLAB (Delorme & Makeig, 2004) toolboxes. Raw EEG data was referenced offline to the average of all channels. EEG signals were filtered using an IIR filter with a bandwidth of .01–50 Hz. Eye artifacts were removed by independent component analysis (Jung et al., 2000). Epochs were time-locked to the onset of the go-correct visual stimuli. The sampling epoch for each trial was 1,200 ms, including a 200 ms pre-stimulus period that was used to baseline correct the epoch. Trials were discarded from the analyses if they contained baseline drift or if the amplitude of movement artifacts was ± 90 μV. Individual channels responsible for rejecting greater than 20% of trials were interpolated using EEGLABs interpolation function.
2.5. Data Analysis
2.5.1. Determining off-task and on-task blocks.
Responses to thought probes were on a continuous scale in order to reduce framing effects such as acquiescence and satisficing (Weinstein, 2018; Weinstein et al., 2018); however, categorization was necessary in order to complete analyses which required a dichotomous assignment. This method is consistent with other studies (Christoff et al., 2009; Kam et al., 2021; Kirschner et al., 2012; Mills et al., 2018; Qin et al., 2011). Each block was categorized as off-task or on-task based on the participant’s response to the first thought probe which asked “were you thinking about something other than what you were doing?” Blocks preceding responses of 1–3.5 on that thought probe were categorized as on-task blocks. Blocks preceding responses of 4.5–7 on that thought probe were categorized as off-task blocks. Blocks preceding responses between 3.5 and 4.5 were not categorized due to the ambiguous nature of a response that lies in the middle of this scale similar to prior studies (Christoff et al., 2009; Mills et al., 2018). To measure frequency of off-task thought, the percentage of blocks reported as off-task were calculated.
For off-task blocks, responses to the thought probe “were your thoughts moving about freely?” were also dichotomized. Responses between 1 and 3.5 were coded as constrained off-task thought, while responses between 4.5 and 7 were coded as freely moving off-task thought. For each participant, the percentage of self-reported constrained and freely moving off-task though were calculated.
Trials that occurred 6 seconds (5 trials) prior to a thought probe were used in behavioral and EEG analyses. While many other studies tend to use a slightly larger time window (e.g., 10–20 seconds), this is often to balance capturing the reported attentional state with the need to have adequate numbers of trials for reliable EEG/ERP analysis (Baird et al., 2014; Kam et al., 2012, 2021; Smallwood et al., 2008). However, there is no such restriction for EEG complexity analysis, rather reliability of complexity measures is dependent on the number of data points in the time-series with a recommended low limit of 128 time points. In the current study, we have 500 time points per epoch (1 second sampled at 500 Hz) (Lutzenberger et al., 1995). Since it is acknowledged that a smaller time window would more reliably capture the subsequent attentional state (Kam et al., 2011; Smallwood et al., 2008), a slightly smaller time window was used here. Trials that occurred in an off-task block were considered off-task trials while trials that occurred within an on-task block were considered on-task trials (Kam et al., 2012, 2013, 2021; Smallwood et al., 2008). See Figure 1 for a depiction.
2.5.2. Task performance.
Mean reaction time (RT), standard deviation of reaction time (SDRT; a measure of reaction time variability), errors of omission, and errors of commission were calculated for the overall task. Additionally, RT, SDRT, and errors of omission were also calculated separately for off-task and on-task trials. Errors of commission could not be calculated for off-task and on-task trials since there were no no-go stimuli within the 6 seconds prior to the thought probes.
2.5.3. EEG Complexity.
EEG segments were averaged and the 200 ms baseline was not included when calculating complexity values. Complexity was calculated for off-task and on-task trials separately. Because complexity is sensitive to signal noise (Skinner et al., 1994; Stam, 2005), 30 epochs were randomly selected from each condition for each subject to ensure more similar signal-to-noise ratio in the signals and as has been used in other work (Müller & Lindenberger, 2012).
Complexity values were calculated with the Skinner’s algorithm to calculate pointwise correlation dimension (PD2) (Skinner et al., 1992, 1994) using the Dataplore software package (Datan Software and Analysis GmbH, Teltow, Germany). PD2 is a mathematical measure derived from non-linear system theory that has frequently been used to measure overall complexity of EEG brain dynamics, particularly during various attentional states (Lutzenberger et al., 1992; Mölle et al., 1996, 1999). First the system dynamics were reconstructed with the time delay (τ) of 1 (2 ms) consistent with similar work (Müller & Lindenberger, 2012). The maximum embedding dimension was set to 12 (Aftanas et al., 1998). The dimensionality of the resulting attractor was calculated using the following formula: PD2(i) = logC(r,i)/log(r). The pointwise correlation integral (C(r,i)) was be calculated based on:
where r is the radius of the state space neighborhood around x, and are the state space coordinates with the delay τ, N is the length of the signal, and θ is the Heavyside function defined as:
The PD2 value is the dimension of an attractor of the time-series which reflects the system’s dynamic complexity. A PD2 value was calculated for the signal generated from each electrode across the scalp and an average was created to reflect global complexity (i.e., complexity across the entire scalp) (Ibáñez-Molina & Iglesias-Parro, 2016).
2.5.4. ADHD Symptom Counts.
Symptom counts were determined by combining participant ratings and informant ratings on the BAARS using an “OR” algorithm such that a symptoms was counted if either the participant OR the informant reported it as present (Pelham et al., 2005). The BAARS has high internal consistency for self-report (Cronbach’s alpha = 0.92) and good interobserver agreement (Cronbach’s alpha = 0.67–0.70) as well as high test-retest reliability over a 2–3 week period (Cronbach’s alpha = 0.75) (Barkley, 2011).
2.6. Statistical Analysis
2.6.1. Behavioral data.
To determine whether those with ADHD engaged in a larger proportion of freely moving to constrained off-task thought as compared to controls, the percentage of constrained and freely moving off-task thoughts were submitted to a 2×2 linear mixed model with off-task thought type (constrained vs. freely moving) as the within-subjects variable and group (ADHD vs. control) as the between-subjects variable.
2.6.2. EEG data.
To determine whether those with ADHD engaged in a larger proportion of freely moving off-task thought than control subjects, complexity values were submitted to a 2×2 linear mixed model with attentional state (off-task vs. on-task) as the within-subjects variable and group (ADHD vs. control) as the within-subjects variable.
2.6.3. Assessing the relationship between EEG and self-report measures.
In order to gain further insight into the relationship between EEG complexity and aspects of off-task thought, linear regressions were used with EEG complexity (regardless of attentional state) as the independent variable and percentage of off-task thought or percentage of freely-moving thought as the dependent variable.
2.6.4. Predicting impairment and symptom severity.
To determine the relationship between off-task thought and impairment in ADHD, linear regression analyses were used with the measurement of off-task thought (percentage off-task or percentage freely moving and EEG complexity) as the independent variable and ADHD impairment or symptom severity (global score from the WFIRS-S or total ADHD symptoms) as the dependent variable. Four separate regression models were run: 1) frequency of off-task thought predicting impairment 2) frequency of off-task thought predicting symptom severity; 3) frequency of freely moving thought and EEG complexity predicting impairment; 4) frequency of freely moving thought and EEG complexity predicting symptom severity. These regressions were run within the ADHD group to specifically examine whether within-group heterogeneity in symptom severity is related to differences in off task thought.
2.6.5. Missing data.
Participants needed 30 clean trials in order to calculate complexity measures. 11 participants were missing off-task complexity data (nADHD = 5; nControl = 7) and 14 were missing on-task complexity data (nADHD = 8; nControl = 6). Of those 11 and 14 participants, 5 participants never reported being on-task (nADHD =1; nControl = 4) and 3 participants never reported being off-task (nADHD =3) and were therefore missing behavioral data in the on- or off-task conditions, respectively. Linear mixed models were used here due to their ability to better accommodate missing data using pairwise (as opposed to listwise deletion).
3. Results
3.1. Participants
79 individuals participated in this study (40 controls, 39 ADHD). 82.3% of the participants identified as being Caucasian, 8.9% as Multiracial, 6.3% as Asian, 1.3% as Black, and 1.3% as Pacific Islander. Additionally, 6.3% identified as Hispanic. Distribution of race and ethnicity did not differ between ADHD and control groups. See Table 1 for other participant demographics and clinical scores.
Table 1:
Demographic information and clinical scores
| Control | ADHD | F | Effect Size (95% CI) | |
|---|---|---|---|---|
| n | 40 | 39 | ||
| Age (years) | 28.67 (4.97) | 29.71 (5.69) | 0.72 | 0.20 (−1.40 – 3.48) |
| IQ | 116.50 (11.33) | 111.44 (14.37) | 3.03 | 0.40 (−10.85 – 0.73) |
| Sex (male:female) | 18:22 | 20:19 | X2=0.31 | |
| Education (years) | 17.18 (2.67) | 15.87 (2.41) | 5.18* | 0.51 (−2.44 – −0.16) |
| Sleepiness | 1.63 (0.67) | 1.92 (0.62) | 4.20* | 0.46 (0.009–.59) |
| Inattention Symptoms | ||||
| ACDS | 0.13 (0.52) | 5.54 (2.65) | 156.17** | 2.90 (4.55–6.28) |
| BAARS (self-report) | 0.10 (0.38) | 5.26 (2.22) | 209.43** | 3.30 (4.45–5.87) |
| BAARS (informant report) | 0.51 (1.43) | 4.05 (2.78) | 49.54** | 1.64 (2.54 – 4.54) |
| Hyperactivity/impulsivity Symptoms | ||||
| ACDS | 0.05 (0.32) | 4.16 (2.70) | 88.95** | 2.19 (3.24 – 4.98) |
| BAARS (self-report) | 0.28 (0.51) | 4.15 (2.37) | 102.56** | 2.31 (3.12 – 4.64) |
| BAARS (informant report) | 0.51 (1.48) | 2.84 (2.56) | 21.81** | 1.09 (1.27 – 3.17) |
| Other Psychiatric Disorders | ||||
| Major Depressive Disorder (met criteria:did not meet criteria) | 8:32 | 22:17 | X2=11.11** | |
| Generalized Anxiety Disorder (met criteria:did not meet criteria) | 1:39 | 7:32 | X2=5.18* | |
| Medication Use | ||||
| Prescribed Stimulant Medication (yes:no) | 0:40 | 21:18 | X2=29.34** | |
| Prescribed SSRI/SNRI (yes:no) | 0:40 | 2:37 | X2=2.11 |
p < .05;
p < .001
ACDS = Adult ADHD Clinical Diagnostic Scale; BAARS = Barkley Adult ADHD Rating Scale; SSRI = selective serotonin reuptake inhibitor; SNRI = serotonin norepinephrine reuptake inhibitor
All symptom counts are for current symptoms
Sleepiness measured from the Stanford Sleepiness Scale and ranged from 1 (wide awake) to 4 (somewhat foggy)
3.2. Task Performance
As expected, on the entire task (regardless of on/off-task report) those with ADHD had significantly more variable reaction time, more errors of commission and omission, as well as poorer accuracy. See Table 2. Of note, these results did not change when adding off-task thought frequency as a covariate.
Table 2.
Task performance
| Control | ADHD | F | Effect Size (95% CI) | |
|---|---|---|---|---|
| RT (ms) | 365.61 (38.81) | 362.93 (47.85) | 0.07 | 0.06 (−22.28–16.92) |
| SDRT (ms) | 79.13 (22.54) | 88.94 (26.60) | 3.10† | 0.40 (1.28 – −20.91) |
| errors of commission (%) | 3.82 (1.55) | 4.81 (1.62) | 7.50** | 0.62 (0.27 – 1.70) |
| errors of omission (%) | 1.81 (3.08) | 4.17 (6.79) | 3.99* | 0.45 (0.006 – 4.72) |
| accuracy (%) | 94.36 (4.18) | 91.02 (7.63) | 5.84* | 0.54 (−6.10 – −0.59) |
| Off-task trials only | ||||
| RT | 365.22 (48.04) | 350.13 (48.04) | 1.83 | 0.31 (−38.27 – 6.44) |
| SDRT | 34.94 (15.73) | 37.61 (22.03) | 0.36 | 0.14 (0.46 – 14.54) |
| errors of omission | 2.17 (4.11) | 6.23 (9.04) | 6.08* | 0.58 (0.45 – 7.15) |
| accuracy | 97.83 (4.11) | 93.77 (9.04) | 6.08* | 0.58 (−7.15 – −0.45) |
| On-task trials only | ||||
| RT | 376.14 (46.95) | 365.82 (56.50) | 0.78 | 0.20 (−40.36 – 8.01) |
| SDRT | 26.83 (13.26) | 29.22 (19.20) | 0.41 | 0.14 (−2.47 – 14.11) |
| errors of omission | 1.33 (2.32) | 3.56 (6.01) | 4.85* | 0.49 (3.00 – 39.16) |
| accuracy | 98.67 (2.32) | 96.44 (6.01) | 4.85* | 0.49 (−39.16 – −3.00) |
p < 0.1
p < .05;
p < .01
A series of 2 attentional state (off-task vs. on-task trials) by 2 group (ADHD vs. control) linear mixed model revealed significant attentional state effects for RT (F(1,72.17) = 11.64, p = 0.001), SDRT (F(1,76.01) = 8.92, p = 0.004), and errors of omission (F(1,73.77) = 11.14, p = 0.001). Faster RTs, larger SDRTs, and more errors of omission occurred during off-task trials. There was main effect of group for errors of omission (F(1,68) = 6.46, p = 0.01). Those with ADHD made more errors of omission than those without ADHD. There were no other main effects of group (all ps > 0.16) and no group × attentional state interactions were significant (all ps > 0.11).
3.3. Thought probes
The number of blocks removed from analyses (responses that occurred between 3.5 and 4.5) were minimal. On average, 7 blocks were removed per participant for the thought probe assessing off-task thought and 3 blocks were removed per participant for the thought probe assessing freely moving thought. The number of blocks removed for each of the thought probes did not differ between ADHD and control groups (all ps > 0.76).
As expected, those with ADHD reported more off-task thought on the SART (M = 59.77% of trials) than controls (M = 44.62% of trials) (F(1,76) = 6.40, p = 0.01). A 2 attentional state (constrained blocks vs. freely moving blocks) by 2 group (ADHD vs. control) linear mixed model was run to determine whether adults with ADHD engaged in proportionally more freely moving off-task thought than controls (group × attentional state interaction). A main effect of attentional state demonstrated that all participants reported engaging in more freely moving than constrained off-task thought (F(1,73.00) = 51.45, p < 0.001). This was qualified by a group × attentional state interaction (F(1,73.00) = 4.63, p = .04). While both the control and ADHD group demonstrated more freely moving than constrained thought, this effect was of greater magnitude in the ADHD group (F1,38) = 71.50, p < 0.001, d = 5.86) than in the control group (F1,35) = 8.67, p = 0.006, d = 2.71). These results support the hypothesis that those with ADHD engaged in a greater proportion of freely moving off-task thought than controls. See Figure 2 for a depiction of the results.
Figure 2.
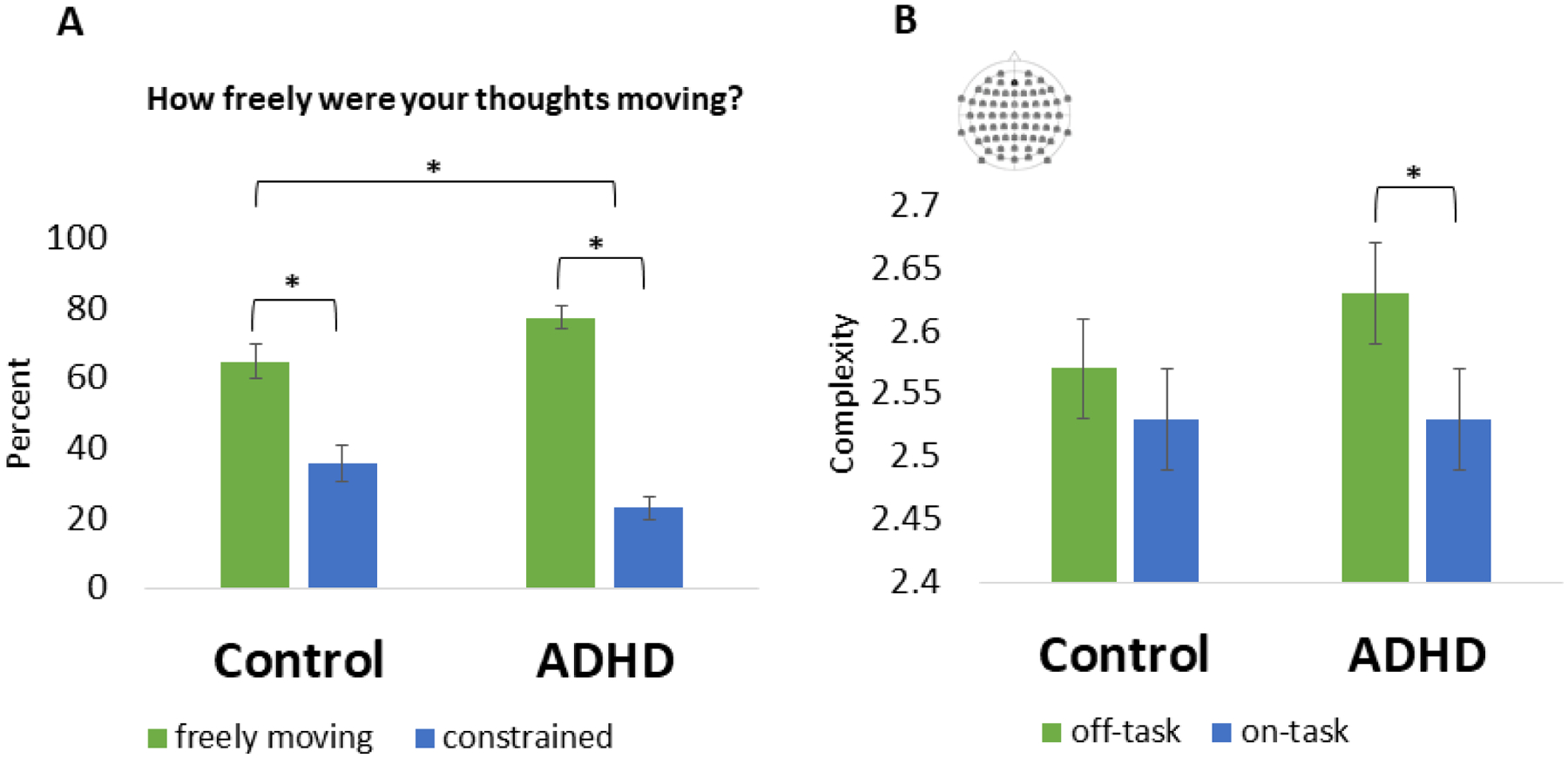
(A) Bar graph representing the percentage of thought probes responded to as either freely moving of constrained for Control and ADHD groups. (B) Bar graph of complexity scores during off-task and on-task trials for Control and ADHD groups. Complexity was calculated across the entire scalp. The distribution of the electrodes is depicted in the upper left corner.
3.4. Complexity
3.4.1. SART.
2 attentional state (off-task vs. on-task) by 2 group (ADHD vs. control) linear mixed models were used to determine whether the EEG complexity for off-task and on-task trials differed between those with and without ADHD during the SART.
As expected, there was a significant effect of attentional state (F(1,59.77) = 10.04, p = 0.002, d = 0.35) where complexity was greater during off-task than on-task trials. There was also an interaction between group and attentional state (F(1,59.77) = 4.96, p = 0.03). In line with the hypothesis, the attentional state effect for the control group did not reach significance (F(1,27.65) = 0.46, p = 0.50, d = 0.20), whereas in the ADHD group, complexity while off-task was greater than complexity while on-task (F(1,31.62) = 10.79, p = 0.002, d = 0.48). See Figure 3 for a depiction of results.
3.5. Relationships across variables
3.5.1. Complexity and thought probes.
EEG complexity regardless of attentional state (i.e., EEG complexity over the entire task) was positively related to the percentage of time participants reported being off-task (β = 0.28, p = 0.02) as well as the percentage of time they reported being engaged in freely moving thought (β = 0.29, p = 0.01).
3.5.1. ADHD impairment and off-task thought.
Within the ADHD group, neither frequency of off-task thought nor freely moving off-task thought predicted ADHD related impairment as measured on the WFIRS-S (all ps > 0.22).
However, frequency of off-task thought marginally predicted ADHD symptom severity (β = 0.31, p = 0.06). For freely moving thought, the overall model for the multiple regression was not significant (F, (1,35) = 2.39, p = 0.11). See Table 3 for a full output of these models.
Table 3.
Off-task thought frequency, freely moving thought frequency, and EEG complexity prediction of impairment and symptom severity
| β | p | F | df | p | R2 | |
|---|---|---|---|---|---|---|
| Global Impairment | ||||||
| Off-task frequency | −0.20 | 0.22 | 1.53 | 1,37 | 0.22 | 0.04 |
| Global Impairment | ||||||
| Overall model | 0.60 | 2,35 | 0.55 | 0.04 | ||
| Freely moving frequency | −0.12 | 0.31 | ||||
| EEG complexity | 0.07 | 0.70 | ||||
| ADHD symptom severity | ||||||
| Off-task frequency | 0.31 | 0.06 | 3.90 | 1,37 | 0.06 | 0.10 |
| ADHD symptom severity | ||||||
| Overall model | 2.39 | 2,35 | 0.11 | 0.13 | ||
| Freely moving frequency | 0.24 | 0.14 | ||||
| EEG complexity | 0.25 | 0.14 |
4. Discussion
Frequency of off-task thought is higher in ADHD than typical development (Franklin et al., 2017; Mowlem et al., 2016; Seli et al., 2015; Shaw & Giambra, 1993); however, clarifying whether off-task thought is qualitatively different in ADHD as well is relatively poorly studied. The current work aimed to determine whether those with ADHD engage not only in quantitatively more off-task thought, but also qualitatively different (i.e., the features of off-task thought are different). Specifically focusing on if those with ADHD would engage in proportionally more freely moving off-task thought than those without. Consistent with our hypotheses, results of both self-report and neurophysiological measures confirmed that adults with ADHD engage in more freely moving off-task thought than controls. This work demonstrates for the first time that ADHD-related difficulties maintaining focused attention are seen not just in externally-focused attention, but also in internally-focused attention as reflected by an increase in freely moving off-task thought.
As expected, those with ADHD performed worse on the SART than controls (Willcutt et al., 2005) and all participants performed more poorly during off- vs. on-task trials (Bozhilova, et al., 2020; McVay & Kane, 2009, 2012). However, hypotheses that off-task thought would more negatively affect cognitive performance in the ADHD group were not supported. Current findings may be due to the relatively low attentional demand of the task as off-task thought has been shown to differentially impact performance in ADHD in studies employing a higher load task (Bozhilova et al., 2020, 2021). Future work should continue to investigate the role of off-task thought on task performance measures in ADHD.
Although results confirm different patterns of off-task thought in those with ADHD as compared to typically developing adults, more research is needed to understand whether this is an epiphenomenal feature of the disorder or whether it is directly related to core features of the disorder. Our own results did not support strong associations between off-task thoughts and ADHD symptoms or ADHD-related impairment. Impairment ratings, in particular, were based only on self-report and use of other informants to assess impairment will be important. Another possibility is that the patterns of thought observed here reflect a type of cognitive strength in ADHD and future studies focused on whether this type of thought supports increased creativity or other positive traits in ADHD will also be important.
4.1. Contributions to the field of off-task thought
Current findings of the percentage of off-task thought engaged in by control participants (~45%) is consistent with lab-based and experience-sampling studies with off-task thought occurring ~50% of the time (Killingsworth & Gilbert, 2010; Smallwood & Schooler, 2015). Similarly, although a more limited body of research. Frequency of off-task thought in ADHD in the current study (~60%) is comparable with what is reported in the literature (50–70%) (Bozhilova, et al., 2020; Van den Driessche et al., 2017).
The relative proportions of freely moving and constrained off-task thought in typically-developing adults seen in the lab for the current study is consistent with prior work using probes in daily life (Mills et al., 2018). We further demonstrate that, at least in the lab, this effect is even larger in ADHD. Findings add to an extremely limited number of studies directly examining the freely moving nature of off-task thought and suggest that off-task thought is largely freely moving under both lab-based and real-world conditions. Despite off-task thought being largely encompassed by freely moving thought (~70% of the time) the remaining 30% of reports were those of constrained thoughts. Restricting off-task thought to be defined as freely moving thought would mean misclassifying or neglecting to capture 30% percent of off-task thought.
The current findings also add to the limited body of work suggesting that specific aspects of off-task thought are more prominent in ADHD. Other studies, although not focused on the constrained to freely moving dimension of thought, have examined whether off-task thought in ADHD is automatic or deliberate and have found that those with ADHD engage in more automatic/unintentional off-task thought than those without ADHD (Arabacı & Parris, 2018; Seli et al., 2015). Clarifying and classifying specific qualities of off-task thought will continue to be a critical step for understanding the relationship between thought and psychopathology.
4.2. Limitations & Future Directions
One potential issue with the thought probe method is that participants become aware of the content that they are being evaluated on which could result in a bias in their responses. With the current experimental design, it is not possible to eliminate this potential bias; however, the convergence of self-report with physiology is reassuring. Identifying non-self-report-based measures of specific aspects of off-task thought (e.g., activation of the medial temporal lobe subcomponent of the default mode network (Christoff et al., 2016)) can inform future studies and potentially help mitigate participant reporting biases.
The neural mechanisms driving EEG complexity measures are widely debated (Tononi et al., 1998), however; studies strongly suggest that this metric can be used to determine between constrained and freely moving attentional states (Bob et al., 2011; Ibáñez-Molina & Iglesias-Parro, 2014, 2016; Iglesias-Parro et al., 2020; Liu et al., 2013; Mölle et al., 1996, 1999). In the current study, EEG complexity was related to both occurrence of off-task thought as well as freely moving thought calling into question whether this is a measurement that reflects off-task thought in general, or freely moving thought specifically. Because a majority of off-task thought IS freely moving, it is difficult to disentangle these two dimensions in the current study. However, if EEG complexity merely reflected off-task thought, we would expect to see increased complexity during off-task thought in the control group as well as the ADHD group, which we did not find. Rather, the complexity findings parallel the behavioral findings suggesting increased freely moving off-task thought in ADHD. In order to further solidify this findings, future work should use other neurophysiological metrics that might reflect freely moving thought (e.g., alpha power variability (Kam et al., 2021) as well as attentional fluctuations (e.g., long-range temporal correlation (Irrmischer et al., 2018).
This study focused on a single dimension of off-task thought: freely moving to constrained. However, thought does not vary along a single dimension and future work should consider investigating how multiple dimensions of thought (e.g., freely moving to constrained and deliberate to automatic) interact to contribute to psychopathology both within and beyond ADHD. Future work investigating whether stimulant medication alters the dynamics of off-task thought in those with ADHD will also be informative. The current work focused specifically on adults with ADHD. This is a critical, and understudied, population; however, this disorder is prevalent across the lifespan. While there is some work investigating off-task thought in children and adolescents with ADHD (Frick et al., 2020; Van den Driessche et al., 2017), additional work is needed to fully understand potential developmental changes in dimensions of off-task thought. The predominantly Caucasian sample in the current study is representative of the greater prevalence of ADHD in White adults as compared to other racial groups (R. Kessler, 2006). However, there are recognized socially-influenced issues of quantifying rates of ADHD (as well as other psychiatric disorders) in minority populations (Chung et al., 2019; Shi et al., 2021). As such, future work should aim to increase the racial diversity of their participants in order to obtain data applicable to all humans rather than a select subset.
4.3. Conclusions
Off-task thought is not only more frequent in those with ADHD, but is also less constrained to a single topic. Both quantitative and qualitative differences are likely to be important for understanding ADHD-related impairment. An increased understanding of how off-task thought occurs in ADHD has the potential to clarify mechanisms underlying psychopathology and provide novel treatment targets to reduce maladaptive thought patterns.
Supplementary Material
Highlights.
Off-task thought occurs at high rates and is related to impairment in ADHD
It’s unclear which dimensions of off-task thought are more prevalent in ADHD
Self-report and neurophysiology were used to measure dimensions of off-task thought
Adults with ADHD engaged in a greater proportion of freely moving off-task thought
Off-task thought is quantitatively and qualitatively different in ADHD
Acknowledgments
The authors would like to thank the participants in this study, as well as Brian Shirley, Libby Nousen, Rebecca Feldman, Evan Challem, Eleanor Battison, Hanna Gustafsson, Greg Simpson, and Rajesh Venkatachalapathy for their support in completing this work.
Funding
This study was funded by the National Center for Advancing Translational Sciences (TL1 TR002371; PI: Alperin). Sample recruitment was partially supported by the National Institute of Mental Health (R44 MH099709; PI: Simpson). Karalunas’ time was supported by K23 MH108656.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Of note, three additional probes were administered evaluating asking “were you thinking about your surroundings?”, “How negative or positive were your thoughts?”, and “How strong was that emotion?”. However, they were not analyzed in the current work.
This included the exclusion of two control participants who were not confident in their responses to the probe asking about freely moving thought. The exclusion of these participants from all analyses did not alter any of the reported results.
References
- Adamou M, Arif M, Asherson P, Aw T-C, Bolea B, Coghill D, Guðjónsson G, Halmøy A, Hodgkins P, Müller U, Pitts M, Trakoli A, Williams N, & Young S (2013). Occupational issues of adults with ADHD. BMC Psychiatry, 13, 59. 10.1186/1471-244X-13-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler L, & Spencer T (2004). The Adult ADHD Clinical Diagnostic Scale (ACDS): Vol. 1.2 New York University School of Medicine. [Google Scholar]
- Aftanas LI, Lotova NV, Koshkarov VI, Makhnev VP, Mordvintsev YN, & Popov SA (1998). Non-linear dynamic complexity of the human EEG during evoked emotions. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 28(1), 63–76. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (Ed.). (2013). Diagnostic and statistical manual of mental disorders: DSM-5 (5th ed). American Psychiatric Association. [Google Scholar]
- Arabacı G, & Parris BA (2018). Probe-caught spontaneous and deliberate mind wandering in relation to self-reported inattentive, hyperactive and impulsive traits in adults. Scientific Reports, 8(1), 4113. 10.1038/s41598-018-22390-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird B, Smallwood J, Lutz A, & Schooler JW (2014). The Decoupled Mind: Mind-wandering Disrupts Cortical Phase-locking to Perceptual Events. Journal of Cognitive Neuroscience, 26(11), 2596–2607. 10.1162/jocn_a_00656 [DOI] [PubMed] [Google Scholar]
- Baird B, Smallwood J, Mrazek MD, Kam JWY, Franklin MS, & Schooler JW (2012). Inspired by distraction: Mind wandering facilitates creative incubation. Psychological Science, 23(10), 1117–1122. 10.1177/0956797612446024 [DOI] [PubMed] [Google Scholar]
- Barkley RA (2011). Barkley Adult ADHD Rating Scale-IV (BAARS-IV). Guilford Press. [Google Scholar]
- Biederman J, Faraone SV, Spencer T, Wilens T, Norman D, Lapey KA, Mick E, Lehman BK, & Doyle A (1993). Patterns of psychiatric comorbidity, cognition, and psychosocial unctioning in adults with attention deficit hyperactivity disorder. The American Journal of Psychiatry, 150(12), 1792–1798. 10.1176/ajp.150.12.1792 [DOI] [PubMed] [Google Scholar]
- Biederman J, Lanier J, DiSalvo M, Noyes E, Fried R, Woodworth KY, Biederman I, & Faraone SV (2019). Clinical correlates of mind wandering in adults with ADHD. Journal of Psychiatric Research, 117, 15–23. 10.1016/j.jpsychires.2019.06.012 [DOI] [PubMed] [Google Scholar]
- Biederman Joseph, Faraone SV, Spencer TJ, Mick E, Monuteaux MC, & Aleardi M (2006). Functional impairments in adults with self-reports of diagnosed ADHD: A controlled study of 1001 adults in the community. The Journal of Clinical Psychiatry, 67(4), 524–540. [DOI] [PubMed] [Google Scholar]
- Biederman Joseph, Fitzgerald M, Uchida M, Spencer TJ, Fried R, Wicks J, Saunders A, & Faraone SV (2017). Towards operationalising internal distractibility (Mind Wandering) in adults with ADHD. Acta Neuropsychiatrica, 29(06), 330–336. 10.1017/neu.2016.70 [DOI] [PubMed] [Google Scholar]
- Bioulac S, Sagaspe P, Micoulaud-Franchi J-A, Altena E, Taillard J, Schro der C, Bouvard M-P, Fabrigoule C, & Philip P (2016). Objective Level of Alertness and Inhibitory Control Predict Highway Driving Impairment in Adults With ADHD. Journal of Attention Disorders. 10.1177/1087054716633751 [DOI] [PubMed] [Google Scholar]
- Bob P, Golla M, Epstein P, & Konopka L (2011). EEG complexity and attentional processes related to dissociative states. Clinical EEG and Neuroscience, 42(3), 175–179. 10.1177/155005941104200306 [DOI] [PubMed] [Google Scholar]
- Bozhilova N, Cooper R, Kuntsi J, Asherson P, & Michelini G (2020). Electrophysiological correlates of spontaneous mind wandering in attention-deficit/hyperactivity disorder. Behavioural Brain Research, 391, 112632. 10.1016/j.bbr.2020.112632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozhilova N, Kuntsi J, Rubia K, Michelini G, & Asherson P (2021). Electrophysiological modulation of sensory and attentional processes during mind wandering in attention-deficit/hyperactivity disorder. NeuroImage: Clinical, 29, 102547. 10.1016/j.nicl.2020.102547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozhilova N, Michelini G, Jones C, Kuntsi J, Rubia K, & Asherson P (2020). Context Regulation of Mind Wandering in ADHD. Journal of Attention Disorders, 108705472095671. 10.1177/1087054720956714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozhilova NS, Michelini G, Kuntsi J, & Asherson P (2018). Mind wandering perspective on attention-deficit/hyperactivity disorder. Neuroscience & Biobehavioral Reviews, 92, 464–476. 10.1016/j.neubiorev.2018.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canu WH, Hartung CM, Stevens AE, & Lefler EK (2016). Psychometric Properties of the Weiss Functional Impairment Rating Scale: Evidence for Utility in Research, Assessment, and Treatment of ADHD in Emerging Adults. Journal of Attention Disorders, 108705471666142. 10.1177/1087054716661421 [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, & Schooler JW (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of the United States of America, 106(21), 8719–8724. 10.1073/pnas.0900234106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Irving ZC, Fox KCR, Spreng RN, & Andrews-Hanna JR (2016). Mind-wandering as spontaneous thought: A dynamic framework. Nature Reviews Neuroscience, 17(11), 718–731. 10.1038/nrn.2016.113 [DOI] [PubMed] [Google Scholar]
- Christoff K, Mills C, Andrews-Hanna JR, Irving ZC, Thompson E, Fox KCR, & Kam JWY (2018). Mind-Wandering as a Scientific Concept: Cutting through the Definitional Haze. Trends in Cognitive Sciences, 22(11), 957–959. 10.1016/j.tics.2018.07.004 [DOI] [PubMed] [Google Scholar]
- Chung W, Jiang S-F, Paksarian D, Nikolaidis A, Castellanos FX, Merikangas KR, & Milham MP (2019). Trends in the Prevalence and Incidence of Attention-Deficit/Hyperactivity Disorder Among Adults and Children of Different Racial and Ethnic Groups. JAMA Network Open, 2(11), e1914344. 10.1001/jamanetworkopen.2019.14344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, & Makeig S (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Elbert T, Ray WJ, Kowalik ZJ, Skinner JE, Graf KE, & Birbaumer N (1994). Chaos and physiology: Deterministic chaos in excitable cell assemblies. Physiological Reviews, 74(1), 1–47. [DOI] [PubMed] [Google Scholar]
- Fayyad J, Sampson NA, Hwang I, Adamowski T, Aguilar-Gaxiola S, Al-Hamzawi A, Andrade LHSG, Borges G, de Girolamo G, Florescu S, Gureje O, Haro JM, Hu C, Karam EG, Lee S, Navarro-Mateu F, O’Neill S, Pennell B-E, Piazza M, … WHO World Mental Health Survey Collaborators. (2017). The descriptive epidemiology of DSM-IV Adult ADHD in the World Health Organization World Mental Health Surveys. Attention Deficit and Hyperactivity Disorders, 9(1), 47–65. 10.1007/s12402-016-0208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin MS, Mrazek MD, Anderson CL, Johnston C, Smallwood J, Kingstone A, & Schooler JW (2017). Tracking Distraction. Journal of Attention Disorders, 21(6), 475–486. 10.1177/1087054714543494 [DOI] [PubMed] [Google Scholar]
- Frick MA, Asherson P, & Brocki KC (2020). Mind‐wandering in children with and without ADHD. British Journal of Clinical Psychology, 59(2), 208–223. 10.1111/bjc.12241 [DOI] [PubMed] [Google Scholar]
- Hoddes E, Zarcone V, & Dement WC (1972). Development and use of Stanford Sleepiness scale (SSS). Psychophysiology, 9(1). [Google Scholar]
- Hoffmann F, Banzhaf C, Kanske P, Bermpohl F, & Singer T (2016). Where the depressed mind wanders: Self-generated thought patterns as assessed through experience sampling as a state marker of depression. Journal of Affective Disorders, 198, 127–134. 10.1016/j.jad.2016.03.005 [DOI] [PubMed] [Google Scholar]
- Huang-Pollock CL, Karalunas SL, Tam H, & Moore AN (2012). Evaluating vigilance deficits in ADHD: A meta-analysis of CPT performance. Journal of Abnormal Psychology, 121(2), 360–371. 10.1037/a0027205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez-Molina AJ, & Iglesias-Parro S (2014). Fractal characterization of internally and externally generated conscious experiences. Brain and Cognition, 87, 69–75. 10.1016/j.bandc.2014.03.002 [DOI] [PubMed] [Google Scholar]
- Ibáñez-Molina AJ, & Iglesias-Parro S (2016). Neurocomputational Model of EEG Complexity during Mind Wandering. Frontiers in Computational Neuroscience, 10. 10.3389/fncom.2016.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias-Parro S, Soriano MF, Prieto M, Rodríguez I, Aznarte JI, & Ibáñez-Molina AJ (2020). Introspective and Neurophysiological Measures of Mind Wandering in Schizophrenia. Scientific Reports, 10(1), 4833. 10.1038/s41598-020-61843-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irrmischer M, Poil S-S, Mansvelder HD, Intra FS, & Linkenkaer-Hansen K (2018). Strong long-range temporal correlations of beta/gamma oscillations are associated with poor sustained visual attention performance. European Journal of Neuroscience, 48(8), 2674–2683. 10.1111/ejn.13672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam JWY, Dao E, Blinn P, Krigolson OE, Boyd LA, & Handy TC (2012). Mind wandering and motor control: Off-task thinking disrupts the online adjustment of behavior. Frontiers in Human Neuroscience, 6. 10.3389/fnhum.2012.00329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam JWY, Dao E, Farley J, Fitzpatrick K, Smallwood J, Schooler JW, & Handy TC (2011). Slow fluctuations in attentional control of sensory cortex. Journal of Cognitive Neuroscience, 23(2), 460–470. 10.1162/jocn.2010.21443 [DOI] [PubMed] [Google Scholar]
- Kam JWY, Dao E, Stanciulescu M, Tildesley H, & Handy TC (2013). Mind Wandering and the Adaptive Control of Attentional Resources. Journal of Cognitive Neuroscience, 25(6), 952–960. 10.1162/jocn_a_00375 [DOI] [PubMed] [Google Scholar]
- Kam JWY, Irving ZC, Mills C, Patel S, Gopnik A, & Knight RT (2021). Distinct electrophysiological signatures of task-unrelated and dynamic thoughts. Proceedings of the National Academy of Sciences of the United States of America, 118(4). 10.1073/pnas.2011796118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R (2006). The Prevalence and Correlates of Adult ADHD in the United States: Results From the National Comorbidity Survey Replication. American Journal of Psychiatry, 163(4), 716. 10.1176/appi.ajp.163.4.716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K, Spencer T, Ustun TB, Walters EE, & Zaslavsky AM (2006). The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. The American Journal of Psychiatry, 163(4), 716–723. 10.1176/ajp.2006.163.4.716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killingsworth MA, & Gilbert DT (2010). A wandering mind is an unhappy mind. Science (New York, N.Y.), 330(6006), 932. 10.1126/science.1192439 [DOI] [PubMed] [Google Scholar]
- Kirschner A, Kam JWY, Handy TC, & Ward LM (2012). Differential Synchronization in Default and Task-Specific Networks of the Human Brain. Frontiers in Human Neuroscience, 6. 10.3389/fnhum.2012.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooij JJS, Marije Boonstra A, Swinkels SHN, Bekker EM, de Noord I, & Buitelaar JK (2008). Reliability, validity, and utility of instruments for self-report and informant report concerning symptoms of ADHD in adult patients. Journal of Attention Disorders, 11(4), 445–458. 10.1177/1087054707299367 [DOI] [PubMed] [Google Scholar]
- Kucyi A (2018). Just a thought: How mind-wandering is represented in dynamic brain connectivity. NeuroImage, 180(Pt B), 505–514. 10.1016/j.neuroimage.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Kuntsi J, & Klein C (2012). Intraindividual variability in ADHD and its implications for research of causal links. Current Topics in Behavioral Neurosciences, 9, 67–91. 10.1007/7854_2011_145 [DOI] [PubMed] [Google Scholar]
- Liu T, Yan N, Chen Y, & Wang J (2013). Multichannel linear descriptors analysis for sustained attention-related electroencephalography: NeuroReport, 24(11), 631–635. 10.1097/WNR.0b013e3283639396 [DOI] [PubMed] [Google Scholar]
- Lopez-Calderon J, & Luck SJ (2014). ERPLAB: An open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience, 8, 213. 10.3389/fnhum.2014.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutzenberger W, Elbert T, Birbaumer N, Ray WJ, & Schupp H (1992). The scalp distribution of the fractal dimension of the EEG and its variation with mental tasks. Brain Topography, 5(1), 27–34. [DOI] [PubMed] [Google Scholar]
- Lutzenberger W, Preissl H, & Pulvermüller F (1995). Fractal dimension of electroencephalographic time series and underlying brain processes. Biological Cybernetics, 73(5), 477–482. [DOI] [PubMed] [Google Scholar]
- McVay JC, & Kane MJ (2009). Conducting the train of thought: Working memory capacity, goal neglect, and mind wandering in an executive-control task. Journal of Experimental Psychology: Learning, Memory, and Cognition, 35(1), 196–204. 10.1037/a0014104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, & Kane MJ (2012). Drifting from slow to “d’oh!”: Working memory capacity and mind wandering predict extreme reaction times and executive control errors. Journal of Experimental Psychology: Learning, Memory, and Cognition, 38(3), 525–549. 10.1037/a0025896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills C, Porter AR, Andrews-Hanna JR, Christoff K, & Colby A (2021). How task-unrelated and freely moving thought relate to affect: Evidence for dissociable patterns in everyday life. Emotion. 10.1037/emo0000849 [DOI] [PubMed] [Google Scholar]
- Mills C, Raffaelli Q, Irving ZC, Stan D, & Christoff K (2018). Is an off-task mind a freely-moving mind? Examining the relationship between different dimensions of thought. Consciousness and Cognition, 58, 20–33. 10.1016/j.concog.2017.10.003 [DOI] [PubMed] [Google Scholar]
- Mittner M, Boekel W, Tucker AM, Turner BM, Heathcote A, & Forstmann BU (2014). When the Brain Takes a Break: A Model-Based Analysis of Mind Wandering. Journal of Neuroscience, 34(49), 16286–16295. 10.1523/JNEUROSCI.2062-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölle M, Marshall L, Lutzenberger W, Pietrowsky R, Fehm HL, & Born J (1996). Enhanced dynamic complexity in the human EEG during creative thinking. Neuroscience Letters, 208(1), 61–64. [DOI] [PubMed] [Google Scholar]
- Mölle M, Marshall L, Wolf B, Fehm HL, & Born J (1999). EEG complexity and performance measures of creative thinking. Psychophysiology, 36(1), 95–104. [DOI] [PubMed] [Google Scholar]
- Mooneyham BW, & Schooler JW (2013). The costs and benefits of mind-wandering: A review. Canadian Journal of Experimental Psychology = Revue Canadienne De Psychologie Experimentale, 67(1), 11–18. 10.1037/a0031569 [DOI] [PubMed] [Google Scholar]
- Mowlem FD, Skirrow C, Reid P, Maltezos S, Nijjar SK, Merwood A, Barker E, Cooper R, Kuntsi J, & Asherson P (2016). Validation of the Mind Excessively Wandering Scale and the Relationship of Mind Wandering to Impairment in Adult ADHD. Journal of Attention Disorders. 10.1177/1087054716651927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrazek MD, Phillips DT, Franklin MS, Broadway JM, & Schooler JW (2013). Young and restless: Validation of the Mind-Wandering Questionnaire (MWQ) reveals disruptive impact of mind-wandering for youth. Frontiers in Psychology, 4, 560. 10.3389/fpsyg.2013.00560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller V, & Lindenberger U (2012). Lifespan differences in nonlinear dynamics during rest and auditory oddball performance. Developmental Science, 15(4), 540–556. 10.1111/j.1467-7687.2012.01153.x [DOI] [PubMed] [Google Scholar]
- Pelham WE, Fabiano GA, & Massetti GM (2005). Evidence-based assessment of attention deficit hyperactivity disorder in children and adolescents. Journal of Clinical Child and Adolescent Psychology, 34(3), 449–476. 10.1207/s15374424jccp3403_5 [DOI] [PubMed] [Google Scholar]
- Qin J, Perdoni C, & He B (2011). Dissociation of Subjectively Reported and Behaviorally Indexed Mind Wandering by EEG Rhythmic Activity. PLoS ONE, 6(9), e23124. 10.1371/journal.pone.0023124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen P, & Gillberg C (2000). Natural outcome of ADHD with developmental coordination disorder at age 22 years: A controlled, longitudinal, community-based study. Journal of the American Academy of Child and Adolescent Psychiatry, 39(11), 1424–1431. 10.1097/00004583-200011000-00017 [DOI] [PubMed] [Google Scholar]
- Robertson IH, Manly T, Andrade J, Baddeley BT, & Yiend J (1997). “Oops!”: Performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia, 35(6), 747–758. [DOI] [PubMed] [Google Scholar]
- Schubert A-L, Frischkorn GT, & Rummel J (2020). The validity of the online thought-probing procedure of mind wandering is not threatened by variations of probe rate and probe framing. Psychological Research, 84(7), 1846–1856. 10.1007/s00426-019-01194-2 [DOI] [PubMed] [Google Scholar]
- Seli P, Carriere JSA, Levene M, & Smilek D (2013). How few and far between? Examining the effects of probe rate on self-reported mind wandering. Frontiers in Psychology, 4. 10.3389/fpsyg.2013.00430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seli P, Kane MJ, Metzinger T, Smallwood J, Schacter DL, Maillet D, Schooler JW, & Smilek D (2018). The Family-Resemblances Framework for Mind-Wandering Remains Well Clad. Trends in Cognitive Sciences, 22(11), 959–961. 10.1016/j.tics.2018.07.007 [DOI] [PubMed] [Google Scholar]
- Seli P, Kane MJ, Smallwood J, Schacter DL, Maillet D, Schooler JW, & Smilek D (2018). Mind-Wandering as a Natural Kind: A Family-Resemblances View. Trends in Cognitive Sciences, 22(6), 479–490. 10.1016/j.tics.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seli P, Smallwood J, Cheyne JA, & Smilek D (2015). On the relation of mind wandering and ADHD symptomatology. Psychonomic Bulletin & Review, 22(3), 629–636. 10.3758/s13423-014-0793-0 [DOI] [PubMed] [Google Scholar]
- Shaw GA, & Giambra L (1993). Task-unrelated thoughts of college students diagnosed as hyperactive in childhood. Developmental Neuropsychology, 9(1), 17–30. 10.1080/87565649309540541 [DOI] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, & Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry, 59 Suppl 20, 22–33;quiz 34–57. [PubMed] [Google Scholar]
- Shi Y, Hunter Guevara LR, Dykhoff HJ, Sangaralingham LR, Phelan S, Zaccariello MJ, & Warner DO (2021). Racial Disparities in Diagnosis of Attention-Deficit/Hyperactivity Disorder in a US National Birth Cohort. JAMA Network Open, 4(3), e210321. 10.1001/jamanetworkopen.2021.0321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley MH, Pelham WE, Molina BSG, Gnagy EM, Waxmonsky JG, Waschbusch DA, Derefinko KJ, Wymbs BT, Garefino AC, Babinski DE, & Kuriyan AB (2012). When diagnosing ADHD in young adults emphasize informant reports, DSM items, and impairment. Journal of Consulting and Clinical Psychology, 80(6), 1052–1061. 10.1037/a0029098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner JE, Molnar M, & Tomberg C (1994). The point correlation dimension: Performance with nonstationary surrogate data and noise. Integrative Physiological and Behavioral Science: The Official Journal of the Pavlovian Society, 29(3), 217–234. [DOI] [PubMed] [Google Scholar]
- Skinner JE, Molnar M, Vybiral T, & Mitra M (1992). Application of chaos theory to biology and medicine. Integrative Physiological and Behavioral Science: The Official Journal of the Pavlovian Society, 27(1), 39–53. [DOI] [PubMed] [Google Scholar]
- Smallwood J (2013). Distinguishing how from why the mind wanders: A process–occurrence framework for self-generated mental activity. Psychological Bulletin, 139(3), 519–535. 10.1037/a0030010 [DOI] [PubMed] [Google Scholar]
- Smallwood J, Beach E, Schooler JW, & Handy TC (2008). Going AWOL in the brain: Mind wandering reduces cortical analysis of external events. Journal of Cognitive Neuroscience, 20(3), 458–469. 10.1162/jocn.2008.20037 [DOI] [PubMed] [Google Scholar]
- Smallwood J, & Schooler JW (2015). The Science of Mind Wandering: Empirically Navigating the Stream of Consciousness. Annual Review of Psychology, 66(1), 487–518. 10.1146/annurev-psych-010814-015331 [DOI] [PubMed] [Google Scholar]
- Smilek D, Carriere JSA, & Cheyne JA (2010). Failures of sustained attention in life, lab, and brain: Ecological validity of the SART. Neuropsychologia, 48(9), 2564–2570. 10.1016/j.neuropsychologia.2010.05.002 [DOI] [PubMed] [Google Scholar]
- Smith AC, Brosowsky NP, Ralph BCW, Smilek D, & Seli P (2021). Re-examining the effect of motivation on intentional and unintentional task-unrelated thought: Accounting for thought constraint produces novel results. Psychological Research. 10.1007/s00426-021-01487-5 [DOI] [PubMed] [Google Scholar]
- Stam CJ (2005). Nonlinear dynamical analysis of EEG and MEG: Review of an emerging field. Clinical Neurophysiology, 116(10), 2266–2301. 10.1016/j.clinph.2005.06.011 [DOI] [PubMed] [Google Scholar]
- Tononi G, Edelman GM, & Sporns O (1998). Complexity and coherency: Integrating information in the brain. Trends in Cognitive Sciences, 2(12), 474–484. [DOI] [PubMed] [Google Scholar]
- Van den Driessche C, Bastian M, Peyre H, Stordeur C, Acquaviva É, Bahadori S, Delorme R, & Sackur J (2017). Attentional Lapses in Attention-Deficit/Hyperactivity Disorder: Blank Rather Than Wandering Thoughts. Psychological Science, 28(10), 1375–1386. 10.1177/0956797617708234 [DOI] [PubMed] [Google Scholar]
- van Lieshout M, Luman M, Twisk JWR, van Ewijk H, Groenman AP, Thissen AJAM, Faraone SV, Heslenfeld DJ, Hartman CA, Hoekstra PJ, Franke B, Buitelaar JK, Rommelse NNJ, & Oosterlaan J (2016). A 6-year follow-up of a large European cohort of children with attention-deficit/hyperactivity disorder-combined subtype: Outcomes in late adolescence and young adulthood. European Child & Adolescent Psychiatry, 25(9), 1007–1017. 10.1007/s00787-016-0820-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varao-Sousa TL, & Kingstone A (2019). Are mind wandering rates an artifact of the probe-caught method? Using self-caught mind wandering in the classroom to test, and reject, this possibility. Behavior Research Methods, 51(1), 235–242. 10.3758/s13428-018-1073-0 [DOI] [PubMed] [Google Scholar]
- Weinstein Y (2018). Mind-wandering, how do I measure thee with probes? Let me count the ways. Behavior Research Methods, 50(2), 642–661. 10.3758/s13428-017-0891-9 [DOI] [PubMed] [Google Scholar]
- Weinstein Y, De Lima HJ, & van der Zee T (2018). Are you mind-wandering, or is your mind on task? The effect of probe framing on mind-wandering reports. Psychonomic Bulletin & Review, 25(2), 754–760. 10.3758/s13423-017-1322-8 [DOI] [PubMed] [Google Scholar]
- Weiss MD, McBride NM, Craig S, & Jensen P (2018). Conceptual review of measuring functional impairment: Findings from the Weiss Functional Impairment Rating Scale. Evidence Based Mental Health, 21(4), 155–164. 10.1136/ebmental-2018-300025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, & Pennington BF (2005). Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry, 57(11), 1336–1346. 10.1016/j.biopsych.2005.02.006 [DOI] [PubMed] [Google Scholar]
- Zucker M, Morris MK, Ingram SM, Morris RD, & Bakeman R (2002). Concordance of self- and informant ratings of adults’ current and childhood attention-deficit/hyperactivity disorder symptoms. Psychological Assessment, 14(4), 379–389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


